Abstract
With refinement in ultrasound technology, detection of fetal structural abnormalities has improved and there have been detailed reports of the natural history and expected outcomes for many anomalies. The ability to either reassure a high-risk woman with normal intrauterine images or offer comprehensive counseling and offer options in cases of strongly suspected lethal or major malformations has shifted prenatal diagnoses to the earliest possible gestational age. When indicated, scans in early gestation are valuable in accurate gestational dating. Stricter sonographic criteria for early nonviability guard against unnecessary intervention. Most birth defects are without known risk factors, and detection of certain malformations is possible in the late first trimester. The best time for a standard complete fetal and placental scan is 18–20 weeks. In addition, certain soft anatomic markers provide clues to chromosomal aneuploidy risk. Maternal obesity and multifetal pregnancies are now more common and further limit early gestation visibility. Other advanced imaging techniques during early gestation in select cases of suspected malformations include fetal echocardiography and magnetic resonance imaging.
Keywords: aneuploidy, early ultrasonography, malformations, nonviability, prenatal diagnosis
Introduction
In the United States, approximately 2 to 3 percent of newborn infants have a major structural malformation (Dolk et al., 2010). By age 5, another 3 percent are diagnosed with a malformation. The etiology is unknown in about two-thirds of cases (Schardein et al., 2000; Wlodarczyk et al., 2011). Importantly, teratogen-induced birth defects, which include those caused by medications, chemicals, viruses, environmental agents, and physical factors, account for a very low percent of all birth defects.
Historically, the first trimester extends through completion of 14 weeks from onset of the last menses, and the second trimester through 28 weeks. The clinical use of trimesters to describe a specific pregnancy is too imprecise, and clinicians designate gestational age using completed weeks and days. The pre-implantation period is the two weeks from fertilization to implantation and has traditionally been called the “all or none” period. The embryonic period is from the fourth through the 10th week from the last menses (two to eight weeks from fertilization). It encompasses the period of organogenesis and is thus the most crucial period with regard to maternal exposures with teratogenic potential. Growth, maturation, and functional development continue after 10 weeks, when certain organs (brain, heart, gonads, hematologic) remain vulnerable.
Since the advent of fetal ultrasonography in the 1960s, the average number of imaging studies per pregnancy has increased. Substantial advances in magnification imaging and signal processing improved the ability to visualize embryonic and early fetal anatomy. Significant practice variation exists in the frequency and performance of ultrasonography during pregnancy. Innovations in imaging, such as echocardiography and magnetic resonance imaging (MRI), have added to early gestation evaluation in select cases.
The abilities to reassure a high-risk woman about normal fetal findings and to offer comprehensive counselling with the option to terminate in cases of strongly suspected lethal or major malformations have shifted prenatal diagnosis to the earliest possible gestational age. The objective of the present report is to review current literature about the diagnostic accuracy of imaging techniques in the detection of birth defects in early gestation. We further explore criteria for nonviability, and types of structural defects visible using a variety of imaging techniques.
Very Early Intrauterine Landmarks and Criteria for Nonviability
Ultrasonography is believed to be safe in early pregnancy when used appropriately (Ultrasonography, 2009). Benefits and limitations of early ultrasonography should be discussed with all patients in the absence of specific indications such as uncertain dating, vaginal bleeding, poor reproductive history, or potential teratogen exposure. Over at least the past two decades, intravaginal ultrasonography and quantitative measurement of maternal serum human chorionic gonadotropin (hCG) levels have been mainstays in the diagnosis and management of early pregnancy problems which may result from an abnormal conceptus or implantation (Bree et al., 1989). For either an intrauterine pregnancy of uncertain viability or a pregnancy of unknown location, the consequence of a false positive diagnosis of nonviability may be dire. Overzealous medical or surgical intervention can eliminate or severely damage a viable intrauterine pregnancy (Doubilet et al., 2013).
The pattern of early intrauterine development, as seen on transvaginal ultrasonography, is fairly predictable (Goldstein et al., 1991). Landmarks in early embryo development are shown in Table 1. The gestational sac, first seen at approximately 5 weeks from the last menses, appears as a small fluid-filled cyst with rounded edges and no visible contents. Much becomes visible at about 6 weeks in the central echogenic portion of the uterus. The yolk sac, an inside circular structure about 3 to 5 mm in diameter, makes its appearance at about 5 ½ to 6 weeks of gestation. The embryonic pole is seen adjacent to the yolk sac by 7 weeks, at which time the heartbeat is viewed as a flickering motion. In general, the most accurate means for determining the estimated due date is by measuring the embryonic pole or crown rump length (CRL).
Table 1.
Early visible intrauterine landmarks according to last menstrual dating
| Menstrual age (wks) | Gestational sac diameter (cm) | Yolk sac | Embryo and heart motion | Crown rump length |
|---|---|---|---|---|
| 5 | 1 | − | − | − |
| 6 | 1.5 | + | + | ± |
| 7 | 2 | + | + | + |
| 8 | 3 | + | + | + |
| 9–11 | 4–5 | + | + | + |
Variations from this early sequence of events are worrisome or, if major, definitive for early pregnancy failure. A diagnosis of early pregnancy failure was defined by the Society of Radiologists Multispecialty Panel (Doubilet et al., 2013) as having any of the following criteria: 1) absence of a visible embryo by the time the gestational sac had grown to a 25 mm diameter, 2) absence of an embryo with heartbeat by two weeks after seeing a gestational sac without a yolk sac, 3) absence of a visible embryo with heartbeat by two weeks after seeing a yolk sac, or 4) absence of cardiac activity by the time the embryo reached at least a 7 mm CRL measurement.
Ultrasonography at 11–14 weeks to screen for certain structural anomalies
Early diagnosis of a wide range of fetal structural anomalies can be suspected on transvaginal, transabdominal, or both examinations as early as 11–14 weeks gestation (Economides, 1999; Timor-Tritsch et al., 2004; Souka et al., 2004; den Hollander et al., 2002; Dugoff et al 2002). Screening for aneuploidy assessment at this time, as recommended by the American College of Obstetricians and Gynecologists, is an excellent time for the earliest detection of certain major anomalies (Ultrasonography, 2009; den Hollander et al., 2002). Several studies offer encouragement about the early diagnosis of major anomalies among fetuses with increased nuchal translucency (3 mm or greater), regardless whether the karyotype is normal or abnormal (Whitlow and Economides, 1998; Ghi et al., 2001; Hyett et al., 1997; Souka et al., 2001; McAuliffe et al., 2005). As the size of the nuchal translucency increases, so does the risk for fetal aneuploidy, other genetic diseases and syndromes, and cardiac or other major malformations.
Rossi and colleagues reviewed the literature about the efficacy of ultrasonography at 11 to 14 weeks gestation to identify structural anomalies (Rossi et al., 2013). Their findings from 19 studies involving 78,002 fetuses revealed a malformation prevalence of 12 per 1,000 pregnancies. Half of all cases with malformations were viewed sonographically. Heart defects were the most common and involved 45% of affected fetuses. Summarized in Table 2 are detection rates of certain fetal malformations between 11 to 14 weeks (Rossi et al., 2013). The highest rate was achieved for neck anomalies (92%), while only one-third of face, genitourinary anomalies, and limb anomalies were viewed. Fetuses with multiple defects were identified more frequently than those with isolated malformations (60% versus 44%). Detection rates were highest with transabdominal and transvaginal imaging techniques combined rather than either alone. Pregnancies at high risk (e.g., diabetes, alcohol, morbid obesity, monochorionic twins, prior affected fetus, antiepileptic medication) had a higher malformation detection rate than in an unselected population (65% versus 50%).
Table 2.
Detection Rates for Certain Fetal Malformations during 11 to 14 week Ultrasound Imaging
| Detection rate | Examples of malformations |
|---|---|
| 100% | Acrania, anencephaly, ectopia cordis, encephalocele |
| 50–99% | Cystic hygroma, double-outlet right ventricular flow, Fallot, gastroschisis, omphalocele, holoprosencephaly, hypoplastic left heart syndrome, limb reduction, mega-cystis, polydactyly, septal defects, transposition of great vessels, valvular disease |
| 1–49% | Spina bifida, hydrocephalus, skeletal dysplasia, facial cleft, Dandy-Walker, aortic coarctation, arthyrogryposis |
| 0% | Corpus callosum agenesia, bladder exstrophy, cyst adenomatoid malformation, cerebellar hypoplasia, duodenal atresia, hydronephrosis, renal agenesis, duplex kidneys, bowel obstruction, extra-lobar sequestration |
Adapted from Rossi et al., 2013
Role of First Trimester Fetal Echocardiography
The traditional time to perform fetal echocardiography is at 18 to 22 weeks gestation (American Institute Ultrasound in Medicine, 2013). However, there are certain circumstances in which an earlier fetal echocardiography may be warranted such as a first-degree relative with a significant congenital heart disease, known or suspected fetal chromosomal abnormality, major extracardiac anomaly, or teratogen exposure. In general, early fetal echocardiography should be reserved for high-risk patients and performed at experienced centers. Regardless of expertise, cardiac anatomy should be reassessed with a second trimester fetal echocardiogram.
Sonographic markers of congenital heart disease that may be detected at the first trimester include increased nuchal translucency, abnormal ductus venosus waveform, and tricuspid regurgitation. The prevalence of major congenital heart disease is known to increase as the nuchal translucency measurement reaches or exceeds the 95th percentile (Khalil and Nicolaides, 2013). In order not to overwhelm resources, experts have recommended an absolute cut-off of 3.0 to 3.5 mm or the 95th to 99th percentile for gestational age be used as an indication for fetal echocardiography (ACOG, 1999; Foy et al, 2013). Universal nuchal translucency screening can identify about 30 percent of fetuses with major congenital heart disease in the general population. (Simpson et al, 2007). Patients found to have increased nuchal translucency at 11 to 13 weeks often prefer fetal echocardiography before 14 weeks rather than waiting one to two months for a standard second trimester study.
The ductus venosus plays a major role in the regulation of the fetal circulation with forward flow throughout the entire cardiac cycle to ensure oxygenated blood reaches the left side of the fetal heart and fetal brain. Absence or reversed a-wave in the ductus venosus waveform has been associated with major fetal heart anomalies (Khalil and Nicolaides, 2013). While ductus venosus evaluation is not a routine screening test, it can increase the detection rate of major congenital heart disease in cases of nuchal translucency >99th percentile from <30 percent to 40 percent (Martinez et al, 2010). Screening for presence of tricuspid regurgitation is also not routine. It can be a normal finding in the first trimester that is usually mild and decreases with gestational age. Severe or persistent tricuspid regurgitation, detected by color flow mapping or Doppler velocimetry, may be a marker for fetal aneuploidy as well as congenital heart disease. It is estimated that 30 to 50 percent of fetuses with significant tricuspid regurgitation, and a normal karyotype will have structural heart anomalies (Persico et al, 2011; Khalil and Nicolaides, 2013).
In addition to sonographic markers and historical factors, unexpected findings at the time of first trimester risk assessment such as major malformations, cystic hygroma, and monochorionic twins are additional risk factors for congenital heart disease (AIUM, 2013). In the past, fetal echocardiography was often postponed until after fetal karyotyping to limit unnecessary studies on patients who might opt to terminate pregnancies with confirmed chromosomal abnormalities. With the availability of noninvasive cell-free fetal DNA analysis from maternal blood, timely use of fetal echocardiography in fetuses without trisomy 21, 18 and 13 can be scheduled.
With experience, major cardiac lesions can be reliably detected prior to 14 weeks gestation. Figure 2 displays atrioventricular septal defects, hypoplastic left heart syndrome, large ventricular septal defects, and chamber disproportion (Huggon et al, 2002). Early cardiac imaging should include standard views of the fetal heart such as a four-chamber view, left and right ventricular outflow tracts, three-vessel-trachea view, aortic and ductal arches, superior and inferior vena cava, and pulmonary veins. (Figure 3) Cardiac imaging has been shown to steadily improve from 10 to 14 weeks gestation and a transvaginal approach can significantly improve visualization (Haak and van Vugt, 2003). It is estimated that a complete fetal cardiac exam is feasible in over half of cases in less than 10 minutes, with a four-chamber view being detected 100% of the time and a three-vessel view visualized in only 55% of cases (Abu-Rustum et al, 2011). Despite its feasibility, imaging in the first trimester is more difficult than at 18 to 22 weeks gestation. Overall, standard cardiac views can be obtained in >75 percent of early echocardiograms compared to >85 percent in those performed at mid-gestation (Moon-Grady et al, 2012).
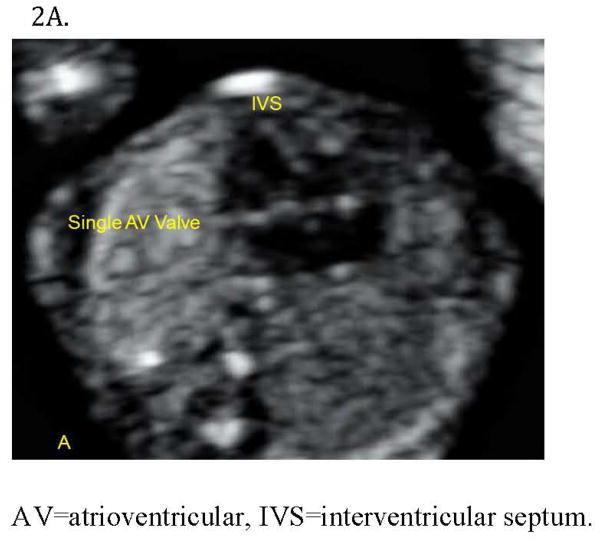
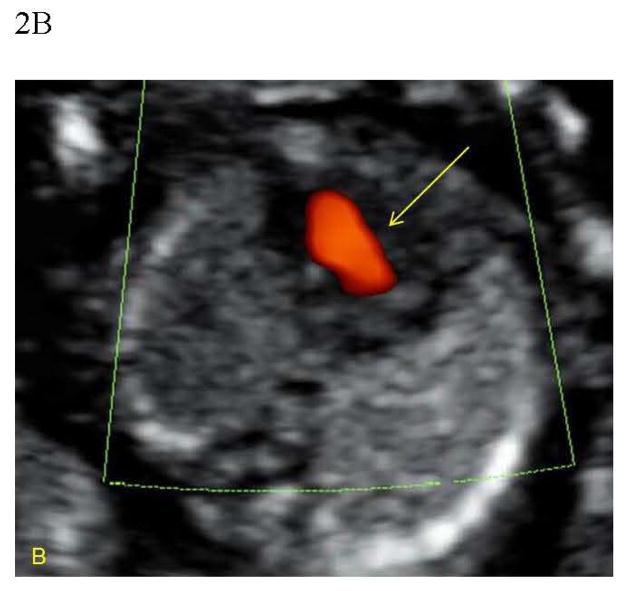
Figure 2.
Figure 2A. Complete atrioventricular (AV) septal defect diagnosed at 13 weeks in a fetus with Down Syndrome. The four-chamber view demonstrates a deficient crux with an inlet ventricular septal (IVS) defect and an atrial septum primum defect.
Figure 2B. Color Doppler demonstrates central flow across a common atrioventricular valve (arrow).
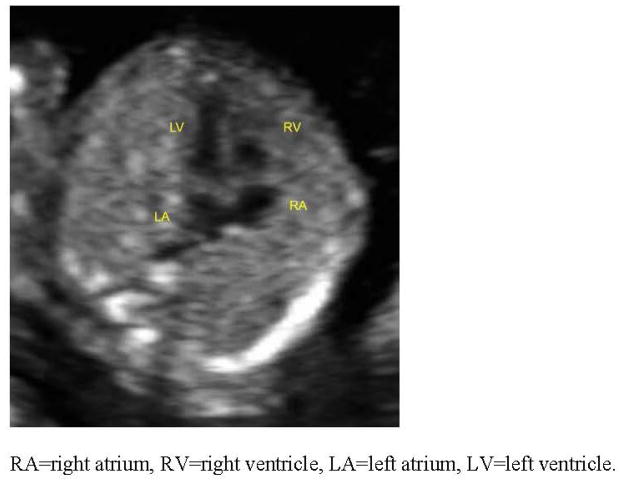
Figure 3.
Normal four chamber view of the fetal heart at 12 weeks gestation. (RA=right atrium, RV=right ventricle, LA=left atrium, LV=left ventricle).
The sensitivity of first trimester fetal echocardiography for major congenital heart disease varies from 10 percent in low-risk populations to over 50 percent in high-risk groups (Haak and van Vugt, 2003; Volpe et al, 2011; Rossi and Prefumo, 2013). There are recognized limitations of early echocardiography, however. Valvular stenosis, coarctation of the aorta, cardiac tumors, and cardiomyopathy may develop later in fetal life and not be visible at <14 weeks gestation. In contrast, the early detection of defects that may resolve in utero such as ventricular septal defects may cause unnecessary parental stress and pregnancy interventions. At early gestational ages, particularly in cases of maternal obesity or prior abdominal surgery, the transabdominal approach may be insufficient so transvaginal imaging may be necessary to acquire interpretable views of the fetal heart (Huggon et al, 2002). Finally, early echocardiography has a lower diagnostic accuracy with lower detection rate of major cardiac anomalies and a higher false positive rate (Haak and van Vugt, 2003). The performance of early echocardiography as a screening and diagnostic tool needs further evaluation, particularly when applied to the general population.
Value of the Routine 18 to 20 Week Fetal Ultrasound
Several large studies and systematic reviews have reported a wide detection rate (16–44%) of malformations before 24 weeks, especially higher if lethal or major anomalies (National Collaborating Center, 2008; Whitworth et al., 2010). Multiple organizations such as the American College of Obstetricians and Gynecologists, Royal College of Obstetricians and Gynaecologists, and Society of Obstetricians and Gynaecologists of Canada have concluded that previable second-trimester ultrasonography should be offered routinely and follow specific guidelines (Ultrasonography, 2009; National Collaborating Center, 2008; Cargill et al., 2009). A fetal imaging workshop hosted by the National Institute of Child Health and Human Development in 2012 concurred that at least one ultrasound study should be offered to all pregnant women between 18 and 20 weeks (Reddy et al., 2014).
This period allows for optimal early evaluation of fetal anatomy, soft anatomic markers that suggest aneuploidy, diagnosis of multiple gestation and chorionicity, reassessment of gestational dating, and presence of any abnormal placentation. Fetal anatomy components of a standard examination at 18 to 20 weeks involve measurements taken in an orderly manner of the head, face, neck, heart, abdomen, spine, extremities, and genital regions. Evaluation of the maternal pelvic anatomy includes cervical length when risk factors are positive for preterm delivery.
Minor or soft sonographic findings associated with aneuploidy, most commonly Down syndrome, were first reported in the 1980s (Reddy et al., 2014). Table 3 lists those soft markers sought during targeted ultrasound examinations between 18 and 20 weeks. Searching for these markers is helpful to recalculate the aneuploidy risk so that individuals may determine whether they wish to pursue amniocentesis for chromosome or microarray analysis. If a risk assessment was not undertaken before 18 weeks, then maternal blood could be obtained for either biochemical or quad screening (hCG, estriol, inhibin, alpha fetoprotein) or noninvasive prenatal testing (cell-free DNA).
Table 3.
Follow-up of Isolated Second-Trimester Ultrasonographic Soft Markers for Down Syndrome Beyond a Targeted Ultrasonogram
| Soft marker | Other considerations and follow-up |
|---|---|
| Choroid plexus cyst | None* |
| Echogenic cardiac focus | None* |
| Nuchal fold thickening | Genetic counseling |
| Absent/hypoplastic nasal bone | Genetic counseling |
| Pyelectasis ≥4 mm up to 20 weeks |
32-week ultrasonography to assess kidneys and urinary collecting system |
| Echogenic bowel | Genetic counseling 32-week ultrasonography to assess growth and bowel |
| Shortened long bone | Consider third-trimester ultrasonography to assess growth* |
If isolated finding and aneuploidy screening reveals low-risk
Modified from Reddy, et al., 2014
Recommendations for management in the presence of a soft marker were also offered at the fetal imaging workshop (Reddy et al., 2014). Further genetic counseling with or without a repeat ultrasound examination for growth were common suggestions. Exceptions to further evaluation would be patient preference after counseling or isolated evidence of either a choroid plexus cyst or an echogenic intracardiac focus in the absence of an elevated a priori risk for fetal aneuploidy (Bernier et al., 2005; Nyberg et al., 2001; Bromley et al., 2002a). Evidence of an absent or hypoplastic nasal bone is one of the most sensitive markers for Down syndrome (Bromley et al., 2002b; Cicero et al., 2003; Sonek et al., 2006).
A nuchal fold thickness of 6 mm or greater at 15 to 20 weeks prompts evaluation of the fetal heart at the same time (Nyberg et al., 2001; Bromley et al., 2002). An echogenic bowel is associated with fetal growth restriction, congenital infection (especially cytomegalovirus), intra-amniotic bleeding, cystic fibrosis, and gastrointestinal obstruction (Sepulveda and Sebire, 2000). Examples in which follow-up in the third-trimester (usually 32 weeks) is recommended after the 18 to 20 week scan would be mild renal pyelectasis or either abnormally short or long bones to search for any anatomic progression of the condition, interval fetal growth, and rule out other structural abnormalities (Reddy et al., 2014).
Adjunctive Role of Magnetic Resonance Imaging
Ultrasound is, and will remain, the primary modality for imaging the fetus and maternal anatomic structures during pregnancy. While ultrasound is safe and typically available, the imaging modality does have limitations for prenatal diagnosis. Certain fetal anomalies cannot be adequately evaluated by ultrasound, and magnetic resonance imaging (MRI) may improve the ability to provide accurate information for prenatal counseling. An MRI scan can overcome limitations of ultrasound including beam attenuation by maternal adipose tissue, shadowing artifacts due to osseous structures, suboptimal fetal positioning, and oligohydramnios. MRI scanning also provides greater tissue contrast and a larger field of view than two or three- dimensional ultrasound.
After fetal MRI was introduced in the 1990s, the development of ultrafast techniques decreased artifacts due to fetal movement and improved visualization of fetal anatomy was possible (Girard et al., 2001). MRI scanning technology has continued to improve and over the last 15 years, its use in fetal medicine has expanded. An MRI scan is considered safe during pregnancy, as it utilizes changing electromagnetic fields and not radiation. There have been no proven harmful effects to the developing human fetus from limited exposure to MRI (Baker 1994, Kok 2004, American College of Radiology, 2010). Specific techniques for fetal MRI have been described in detail in prior publications (American College of Radiology, 2010; Reddy 2014).
A detailed fetal ultrasound examination should always precede MRI scan, in screening for structural abnormalities. There is likely limited benefit to fetal MRI before 18 weeks gestation due to the small fetal size and fetal motion artifact (Reddy et al., 2014). Following a fetal anatomic ultrasound at 18–20 weeks, an MRI at 20–22 weeks may improve evaluation and parental counseling regarding certain known or suspected anomalies (Reddy et al., 2014). Later third trimester fetal MRI is the optimal time for assessment of cerebral cortical development and any airway obstruction when planning for delivery (Reddy et al., 2014).
For certain lethal congenital anomalies, such as anencephaly, a two-dimensional ultrasound is sufficient to determine prognosis. Three-dimensional scanning contributes to characterizing certain structural defects (e.g., spina bifida, abdominal wall defects, facial clefting, club feet) For other structural abnormalities, fetal MRI may aid in the diagnosis and define prognosis for parents and care providers. Most research in fetal MRI thus far has been conducted for fetal brain abnormalities and facial or neck masses that may impact delivery planning. The joint Fetal Imaging Workshop suggested that indications for further evaluation with a fetal MRI include identification of ventriculomegaly, midline defects such as agenesis of the corpus callosum, posterior fossa anomalies and cerebral cortical malformations (Reddy et al., 2014). An MRI scan may also be used in select cases to screen fetuses with familial risk for brain abnormalities that may not be identified with routine ultrasound such as lissencephaly, dysgenesis of the corpus callosum, or changes associated with tuberous sclerosis such as brain tubers and subependymal nodules. In a systematic review of additional value in the prenatal diagnosis of central nervous system (CNS) anomalies, fetal MRI found CNS anomalies in 18.4% of cases, was observed to provide additional information in 22.1% of cases and in 30% of fetuses MRI findings were so different from ultrasound that the clinical management changed (Rossi et al., 2012). When a fetal cervicofacial mass is diagnosed sonographically, an MRI can delineate the extent of the mass and degree of airway obstruction if present (MacArthur 2012). The information obtained may be utilized for delivery planning, in particular if an ex-utero intrapartum treatment (EXIT) procedure is indicated. An MRI is also used at specialized centers for fetal therapy as an adjunct to confirm diagnosis and plan potential fetal surgery.
Many other fetal body systems have been evaluated with MRI. Imaging complex genitourinary tract abnormalities including bladder exstrophy, cloacal exstrophy, and lower urinary tract obstructions may be better defined using an MRI (Chauvin et al., 2012). MRI has also been used to predict residual lung volume and neonatal survival in the setting of chest masses and congenital diaphragmatic hernias (Bebbington et al., 2014; Zamora et al., 2014). MRI scanning is believed to be less useful for evaluating abdominal wall defects. Due to motion artifact from the rapid fetal heart rate, fetal cardiac evaluation with MRI is currently limited.
While the role for MRI in prenatal diagnosis may expand in the future, it is unlikely to become a primary imaging technique for fetuses. Magnetic resonance equipment is expensive, availability is limited, and it is critical to have appropriately trained staff to read and interpret fetal images. Fetal anatomy and pathophysiology can differ from that of the newborn, pediatric and adult population (Reddy et al., 2014), thus a clear understanding and knowledge of this is imperative. The joint Fetal Imaging Workshop outlined a research agenda for future areas to investigate the usefulness of MRI in prenatal diagnosis (Reddy et al., 2014). As data on its use accumulates, congenital malformations in which fetal MRI can aid in assessment will be further defined (Reddy et al., 2014). A post-mortom MRI holds hope as an alternative to conventional autopsy for detection of the cause of death or any major pathologic abnormality after death of the fetus (Thayyil et al., 2013). It can add information when the parents decline autopsy.
Precautions and Special Considerations with Imaging Studies in Early Gestation
Experience of staff, imaging equipment, and maternal characteristics influence accuracy in reporting fetal anomalies, especially in the late first-trimester (Timor-Tritsch et al., 2004; Syngelaki et al., 2011). Skillsets required in imaging and high costs in terms of time and equipment improve detection rates. Select attention by the operator may be focused more on specific organs such as the fetal heart that may enhance accuracy of the imaging study. The presence of associated anomalies and maternal risk factors for fetal structural abnormalities (e.g., diabetes, morbid obesity, alcohol, prior affected infant, race/ethnicity) stimulate more attention. Fetal positioning often prohibits complete visualization, especially during transabdominal imaging alone. Maternal obesity is associated with at least a 20% lower detection of fetal anomalies and a need for repeat imaging compared with women with a normal body mass index (Dashe et al., 2009; Tsai et al., 2010). In addition, an inverse relation between the body mass index and detection of the soft sonographic findings of an echogenic intra-cardiac focus (EIF) and echogenic bowel was reported as the possible reason of the higher incidence of EIF in Asian pregnant women (Bornstein et al., 2010) and should be taken into account when counseling patients (Borgida et al., 2005). Although an MRI may overcome some of the limitations of ultrasound, experience of those interpreting the images can also influence accuracy in describing fetal anomalies.
The natural history of fetal malformations plays a very important role in the detection of structural anomalies with early ultrasonography. Examples of normal morphologic differentiation in the first trimester include midgut rotation up to 13 weeks of gestation, small ventricular defects that commonly undergo spontaneous closure, and hydronephrosis (Rossi et al., 2012). Detection of hydrocephalus in the first trimester is very unlikely because of the customary large lateral ventricular width to calvarium ratio. Development of the fetal cerebellum and corpus callosum may not be complete until 18 weeks gestation; thus, premature diagnosis of abnormal formation should be avoided. In contrast, the normal appearance of cardiac anatomy at any time of pregnancy does not exclude heart defects that may develop with advancing gestational age or even postnatally.
Twins account for over 3% of all live births in the United States, and the combined risk of structural anomalies is higher in twins than among singletons (Martin et al., 2012). Determination of chorionicity of multifetal gestations is preferably done in the late first trimester. Because of the high-risk for malformations and twin-twin transfusion with monochorionic twins, ultrasound scans every 2 weeks should be considered starting as early as 16 weeks gestation (Society of Maternal Fetal Medicine, 2013). While malformation rates may not be increased in each fetus of dizygotic twins, deformations would be more common than for singletons intrauterine due to crowding.
If any component of the ultrasound examination is not visualized adequately beyond the first trimester, it should be documented in the report. The clinical utility and cost effectiveness of follow-up scans have not been established when parts are not well seen in low-risk patients with an otherwise normal survey (Reddy et al., 2014). It seems reasonable to consider repeating the exam in 2 to 4 weeks or referring to a tertiary perinatal center. Cost containment is important to acknowledge.
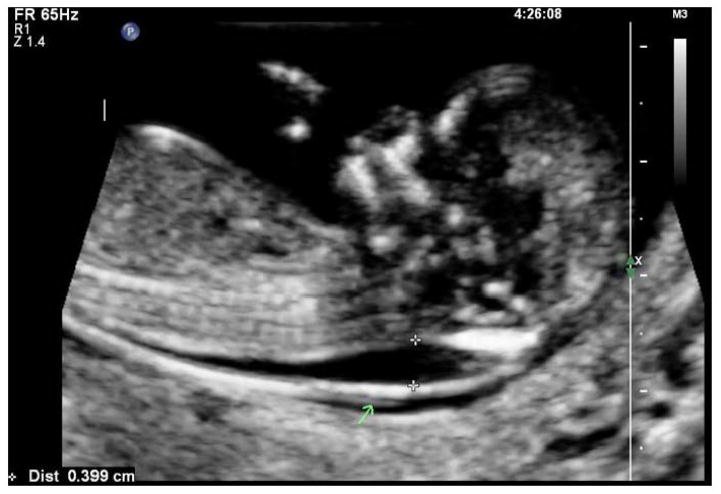
Figure 1.
Increased nuchal translucency in 12 week 1 day fetus
Acknowledgments
Dr. Rayburn’s effort is supported by the NIH/NIAAA 1 R01 AA0218771-01 grant
The authors thank Drs. Ludmilla Bakhireva, Rebecca Hall, and Luis Izquierdo for their assistance with tables and figures and their critical comments on the manuscript.
Footnotes
Presented at the March of Dimes Symposium, 54th Annual Meeting, The Teratology Society, meeting, Bellevue, WA June 30, 2014
None of the authors of this manuscript have any conflict of interest.
References
- Abu-Rustum RS, Ziade MF, Abu-Rustum SE. Learning curve and factors influencing the feasibility of performing fetal echocardiography at the time of the first-trimester scan. J Ultrasound Med. 2011;30:695–700. doi: 10.7863/jum.2011.30.5.695. [DOI] [PubMed] [Google Scholar]
- American College of Radiology Practice Guideline. ACR-SPR Practice Guideline for the Safe and Optimal Performance of Fetal Magnetic Resonance Imaging (MRI) Resolution. 2010;13:1–12. [Google Scholar]
- ACOG Committee Opinion. First trimester screening for fetal anomalies with nuchal translucency. Number 223, October 1999. Int J Gynaecol Obstet. 2000;68:71–72. [PubMed] [Google Scholar]
- American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of fetal echocardiography. J Ultrasound Med. 2013;32:1067–1082. doi: 10.7863/ultra.32.6.1067. [DOI] [PubMed] [Google Scholar]
- Baker PN, Johnson IR, Harvey PR, Gowland PA, Mansfield P. A three-year follow-up of children imaged in utero with echo planar magnetic resonance. Am J Obstet Gynecol. 1994;170 (1 Pt 1):32–33. doi: 10.1016/s0002-9378(94)70379-5. [DOI] [PubMed] [Google Scholar]
- Bebbington M, Victoria T, Danzer E, Moldenhauer J, Khalek N, Johnson M, Hedrick H, Adzick NS. Comparison of ultrasound and magnetic resonance imaging parameters in predicting survival in isolated left-sided congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2014;46(6):670–4. doi: 10.002/uog.13271. Epub 2014 May 5. [DOI] [PubMed] [Google Scholar]
- Bernara J-P, Cuckle H, Stirnenann J, Salomon J, Ville Y. Screening for fetal spina bifida by ultrasound examination in the first trimester of pregnancy using fetal biparietal diameter. Am J Obstet Gynecol. 2012;207:306, e1–5. doi: 10.1016/j.ajog.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Bernier FP, Crawford SG, Dewey D. Developmental outcome of children who had choroid plexus cysts detected prenatally. Prenat Diagn. 2005;25:322–326. doi: 10.1002/pd.1135. [DOI] [PubMed] [Google Scholar]
- Borgida A, Maffeo C, Gianferarri E, Bolnick A, Zelop C, Egan T. Frequency of echogenic intracardiac focus by race/ethnicity in euploid fetuses. J Mat Fetal neon Med. 2005;18:65–66. doi: 10.1080/14767050500073100. [DOI] [PubMed] [Google Scholar]
- Bree RL, Edwards M, Böhm-Velez M, Beyler S, Roberts J, Mendelson EB. Transvaginal sonography in the evaluation of normal early pregnancy: correlation with hCG level. AJR Am J Roentgenol. 1989;153:75–79. doi: 10.2214/ajr.153.1.75. [DOI] [PubMed] [Google Scholar]
- Bromley B, Lieberman E, Shipp TD, Benacerraf BR. The genetic sonogram: a method of risk assessment for Down syndrome in the second trimester. J Ultrasound Med. 2002a;21:1087–96. doi: 10.7863/jum.2002.21.10.1087. [DOI] [PubMed] [Google Scholar]
- Bornstein E, Sheiner E, Barnhara Y, McKeanna C, Binder D, Divon M. The association of maternal BMI with fetal echogenic intracardiac foci and echogenic bowel. J Matern Fetal Neonatal Med. 2010;23:781–784. doi: 10.3109/14767050903314176. [DOI] [PubMed] [Google Scholar]
- Bromley B, Lieberman E, Shipp TD, Benacerraf BR. Fetal nose bone length: a marker for Down syndrome in the second trimester. J Ultrasound Med. 2002b;21:1387–94. doi: 10.7863/jum.2002.21.12.1387. [DOI] [PubMed] [Google Scholar]
- Cargill Y, Morin L, Bly S, Butt K, Denis N, Gagnon R, et al. Content of a complete routine second trimester obstetrical ultrasound examination and report. J Obstet Gynaecol Can. 2009;31:272–5. 276–280. doi: 10.1016/S1701-2163(16)34127-5. [DOI] [PubMed] [Google Scholar]
- Chaoui R, Behoit B, Mitkowska-Wozniak H, Heling K, Nicolaides Assessment of intracranial translucency (IT) in the detection of spina bifida at the 11–13 weeks scan. Ultrasound Obstet Gynecol. 2009;34:249–252. doi: 10.1002/uog.7329. [DOI] [PubMed] [Google Scholar]
- Chauvin NA, Epelman M, Victoria T, Johnson AM. Complex genitourinary abnormalities on fetal MRI: imaging findings and approach to diagnosis. AJR Am J Roentgenal. 2012;199(2):W222–231. doi: 10.2214/AJR.11.7761. [DOI] [PubMed] [Google Scholar]
- Cicero S, Sonek JD, McKenna DS, Croom CS, Johnson L, Nicolaides KH. Nasal bone hypoplasia in trisomy 21 at 15e22 weeks’ gestation. Ultrasound Obstet Gynecol. 2003;21:15–18. doi: 10.1002/uog.19. [DOI] [PubMed] [Google Scholar]
- Dashe JS, McIntire DD, Twickler DM. Effect of maternal obesity on the ultrasound detection of anomalous fetuses. Obstet Gynecol. 2009;113:1001–1007. doi: 10.1097/AOG.0b013e3181a1d2f5. [DOI] [PubMed] [Google Scholar]
- den Hollander NS, Wessels MW, Niermeijer MF, Los FJ, Wladimiroff JW. Early fetal anomaly scanning in a population at increased risk of abnormalities. Ultrasound Obstet Gynecol. 2002;19:570–574. doi: 10.1046/j.1469-0705.2002.00649.x. [DOI] [PubMed] [Google Scholar]
- Doubilet P, Benson C, Bourne T, Blaivas M. Diagnostic criteria for nonviable pregnancy early in the first trimester for the Society of Radiologists in Ultrasound Multispecialty Panel on early first trimester diagnosis of miscarriage and exclusion of a viable intrauterine pregnancy. N Engl J Med. 2013;369:1443–1451. doi: 10.1056/NEJMra1302417. [DOI] [PubMed] [Google Scholar]
- Dugoff L. Ultrasound diagnosis of structural abnormalities in the first trimester. Prenat Diagn. 2002;22:316–320. doi: 10.1002/pd.309. [DOI] [PubMed] [Google Scholar]
- Economides DL. Early pregnancy screening for fetal abnormalities. Ultrasound Obstet Gynecol. 1999;13:81–83. doi: 10.1046/j.1469-0705.1999.13020081.x. [DOI] [PubMed] [Google Scholar]
- Foy PM, Wheller JJ, Samuels P, Evans KD. Evaluation of the fetal heart at 14 to 18 weeks gestation in fetuses with a screening nuchal translucency greater than or equal to the 95th percentile. J Ultrasound Med. 2013;32:1713–1719. doi: 10.7863/ultra.32.10.1713. [DOI] [PubMed] [Google Scholar]
- Ghi T, Huggon IC, Zosmer N, Nicolaides KH. Incidence of major structural cardiac defects associated with increased nuchal translucency but normal karyotype. Ultrasound Obstet Gynecol. 2001;18:610–614. doi: 10.1046/j.0960-7692.2001.00584.x. [DOI] [PubMed] [Google Scholar]
- Girard N, Raybaud C, Gambarelli D, Figarella-Branger D. Fetal brain MR imaging. Magn Reson Imaging Clin N Am. 2001;9:19–56. [PubMed] [Google Scholar]
- Goldstein I, Zimmer EA, Tamir A, Peretz BA, Paldi E. Evaluation of normal gestational sac growth: appearance of embryonic heartbeat and embryo body movements using the transvaginal technique. Obstet Gynecol. 1991;77:885–888. [PubMed] [Google Scholar]
- Haak MC, van Vugt JM. Echocardiography in early pregnancy: review of literature. J Ultrasound Med. 2003;22:271–280. doi: 10.7863/jum.2003.22.3.271. [DOI] [PubMed] [Google Scholar]
- Huggon IC, Ghi T, Cook AC, Zosmer N, Allan LD, Nicolaides KH. Fetal cardiac abnormalities identified prior to 14 weeks gestation. Ultrasound Obstet Gynecol. 2002;20:22–29. doi: 10.1046/j.1469-0705.2002.00733.x. [DOI] [PubMed] [Google Scholar]
- Hyett JA, Perdu M, Sharland GK, Snijders RS, Nicolaides KH. Increased nuchal translucency at 10–14 weeks of gestation as a marker for major cardiac defects. Ultrasound Obstet Gynecol. 1997;10:242–246. doi: 10.1046/j.1469-0705.1997.10040242.x. [DOI] [PubMed] [Google Scholar]
- Johnson B, Simpson LL. Screening for congenital heart disease: a move toward earlier echocardiography. Am J Perinatol. 2007;24:449–56. doi: 10.1055/s-2007-986681. [DOI] [PubMed] [Google Scholar]
- Khalil A, Nicolaides KH. Fetal heart defects: potential and pitfalls of first-trimester detection. Semin Fetal Neonatal Med. 2013;18:251–260. doi: 10.1016/j.siny.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Kok RD, de Vries MM, Heerschap A, van den Berg PP. Absence of harmful effects of magnetic resonance exposure at 1.5 T in utero during the third trimester of pregnancy: a follow-up study. Magn Reson Imaging. 2004;22(6):851–854. doi: 10.1016/j.mri.2004.01.047. [DOI] [PubMed] [Google Scholar]
- MacArthur CJ. Prenatal diagnosis of fetal cervicofacial anomalies. Curr Opin Otolaryngol Head Neck Surg. 2012;20(6):482–490. doi: 10.1097/MOO.0b013e3283582e21. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJ. Three decades of twin births in the United States, 1980 – 2009. NCHS Data Brief. 2012;80:1–8. [PubMed] [Google Scholar]
- Martinez JM, Comas M, Borrell A, Bennasar M, Gomez O, Puerto B, Gratacos E. Abnormal first trimester ductus venosus blood flow: a marker of cardiac defects in fetuses with normal fetal karyotype and nuchal translucency. Ultrasound Obstet Gynecol. 2010;35:267–272. doi: 10.1002/uog.7544. [DOI] [PubMed] [Google Scholar]
- McAuliffe FM, Fong KW, Toi A, Chitayat D, Keating S, Johnson JA. Ultrasound detection of fetal anomalies in conjunction with first-trimester nuchal translucency screening: a feasibility study. Am J Obstet Gynecol. 2005;193:1260–1265. doi: 10.1016/j.ajog.2005.06.075. [DOI] [PubMed] [Google Scholar]
- Moon-Grady A, Shahanavaz S, Brook M, Rodriguez H, Hornberger LK. Can a complete fetal echocardiogram be performed at 12 to 16 weeks gestation? J Am Soc Echocardiogr. 2012;25:1342–1352. doi: 10.1016/j.echo.2012.09.003. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Women’s and Children’s Health. Antenatal care: routine care for the healthy pregnant woman. London, UK: RCOG Press; 2008. [PubMed] [Google Scholar]
- Nyberg DA, Souter VL, El-Bastawissi A, Young S, Luthhardt F, Luthy DA. Isolated sonographic markers for detection of fetal Down syndrome in the second trimester of pregnancy. J Ultrasound Med. 2001;20:1053–1063. doi: 10.7863/jum.2001.20.10.1053. [DOI] [PubMed] [Google Scholar]
- Persico N, Moratalla J, Lombardi Cm, Zidere V, Allan L, Nicolaides KH. Fetal echocardiography at 11–13 weeks by transabdominal high-frequency ultrasound. Ultrasound Obstet Gynecol. 2011;37:296–301. doi: 10.1002/uog.8934. [DOI] [PubMed] [Google Scholar]
- Reddy UM, Abuhamad AZ, Levine D, Saade GR. Fetal Imaging Workshop Invited Participants. Fetal imaging: Executive Summary of a Joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, American College of Radiology, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound Fetal Imaging Workshop. Am J Obstet Gynecol. 2014;210(5):387–397. doi: 10.1016/j.ajog.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Rossi AC, Prefumo F. Accuracy of ultrasonography at 11–14 weeks of gestation for detection of fetal structural anomalies: a systematic review. Obstet Gynecol. 2013;122(6):1160–1167. doi: 10.1097/AOG.0000000000000015. [DOI] [PubMed] [Google Scholar]
- Rossi C, Prefumo F. The additional value of fetal magnetic resonance imaging in the prenatal diagnosis of central nervous system anomalies: a systematic review of the literature. Ultrasound Obstet Gynecol. 2014 Jun 2; doi: 10.1002/uog.13429. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Sepulveda W, Sebire NJ. Fetal echogenic bowel: a complex scenario. Ultrasound Obstet Gynecol. 2000;16:510–514. doi: 10.1046/j.1469-0705.2000.00322.x. [DOI] [PubMed] [Google Scholar]
- Society for Maternal-Fetal Medicine. Simpson LL. Twin-twin transfusion syndrome. Am J Obstet Gynecol. 2013;208(1):3–10. doi: 10.1016/j.ajog.2012.10.880. [DOI] [PubMed] [Google Scholar]
- Simpson LL, Malone FD, Bianchi DW, Ball RH, Nyberg DA, Comstock CH, Saade G, Eddleman K, Gross SJ, Dugoff L, Craigo SD, Timor-Tritsch IE, Carr SR, Wolfe HM, Tripp T, D’Alton ME. Nuchal translucency and the risk of congenital heart disease. Obstet Gynecol. 2007;109:376–383. doi: 10.1097/01.AOG.0000250473.99575.72. [DOI] [PubMed] [Google Scholar]
- Sonek JD, Cicero S, Neiger R, Nicolaides KH. Nasal bone assessment in prenatal screening for trisomy 21. Am J Obstet Gynecol. 2006;195:1219–1230. doi: 10.1016/j.ajog.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Souka AP, Krampl E, Bakalis S, Heath V, Nicolaides KH. Outcome of pregnancy in chromosomally normal fetuses with increased nuchal translucency in the first trimester. Ultrasound Obstet Gynecol. 2001;18:9–17. doi: 10.1046/j.1469-0705.2001.00454.x. [DOI] [PubMed] [Google Scholar]
- Souka AP, Pilalis A, Kavalakis Y, Kosmas Y, Antsaklis P, Antsaklis A. Assessment of fetal anatomy at the 11–14-week ultrasound examination. Ultrasound Obstet Gynecol. 2004;24:730–734. doi: 10.1002/uog.1775. [DOI] [PubMed] [Google Scholar]
- Syngelaki A, Chelemen T, Dagklis T, Allan L, Nicolaides KH. Challenges in the diagnosis of fetal non-chromosomal abnormalities at 11–13 weeks. Prenat Diagn. 2011;31:90–102. doi: 10.1002/pd.2642. [DOI] [PubMed] [Google Scholar]
- Thayyil S, Sebire N, Chitty L, Wade A, Chong W, Olsen O, et al. Post-mortem MRI versus conventional autopsy in fetuses and children: a prospective validation study. Lancet. 2013;382:223–233. doi: 10.1016/S0140-6736(13)60134-8. [DOI] [PubMed] [Google Scholar]
- Timor-Tritsch IE, Bashiri A, Monteagudo A, Arsland AA. Qualified and trained sonographers in the US can perform early fetal anatomy scans between 11 and 14 weeks. Am J Obstet Gynecol. 2004;191:1247–1252. doi: 10.1016/j.ajog.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Tsai LJ, Ho M, Pressman EK, Thornburg LL. Ultrasound screening for fetal aneuploidy using soft markers in the overweight and obese gravida. Prenatal Diagn. 2010;30:821–826. doi: 10.1002/pd.2554. [DOI] [PubMed] [Google Scholar]
- Ultrasonography in pregnancy. ACOG Practice Bulletin No. 101. 2009. American College of Obstetricians and Gynecologists. Obstet Gynecol. 113:451–461. doi: 10.1097/AOG.0b013e31819930b0. [DOI] [PubMed] [Google Scholar]
- Volpe P, Ubaldo P, Volpe N, Campobasso G, De Robertis V, Tempesta A, Volpe G, Rembouskos G. Fetal cardiac evaluation at 11–14 weeks by experience obstetricians in a low-risk population. Prenat Diagn. 2011;31:1054–1061. doi: 10.1002/pd.2831. [DOI] [PubMed] [Google Scholar]
- Whitlow BJ, Economides DL. The optimal gestational age to examine fetal anatomy and measure nuchal translucency in the first trimester. Ultrasound Obstet Gynecol. 1998;11:258–261. doi: 10.1046/j.1469-0705.1998.11040258.x. [DOI] [PubMed] [Google Scholar]
- Whitworth M, Bricker L, Neilson JP, Dowswell T. Ultrasound for fetal assessment in early pregnancy. The Cochrane Database of Systematic Reviews. 2010:CD007058. doi: 10.1002/14651858.CD007058.pub2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczk BJ, Palacios AM, Chapa, et al. Genetic basis of susceptibility to teratogen induced birth defects. Am J Med Genet C Semin Med Genet. 2011;157:215–220. doi: 10.1002/ajmg.c.30314. [DOI] [PubMed] [Google Scholar]
- Zamora IJ, Sheikh F, Cassady CI, Olutoye OO, Mehollin-Ray AR, Ruano R, Lee TC, Welty SE, Belfort MA, Ethun CG, Kim ME, Cass Dl. Fetal MRI lung volumes are predictive of perinatal outcomes in fetuses with congenital lung masses. J Pediatr Surg. 2014;49(6):853–858. doi: 10.1016/j.jpedsurg.2014.01.012. [DOI] [PubMed] [Google Scholar]