Abstract
Solid-state NMR studies of sedimented soluble proteins has been recently developed as an attractive approach for overcoming the size limitations of solution NMR spectroscopy and bypassing the need for sample crystallization or precipitation (Bertini et al. 2011). Inspired by the potential benefits of this method, we have investigated the ability to sediment lipid bilayer nanodiscs reconstituted with a membrane protein. In this study, we show that nanodiscs containing the outer membrane protein Ail from Yersinia pestis can be sedimented for solid-state NMR structural studies, without the need for precipitation or lyophilization. Optimized preparations of Ail in phospholipid nanodiscs support both the structure and the fibronectin binding activity of the protein. The same sample can be used for solution NMR, solid-state NMR and activity assays, facilitating structure-activity correlation experiments across a wide range of timescales.
Keywords: nanodisc, NMR, sedimentation, membrane protein, Ail, yersinia
INTRODUCTION
The preparation of natively folded and active membrane proteins is a key requirement for structural studies aimed at correlating molecular structure with biological function (Zhou and Cross 2013). NMR spectroscopy is particularly well-suited for examining protein structure and dynamics in samples that are very close to their functional environments, and for establishing structure-activity correlations by direct spectroscopic detection of ligand binding events or conformational changes (Shuker et al. 1996). However, while it is relatively straightforward to mimic the homogeneous physical and chemical properties of the isotropic aqueous environment surrounding soluble proteins, reconstituting a heterogeneous environment that mimics the anisotropic properties of the lipid bilayer membrane presents additional challenges. For example, even in cases where detergent micelles support native fold, the interaction of detergents with water-exposed regions of membrane proteins can interfere with ligand-binding activity precluding parallel structure-activity studies aimed at understanding biological function.
Progress on NMR structure determination of membrane proteins is documented in the protein data bank (PDB) and highlighted in the database of membrane proteins structures determined by NMR (Warschawski). Solid-state NMR methods can be used for proteins in a variety of lipid sample environments including: liposomes, supported planar lipid bilayers, macrodiscs, lipid-detergent bicelles, precipitated lipids or nanodisc preparations (Drechsler and Separovic 2003; McDermott 2009; Sharma et al. 2010; Maltsev and Lorigan 2011; Park et al. 2011; Durr et al. 2012; Franks et al. 2012; Hong et al. 2012; Orwick-Rydmark et al. 2012; Park et al. 2012; Ding et al. 2013; Gopinath et al. 2013; Loquet et al. 2013; Mors et al. 2013; Murray et al. 2013; Ni et al. 2013; Tang et al. 2013; Ullrich and Glaubitz 2013; Wang et al. 2013; Weingarth and Baldus 2013; Sackett et al. 2014). Solution NMR studies can be performed on membrane proteins in detergent micelles (Arora and Tamm 2001; Fernandez and Wuthrich 2003; Sanders and Sonnichsen 2006; Chill et al. 2007; Poget and Girvin 2007; Prosser et al. 2007; Teriete et al. 2007; Hiller and Wagner 2009; Kim et al. 2009; Zhou et al. 2008; Berardi et al. 2011; Wang and Tjandra 2013; Fox et al. 2014) and, more recently, lipid bilayer nanodiscs are being used effectively for solution NMR studies of membrane proteins (Gluck et al. 2009; Raschle et al. 2009; Shenkarev et al. 2009; Shenkarev et al. 2010; Etzkorn et al. 2013; Hagn et al. 2013; Shenkarev et al. 2013; Tzitzilonis et al. 2013; Bibow et al. 2014; Susac et al. 2014).
The ability to perform NMR studies on one sample in both the solid-state and in solution could extend the range of dynamics and functional properties that can be probed by NMR for a given protein. The recent demonstration, by Bertini and coworkers (Bertini et al. 2011; Bertini et al. 2012a), that large soluble proteins can be sedimented either in situ, by the G forces active during magic angle spinning (MAS) solid-state NMR experiments, or by ultracentrifugation, before transfer to the MAS rotor, provide an avenue for this approach. As noted by the authors (Bertini et al. 2011), “Ultracentrifuged proteins are usually well behaved. The sediment resolubilizes as soon as the centrifugal force is removed, and the protein generally maintains its native state throughout”. Evidence of protein functionality in the sedimented state is provided by equilibrium sedimentation in many studies. Conversely, precipitation and lyophilization can compromise biological function.
Inspired by this work we have examined the ability to sediment nanodiscs, containing the outer membrane protein Ail, for solid-state NMR studies. Previous solid-state NMR studies of proteins in nanodiscs used precipitation with polyethylene glycol (PEG) or lyophilization to pack nanodisc samples in the limited volume of a MAS rotor (Li et al. 2006; Kijac et al. 2007; Mors et al. 2013; Kijac et al. 2010; Boettcher et al. 2011). However, PEG precipitation has been reported to disrupt nanodiscs prepared with 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) (Kijac et al. 2010). Furthermore, precipitation and lyophilization are not always compatible with protein activity.
Ail is a virulence factor from Yersinia pestis, that mediates bacterial cell adhesion to human cells and endows the bacterium with resistance to human innate immunity. The interactions of Y. pestis Ail with the extracellular matrix proteins fibronectin and laminin have been shown to be important for mediating bacterial cell adhesion (Tsang et al. 2010; Yamashita et al. 2011; Tsang et al. 2012). Amino acid residues in the protein’s four extracellular loops (EL1-EL4) have been shown to play important roles in the adhesion of Y. pestis and the related Yersinia species Y. enterocolitica (Miller et al. 2001; Tsang et al. 2013) as well as in the invasion and serum resistance of Y. enterocolitica (Miller et al. 2001).
The crystal structure of Ail has been determined in tetraethylene glycol monooctyl ether (C8E4) at high resolution (Yamashita et al. 2011). Ail adopts an eight-stranded β-barrel conformation similar to that of its homolog OmpX. However, several residues in the functionally important loops EL2 and EL3 are disordered and incompletely resolved in the crystal structure, and little is known about the loop-mediated interactions of Ail with its human host partners.
Recently, we assigned the solution NMR spectrum of Ail in n-decyl-phosphocholine (DePC) micelles and showed that the protein adopts the correct β-barrel fold in this detergent (Ding et al. 2015). However, we found that the high detergent concentration required for high-resolution NMR spectroscopy is not compatible with ligand binding activity, precluding NMR mapping experiments aimed at correlating structure with activity. By contrast, the protein embedded in lipid bilayer nanodiscs is fully functional and also yields well-resolved solution NMR spectra. However, these spectra have broader lines and may not be amenable to chemical shift mapping studies with large protein ligands of Ail, such as fibronectin, a large disulfide-linked homodimer of 500 kD subunits.
Here we show that it is possible to sediment phospholipid nanodiscs reconstituted with Ail for solid-state NMR experiments. Optimized preparations of Ail in nanodiscs support both activity and structure, and can be used for parallel activity and NMR studies on exactly the same samples. Sedimentation in an ultracentrifuge yields dense transparent preparations, highly enriched in Ail nanodiscs, that can be used for solid-state NMR experiments aimed at obtaining correlated structural and activity information.
MATERIALS AND METHODS
Expression and purification of membrane scaffold proteins
Two variants of the membrane scaffold protein (MSP), needed for nanodisc assembly, were expressed and purified as described previously: MSP1E3D1, which contains three additional helices (repeats of helices 4, 5 and 6) compared to the MSP1D1 sequence (Denisov et al. 2007), and MSP1D1-Δh5, which lacks helix 5 of MSP1D1 (Hagn et al. 2013). The pET-28a MSP1E3D1 plasmid was obtained from Addgene (Addgene plasmid 20066). The nucleotide encoding MSP1D1Δh5 was obtained from GenScript and cloned into plasmid pET-28a (EMD) as described (Ding et al. 2015). The MSPs were purified by Ni-affinity chromatography and the C-terminal His tags were removed by proteolysis with tobacco etch virus.
Expression and purification of Ail
Wild-type Ail and C-terminal His-tagged Ail (Ail-His) were cloned in the E. coli plasmid pET-30b, expressed and purified as described previously (Ding et al. 2015). The expressed amino acid sequences of Ail and Ail-His begin with an extra N-terminal methionine before residue Glu1 of the native sequence. The sequence of Ail terminates with the native residue Phe156, while Ail-His includes 33 additional C-terminal residues from the His tag of the plasmid vector. For 15N and 13C labeling of Ail, bacteria were grown in M9 medium containing 1 g/L of U-99%15NH4Cl and 2 g/L of U-99%13C-glucose (Cambridge Isotope Laboratories) as the sole sources of N and C atoms.
Preparation of Ail in micelles, vesicles and nanodiscs and functional assays
Ail was refolded in n-decyl-phosphocholine (DePC; Anatrace) and samples were prepared for solution NMR in DePC micelles, phospholipid liposomes and phospholipid nanodiscs, as described (Ding et al. 2015). Protein folding was assessed by monitoring the shift in SDS-PAGE apparent molecular weight that correlates with the transition from unfolded to folded states of transmembrane β-barrels (Tamm et al. 2004) and by NMR spectroscopy as illustrated in the text. The DePC concentration was estimated by monitoring the intensity of the 1H NMR peak from the trimethylamino protons at 3.15 ppm. The optimal Ail/MSP/lipid ratio for Ail-containing nanodiscs was determined as described (Ding et al. 2015), by screening with size exclusion chromatography (Superdex 75 10/300 GL column, GE Healthcare), performed in nanodisc buffer (20 mM Tris-Cl, pH 7.5, 100 mM NaCl, 1 mM EDTA) and monitored by detecting the UV absorbance at 280 nm of Ail and MSP. Enzyme-linked immunosorbent assays (ELISA) were performed as described (Ding et al. 2015), using Ail-His incorporated in MSP1D1Δh5 nanodiscs, MSP1E3D1 nanodiscs or DePC micelles.
Nanodisc sedimentation
Nanodisc sedimentation experiments were performed using a Beckman Airfuge equipped with an A/100-30 rotor, at room temperature and 90,000 rpm (160,000 × g). The extent of sedimentation was assessed by measuring the UV absorbance at 280 nm (A280) of the supernatant 170 μL fraction and bottom 50 μL fraction, obtained in the 240 μL centrifuge tube.
Samples for NMR spectroscopy
The samples of Ail in micelles, for solution NMR studies, contained 0.5 mM Ail (4 mg), 170 mM DePC and NMR buffer (20 mM Na-PO4, pH 6.8, 5 mM NaCl, 1 mM EDTA, 10% D2O), in a volume of 450 μL. The samples of Ail in MSP1D1Δh5 nanodiscs, for solution NMR studies, contained 0.5 mM Ail (4 mg), 37.5 mM DMPC (11.5 mg), 12.5 mM DMPG (3.9 mg) and NMR buffer, in a volume of 450 μL. The samples of Ail in sedimented nanodiscs, for solid-state NMR studies, contained 5.5 mM Ail (3.5 mg), 825 mM DMPC (20 mg), 275 mM DMPG (6.8 mg) and NMR buffer, in a volume of 36 μL inside the MAS rotor. The samples of Ail in proteoliposomes for solid-state NMR studies contained 2.4 mM Ail (1.5 mg), 740 mM DMPC (18 mg) and NMR buffer, in a volume of 36 μL inside the MAS rotor.
NMR experiments
Solution NMR experiments were performed on a Bruker AVANCE 600 MHz spectrometer equipped with a 1H/15N/13C triple-resonance cryoprobe. TROSY-based (Pervushin et al. 1997; Salzmann et al. 1998) 1H-15N correlation NMR spectra were obtained with 16 transients for 2H/15N labeled Ail in nanodiscs, or 8 transients for 2H/15N labeled Ail in DePC, for each of 256 t1 points. Backbone CA and CB chemical shifts were taken from the deposited (BMRB accession: 25281) resonance assignments for Ail in 170 mM DePC (Ding et al. 2015).
Solid-state NMR studies were performed on a Bruker AVANCE 500 MHz spectrometer equipped with a Bruker 1H/15N/13C, 4 mm, Efree MAS probe and a home-built 1H/15N, 5 mm Efree, static probe, or on a Bruker AVANCE 700 MHz spectrometer equipped with a home-built 1H/15N/13C, 3.2 mm, Efree MAS probe.
One-dimensional 31P spectra were acquired using a Hahn echo pulse sequence with continuous wave 1H decoupling. One-dimensional 13C and 15N spectra were acquired on the 500 and 700 MHz spectrometers, using CP (cross polarization) (Pines et al. 1973) or INEPT (Insensitive Nuclei Enhancement by Polarization Transfer) (Morris and Freeman 1979) pulse sequences. CP spectra were obtained with 1H-13C or 1H-15N contact times of 400 μs or 2 ms and TPPM or SWf-TPPM sequences for 1H decoupling. INEPT 15N spectra were obtained with a magnetization transfer time of 2.5 ms. INEPT 13C spectra were obtained with a transfer time of 1.67 ms and a 1.11 ms refocusing delay.
Two-dimensional solid-state NMR 13C-13C correlation spectra were acquired on the 700 MHz spectrometer, using PDSD (proton driven spin diffusion) (Szeverenyi et al. 1982), with a 1H-13C contact time of 400 μs, a mixing time of 50 ms and SWf-TPPM 1H decoupling. A total of 32 transients (sedimented nanodiscs) or 512 transients (proteoliposomes) were acquired for each of 1,024 t1 points.
The NMR data were processed using NMRPipe (Delaglio et al. 1995) and analyzed using NMRView (Johnson and Blevins 1994).
RESULTS AND DISCUSSION
Preparation and solution NMR of Ail nanodiscs
Y. pestis Ail can be refolded in a variety of detergent and lipid samples suitable for solution and solid-state NMR studies (Plesniak et al. 2011; Ding et al. 2013; Ding et al. 2015). To examine the usefulness of Ail nanodiscs for structural and ligand-binding activity studies, we prepared two types of samples with different nanodisc sizes. The shorter scaffold protein, MSP1D1Δh5, has been developed specifically for the preparation of smaller nanodiscs to facilitate solution NMR studies of membrane proteins (Hagn et al. 2013). While the longer the longer scaffold protein, MSP1E3D1, has been developed for biochemical studies of larger membrane protein and their complexes (Denisov et al. 2007).
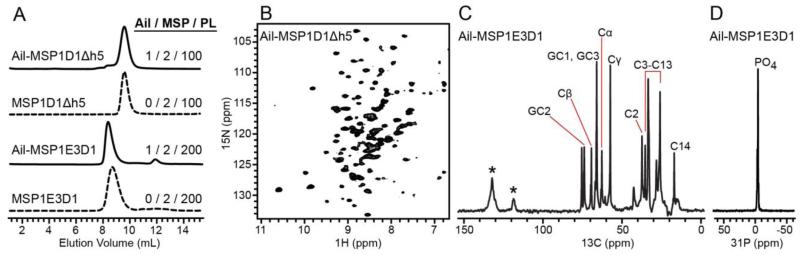
Optimal preparations of Ail in MSP1D1Δh5 nanodiscs were obtained with a molar ratio of 1/2/100 (Ail / MSP / phospholipid) (Ding et al. 2015). Analysis by size exclusion chromatography (Fig. 1A) shows that these preparations have a narrow elution profile and homogeneity similar to empty nanodiscs. Indeed, 15N labeled Ail incorporated in MSP1D1Δh5 nanodiscs yields a well-dispersed 1H/15N correlation NMR spectrum with many well-resolved peaks of homogeneous intensity (Fig. 1B). Although the 1H and 15N resonance lines are broader than those observed in the spectra of Ail in DePC micelles (Fig. 2B), the nanodisc and micelle spectra also share many similarities, indicating that the protein adopts a similar fold in the two environments. Many peaks, including several from Gly, Trp and Phe residues, appear at nearly identical positions and can be tentatively assigned to specific sites by comparison with the assigned spectrum of Ail in DePC (BMRB accession: 25281).
Figure 1. Characterization of Ail nanodiscs.
(A) Size exclusion chromatography of Ail nanodiscs (solid line) or empty nanodiscs (dashed lines) with optimized molar ratios of Ail to MSP to phospholipid (Ail / MSP / PL). (B) TROSY 1H-15N correlation NMR spectrum of 15N labeled Ail in MSP1D1Δh5 nanodiscs in solution (0.5 mM Ail). (C) INEPT 13C NMR spectrum of 13C labeled Ail in MSP1E3D1 nanodiscs in solution (0.5 mM Ail). Assigned lipid signals were derived from published data (Oldfield and Chapman 1971; Birdsall et al. 1972; Levine et al. 1972; Haberkorn et al. 1978); asterisks indicate signals from protein aromatic sites. (D) Hahn echo 31P-detected 1H-decoupled spectrum of Ail-MSP1E3D1 nanodiscs in solution (0.5 mM Ail).
Figure 2. Fibronectin-bnding activity of Ail in nanodiscs and detergent.
(A) Purified refolded Ail-His in DePC or in nanodiscswas added at increasing concentrations to fibronectin-coated plates and incubated overnight. Binding was detected by ELISA using a mouse anti-His antibody. Ail-His in MSP1D1Δh5 or MSP1E3D1 nanodiscs bind fibronectin (black solid lines). Ail-His in 170 mM DePC (green) does not bind fibronectin. Empty nanodiscs lacking Ail-His were probed with anti-ApoA1 antibody and do not bind fibronectin (black dotted line). Each point in each data set represents the average of three experiments. (B) TROSY 1H-15N correlation NMR spectrum of Ail in 170 mM DePC solution (0.5 mM Ail). (C) TROSY 1H-15N correlation NMR spectra of Ail in MSP1D1Δh5 nanodiscs in solution obtained in the absence of detergent (black), and after addition of 4 mM DePC (orange) or 11 mM DePC (green).
Homogenous preparations were also obtained for Ail in MSP1E3D1 nanodiscs, at a molar ratio of 1/2/200 (Ail / MSP / phospholipid). Consistent with the larger size of these nanodiscs, size exclusion chromatography shows that they elute significantly faster than their smaller MSP1D1Δh5 counterparts, at an elution volume that is similar to that of empty MSP1E3D1 nanodiscs (Fig. 1A).
The larger size of these nanodiscs prevents protein signals to be detected in the solution NMR spectra of 13C labeled Ail. The 13C INEPT spectrum (Fig. 1C) is dominated by signals from the natural abundance 13C in the phospholipids, while only weak signals from protein aromatic side chain sites are visible. Hydrodynamic diameters in the range of 14 nm have been reported for nanodiscs prepared with MSP1E3D1 (Denisov et al. 2007). The solid-state NMR 31P spectrum of the nanodisc lipids displays a single sharp peak at the isotropic frequency of the lipid and buffer phosphate groups (Fig. 1D). The spectrum shows no sign of the characteristic, motionally averaged powder pattern, that is observed for liquid crystalline phospholipid bilayer assemblies larger than ~150 nm (Burnell et al. 1980). These results are consistent with the known effects of phospholipid dynamics on their NMR spectra, as described in an extensive body of seminal papers in this area (Oldfield and Chapman 1971; Birdsall et al. 1972; Levine et al. 1972; Bloom et al. 1977; Seelig 1977; Bloom et al. 1978; Haberkorn et al. 1978; Seelig 1978; Forbes et al. 1988; Oldfield et al. 1987; Auger 2000; Macdonald et al. 2013).
Molecular dynamics simulations indicate that OmpX is surrounded by well over a full ring of phospholipids, when it’s embedded in a nanodisc formed with MSP1D1Δh5 and 40 molecules of DMPC per bilayer leaflet, consistent with thermal unfolding experiments showing that these smaller nanodiscs provide a stable environment for the protein (Hagn et al. 2013). In the case of Ail, we found that MSP1D1Δh5 nanodiscs with 50 phospholipids per bilayer leaflet have the highest homogeneity and best solution NMR characteristics (Ding et al. 2015) and, since 40 lipids were sufficient for stabilizing OmpX, we conclude that 50 must also provide a stable membrane environment for Ail. For an Ail cross-barrel diameter of ~2 nm, estimated from the crystal structure (Yamashita et al. 2011), about 7 molecules of DMPC, each with a surface area of 0.6 nm2, would be needed to form a single ring of lipids around the circumference of the barrel. The larger nanodiscs, prepared with MSP1E3D1, contained 100 phospholipid molecules per bilayer leaflet (Fig. 1), and therefore enable a membrane of 5 rings of phospholipid to form around the embedded protein. This 1/200 protein/lipid ratio is in the low range of what is typically used in solid-state NMR studies of membrane proteins in liposomes, oriented bicelles, and oriented planar bilayers (Das et al. 2013).
Ligand-binding activity of Ail in nanodiscs and effect of detergent
We next tested the ability of Ail to bind fibronectin-coated surfaces in ELISAs. The data in Fig. 2A (black) show that the fibronectin binding activity of Ail can be reconstituted in smaller MSP1D1Δh5 nanodiscs as well as the larger MSP1E3D1 nanodiscs; in both cases, Ail exhibits concentration-dependent binding to fibronectin-coated plates. The activity can be fully ascribed to Ail since assays performed with empty nanodiscs yield no ELISA signal. Conversely, no fibronectin binding is detected for Ail in 170 mM DePC (Fig. 2A, green), even though this detergent concentration is optimal for solution NMR spectroscopy, and the 1H-15N correlation spectrum of Ail in 170 mM DePC has narrow, homogenous resonance linewidths and large chemical shift dispersions, consistent with conformational order and relatively uniform backbone dynamics across the entire length of the polypeptide (Fig. 2B).
Recently (Ding et al. 2015), we reported that Ail adopts its native eight-stranded β-barrel fold in DePC micelles, with a conformation that is very similar to the crystal structure (Yamashita et al. 2011) and backbone dynamics that are consistent with flexibility in the functionally important extracellular loops. However, we also found that the fibronectin-binding activity of Ail is disrupted at the high detergent concentrations required for NMR studies. The data in Fig. 2C show that addition of even small amounts of DePC to Ail in nanodiscs has a profound effect on the NMR spectrum, with peaks from the water-exposed loops affected to a greater extent (Fig. 2C). For example, the signal from G60, situated in the second extracellular loop (EL2) of Ail, is perturbed by as little as 4 mM DePC, a concentration well below the critical micelle concentration of 11 mM DePC. On the other hand, signals from G76 and G153 in the transmembrane β-barrel are not highly affected. Since the extracellular loops of Ail are involved in mediating its interaction with fibronectin, the disruption of fibronectin binding in detergent micelles appears to be caused by the interaction of monomeric detergent molecules with key water-exposed sites on Ail and, possibly, also on fibronectin. Thus we conclude that although both detergents and nanodiscs support the native structure of Ail, detergents interfere with ligand binding activity, precluding parallel structure-activity correlation studies.
It is well known that detergents can have adverse effects on membrane protein structure and function (Zhou and Cross 2013). Monomeric detergent exists in equilibrium with micelles, and is sometimes found associated with crystallized membrane proteins, including Ail (Yamashita et al. 2011). Recent studies have shown that the extracellular loops of outer membrane β-barrels are more susceptible to the surrounding environment than their transmembrane regions. For example, the detergent environment has been reported to affect the structures and dynamics of the water-exposed loops of OmpX, OmpA and Opa to greater extent than their membrane-embedded β-barrels (Hagn et al. 2013; Susac et al. 2014; Fox et al. 2014).
Small nanodiscs prepared with MSP1D1Δh5 and MSP1D1 have been used for solution NMR studies of membrane proteins, including structure determination of the outer membrane proteins OmpX and OmpA (Hagn et al. 2013; Susac et al. 2014). Nanodiscs are detergent-free and provide a soluble phospholipid bilayer environment that retains the essential anisotropic physical and chemical properties of biological membranes (Ritchie et al. 2009). Furthermore, compared to spherical liposomes, the open configuration of nanodiscs facilitates structure-activity studies where the addition of ligands is detected by direct spectroscopic comparisons.
However, nanodisc samples may not be generally suitable for solution NMR studies of all membrane proteins, and are likely to be inappropriate for chemical shift mapping studies with Ail and its high molecular weight human ligands, such as fibronectin. While the increased size of such complexes may be prohibitive for solution NMR spectroscopy, solid-state NMR has no physical size limitations and can be used with larger or motionally restricted lipid bilayer assemblies for structure-activity studies of membrane proteins in detergent-free native-like samples.
Solid-state NMR of sedimented Ail nanodiscs
We first tested sedimentation of 15N labeled Ail in the MSP1D1Δh5 nanodiscs that yield high-resolution solution NMR spectra at the relatively dilute concentration of 0.5 mM Ail. Rather then attempting to sediment the sample in situ, as described originally by Bertini and coworkers (Bertini et al. 2011), we centrifuged the sample before transferring it to the MAS rotor, as described for soluble proteins (Bertini et al. 2012b; Gardiennet et al. 2012), so as to obtain a higher concentration of isotopically labeled protein for NMR studies.
Centrifugation in the A/100-30 rotor of a Beckman Airfuge at 160,000 × g, for 4 hours yielded a dense viscous, visually transparent sediment in the bottom 50 μL fraction of the 240 μL centrifuge tube, overlaid by a fluid top fraction with the consistency of water. When this sedimented fraction was transferred to the MAS rotor for solid-state NMR experiments, abundant 15N signal could be detected by 1H CP with a slow spinning frequency of 5 kHz (Fig. 3A). However, no signal was detected with an INEPT experiment (Fig. 3B). INEPT pulse sequences have been used to detect fast lipid and protein dynamics (Andronesi et al. 2005; Warschawski and Devaux 2005). Therefore, the data in Fig. 3 indicate that the rotational motion of the nanodiscs is sufficiently restricted for solid-state NMR studies.
Figure 3. NMR spectra of Ail-MSP1D1Δh5 nanodiscs obtained after sedimentation (A, B) and subsequent redilution (C).
(A, B) Solid-state NMR 15N spectra of sedimented Ail nanodiscs obtained with CP (A) or INEPT (B) under 5 kHz MAS, at 25°C. (C) TROSY 1H-15N correlation solution NMR spectra obtained for Ail nanodiscs before sedimentation (black; 0.5 mM Ail) and after re-dilution of the sedimented sample (red; 0.5 mM Ail) at 45°C.
Dilution of the sedimented fraction back to the original 0.5 mM concentration, yielded a solution NMR 1H/15N spectrum that is essentially identical to the original spectrum obtained prior to centrifugation (Fig. 3C). This result, together with the transparent appearance of the sample after sedimentation, leads us to conclude that concentration of the Ail-MSP1D1Δh5 nanodiscs is not due to nonspecific precipitation or aggregation but rather to sedimentation under the high G forces of the centrifuge.
Encouraged by this result we performed a similar experiment with 13C labeled Ail incorporated in the larger MSP1E3D1 nanodiscs. As observed for the smaller nanodiscs, centrifugation at 160,000 × g, for 18 hrs yielded a transparent, viscous sediment at the bottom of the centrifuge tube. UV absorbance measurements indicate that the bottom 50 μL fraction is enriched in protein by a factor of 22 compared to the top fluid fraction (Table 1).
Table 1.
Sedimentation efficiency of nanodisc samples. Measurements were made after centrifugation in the A/100-30 rotor of a Beckman Airfuge (90,000 rpm room temperature 18 hrs)
| Sample | initial sample | top fractiona | sedimented fractiona |
% enrichment in sedimented fraction |
|||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Vi (μl) | A280 - i | Vtop (mL) |
A280 - top | Vsed (μL) | A280 - sed | ||
|
| |||||||
| MSP1D1Δh5 | 200 | 0.891 | 150 | 0.890 | 50 | 0.89 | 0 |
| Ail - MSP1D1Δh5 | 220 | 0.331 | 170 | 0.095 | 50 | 0.96 | 71 |
| MSP1E3D1 | 135 | 0.550 | 90 | 0.433 | 45 | 0.73 | 21 |
| Ail - MSP1E3D1 | 200 | 0.643 | 150 | 0.104 | 50 | n.d.a | 84 |
Value not determined.
This sedimented fraction gave an excellent one dimensional 13C MAS solid-state NMR spectrum by through-space 1H-13C CP (Fig. 4A). On the other hand, through-bond transfer of polarization by INEPT gave a 13C spectrum that contains only signals from the natural abundance 13C in the phospholipids (Fig. 4B), and is essentially identical to the 13C INEPT spectrum of the diluted nanodiscs observed by solution NMR without MAS (Fig. 1C). The 31P NMR spectrum of this sedimented fraction (Fig. 4C) now displays the powder pattern characteristic of liquid crystalline phospholipids in a bilayer arrangement (additional signal at the isotropic 31P chemical shift frequency arises from Na phosphate in the NMR buffer), further confirming that the rotational motion of the sedimented nanodiscs is sufficiently restricted to enable observation by solid-state NMR spectroscopy.
Figure 4. One-dimensional solid-state NMR spectra of sedimented Ail-MSP1E3D1 nanodiscs (5.5 mM Ail) obtained at 25°C (A-C) or 10°C (D-F).
(A, D) 13C spectra obtained with CP (black) or DP (red) and 10 kHz MAS. (B, E) 13C spectra obtained with INEPT at 10 kHz MAS. (C, F) 31P spectra obtained with DP for static sample. Isotropic signal intensity (~0 ppm) is attributed to the presence of Na phosphate in the NMR buffer.
Similar experiments performed at 10°C, show that the CP spectrum (Fig. 4D, black) is enhanced by a factor of 2 compared to its counterpart obtained by direct 13C polarization (Fig. 4D, red), further indicating that the dipolar interactions are not averaged by fast rotational motions and can be exploited to enhance the NMR signal by CP, as intended (Pines et al. 1973). The broadening seen at the edges of the 31P powder pattern acquired at 10°C (Fig. 4F) is consistent with the phospholipid bilayer transition from liquid crystalline to gel phase, which occurs at 23°C for DMPC and DMPG. Thus, the data show that the sedimented nanodiscs are motionally restricted and exhibit the correct properties of intact well-behaved phospholipid bilayers.
Furthermore, the two-dimensional 13C correlation spectrum (Fig. 5) shows that Ail maintains its structure in the sedimented Ail MSP1E3D1 nanodiscs. The spectrum, obtained at 27°C, above the phase transition of the lipids DMPC and DMPG, displays several resolved peaks from 13C labeled Ail, as well as intense diagonal intensity from the natural abundance 13C of the lipids. Equally good spectra were obtained at 27°C, 5°C and 0°C.
Figure 5. Two-dimensional solid-state NMR 13C correlation spectra of Ail in sedimented MSP1E3D1 nanodiscs (black; 5.5 mM Ail) or proteoliposomes (red; 2.4 mM Ail).
Spectra were recorded at 27°C (for sedimented nanodiscs) or 0°C (for liposomes), with 10 kHz MAS, using PDSD with 32 scans for sedimented nanodiscs or 512 scans for liposomes. (A) Boxes highlight signals with chemical shifts characteristic for Ala, Ile, Pro and Thr residues. (B) Selected region of the spectra showing the Cα-Cβ correlations derived from the assigned spectrum of Ail in 170 mM DePC (green). (C, D) Expanded regions of the spectra.
The spectral resolution is comparable to that obtained with Ail in proteoliposomes at 0°C (Ding et al. 2013), with many peaks at identical or similar positions. However, while the spectrum of Ail in sedimented nanodiscs was obtained with 32 scans (18 hr acquisition), the spectrum from liposomes was obtained with 512 scans (42 hr acquisition), due to the lower concentration of Ail in the latter. It is possible that the open geometry of nanodiscs affords greater extent of sample concentration and greater filling factor than spherical liposomes, a potentially important benefit associated with their use in NMR experiments.
In the 13C correlation spectrum, peaks from the Ala, Ile, Pro, Ser, and Thr residues are detected at chemical shifts consistent with β-sheet conformation, and several of these signals are well resolved. Comparison with the Cα-Cβ peak positions derived from the chemical shifts assigned in the solution NMR spectrum of Ail in DePC micelles (Fig. 5B) reveals many similarities, demonstrating that the protein adopts the same overall structure in detergent as in lipid bilayers.
Expanded regions of the spectrum show that several peaks can be tentatively assigned by direct comparison with the solution NMR spectrum of Ail in DePC (Fig. 5C). For example, peaks from Cβ-Cα and Cβ-Cγ correlations are observed for all four Thr residues in the protein, and can be tentatively assigned from the corresponding solution NMR resonances. All four Thr Cβ-Cγ peaks have similar intensities. However, the intensities of the Cβ-Cα peaks vary, with strong signals observed for T113 and T147, located in transmembrane β-strands β6 and β8, and weaker signals for T50 in the second extracellular loop and T74 in transmembrane β4.
Similarly, peaks from Cβ-Cα and Cδ-Cα correlations of the four Pro residues can be clearly resolved and tentatively assigned, as can several Cβ-Cα signals from the nine Ala residues (Fig. 5D). The positions of Cβ-Cα peaks from the sixteen Ser residues also correlate well with their solution NMR chemical shifts measured in DePC (Fig. 5C). Furthermore, peaks correlating Cδ and Cγ to Cβ side chain sites are detected for the seven Ile residues of Ail (Fig. 5A). The ability to resolve peaks from extracellular loop sites (e.g. T50, D23) will be particularly useful for chemical shift mapping experiments aimed at characterizing the interactions of Ail with its ligands.
Sedimentation properties of nanodiscs
As described by Bertini and coworkers (Bertini et al. 2011; Bertini et al. 2012a), it is possible to estimate the sedimentation properties of a molecular species in a solvent using equations derived over the years by the large field of protein ultracentrifugation (Laue and Stafford 1999). To understand the sedimentation behavior of nanodiscs, we used these equations to calculate various hydrodynamic parameters (Table 2). The time (tSED) required for sedimentation was estimated using the Svedberg equation, tSED = 2.533 × 1011 (k/S), where the k factor reflects the rotor’s pelleting efficiency and depends on the rotor’s geometry and angular velocity, while the sedimentation coefficient, S, depends on the hydrodynamic properties of the nanodisc assembly.
Table 2.
Calculated sedimentation properties of empty and Ail-containing nanodiscs.
| MSP1D1Δh5 | Ail - MSP1D1Δh5 | MSP1E3D1 | Ail - MSP1E3D1 | |
|---|---|---|---|---|
|
| ||||
| nAil | 0 | 1 | 0 | 1 |
| nMSP | 2 | 2 | 2 | 2 |
| nPC | 70 | 70 | 140 | 140 |
| nPG | 30 | 30 | 60 | 60 |
| Mn (g/mol) a | 107,097 | 124,688 | 196,205 | 213,797 |
| vn (mL/g) b | 0.92 | 0.90 | 0.93 | 0.92 |
| MB (g/mol) c | 7,431 | 10,402 | 11,824 | 14,795 |
| Rh (nm) d | 4.6 | 4.6 | 7.0 | 7.0 |
| f (g/sec) e | 0.89 × 10−7 | 0.89 × 10−7 | 1.35 × 10−7 | 1.35 × 10−7 |
| S (S) f | 1.35 | 1.89 | 1.41 | 1.76 |
| tSED (hr) g | 16.1 | 11.5 | 15.4 | 12.3 |
The molecular weight of the nanodisc (Mn) was estimated from the number of molecules (n) and molecular weights (M) of the individual protein and lipids in the nanodisc, as: Mn = nAil MAil + nMSP MMSP + nPC MDMPC + nPG MDMPG, where MAil = 17,592 Da; MMSPID1Δh5 = 19,488 Da; MMSP1E3D1 = 29,982 Da; MDMPC = 678 Da and MDMPG = 689 Da.
The partial specific volume of the nanodisc (Vn) was estimated from the partial specific volumes of protein (vp = 0.82 mL/g) (Ferella et al. 2013), DPMC (vDMPC = 0.98 mL/g) (Nagle and Tristram-Nagle 2000) and DMPG (vDMPG = 0.94 mL/g) (Pan et al. 2012), as: Vn = (Vp nAil MAil + vp nMSP MMSP + VDMPC nPC MDMPC + VDMPG nPG MDMPG) / Mn.
The buoyant mass of the nanodisc (MB) was estimated from Mn, vn and the solvent’s density (ρsol = 1.014 g/mL), as: MB = Mn (1 − vn ρsol). The value of ρsol for NMR buffer was estimated using the SEDNTERP software.
Values of the hydrodynamic radius (Rh) were taken from experimental size exclusion chromatography analyses of MSP1D1Δh5 (Hagn et al. 2013) and MSP1E3D1 (Denisov et al. 2007) nanodiscs.
The frictional coefficient (f) was derived from Rh and the viscosity of the solvent (ηsol = 0.01 g/mL), as: f = 6π ηsol Rh. The value of ηsol for NMR buffer was estimated using the SEDNTERP software.
The sedimentation coefficient (S) was estimated from Avogadro’s number (NA), MB and f, as: S = (MB / f) × (1013 / NA), where the factor 1013/NA yields S in Svedberg units (S), defined as 1S = 10−13 sec.
tsED = 2.533 × 1011 (k/S), where k = 21.7 for the A-100/30 Beckman Airfuge rotor.
The air-driven centrifuge rotor that we used to obtain nanodisc sedimentation has a very favorable pelleting efficiency, with a k factor of 21.7 achieved at 90,000 rpm (160,000 × g), near the rotor’s maximum angular velocity of 92,000 rpm. Furthermore, the small 240 μL volume of each of the six centrifuge tubes that fit the rotor helps minimize sample loss. The value of S for each nanodisc was derived from the known hydrodynamic properties of proteins and lipids in water, the stoichiometry and molecular weights of the protein and lipid nanodisc components, and measurements of the hydrodynamic radii of MSP1D1Δh5 and MSP1E3D1 nanodiscs obtained by size exclusion chromatography (Hagn et al. 2013; Denisov et al. 2007).
Although the values in Table 2 are theoretical, the calculated values are well within the range observed experimentally in analytical centrifugation experiments with lipid nanodiscs. The estimates of partial specific volume (Vn) agree with data reported by Sykes and coworkers on ApoE3-DMPG lipoparticles (Raussens et al. 2000) and by Grisshammer and coworkers on MSP1D1 nanodiscs (Inagaki et al. 2013).
The hydrodynamic properties of lipid assemblies are governed by phospholipid composition, as the specific nature of hydrocarbon chains and polar headgroups influence the partial specific volumes of phospholipids. The estimates in Table 2 illustrate how the sedimentation properties of nanodiscs are affected, not only by the molecular weight of the entire assembly, but also, significantly, by the higher specific volume (low density) relative to protein) of the lipid molecules in the bilayer. It is the balance of these properties that determines whether the species will sediment, float or remain dispersed solution.
In both MSP1D1Δh5 and MSP1E3D1 nanodiscs the incorporation of Ail decreases the overall specific volumes and the predicted sedimentation times. The sedimentation efficiency will be further enhanced by the addition of ligands that bind the nanodisc-embedded protein, such as fibronectin, in the case of Ail. The trend of these theoretical predictions are in line with our observations (Table 1) that Ail-containing MSP1D1Δh5 nanodiscs could be sedimented by Airfuge centrifugation, while their empty counterparts could not.
CONCLUSIONS
In summary, our results show that nanodiscs containing the outer membrane protein Ail can be sedimented by centrifugation and transferred to a rotor for solid-state NMR experiments. The nanodisc samples can be diluted back to their original concentration for solution NMR studies, indicating that neither the protein or lipid bilayer structures are disrupted by sedimentation. Furthermore, since the protein is active in the nanodiscs the same samples can be used for activity assays, solution NMR and solid-state NMR experiments, facilitating investigation of the structure, and activity properties across a wide range of dynamics time scales, without having to add potentially damaging detergents or precipitants, and without the need to lyophilize the sample.
ACKNOWLEDGMENTS
We thank Bibhuti Das, Chris Grant, Chin Wu and Stan Opella for assistance with solid-state NMR experiments. This research was supported by grants from the National Institutes of Health (GM100265; P41 EB002031; P30 CA030199).
REFERENCES
- Andronesi OC, Becker S, Seidel K, Heise H, Young HS, Baldus M. Determination of membrane protein structure and dynamics by magic-angle-spinning solid-state NMR spectroscopy. J Am Chem Soc. 2005;127(37):12965–12974. doi: 10.1021/ja0530164. doi:10.1021/ja0530164. [DOI] [PubMed] [Google Scholar]
- Arora A, Tamm LK. Biophysical approaches to membrane protein structure determination. Curr Opin Struct Biol. 2001;11(5):540–547. doi: 10.1016/s0959-440x(00)00246-3. doi:S0959-440X(00)00246-3 [pii] [DOI] [PubMed] [Google Scholar]
- Auger M. Biological membrane structure by solid-state NMR. Curr Issues Mol Biol. 2000;2(4):119–124. [PubMed] [Google Scholar]
- Berardi MJ, Shih WM, Harrison SC, Chou JJ. Mitochondrial uncoupling protein 2 structure determined by NMR molecular fragment searching. Nature. 2011;476(7358):109–113. doi: 10.1038/nature10257. doi:10.1038/nature10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini I, Engelke F, Gonnelli L, Knott B, Luchinat C, Osen D, Ravera E. On the use of ultracentrifugal devices for sedimented solute NMR. J Biomol NMR. 2012a;54(2):123–127. doi: 10.1007/s10858-012-9657-y. doi:10.1007/s10858-012-9657-y. [DOI] [PubMed] [Google Scholar]
- Bertini I, Engelke F, Luchinat C, Parigi G, Ravera E, Rosa C, Turano P. NMR properties of sedimented solutes. Phys Chem Chem Phys. 2012b;14(2):439–447. doi: 10.1039/c1cp22978h. doi:10.1039/c1cp22978h. [DOI] [PubMed] [Google Scholar]
- Bertini I, Luchinat C, Parigi G, Ravera E, Reif B, Turano P. Solid-state NMR of proteins sedimented by ultracentrifugation. Proc Natl Acad Sci U S A. 2011;108(26):10396–10399. doi: 10.1073/pnas.1103854108. doi:10.1073/pnas.1103854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibow S, Carneiro MG, Sabo TM, Schwiegk C, Becker S, Riek R, Lee D. Measuring membrane protein bond orientations in nanodiscs via residual dipolar couplings. Protein Sci. 2014 doi: 10.1002/pro.2482. doi:10.1002/pro.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsall NJM, Feeney J, Lee AG, Levine YK, Metcalfe JC. Dipalmitoyl-lecithin: assignment of the 1H and 13C nuclear magnetic resonance spectra, and conformational studies. Journal of the Chemical Society, Perkin Transactions. 1972;2(10):1441–1445. doi:10.1039/P29720001441. [Google Scholar]
- Bloom M, Burnell EE, MacKay AL, Nichol CP, Valic MI, Weeks G. Fatty acyl chain order in lecithin model membranes determined from proton magnetic resonance. Biochemistry. 1978;17(26):5750–5762. doi: 10.1021/bi00619a024. doi:10.1021/bi00619a024. [DOI] [PubMed] [Google Scholar]
- Bloom M, Burnell EE, Roeder SBW, Valic MI. Nuclear magnetic resonance line shapes in lyotropic liquid crystals and related systems. The Journal of Chemical Physics. 1977;66(7):3012–3020. doi:doi: http://dx.doi.org/10.1063/1.434314. [Google Scholar]
- Boettcher JM, Davis-Harrison RL, Clay MC, Nieuwkoop AJ, Ohkubo YZ, Tajkhorshid E, Morrissey JH, Rienstra CM. Atomic view of calcium-induced clustering of phosphatidylserine in mixed lipid bilayers. Biochemistry. 2011;50(12):2264–2273. doi: 10.1021/bi1013694. doi:10.1021/bi1013694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell EE, Cullis PR, de Kruijff B. Effects of tumbling and lateral diffusion on phosphatidylcholine model membrane 31P-NMR lineshapes. Biochim Biophys Acta. 1980;603(1):63–69. doi: 10.1016/0005-2736(80)90391-0. [DOI] [PubMed] [Google Scholar]
- Chill JH, Louis JM, Delaglio F, Bax A. Local and global structure of the monomeric subunit of the potassium channel KcsA probed by NMR. Biochim Biophys Acta. 2007;1768(12):3260–3270. doi: 10.1016/j.bbamem.2007.08.006. doi:10.1016/j.bbamem.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N, Murray DT, Cross TA. Lipid bilayer preparations of membrane proteins for oriented and magic-angle spinning solid-state NMR samples. Nat Protoc. 2013;8(11):2256–2270. doi: 10.1038/nprot.2013.129. doi:10.1038/nprot.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6(3):277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Denisov IG, Baas BJ, Grinkova YV, Sligar SG. Cooperativity in cytochrome P450 3A4: linkages in substrate binding, spin state, uncoupling, and product formation. J Biol Chem. 2007;282(10):7066–7076. doi: 10.1074/jbc.M609589200. doi:10.1074/jbc.M609589200. [DOI] [PubMed] [Google Scholar]
- Ding Y, Fujimoto LM, Yao Y, Plano GV, Marassi FM. Influence of the lipid membrane environment on structure and activity of the outer membrane protein Ail from Yersinia pestis. Biochim Biophys Acta. 2015;1848(2):712–720. doi: 10.1016/j.bbamem.2014.11.021. doi: http://dx.doi.org/10.1016/j.bbamem.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Yao Y, Marassi FM. Membrane protein structure determination in membrana. Acc Chem Res. 2013;46(9):2182–2190. doi: 10.1021/ar400041a. doi:10.1021/ar400041a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler A, Separovic F. Solid-state NMR structure determination. IUBMB Life. 2003;55(9):515–523. doi: 10.1080/15216540310001622740. doi:10.1080/15216540310001622740. [DOI] [PubMed] [Google Scholar]
- Durr UH, Gildenberg M, Ramamoorthy A. The magic of bicelles lights up membrane protein structure. Chem Rev. 2012;112(11):6054–6074. doi: 10.1021/cr300061w. doi:10.1021/cr300061w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzkorn M, Raschle T, Hagn F, Gelev V, Rice AJ, Walz T, Wagner G. Cell-free expressed bacteriorhodopsin in different soluble membrane mimetics: biophysical properties and NMR accessibility. Structure. 2013;21(3):394–401. doi: 10.1016/j.str.2013.01.005. doi:10.1016/j.str.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferella L, Luchinat C, Ravera E, Rosato A. SedNMR: a web tool for optimizing sedimentation of macromolecular solutes for SSNMR. J Biomol NMR. 2013;57(4):319–326. doi: 10.1007/s10858-013-9795-x. doi:10.1007/s10858-013-9795-x. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Wuthrich K. NMR solution structure determination of membrane proteins reconstituted in detergent micelles. FEBS Lett. 2003;555(1):144–150. doi: 10.1016/s0014-5793(03)01155-4. [DOI] [PubMed] [Google Scholar]
- Forbes J, Bowers J, Shan X, Moran L, Oldfield E, Moscarello MA. Some new developments in solid-state nuclear magnetic resonance spectroscopic studies of lipids and biological membranes, including the effects of cholesterol in model and natural systems. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases. 1988;84(11):3821–3849. doi:10.1039/F19888403821. [Google Scholar]
- Fox DA, Larsson P, Lo RH, Kroncke BM, Kasson PM, Columbus L. The Structure of the Neisserial outer membrane protein Opa: Loop flexibility essential to receptor recognition and bacterial engulfment. J Am Chem Soc in press. 2014 doi: 10.1021/ja503093y. doi:10.1021/ja503093y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks WT, Linden AH, Kunert B, van Rossum BJ, Oschkinat H. Solid-state magic-angle spinning NMR of membrane proteins and protein-ligand interactions. Eur J Cell Biol. 2012;91(4):340–348. doi: 10.1016/j.ejcb.2011.09.002. doi:10.1016/j.ejcb.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Gardiennet C, Schutz AK, Hunkeler A, Kunert B, Terradot L, Bockmann A, Meier BH. A sedimented sample of a 59 kDa dodecameric helicase yields high-resolution solid-state NMR spectra. Angew Chem Int Ed Engl. 2012;51(31):7855–7858. doi: 10.1002/anie.201200779. doi:10.1002/anie.201200779. [DOI] [PubMed] [Google Scholar]
- Gluck JM, Wittlich M, Feuerstein S, Hoffmann S, Willbold D, Koenig BW. Integral membrane proteins in nanodiscs can be studied by solution NMR spectroscopy. J Am Chem Soc. 2009;131(34):12060–12061. doi: 10.1021/ja904897p. doi:10.1021/ja904897p. [DOI] [PubMed] [Google Scholar]
- Gopinath T, Mote KR, Veglia G. Sensitivity and resolution enhancement of oriented solid-state NMR: application to membrane proteins. Prog Nucl Magn Reson Spectrosc. 2013;75:50–68. doi: 10.1016/j.pnmrs.2013.07.004. doi:10.1016/j.pnmrs.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberkorn RA, Herzfeld J, Griffin RG. High resolution phosphorus-31 and carbon-13 nuclear magnetic resonance spectra of unsonicated model membranes. J Am Chem Soc. 1978;100(4):1296–1298. doi:10.1021/ja00472a048. [Google Scholar]
- Hagn F, Etzkorn M, Raschle T, Wagner G. Optimized phospholipid bilayer nanodiscs facilitate high-resolution structure determination of membrane proteins. J Am Chem Soc. 2013;135(5):1919–1925. doi: 10.1021/ja310901f. doi:10.1021/ja310901f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller S, Wagner G. The role of solution NMR in the structure determinations of VDAC-1 and other membrane proteins. Curr Opin Struct Biol. 2009;19(4):396–401. doi: 10.1016/j.sbi.2009.07.013. doi:10.1016/j.sbi.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Zhang Y, Hu F. Membrane protein structure and dynamics from NMR spectroscopy. Annu Rev Phys Chem. 2012;63:1–24. doi: 10.1146/annurev-physchem-032511-143731. doi:10.1146/annurev-physchem-032511-143731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki S, Ghirlando R, Grisshammer R. Biophysical characterization of membrane proteins in nanodiscs. Methods. 2013;59(3):287–300. doi: 10.1016/j.ymeth.2012.11.006. doi:10.1016/j.ymeth.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Blevins RA. NMR View: A computer program for the visualization and analysis of NMR data. J Biomol NMR. 1994;4(5):603–614. doi: 10.1007/BF00404272. doi:10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- Kijac A, Shih AY, Nieuwkoop AJ, Schulten K, Sligar SG, Rienstra CM. Lipid-protein correlations in nanoscale phospholipid bilayers determined by solid-state nuclear magnetic resonance. Biochemistry. 2010;49(43):9190–9198. doi: 10.1021/bi1013722. doi:10.1021/bi1013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijac AZ, Li Y, Sligar SG, Rienstra CM. Magic-angle spinning solid-state NMR spectroscopy of nanodisc-embedded human CYP3A4. Biochemistry. 2007;46(48):13696–13703. doi: 10.1021/bi701411g. doi:10.1021/bi701411g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Howell SC, Van Horn WD, Jeon YH, Sanders CR. Recent Advances in the Application of Solution NMR Spectroscopy to Multi-Span Integral Membrane Proteins. Prog Nucl Magn Reson Spectrosc. 2009;55(4):335–360. doi: 10.1016/j.pnmrs.2009.07.002. doi:10.1016/j.pnmrs.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue TM, Stafford WF., 3rd Modern applications of analytical ultracentrifugation. Annu Rev Biophys Biomol Struct. 1999;28:75–100. doi: 10.1146/annurev.biophys.28.1.75. doi:10.1146/annurev.biophys.28.1.75. [DOI] [PubMed] [Google Scholar]
- Levine YK, Birdsall NJ, Lee AG, Metcalfe JC. 13 C nuclear magnetic resonance relaxation measurements of synthetic lecithins and the effect of spin-labeled lipids. Biochemistry. 1972;11(8):1416–1421. doi: 10.1021/bi00758a014. [DOI] [PubMed] [Google Scholar]
- Li Y, Kijac AZ, Sligar SG, Rienstra CM. Structural analysis of nanoscale self-assembled discoidal lipid bilayers by solid-state NMR spectroscopy. Biophys J. 2006;91(10):3819–3828. doi: 10.1529/biophysj.106.087072. doi:10.1529/biophysj.106.087072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loquet A, Habenstein B, Lange A. Structural Investigations of Molecular Machines by Solid-State NMR. Acc Chem Res. 2013;46(9):2070–2079. doi: 10.1021/ar300320p. doi:10.1021/ar300320p. [DOI] [PubMed] [Google Scholar]
- Macdonald PM, Saleem Q, Lai A, Morales HH. NMR methods for measuring lateral diffusion in membranes. Chem Phys Lipids. 2013;166:31–44. doi: 10.1016/j.chemphyslip.2012.12.004. doi:10.1016/j.chemphyslip.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Maltsev S, Lorigan GA. Membrane proteins structure and dynamics by nuclear magnetic resonance. Comprehensive Physiology. 2011;1(4):2175–2187. doi: 10.1002/cphy.c110022. doi:10.1002/cphy.c110022. [DOI] [PubMed] [Google Scholar]
- McDermott A. Structure and dynamics of membrane proteins by magic angle spinning solid-state NMR. Annu Rev Biophys. 2009;38:385–403. doi: 10.1146/annurev.biophys.050708.133719. doi:10.1146/annurev.biophys.050708.133719. [DOI] [PubMed] [Google Scholar]
- Miller VL, Beer KB, Heusipp G, Young BM, Wachtel MR. Identification of regions of Ail required for the invasion and serum resistance phenotypes. Mol Microbiol. 2001;41(5):1053–1062. doi: 10.1046/j.1365-2958.2001.02575.x. [DOI] [PubMed] [Google Scholar]
- Morris GA, Freeman R. Enhancement of nuclear magnetic resonance signals by polarization transfer. J Am Chem Soc. 1979;101(3):760–762. doi:10.1021/ja00497a058. [Google Scholar]
- Mors K, Roos C, Scholz F, Wachtveitl J, Dotsch V, Bernhard F, Glaubitz C. Modified lipid and protein dynamics in nanodiscs. Biochim Biophys Acta. 2013;1828(4):1222–1229. doi: 10.1016/j.bbamem.2012.12.011. doi:10.1016/j.bbamem.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Murray DT, Das N, Cross TA. Solid state NMR strategy for characterizing native membrane protein structures. Acc Chem Res. 2013;46(9):2172–2181. doi: 10.1021/ar3003442. doi:10.1021/ar3003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle JF, Tristram-Nagle S. Structure of lipid bilayers. Biochim Biophys Acta. 2000;1469(3):159–195. doi: 10.1016/s0304-4157(00)00016-2. doi:S0304-4157(00)00016-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni QZ, Daviso E, Can TV, Markhasin E, Jawla SK, Swager TM, Temkin RJ, Herzfeld J, Griffin RG. High Frequency Dynamic Nuclear Polarization. Acc Chem Res. 2013;46(9):1933–1941. doi: 10.1021/ar300348n. doi:10.1021/ar300348n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield E, Bowers JL, Forbes J. High-resolution proton and carbon-13 NMR of membranes: why sonicate? Biochemistry. 1987;26(22):6919–6923. doi: 10.1021/bi00396a009. [DOI] [PubMed] [Google Scholar]
- Oldfield E, Chapman D. Carbon-13 pulse Fourier transform N. M. R. of lecithins. Biochem Biophys Res Commun. 1971;43(5):949–953. doi: 10.1016/0006-291x(71)90554-7. [DOI] [PubMed] [Google Scholar]
- Orwick-Rydmark M, Lovett JE, Graziadei A, Lindholm L, Hicks MR, Watts A. Detergent-free incorporation of a seven-transmembrane receptor protein into nanosized bilayer Lipodisq particles for functional and biophysical studies. Nano letters. 2012;12(9):4687–4692. doi: 10.1021/nl3020395. doi:10.1021/nl3020395. [DOI] [PubMed] [Google Scholar]
- Pan J, Heberle FA, Tristram-Nagle S, Szymanski M, Koepfinger M, Katsaras J, Kucerka N. Molecular structures of fluid phase phosphatidylglycerol bilayers as determined by small angle neutron and X-ray scattering. Biochim Biophys Acta. 2012;1818(9):2135–2148. doi: 10.1016/j.bbamem.2012.05.007. doi:10.1016/j.bbamem.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Berkamp S, Cook GA, Chan MK, Viadiu H, Opella SJ. Nanodiscs versus Macrodiscs for NMR of Membrane Proteins. Biochemistry. 2011;50(42):8983–8985. doi: 10.1021/bi201289c. doi:10.1021/bi201289c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Das BB, Casagrande F, Tian Y, Nothnagel HJ, Chu M, Kiefer H, Maier K, De Angelis A, Marassi FM, Opella SJ. Structure of the Chemokine Receptor CXCR1 in Phospholipid Bilayers. Nature. 2012;491(7426):779–783. doi: 10.1038/nature11580. doi:10.1038/nature11580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervushin K, Riek R, Wider G, Wuthrich K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci U S A. 1997;94(23):12366–12371. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines A, Gibby MG, Waugh JS. Proton-enhanced NMR of dilute spins in solids. J Chem Phys. 1973;59:569–590. [Google Scholar]
- Plesniak LA, Mahalakshmi R, Rypien C, Yang Y, Racic J, Marassi FM. Expression, refolding, and initial structural characterization of the Y. pestis Ail outer membrane protein in lipids. Biochim Biophys Acta. 2011;1808(1):482–489. doi: 10.1016/j.bbamem.2010.09.017. doi:10.1016/j.bbamem.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poget SF, Girvin ME. Solution NMR of membrane proteins in bilayer mimics: small is beautiful, but sometimes bigger is better. Biochim Biophys Acta. 2007;1768(12):3098–3106. doi: 10.1016/j.bbamem.2007.09.006. doi:10.1016/j.bbamem.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RS, Evanics F, Kitevski JL, Patel S. The measurement of immersion depth and topology of membrane proteins by solution state NMR. Biochim Biophys Acta. 2007;1768(12):3044–3051. doi: 10.1016/j.bbamem.2007.09.011. doi:10.1016/j.bbamem.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Raschle T, Hiller S, Yu TY, Rice AJ, Walz T, Wagner G. Structural and functional characterization of the integral membrane protein VDAC-1 in lipid bilayer nanodiscs. J Am Chem Soc. 2009;131(49):17777–17779. doi: 10.1021/ja907918r. doi:10.1021/ja907918r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raussens V, Mah MK, Kay CM, Sykes BD, Ryan RO. Structural characterization of a low density lipoprotein receptor-active apolipoprotein E peptide, ApoE3-(126-183) J Biol Chem. 2000;275(49):38329–38336. doi: 10.1074/jbc.M005732200. doi:10.1074/jbc.M005732200. [DOI] [PubMed] [Google Scholar]
- Ritchie TK, Grinkova YV, Bayburt TH, Denisov IG, Zolnerciks JK, Atkins WM, Sligar SG. Chapter 11 - Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 2009;464:211–231. doi: 10.1016/S0076-6879(09)64011-8. doi:10.1016/S0076-6879(09)64011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett K, Nethercott MJ, Zheng Z, Weliky DP. Solid-state NMR spectroscopy of the HIV gp41 membrane fusion protein supports intermolecular antiparallel beta sheet fusion peptide structure in the final six-helix bundle state. J Mol Biol. 2014;426(5):1077–1094. doi: 10.1016/j.jmb.2013.11.010. doi:10.1016/j.jmb.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann M, Pervushin K, Wider G, Senn H, Wuthrich K. TROSY in triple-resonance experiments: new perspectives for sequential NMR assignment of large proteins. Proc Natl Acad Sci U S A. 1998;95(23):13585–13590. doi: 10.1073/pnas.95.23.13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CR, Sonnichsen F. Solution NMR of membrane proteins: practice and challenges. Magn Reson Chem. 2006;44(Spec No:S24-40) doi: 10.1002/mrc.1816. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Sharma M, Yi M, Dong H, Qin H, Peterson E, Busath DD, Zhou HX, Cross TA. Insight into the mechanism of the influenza A proton channel from a structure in a lipid bilayer. Science. 2010;330(6003):509–512. doi: 10.1126/science.1191750. doi:10.1126/science.1191750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkarev ZO, Lyukmanova EN, Butenko IO, Petrovskaya LE, Paramonov AS, Shulepko MA, Nekrasova OV, Kirpichnikov MP, Arseniev AS. Lipid-protein nanodiscs promote in vitro folding of transmembrane domains of multi-helical and multimeric membrane proteins. Biochim Biophys Acta. 2013;1828(2):776–784. doi: 10.1016/j.bbamem.2012.11.005. doi:10.1016/j.bbamem.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Shenkarev ZO, Lyukmanova EN, Paramonov AS, Shingarova LN, Chupin VV, Kirpichnikov MP, Blommers MJ, Arseniev AS. Lipid-protein nanodiscs as reference medium in detergent screening for high-resolution NMR studies of integral membrane proteins. J Am Chem Soc. 2010;132(16):5628–5629. doi: 10.1021/ja9097498. doi:10.1021/ja9097498. [DOI] [PubMed] [Google Scholar]
- Shenkarev ZO, Lyukmanova EN, Solozhenkin OI, Gagnidze IE, Nekrasova OV, Chupin VV, Tagaev AA, Yakimenko ZA, Ovchinnikova TV, Kirpichnikov MP, Arseniev AS. Lipid-protein nanodiscs: possible application in high-resolution NMR investigations of membrane proteins and membrane-active peptides. Biochemistry (Mosc) 2009;74(7):756–765. doi: 10.1134/s0006297909070086. doi:BCM74070933 [pii] [DOI] [PubMed] [Google Scholar]
- Shuker SB, Hajduk PJ, Meadows RP, Fesik SW. Discovering high-affinity ligands for proteins: SAR by NMR. Science. 1996;274(5292):1531–1534. doi: 10.1126/science.274.5292.1531. [DOI] [PubMed] [Google Scholar]
- Susac L, Horst R, Wuthrich K. Solution-NMR Characterization of Outer-Membrane Protein A from E. coli in Lipid Bilayer Nanodiscs and Detergent Micelles. Chembiochem. 2014;15(7):995–1000. doi: 10.1002/cbic.201300729. doi:10.1002/cbic.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeverenyi NM, Sullivan MJ, Maciel GE. Observation of spin exchange by two-dimensional Fourier transform 13C cross-polarization-magic angle spinning. J Magn Reson. 1982;47:462–475. [Google Scholar]
- Tamm LK, Hong H, Liang B. Folding and assembly of beta-barrel membrane proteins. Biochim Biophys Acta. 2004;1666(1-2):250–263. doi: 10.1016/j.bbamem.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Tang M, Comellas G, Rienstra CM. Advanced Solid-State NMR Approaches for Structure Determination of Membrane Proteins and Amyloid Fibrils. Acc Chem Res. 2013;46(9):2080–2088. doi: 10.1021/ar4000168. doi:10.1021/ar4000168. [DOI] [PubMed] [Google Scholar]
- Teriete P, Franzin CM, Choi J, Marassi FM. Structure of the Na,K-ATPase regulatory protein FXYD1 in micelles. Biochemistry. 2007;46(23):6774–6783. doi: 10.1021/bi700391b. doi:10.1021/bi700391b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang TM, Annis DS, Kronshage M, Fenno JT, Usselman LD, Mosher DF, Krukonis ES. Ail protein binds ninth type III fibronectin repeat (9FNIII) within central 120-kDa region of fibronectin to facilitate cell binding by Yersinia pestis. J Biol Chem. 2012;287(20):16759–16767. doi: 10.1074/jbc.M112.358978. doi:10.1074/jbc.M112.358978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang TM, Felek S, Krukonis ES. Ail binding to fibronectin facilitates Yersinia pestis binding to host cells and Yop delivery. Infect Immun. 2010;78(8):3358–3368. doi: 10.1128/IAI.00238-10. doi:10.1128/IAI.00238-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang TM, Wiese JS, Felek S, Kronshage M, Krukonis ES. Ail proteins of Yersinia pestis and Y. pseudotuberculosis have different cell binding and invasion activities. PLoS One. 2013;8(12):e83621. doi: 10.1371/journal.pone.0083621. doi:10.1371/journal.pone.0083621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzitzilonis C, Eichmann C, Maslennikov I, Choe S, Riek R. Detergent/nanodisc screening for high-resolution NMR studies of an integral membrane protein containing a cytoplasmic domain. PLoS One. 2013;8(1):e54378. doi: 10.1371/journal.pone.0054378. doi:10.1371/journal.pone.0054378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich SJ, Glaubitz C. Perspectives in Enzymology of Membrane Proteins by Solid-State NMR. Acc Chem Res. 2013;46(9):2164–2171. doi: 10.1021/ar4000289. doi:10.1021/ar4000289. [DOI] [PubMed] [Google Scholar]
- Wang S, Munro RA, Shi L, Kawamura I, Okitsu T, Wada A, Kim SY, Jung KH, Brown LS, Ladizhansky V. Solid-state NMR spectroscopy structure determination of a lipid-embedded heptahelical membrane protein. Nat Methods. 2013;10(10):1007–1012. doi: 10.1038/nmeth.2635. doi:10.1038/nmeth.2635. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tjandra N. Structural insights of tBid, the caspase-8-activated Bid, and its BH3 domain. J Biol Chem. 2013;288(50):35840–35851. doi: 10.1074/jbc.M113.503680. doi:10.1074/jbc.M113.503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warschawski DE. http://wwwdrorlistcom/nmrhtml.
- Warschawski DE, Devaux PF. 1H-13C polarization transfer in membranes: a tool for probing lipid dynamics and the effect of cholesterol. J Magn Reson. 2005;177(1):166–171. doi: 10.1016/j.jmr.2005.07.011. doi:10.1016/j.jmr.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Weingarth M, Baldus M. Solid-State NMR-Based Approaches for Supramolecular Structure Elucidation. Acc Chem Res. 2013;46(9):2037–2046. doi: 10.1021/ar300316e. doi:10.1021/ar300316e. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Lukacik P, Barnard TJ, Noinaj N, Felek S, Tsang TM, Krukonis ES, Hinnebusch BJ, Buchanan SK. Structural insights into Ail-mediated adhesion in Yersinia pestis. Structure. 2011;19(11):1672–1682. doi: 10.1016/j.str.2011.08.010. doi:10.1016/j.str.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HX, Cross TA. Influences of membrane mimetic environments on membrane protein structures. Annu Rev Biophys. 2013;42:361–392. doi: 10.1146/annurev-biophys-083012-130326. doi:10.1146/annurev-biophys-083012-130326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Cierpicki T, Jimenez RH, Lukasik SM, Ellena JF, Cafiso DS, Kadokura H, Beckwith J, Bushweller JH. NMR solution structure of the integral membrane enzyme DsbB: functional insights into DsbB-catalyzed disulfide bond formation. Mol Cell. 2008;31(6):896–908. doi: 10.1016/j.molcel.2008.08.028. doi:10.1016/j.molcel.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]