Abstract
Synthesis of the major toxin proteins of the diarrheal pathogen, Clostridium difficile, is dependent on the activity of TcdR, an initiation (sigma) factor of RNA polymerase. The synthesis of TcdR and the activation of toxin gene expression are responsive to multiple components in the bacterium’s nutritional environment, such as the presence of certain sugars, amino acids, and fatty acids. This review summarizes current knowledge about the mechanisms responsible for repression of toxin synthesis when glucose or branched-chain amino acids or proline are in excess and the pathways that lead to synthesis of butyrate, an activator of toxin synthesis. The regulatory proteins implicated in these mechanisms also play key roles in modulating bacterial metabolic pathways, suggesting that C. difficile pathogenesis is intimately connected to the bacterium’s metabolic state.
Keywords: Clostridium difficile, toxinogenesis, metabolic regulation, redox state, Stickland metabolism
Introduction
The ability to adapt efficiently to a wide range of environmental changes, especially to alterations in nutritional quality, is a universal trait in the bacterial world. In many pathogenic bacteria, adaptation to an altered environment is often associated with the activation of the transcription of virulence genes; hence, many virulence genes are regulated by environmental and nutritional signals suggesting a tight link between metabolism and virulence.
In Clostridium difficile, production of the two large enterotoxins (TcdA and TcdB), considered to be the primary virulence factors [1, 2], responds to various environmental conditions (at least when tested in the laboratory), including the availability of specific nutrients, temperature changes, and alteration of the redox potential [3-5]. For instance, when cells are grown in rich medium, the toxin genes are transcribed only when the cells enter stationary phase, i.e., when some form of nutrient limitation or accumulation of growth inhibiting substances occurs [6, 7]. In addition, the presence of a rapidly metabolizable carbon source [6, 8] or certain amino acids [9, 10] inhibits toxin gene expression. Once expression of the toxin genes is induced, the toxin proteins accumulate inside the cell and are slowly released over the course of several hours. Table 1 summarizes the environmental factors found to affect toxin production.
Table 1.
Environmental effectors of toxin synthesis.
Some of the molecular mechanisms regulating C. difficile toxin gene expression in response to environmental signals have been elucidated [3, 6, 11-14]. Interestingly, several global regulators of metabolic pathways also regulate toxin gene expression; such co-regulation implies strongly that, from the bacterium’s point of view, expression of some virulence genes serves a purpose that needs to be coordinated with expression of specific pathways. This review summarizes the roles of four regulatory proteins (CcpA, CodY, PrdR, and Rex) that couple the control of metabolism and pathogenesis in response to the presence of specific nutrients. In addition, toxin gene expression is known to respond to proteins (e.g., sigma-H and Spo0A) that play essential roles in regulation of spore formation, another nutrient-sensitive process. Thus, C. difficile has evolved to place the expression of its major virulence factors under the control of a panel of nutrient-sensing regulators that act in cooperative or antagonistic ways to cause the appropriate amount of toxin synthesis under a variety of environmental conditions.
The Pathogenicity Locus (PaLoc)
The genes encoding the two major toxins TcdA (tcdA ) and TcdB (tcdB ) are located within a 19.6 kb pathogenicity locus (PaLoc) (Fig. 1) found only in toxigenic strains of C. difficile [15]. This locus also includes genes encoding an RNA polymerase sigma factor (TcdR) that is essential for toxin gene expression [16], an antagonist of TcdR (named TcdC) [17] and a holin-like protein, TcdE, that is required for efficient release of TcdA and TcdB from the bacteria [18]. Although the PaLoc has some characteristics of a mobile genetic element, it does not appear to be intrinsically mobile and is located at the same site in all toxigenic C. difficile strains [15]. Nevertheless, given that the base composition of the PaLoc differs from that of the genome as a whole, it is likely that the PaLoc was acquired by horizontal transfer. Consistent with this hypothesis, the PaLoc was shown to be transferable by a presumably unlinked conjugation-like mechanism to a non-toxigenic strain, resulting in its conversion to a toxin producer [19]. Variations in the PaLoc region, including insertions, deletions and point mutations, have been used to define 32 toxinotypes among C. difficile isolates in comparison to the reference strain VPI10463 [20].
Fig. 1.
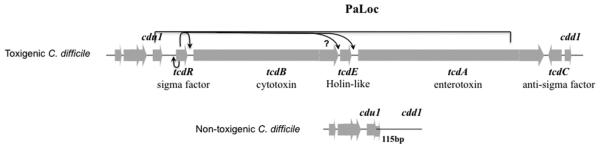
Schematic diagram of PaLoc in toxigenic C. difficile strains. In non-toxigenic strains this region is replaced by a 115-bp sequence. Arrows indicate the positive regulation of tcdR, tcdB and tcdA by σTcdR. cdu1 and cdd1 are similar to genes encoding a transcriptional regulator and a hypothetical protein, respectively, and appear to be of bacteriophage origin. The question mark indicates that the role of TcdR in tcdE transcription is unclear. This figure previously appeared in ref. 18.
When C. difficile cells are grown in rich medium, the tcdR, tcdB, tcdE and tcdA genes are induced in stationary phase. Although tcdA and tcdB are transcribed mainly from their own promoters [6, 7], their transcripts can be found in both polycistronic and monocistronic mRNAs [7]. The promoter regions of tcdA and tcdB do not resemble the canonical σ70 consensus promoters of prokaryotes but show strong similarities to each other and to certain other toxin and bacteriocin gene promoters in several Clostridium species [6, 7, 21].
Toxin gene transcription is activated by multiple sigma factors
The tcdR gene (previously named tcdD or txeR) encodes a basic protein of 22 kDa [15, 22]. Moncrief et al. provided the first evidence that TcdR is a positive regulator of the C. difficile toxin genes when they successfully activated tcdA and tcdB reporter fusions by expressing TcdR in trans in Escherichia coli [22]. Similar effects were seen in C. perfringens [16] and C. difficile [23]. Mani & Dupuy provided the genetic and biochemical evidence that TcdR is required for specific transcription from the tcdA and tcdB promoters and that TcdR functions specifically as a sigma factor of RNA polymerase (RNAP) [16]. TcdR also activates its own expression [23], consistent with the presence in the region upstream of the tcdR gene of two potential promoters for TcdR-dependent transcription (Fig. 2) [23]. Recently, El Meouche et al. identified a SigD-dependent promoter sequence upstream of tcdR (Fig. 2) [24]. Moreover, they showed that SigD, a sigma factor usually associated with motility gene expression, positively controls toxin gene expression [24].
Fig. 2.
Schematic representation of the tcdR promoter region. The arrows indicate transcription start sites activated by σA-, σD- and TcdR-containing forms of RNA polymerase. Blue and pink boxes represent CodY and CcpA binding sites, respectively. Positions of sites are indicated relative to the ATG start codon.
TcdR is a member of a discrete group (Group V) of σ factors within the σ70 family. Other members of Group V include UviA, which directs bacteriocin gene transcription in C. perfringens , BotR and TetR, which activate transcription of the botulinum and tetanus toxin genes, respectively [25], TcsR, which stimulates transcription of the lethal and hemorrhagic toxin genes of Clostridium sordellii [26] and TpeR, the activator of TpeL cytotoxin synthesis in C. perfringens [27]. Interestingly, all of these σ factors are encoded within genetic elements that might have been acquired by horizontal gene transfer. UviA and TetR are encoded on plasmids [28, 29], BotR within bacteriophage genomes in some C. botulinum strains [30], TpeR within an uncharacterized pathogenicity locus [27] and TcsR within a toxin locus that shows signatures of integrative and conjugative elements [26]. The organization of the sigma factor gene and its target gene promoters has also been largely conserved [26, 31]. Moreover, in all cases examined, the sigma factor gene is autoregulated and induced by one or more environmental stresses [26, 31]. Additional homologues of the group V sigma factors can be found in other pathogenic Clostridium spp., such as Clostridium novyi (G. Carter and D. Lyras, personal communication), as well as in non-pathogenic Clostridium spp., such as C. acetobutylicum [31]. The functions of these latter sigma factor homologs remain to be determined.
A negative role for TcdC in toxin expression was first suggested based on the inverse orientation and timing of its expression relative to those of the other members of the PaLoc [7]. This putative role of TcdC in toxin regulation appeared to be reinforced by the emergence of epidemic strains that carry deletions or frameshift mutations in the tcdC gene and produce high levels of both toxins [32, 33].
TcdC is an acidic, membrane-associated protein with a predicted molecular weight of 26-kDa [15, 34]. TcdC can form dimers, which is consistent with the presence of a coiled-coil motif in the middle of the protein, and appears to act as an anti-sigma factor [17, 35]. TcdC negatively regulates C. difficile toxin gene expression by interfering with the ability of the TcdR-containing RNAP to recognize the tcdA and tcdB promoters [17]. In fact, both free TcdR and TcdR-containing holoenzyme are sensitive to TcdC. Once a stable open complex is formed with the tcdA promoter, TcdC cannot disrupt the binding of RNAP to the promoter [17]. Recently, van Leeuwen et al. [35] showed that TcdC could bind to DNA folded into G-quadruplex structures containing repetitive guanine nucleotides, suggesting that TcdC might also act by destabilizing open complex formation before transcription initiation. However, no quadruplex-forming motif with multiple G-stretches is found in the PaLoc [35]. In support of a direct contact between TcdR and TcdC, TcdC was shown to inhibit transcription dependent on alternative sigma factors such as UviA and SigW, but not primary SigA-type factors [17]. This strongly suggests that the mode of action of TcdC is more dependent on the nature of the sigma factor than of the core enzyme. Nevertheless, questions still remain as to how TcdC inactivates or destabilizes the TcdR-containing RNAP holoenzyme.
Although blocking of TcdR-dependent transcription by TcdC in vitro is not in dispute, different experimental approaches have led to conflicting conclusions about the importance of TcdC in regulating toxin expression in living cells. The prevailing model that TcdC is a negative regulator of toxin expression was supported by the finding that introduction of a functional tcdC gene into an epidemic strain that carries a non-functional tcdC gene (M7404, a PCR ribotype NAP1/027 strain) resulted in decreased toxin production and attenuated virulence in a hamster model [36]. However, chromosomal complementation in strain R20291, another PCR ribotype NAP1/027 strain with an inactive tcdC gene, resulted in no discernible effect on toxin expression [37]. Moreover, other studies showed that disruption of the tcdC gene in strain 630ΔErm had little if any effect on toxin expression under the conditions tested [37, 38]. The reasons for the conflicting data may relate to experimental variations, including the strain used or the specific growth conditions, either of which might affect the level of TcdC expression or activity.
Given the complexity of toxin gene expression, the various components of the environment that affect toxin synthesis (Table 1) could do so by altering expression or activity of TcdR, SigD, TcdC, TcdE or the toxin proteins themselves.
Roles of proline and glycine and regulation of Stickland metabolism by PrdR and Rex
In the mid-1930’s, using extracts of Clostridium sporogenes, Stickland and Woods discovered coupled pathways of amino acid metabolism in which oxidative deamination and decarboxylation of one amino acid, generating ATP and NADH, is linked to reduction of d-proline or reductive deamination of glycine [39-41]. The products of d-proline reductase, the proline metabolism complex, [42] are 5-aminovalerate and NAD+; the products of glycine reductase (GR) are ammonium ion, acetate and ATP. C. difficile PR and GR are seleno-enzyme complexes [43] encoded by distinct multi-gene clusters. Jackson et al. [43] suggested that, given the selenium requirement of C. difficile for growth in a defined, basal medium, the selenium-containing reductases (PR and GR) are likely to play a key role in the physiology of the bacteria. In fact, both glycine and L-proline are required for optimal growth in defined media [44-46] and PR is required for optimal growth even in a rich, complex medium containing a multitude of carbon and nitrogen sources [47]. Interestingly, the lack of GR does not affect growth in rich medium and addition of proline to the medium increases expression of PR but decreases expression of GR [43]. The observations that proline and/or a mixture of seven amino acids containing glycine and proline strongly decrease C. difficile toxin yield [3, 10] suggested a role for Stickland metabolism in the regulation of toxin production.
PrdR, an apparent sigma-54-dependent transcriptional activator encoded by a gene located upstream of the PR gene cluster [43, 47], is the mediator of proline-dependent activation of the expression of PR and proline-dependent repression of the toxin gene tcdA and the GR operon during growth in vitro [47]. Interestingly, Janoir et al. also observed inverse regulation of the prd and grd gene clusters during the course of infection of monoxenic mice [48]. Broader effects of proline on C. difficile gene expression were revealed by microarray analysis. These effects are dependent on PrdR and mostly affect reductive pathways (L. Bouillaut, T. Dubois, M. Monot, B. Dupuy and A. L. Sonenshein, manuscript in preparation). If proline is limiting in the growth medium or if PR or PrdR is inactive, alternative pathways for NAD+ regeneration [e.g., glycine reductase, alcohol dehydrogenase, butyrate production from acetyl CoA and succinate] (Fig. 3) are induced (Bouillaut et al., manuscript in preparation). This phenomenon implies a global role for PrdR in the control of reductive pathways. However, is this control direct? Given that a functional PR is required in addition to PrdR for proline-dependent repression of GR and other reductive pathways [47], it seemed possible that such a broad pattern of repression might not be mediated directly by PrdR. Since all of these pathways regenerate NAD+ from NADH, the direct regulator might be a sensor of redox status.
Fig. 3.
Roles of global regulators in controlling expression of pathways for NAD+ regeneration.
Several Gram-positive bacteria (e.g., C. acetobutylicum [49], Streptomyces coelicolor [50], Thermus aquaticus [51], B. subtilis [52, 53], and S. aureus [42]) have a redox-dependent transcriptional repressor (Rex) that plays a key role in regulating anaerobic metabolism. Rex is a DNA binding protein containing a Rossmann fold dinucleotide binding site [51]. Structural studies of T. aquaticus Rex-NADH/NAD+ interactions have shown that binding of NADH induces a conformational change as a result of which binding of Rex to its DNA target site (TTGTGAA[a/t]6TTCACAA) is blocked [51]. In other words, Rex is only active as a DNA-binding protein when the intracellular NADH/NAD+ ratio is low [50]. Rex is found in C. difficile and putative binding sites can be identified upstream of genes involved in alternative pathways for NAD+ regeneration [54]. In fact, a rex null mutant of C. difficile proved to be insensitive to the presence of proline in the medium and to be defective in repression of the GR locus, alcohol dehydrogenase, the acetyl CoA-to-butyrate pathway and the succinate-to-butyrate pathway (Fig. 3). In accord with the general model, binding of C. difficile Rex protein to its target sites was stimulated by NAD+, but inhibited by NADH [Bouillaut et al., manuscript in preparation]. Since C. difficile Rex acts as a repressor of all of its target genes identified to date, Rex dissociates from the operator sites when intracellular NADH concentrations increase relative to NAD+ concentrations, allowing expression of the genes it represses. In addition, because the intracellular concentration of NADH is about 10-fold lower than that of NAD+ and the affinity of Rex for NADH is 10-fold higher than for NAD+, Rex may allow a sensitive control of the expression of NAD+ -regenerating pathways.
Our model for how proline and PrdR influence the activity of Rex and hence the transcription of Rex-repressed genes is the following. Reduction of proline is the most efficient and, therefore, the favored pathway for NAD+ regeneration. (This hypothesis is supported by the fact that PR gene expression is also activated by glucose (see below); glycolysis is a major producer of NADH.) When proline is in excess, PrdR is active and stimulates PR expression; as a result, enough NADH is oxidized to satisfy the cell’s requirements. In the presence of a low ratio of NADH to NAD+, Rex is active as a repressor of the alternative NAD+ regeneration pathways. When proline becomes limiting and the NADH/NAD+ ratio rises, Rex loses activity as a repressor and the alternative pathways are expressed (Fig. 3). Regeneration of NAD+ using these alternative pathways leads to the production of butyrate, a stimulator of toxin synthesis. Therefore, it is tempting to think that butyrate production signals redox stress and nutrient limitation (i.e., proline), as a result of which the cell is programmed to activate toxin synthesis. The molecular mechanism by which butyrate activates toxin synthesis is unknown.
Repression of toxin synthesis by glucose is mediated by CcpA
In complex media, the presence of glucose or other rapidly metabolizable carbon sources, such as fructose or mannitol, leads to a strong decrease of toxin production [6, 10]. The inhibitory effect of glucose is not due to accumulation of acidic metabolites derived from glucose metabolism [6, 10] and was observed in multiple C. difficile strains [6]. Furthermore, the repressive effect of glucose on toxin gene expression occurs at the transcriptional level, consistent with the repression by glucose of tcdR expression [6, 23].
The effects of rapidly metabolizable sugars on toxin gene expression are examples of carbon catabolite repression (CCR). CCR in bacteria has two principal elements: inducer exclusion, usually mediated by a component of the glucose transporter, and transcriptional regulation by a glucose-responsive DNA binding protein. In low G+C Gram-positive bacteria, the major agent of CCR is CcpA, a protein of the Lac repressor family [55]. CcpA usually acts as a repressor of utilization of alternative carbon sources and as a positive regulator of pathways associated with glycolysis, such as carbon overflow pathways. In most Gram-positive bacteria studied to date, the HPr protein, a component of the phosphoenolpruvate-dependent carbohydrate:phosphotransferase system (PTS), is involved in both sugar uptake and the regulatory activity of CcpA [56]. To activate CcpA, HPr must be phosphorylated at serine-46 by HPr kinase/phosphorylase, an enzyme whose activity is stimulated by fructose-1,6-bisphosphate. The CcpA/HPr~P complex then binds to catabolite responsive elements (cre sites) of target genes to affect their expression. Few of these details of CcpA-mediated regulation of carbon metabolism have been established for C. difficile , but the role of CcpA in toxin gene regulation is now well known [11, 57]. That is, no glucose-dependent repression of toxin gene expression was observed in mutant strains defective in genes of the PTS pathway or in ccpA. However, mutations in hprK, the gene for HPr kinase/phosphorylase, do not affect repression of toxin gene expression [11]. CcpA mediates repression of toxin production in vitro by interacting directly with the promoter regions of the PaLoc with the strongest affinity for the promoter region of tcdR (Fig. 2) [57]. The sequence of the CcpA-binding site defined for C. difficile is significantly different than the consensus cre site defined in B. subtilis [57]. Interestingly, although toxin production in the C. difficile, ccpA mutant strain is constitutive with respect to glucose, the level of expression is lower than in the wild-type strain grown without glucose [11]. Moreover, in a glucose-limited defined medium (SDM), toxin production is low compared to high-glucose SDM medium [10], implying that glucose limitation does not induce toxin production. Thus, it appears that one or more other regulators controlled by CcpA might contribute to toxin gene regulation independently of the presence of glucose. In fact, CcpA controls directly or indirectly a large number of regulatory proteins [57] and participates with other global regulators like CodY and Rex (see below) in coordinating metabolism and virulence. As described below, toxin synthesis is induced in the presence of butyric acid and the genes whose products produce butyrate from succinate or acetyl-CoA are regulated by CcpA, CodY and Rex (Fig. 3) [57, 58].
Repression of toxin synthesis by a mixture of amino acids is mediated by CodY
CodY is a global regulator involved in the adaptive response to nutrient limitation in low G+C content Gram-positive bacteria [59]. First, identified as a repressor of the B. subtilis dipeptide permease operon [60], CodY is now known to control hundreds of genes involved in major physiological processes in non-pathogenic (Bacillus [61], Lactococcus [62]) and pathogenic bacteria (Listeria [63]), Streptococcus [64, 65], Bacillus [66, 67] and Staphylococcus [68, 69]). In C. difficile, 146 genes putatively organized in 82 transcription units are overexpressed at least 4-fold in a codY mutant and 19 genes (15 transcription units) are underexpressed [58]. Genome-wide identification of direct CodY targets suggests that CodY controls 52 of the 82 transcription units directly [58]; most target genes are involved in biosynthesis of amino acids, nutrient transport, fermentation pathways, toxin production and spore formation.
Structural studies of B. subtilis CodY revealed that the protein contains two functional domains linked by a rigid α-helical domain [70]. A winged helix-turn-helix domain needed for binding to DNA lies near the C-terminus, and a GAF domain that binds BCAAs is close to the N-terminus [70, 71]. In vitro, the DNA-binding activity of CodY is enhanced by interaction with two types of effectors, branched-chain amino acids (isoleucine, leucine and valine; BCAAs) [71, 72] and GTP [73, 74]. The effects of BCAAs and GTP on CodY affinity for DNA are synergistic [74]. CodY binding sites in all bacteria examined to date are variants of the consensus motif AATTTTCWGAAAATT [64, 75, 76]. The intracellular levels of BCAAs and GTP decrease when nutrients become limiting [77, 78], triggering the release of CodY from its target sites and altering expression of CodY-responsive genes.
The role of BCAAs in activating CodY can explain why isoleucine [79] and a seven-amino acid mixture that includes the BCAAs [10] suppress C. difficile toxin production. In addition, the GTP pool reflects the availability of all amino acids, since, when any amino acid is limiting, the stringent response is induced and the GTP pool drops significantly [80]. In fact, CodY was the first regulator encoded outside the PaLoc found to be involved in toxin regulation. All the genes of the PaLoc, i.e., tcdR, tcdB, tcdA, tcdE and tcdC are derepressed in a C. difficile codY mutant [14, 58]. The amount of toxin A protein released into the culture fluid after 24 hrs of growth is 100-fold higher in the codY mutant strain than in its parent (L. Bouillaut, X. Sun, S. Tzipori and A. L. Sonenshein, unpublished). CodY regulates toxin gene expression by binding to the tcdR promoter region at three different locations with varying affinities (Fig. 2); two of the binding sites overlap with sigma factor-binding regions.
In addition to its direct effect on expression of the toxin gene locus, CodY contributes to repression of toxin synthesis by regulating the synthesis of butyrate. All the transcription units required for butyrate synthesis from acetyl CoA and succinate (Fig. 3) are direct CodY targets [58]. Thus, just as Rex represses most of the same genes in response to the ratio of NAD+ to NADH, CodY contributes to repression when BCAAs and GTP are in excess.
Role of sporulation regulators SigH and Spo0A
During transition from exponential growth phase to stationary phase, complex regulatory circuits are activated to modify metabolism and turn on both toxin synthesis and sporulation. Initiation of spore formation in B. subtilis is controlled by a regulatory cascade consisting of five kinases, two phosphorylated intermediate proteins (Spo0F and Spo0B) and several phosphatases [81]. The end result is the finely tuned activation-by-phosphorylation of Spo0A, a response regulator that activates transcription of several key sporulation genes as well as other stationary phase genes and represses the expression of inhibitors of sporulation. Incompletely defined environmental signals activate the phosphorelay [81]. C. difficile encodes a Spo0A homolog, but does not encode homologs of Spo0F or Spo0B; three orphan histidine kinases are the only factors known to modulate Spo0A activity [12, 13, 82]. As in B. subtilis, not all C. difficile genes affected by Spo0A activation are involved in sporulation. C. difficile Spo0A also contributes to regulation of metabolism and, in some strains, to toxin production. Indeed, glucose uptake, glycolysis and butyrate production (Fig. 3) are underexpressed in a C. difficile spo0A mutant [83]. These effects of a spo0A mutant appear to be strain-specific. Spo0A represses toxin gene expression in two ribotype 027 strains but has no or marginal effects in ribotype 078 and 012 strains [83-86]. The mechanism of the putative regulation of the toxin locus by Spo0A remains to be determined and is likely indirect, since no Spo0A binding sequences have been found upstream of any of the PaLoc genes [12].
Another key element of the transition phase and early sporulation events is the alternative sigma factor, SigH. In C. difficile, SigH is required for expression of spo0A, the genes whose products phosphorylate Spo0A, the butyrate biosynthesis pathway and virulence [13]. Interestingly, the regulation of SigH and Spo0A is reciprocal; SigH modulates spo0A transcription and Spo0A activates sigH expression. The effects of SigH and Spo0A on toxin gene expression may be different, however. That is, in a sigH mutant, the tcdR, tcdA and tcdB genes are overexpressed, implying that SigH controls expression of a gene whose product inhibits toxin gene expression [13]. The mechanistic basis for this apparent inhibition is not known.
Future directions
The complex, environmentally-responsive manner in which C. difficile controls expression of its major virulence factors suggests that the bacterium’s strategy for causing damage to the host is closely tied to its nutritional status (Fig. 4). The use by C. difficile of global metabolic regulators, such as CcpA, CodY and Rex, to control toxin gene expression implies that, from the bacterium’s point of view, virulence is to a great extent a mechanism for improving nutrient availability.
Fig. 4.
Effects of metabolites and regulatory proteins on C. difficile toxin synthesis. The metabolites that activate each regulatory protein are indicated; FBP, fructose-1,6-bisphosphate. Question marks indicate that metabolites affect toxin synthesis via unknown proteins and that some regulators (PrdR, SigH, Spo0A) are likely to affect toxin gene expression indirectly.
The specific metabolites that influence the activities of these regulators can be viewed as indicators of the viability of major metabolic pathways. For instance, CcpA is activated by fructose-1,6-bisphosphate, a key substrate of glycolysis, CodY responds to GTP (an indicator of ability to make RNA and protein) and BCAAs (indicators of ability to make protein and membrane fatty acids), Rex responds to the NADH/NAD+ ratio (an indicator of redox state) and PrdR responds to proline, the substrate of the primary pathway for NAD+ regeneration (Fig. 4). The mechanisms by which C. difficile responds to cysteine, biotin and butyrate remain to be discovered. The role of butyrate in regulating toxin synthesis is of great potential interest. First, C. difficile appears to produce butyrate only when the cells have an excess of NADH, suggesting that stimulation of toxin synthesis by butyrate is a response to a high NADH/NAD+ ratio. Second, butyrate is produced by many inhabitants of the GI tract, suggesting that C. difficile turns on toxin synthesis when in the presence of other butyrate-producing species. The latter hypothesis would seem to run counter to the presumption that C. difficile is only able to colonize the colon when the normal microbiota has been compromised. Finally, butyryl CoA, the precursor of butyrate, is also the precursor of butanol, an inhibitor of toxin synthesis (Figs. 3, 4) [8]. Discovering how the bacterium decides whether to convert butyryl CoA to butanol or to butyrate will reveal an important decision point for C. difficile and would potentially yield new methods of reducing virulence.
For an intestinal pathogen, such as C. difficile, inducing diarrhea is a mechanism for dispersal of the bacteria to new and potentially more supportive environments. Given that it is only the spore form of C. difficile that can survive outside the colon, however, the dispersed bacterial population must have a substantial spore component in order to maintain and spread the organism. This limitation on C. difficile survival would seem to demand that the same population that causes diarrhea include a reasonable number of spore-forming cells. Such an obligation raises a critical unanswered question: Do the same cells in a population produce both toxins and spores or is the population split between toxin producers and spore-formers? The negative correlation between the activity of SigH and the ability of cells to produce toxin suggests that the two processes are antithetical and predicts that a given cell can produce either toxins or spores but not both. However, it is also possible that toxin synthesis and sporulation occur in different time frames, so that a cell might produce toxin before committing to form a spore.
In summary, virtually everything we know about the regulation of the C. difficile PaLoc indicates an intimate connection between metabolism and virulence (Fig. 4). As virulence factors other than the major toxin proteins become identified and verified, it will be interesting to determine how their genes are regulated as well. The answers will tell us the extent to which C. difficile pathogenesis is determined by intracellular metabolite pools.
Acknowledgements
Unpublished work described here was supported by the Institut Pasteur and by a research grant from the US National Institute of General Medical Sciences (R01GM042219). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–9. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–3. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- [3].Karlsson S, Dupuy B, Mukherjee K, Norin E, Burman LG, Akerlund T. Expression of Clostridium difficile toxins A and B and their sigma factor TcdD is controlled by temperature. Infection and immunity. 2003;71:1784–93. doi: 10.1128/IAI.71.4.1784-1793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yamakawa K, Karasawa T, Ikoma S, Nakamura S. Enhancement of Clostridium difficile toxin production in biotin-limited conditions. Journal of medical microbiology. 1996;44:111–4. doi: 10.1099/00222615-44-2-111. [DOI] [PubMed] [Google Scholar]
- [5].Onderdonk AB, Lowe BR, Bartlett JG. Effect of environmental stress on Clostridium difficile toxin levels during continuous cultivation. Applied and environmental microbiology. 1979;38:637–41. doi: 10.1128/aem.38.4.637-641.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dupuy B, Sonenshein AL. Regulated transcription of Clostridium difficile toxin genes. Molecular microbiology. 1998;27:107–20. doi: 10.1046/j.1365-2958.1998.00663.x. [DOI] [PubMed] [Google Scholar]
- [7].Hundsberger T, Braun V, Weidmann M, Leukel P, Sauerborn M, von Eichel-Streiber C. Transcription analysis of the genes tcdA-E of the pathogenicity locus of Clostridium difficile. European journal of biochemistry / FEBS. 1997;244:735–42. doi: 10.1111/j.1432-1033.1997.t01-1-00735.x. [DOI] [PubMed] [Google Scholar]
- [8].Karlsson S, Lindberg A, Norin E, Burman LG, Akerlund T. Toxins, butyric acid, and other short-chain fatty acids are coordinately expressed and down-regulated by cysteine in Clostridium difficile. Infection and immunity. 2000;68:5881–8. doi: 10.1128/iai.68.10.5881-5888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Karasawa T, Maegawa T, Nojiri T, Yamakawa K, Nakamura S. Effect of arginine on toxin production by Clostridium difficile in defined medium. Microbiology and immunology. 1997;41:581–5. doi: 10.1111/j.1348-0421.1997.tb01895.x. [DOI] [PubMed] [Google Scholar]
- [10].Karlsson S, Burman LG, Akerlund T. Suppression of toxin production in Clostridium difficile VPI 10463 by amino acids. Microbiology. 1999;145:1683–93. doi: 10.1099/13500872-145-7-1683. Pt 7. [DOI] [PubMed] [Google Scholar]
- [11].Antunes A, Martin-Verstraete I, Dupuy B. CcpA-mediated repression of Clostridium difficile toxin gene expression. Molecular microbiology. 2011;79:882–99. doi: 10.1111/j.1365-2958.2010.07495.x. [DOI] [PubMed] [Google Scholar]
- [12].Underwood S, Guan S, Vijayasubhash V, Baines SD, Graham L, Lewis RJ, et al. Characterization of the sporulation initiation pathway of Clostridium difficile and its role in toxin production. Journal of bacteriology. 2009;191:7296–305. doi: 10.1128/JB.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Saujet L, Monot M, Dupuy B, Soutourina O, Martin-Verstraete I. The key sigma factor of transition phase, SigH, controls sporulation, metabolism, and virulence factor expression in Clostridium difficile. Journal of bacteriology. 2011;193:3186–96. doi: 10.1128/JB.00272-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dineen SS, Villapakkam AC, Nordman JT, Sonenshein AL. Repression of Clostridium difficile toxin gene expression by CodY. Molecular microbiology. 2007;66:206–19. doi: 10.1111/j.1365-2958.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- [15].Braun V, Hundsberger T, Leukel P, Sauerborn M, von Eichel-Streiber C. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene. 1996;181:29–38. doi: 10.1016/s0378-1119(96)00398-8. [DOI] [PubMed] [Google Scholar]
- [16].Mani N, Dupuy B. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5844–9. doi: 10.1073/pnas.101126598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Matamouros S, England P, Dupuy B. Clostridium difficile toxin expression is inhibited by the novel regulator TcdC. Molecular microbiology. 2007;64:1274–88. doi: 10.1111/j.1365-2958.2007.05739.x. [DOI] [PubMed] [Google Scholar]
- [18].Govind R, Dupuy B. Secretion of Clostridium difficile toxins A and B requires the holin- like protein TcdE. PLoS pathogens. 2012;8:e1002727. doi: 10.1371/journal.ppat.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brouwer MS, Roberts AP, Hussain H, Williams RJ, Allan E, Mullany P. Horizontal gene transfer converts non-toxigenic Clostridium difficile strains into toxin producers. Nature communications. 2013;4:2601. doi: 10.1038/ncomms3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rupnik M. Heterogeneity of large clostridial toxins: importance of Clostridium difficile toxinotypes. FEMS microbiology reviews. 2008;32:541–55. doi: 10.1111/j.1574-6976.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- [21].Hammond GA, Lyerly DM, Johnson JL. Transcriptional analysis of the toxigenic element of Clostridium difficile. Microbial pathogenesis. 1997;22:143–54. doi: 10.1006/mpat.1996.0100. [DOI] [PubMed] [Google Scholar]
- [22].Moncrief JS, Barroso LA, Wilkins TD. Positive regulation of Clostridium difficile toxins. Infection and immunity. 1997;65:1105–8. doi: 10.1128/iai.65.3.1105-1108.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mani N, Lyras D, Barroso L, Howarth P, Wilkins T, Rood JI, et al. Environmental response and autoregulation of Clostridium difficile TxeR, a sigma factor for toxin gene expression. Journal of bacteriology. 2002;184:5971–8. doi: 10.1128/JB.184.21.5971-5978.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].El Meouche I, Peltier J, Monot M, Soutourina O, Pestel-Caron M, Dupuy B, et al. Characterization of the SigD regulon of C. difficile and its positive control of toxin production through the regulation of tcdR. PloS one. 2013;8:e83748. doi: 10.1371/journal.pone.0083748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Raffestin S, Dupuy B, Marvaud JC, Popoff MR. BotR/A and TetR are alternative RNA polymerase sigma factors controlling the expression of the neurotoxin and associated protein genes in Clostridium botulinum type A and Clostridium tetani. Molecular microbiology. 2005;55:235–49. doi: 10.1111/j.1365-2958.2004.04377.x. [DOI] [PubMed] [Google Scholar]
- [26].Sirigi Reddy AR, Girinathan BP, Zapotocny R, Govind R. Identification and characterization of Clostridium sordellii toxin gene regulator. Journal of bacteriology. 2013;195:4246–54. doi: 10.1128/JB.00711-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Carter GP, Larcombe S, Li L, Jayawardena D, Awad MM, Songer JG, et al. Expression of the large clostridial toxins is controlled by conserved regulatory mechanisms. International journal of medical microbiology : IJMM. 2014 doi: 10.1016/j.ijmm.2014.08.008. [DOI] [PubMed] [Google Scholar]
- [28].Finn CW, Jr., Silver RP, Habig WH, Hardegree MC, Zon G, Garon CF. The structural gene for tetanus neurotoxin is on a plasmid. Science. 1984;224:881–4. doi: 10.1126/science.6326263. [DOI] [PubMed] [Google Scholar]
- [29].Garnier T, Cole ST. Characterization of a bacteriocinogenic plasmid from Clostridium perfringens and molecular genetic analysis of the bacteriocin-encoding gene. Journal of bacteriology. 1986;168:1189–96. doi: 10.1128/jb.168.3.1189-1196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Eklund MW, Poysky FT, Reed SM, Smith CA. Bacteriophage and the toxigenicity of Clostridium botulinum type C. Science. 1971;172:480–2. doi: 10.1126/science.172.3982.480. [DOI] [PubMed] [Google Scholar]
- [31].Dupuy B, Matamouros S. Regulation of toxin and bacteriocin synthesis in Clostridium species by a new subgroup of RNA polymerase sigma-factors. Research in microbiology. 2006;157:201–5. doi: 10.1016/j.resmic.2005.11.004. [DOI] [PubMed] [Google Scholar]
- [32].Curry SR, Marsh JW, Muto CA, O'Leary MM, Pasculle AW, Harrison LH. tcdC genotypes associated with severe TcdC truncation in an epidemic clone and other strains of Clostridium difficile. Journal of clinical microbiology. 2007;45:215–21. doi: 10.1128/JCM.01599-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–84. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- [34].Govind R, Vediyappan G, Rolfe RD, Fralick JA. Evidence that Clostridium difficile TcdC is a membrane-associated protein. Journal of bacteriology. 2006;188:3716–20. doi: 10.1128/JB.188.10.3716-3720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].van Leeuwen HC, Bakker D, Steindel P, Kuijper EJ, Corver J. Clostridium difficile TcdC protein binds four-stranded G-quadruplex structures. Nucleic acids research. 2013;41:2382–93. doi: 10.1093/nar/gks1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Carter GP, Douce GR, Govind R, Howarth PM, Mackin KE, Spencer J, et al. The anti- sigma factor TcdC modulates hypervirulence in an epidemic BI/NAP1/027 clinical isolate of Clostridium difficile. PLoS pathogens. 2011;7:e1002317. doi: 10.1371/journal.ppat.1002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cartman ST, Kelly ML, Heeg D, Heap JT, Minton NP. Precise manipulation of the Clostridium difficile chromosome reveals a lack of association between the tcdC genotype and toxin production. Applied and environmental microbiology. 2012;78:4683–90. doi: 10.1128/AEM.00249-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bakker D, Smits WK, Kuijper EJ, Corver J. TcdC does not significantly repress toxin expression in Clostridium difficile 630DeltaErm. PloS one. 2012;7:e43247. doi: 10.1371/journal.pone.0043247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Stickland LH. Studies in the metabolism of the strict anaerobes (genus Clostridium): The oxidation of alanine by Cl. sporogenes. IV. The reduction of glycine by Cl. sporogenes. The Biochemical journal. 1935;29:889–98. doi: 10.1042/bj0290889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Stickland LH. Studies in the metabolism of the strict anaerobes (Genus Clostridium): The reduction of proline by Cl. sporogenes. The Biochemical journal. 1935;29:288–90. doi: 10.1042/bj0290288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Woods DD. Studies in the metabolism of the strict anaerobes (genus Clostridium): Further experiments on the coupled reactions between pairs of amino-acids induced by Cl. sporogenes. The Biochemical journal. 1936;30:1934–46. doi: 10.1042/bj0301934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pagels M, Fuchs S, Pane-Farre J, Kohler C, Menschner L, Hecker M, et al. Redox sensing by a Rex-family repressor is involved in the regulation of anaerobic gene expression in Staphylococcus aureus. Molecular microbiology. 2010;76:1142–61. doi: 10.1111/j.1365-2958.2010.07105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jackson S, Calos M, Myers A, Self WT. Analysis of proline reduction in the nosocomial pathogen Clostridium difficile. Journal of bacteriology. 2006;188:8487–95. doi: 10.1128/JB.01370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Haslam SC, Ketley JM, Mitchell TJ, Stephen J, Burdon DW, Candy DC. Growth of Clostridium difficile and production of toxins A and B in complex and defined media. Journal of medical microbiology. 1986;21:293–7. doi: 10.1099/00222615-21-4-293. [DOI] [PubMed] [Google Scholar]
- [45].Karasawa T, Ikoma S, Yamakawa K, Nakamura S. A defined growth medium for Clostridium difficile. Microbiology. 1995;141:371–5. doi: 10.1099/13500872-141-2-371. Pt 2. [DOI] [PubMed] [Google Scholar]
- [46].Osgood DP, Wood NP, Sperry JF. Nutritional aspects of cytotoxin production by Clostridium difficile. Applied and environmental microbiology. 1993;59:3985–8. doi: 10.1128/aem.59.12.3985-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bouillaut L, Self WT, Sonenshein AL. Proline-dependent regulation of Clostridium difficile Stickland metabolism. Journal of bacteriology. 2013;195:844–54. doi: 10.1128/JB.01492-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Janoir C, Deneve C, Bouttier S, Barbut F, Hoys S, Caleechum L, et al. Adaptive strategies and pathogenesis of Clostridium difficile from in vivo transcriptomics. Infection and immunity. 2013;81:3757–69. doi: 10.1128/IAI.00515-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wietzke M, Bahl H. The redox-sensing protein Rex, a transcriptional regulator of solventogenesis in Clostridium acetobutylicum. Applied microbiology and biotechnology. 2012;96:749–61. doi: 10.1007/s00253-012-4112-2. [DOI] [PubMed] [Google Scholar]
- [50].Brekasis D, Paget MS. A novel sensor of NADH/NAD+ redox poise in Streptomyces coelicolor A3(2) The EMBO journal. 2003;22:4856–65. doi: 10.1093/emboj/cdg453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sickmier EA, Brekasis D, Paranawithana S, Bonanno JB, Paget MS, Burley SK, et al. X- ray structure of a Rex-family repressor/NADH complex insights into the mechanism of redox sensing. Structure. 2005;13:43–54. doi: 10.1016/j.str.2004.10.012. [DOI] [PubMed] [Google Scholar]
- [52].Gyan S, Shiohira Y, Sato I, Takeuchi M, Sato T. Regulatory loop between redox sensing of the NADH/NAD(+) ratio by Rex (YdiH) and oxidation of NADH by NADH dehydrogenase Ndh in Bacillus subtilis. Journal of bacteriology. 2006;188:7062–71. doi: 10.1128/JB.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schau M, Chen Y, Hulett FM. Bacillus subtilis YdiH is a direct negative regulator of the cydABCD operon. Journal of bacteriology. 2004;186:4585–95. doi: 10.1128/JB.186.14.4585-4595.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ravcheev DA, Li X, Latif H, Zengler K, Leyn SA, Korostelev YD, et al. Transcriptional regulation of central carbon and energy metabolism in bacteria by redox-responsive repressor Rex. Journal of bacteriology. 2012;194:1145–57. doi: 10.1128/JB.06412-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fujita Y. Carbon catabolite control of the metabolic network in Bacillus subtilis. Bioscience, biotechnology, and biochemistry. 2009;73:245–59. doi: 10.1271/bbb.80479. [DOI] [PubMed] [Google Scholar]
- [56].Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiology and molecular biology reviews : MMBR. 2006;70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Antunes A, Camiade E, Monot M, Courtois E, Barbut F, Sernova NV, et al. Global transcriptional control by glucose and carbon regulator CcpA in Clostridium difficile. Nucleic acids research. 2012;40:10701–18. doi: 10.1093/nar/gks864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dineen SS, McBride SM, Sonenshein AL. Integration of metabolism and virulence by Clostridium difficile CodY. Journal of bacteriology. 2010;192:5350–62. doi: 10.1128/JB.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sonenshein AL. CodY, a global regulator of stationary phase and virulence in Gram- positive bacteria. Current opinion in microbiology. 2005;8:203–7. doi: 10.1016/j.mib.2005.01.001. [DOI] [PubMed] [Google Scholar]
- [60].Slack FJ, Serror P, Joyce E, Sonenshein AL. A gene required for nutritional repression of the Bacillus subtilis dipeptide permease operon. Molecular microbiology. 1995;15:689–702. doi: 10.1111/j.1365-2958.1995.tb02378.x. [DOI] [PubMed] [Google Scholar]
- [61].Molle V, Nakaura Y, Shivers RP, Yamaguchi H, Losick R, Fujita Y, et al. Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis. Journal of bacteriology. 2003;185:1911–22. doi: 10.1128/JB.185.6.1911-1922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].den Hengst CD, van Hijum SA, Geurts JM, Nauta A, Kok J, Kuipers OP. The Lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. The Journal of biological chemistry. 2005;280:34332–42. doi: 10.1074/jbc.M502349200. [DOI] [PubMed] [Google Scholar]
- [63].Bennett HJ, Pearce DM, Glenn S, Taylor CM, Kuhn M, Sonenshein AL, et al. Characterization of relA and codY mutants of Listeria monocytogenes: identification of the CodY regulon and its role in virulence. Molecular microbiology. 2007;63:1453–67. doi: 10.1111/j.1365-2958.2007.05597.x. [DOI] [PubMed] [Google Scholar]
- [64].Hendriksen WT, Bootsma HJ, Estevao S, Hoogenboezem T, de Jong A, de Groot R, et al. CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization. Journal of bacteriology. 2008;190:590–601. doi: 10.1128/JB.00917-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Malke H, Ferretti JJ. CodY-affected transcriptional gene expression of Streptococcus pyogenes during growth in human blood. Journal of medical microbiology. 2007;56:707–14. doi: 10.1099/jmm.0.46984-0. [DOI] [PubMed] [Google Scholar]
- [66].Hsueh YH, Somers EB, Wong AC. Characterization of the codY gene and its influence on biofilm formation in Bacillus cereus. Archives of microbiology. 2008;189:557–68. doi: 10.1007/s00203-008-0348-8. [DOI] [PubMed] [Google Scholar]
- [67].van Schaik W, Chateau A, Dillies MA, Coppee JY, Sonenshein AL, Fouet A. The global regulator CodY regulates toxin gene expression in Bacillus anthracis and is required for full virulence. Infection and immunity. 2009;77:4437–45. doi: 10.1128/IAI.00716-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pohl K, Francois P, Stenz L, Schlink F, Geiger T, Herbert S, et al. CodY in Staphylococcus aureus: a regulatory link between metabolism and virulence gene expression. Journal of bacteriology. 2009;191:2953–63. doi: 10.1128/JB.01492-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Majerczyk CD, Sadykov MR, Luong TT, Lee C, Somerville GA, Sonenshein AL. Staphylococcus aureus CodY negatively regulates virulence gene expression. Journal of bacteriology. 2008;190:2257–65. doi: 10.1128/JB.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Levdikov VM, Blagova E, Colledge VL, Lebedev AA, Williamson DC, Sonenshein AL, et al. Structural rearrangement accompanying ligand binding in the GAF domain of CodY from Bacillus subtilis. Journal of molecular biology. 2009;390:1007–18. doi: 10.1016/j.jmb.2009.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Villapakkam AC, Handke LD, Belitsky BR, Levdikov VM, Wilkinson AJ, Sonenshein AL. Genetic and biochemical analysis of the interaction of Bacillus subtilis CodY with branched- chain amino acids. Journal of bacteriology. 2009;191:6865–76. doi: 10.1128/JB.00818-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Shivers RP, Sonenshein AL. Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched-chain amino acids. Molecular microbiology. 2004;53:599–611. doi: 10.1111/j.1365-2958.2004.04135.x. [DOI] [PubMed] [Google Scholar]
- [73].Ratnayake-Lecamwasam M, Serror P, Wong KW, Sonenshein AL. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes & development. 2001;15:1093–103. doi: 10.1101/gad.874201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Handke LD, Shivers RP, Sonenshein AL. Interaction of Bacillus subtilis CodY with GTP. Journal of bacteriology. 2008;190:798–806. doi: 10.1128/JB.01115-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Guedon E, Sperandio B, Pons N, Ehrlich SD, Renault P. Overall control of nitrogen metabolism in Lactococcus lactis by CodY, and possible models for CodY regulation in Firmicutes. Microbiology. 2005;151:3895–909. doi: 10.1099/mic.0.28186-0. [DOI] [PubMed] [Google Scholar]
- [76].Belitsky BR, Sonenshein AL. Genetic and biochemical analysis of CodY-binding sites in Bacillus subtilis. Journal of bacteriology. 2008;190:1224–36. doi: 10.1128/JB.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lopez JM, Dromerick A, Freese E. Response of guanosine 5'-triphosphate concentration to nutritional changes and its significance for Bacillus subtilis sporulation. Journal of bacteriology. 1981;146:605–13. doi: 10.1128/jb.146.2.605-613.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Morohashi M, Ohashi Y, Tani S, Ishii K, Itaya M, Nanamiya H, et al. Model-based definition of population heterogeneity and its effects on metabolism in sporulating Bacillus subtilis. Journal of biochemistry. 2007;142:183–91. doi: 10.1093/jb/mvm121. [DOI] [PubMed] [Google Scholar]
- [79].Ikeda D, Karasawa T, Yamakawa K, Tanaka R, Namiki M, Nakamura S. Effect of isoleucine on toxin production by Clostridium difficile in a defined medium. Zentralblatt fur Bakteriologie : international journal of medical microbiology. 1998;287:375–86. doi: 10.1016/s0934-8840(98)80174-6. [DOI] [PubMed] [Google Scholar]
- [80].Ochi K, Kandala J, Freese E. Evidence that Bacillus subtilis sporulation induced by the stringent response is caused by the decrease in GTP or GDP. Journal of bacteriology. 1982;151:1062–5. doi: 10.1128/jb.151.2.1062-1065.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Piggot PJ, Hilbert DW. Sporulation of Bacillus subtilis. Current opinion in microbiology. 2004;7:579–86. doi: 10.1016/j.mib.2004.10.001. [DOI] [PubMed] [Google Scholar]
- [82].Paredes-Sabja D, Shen A, Sorg JA. Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends in microbiology. 2014;22:406–16. doi: 10.1016/j.tim.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Pettit LJ, Browne HP, Yu L, Smits WK, Fagan RP, Barquist L, et al. Functional genomics reveals that Clostridium difficile Spo0A coordinates sporulation, virulence and metabolism. BMC genomics. 2014;15:160. doi: 10.1186/1471-2164-15-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Deakin LJ, Clare S, Fagan RP, Dawson LF, Pickard DJ, West MR, et al. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infection and immunity. 2012;80:2704–11. doi: 10.1128/IAI.00147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mackin KE, Carter GP, Howarth P, Rood JI, Lyras D. Spo0A differentially regulates toxin production in evolutionarily diverse strains of Clostridium difficile. PloS one. 2013;8:e79666. doi: 10.1371/journal.pone.0079666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Rosenbusch KE, Bakker D, Kuijper EJ, Smits WK. C. difficile 630Deltaerm Spo0A regulates sporulation, but does not contribute to toxin production, by direct high-affinity binding to target DNA. PloS one. 2012;7:e48608. doi: 10.1371/journal.pone.0048608. [DOI] [PMC free article] [PubMed] [Google Scholar]





