Abstract
Aims
To examine the association between developmental trajectories of inattention, hyperactivity-impulsivity, and delinquency through childhood and adolescence (ages 8-16) and subsequent binge drinking and marijuana use in early adulthood (age 21).
Design
Prospective naturalistic follow-up of children with attention-deficit/hyperactivity disorder (ADHD) previously enrolled in a randomized controlled trial (RCT). Treatment-phase assessments occurred at 3, 9, and 14 months after randomization; follow-up assessments occurred at 24 months, 36 months, and 6, 8, and 12 years after randomization.
Setting
Secondary analysis of data from the Multimodal Treatment Study of ADHD (MTA), a multi-site RCT comparing the effects of careful medication management, intensive behavior therapy, their combination, and referral to usual community care.
Participants
579 children with DSM-IV ADHD combined type, aged 7.0 and 9.9 years old at baseline (M=8.5, SD=.80).
Measurements
Ratings of inattention, hyperactivity-impulsivity, and delinquency were collected from multiple informants at baseline and through the 8-year follow-up. Self-reports of binge drinking and marijuana use were collected at the 12-year follow-up (M age 21).
Findings
Trajectories of worsening inattention symptoms and delinquency (and less apparent improvement in hyperactivity-impulsivity) were associated with higher rates of early adult binge drinking and marijuana use, compared with trajectories of stable or improving symptoms and delinquency (of 24 comparisons, 22 p-values <.05), even when symptom levels in stable trajectories were high.
Conclusions
Worsening inattention symptoms and delinquency during adolescence are associated with increased-levels of early adult substance use; this pattern may reflect a developmental course of vulnerability to elevated substance use in early adulthood.
Childhood attention-deficit/hyperactivity disorder (ADHD) is associated with increased risk for later substance use and disorder (1-6), especially among children who exhibit disruptive behavior (7,8). Recent meta-analyses show that childhood ADHD is associated with a higher probability of marijuana but not alcohol use in adolescence, and with increased risk for an alcohol or substance use disorder in early adulthood (9,10). However, modest effect sizes across studies suggest there may be substantial heterogeneity in substance use risk. In the current study, we explore whether risk for early adult substance use is explained in part by individual differences in the developmental course of ADHD and delinquency.
Children with diagnosed ADHD often persist with significant symptoms of the disorder; approximately two-thirds continue to meet DSM-IV diagnostic criteria in adolescence (11,12), although symptoms of hyperactivity-impulsivity often decrease. Children whose ADHD symptoms persist may have greater risk for later substance use compared to children whose symptoms diminish (2,13). In the Fast Track Project, a school-based sample of children with disruptive behavior, children whose symptoms initially declined but subsequently increased had the earliest onset of illicit drug use in adolescence (15). Additionally, in the Pittsburgh ADHD Longitudinal Study, higher symptom scores in adolescence related to higher frequency of drinking alcohol in adolescence (16), but individual differences in symptom trajectories were not examined. Studies examining differential patterns of ADHD symptoms over several years are needed to determine whether substance use risk in early adulthood depends on individual differences in the progression of ADHD symptomatology through adolescence. Examining patterns of change during such crucial windows of development may identify key periods of vulnerability.
A 3-year follow-up of children with ADHD in the present sample (17) found three trajectories of change in ADHD symptoms (18). Half (52%) of the sample showed large symptom improvements through the study's 14-month treatment phase that were maintained through the 36-month assessment. One third (34%) showed gradual decline in symptoms over 36 months, and 14% showed large initial declines followed by a return to baseline symptom levels. These patterns predicted adolescent functioning for most measures (e.g., academic performance, social skills, police contacts (19)), providing evidence that patterns of symptom change can identify children who struggle with impairments and behavioral problems in adolescence. Whether such individual differences herald vulnerability to later substance use is unknown.
Nearly all studies of childhood ADHD implicate comorbid conduct problems as a major risk factor for later substance use (2,3,5,11,20). In several studies, risk for adult substance use was highest among children with comorbid psychopathology (7,8,21-23). Still, it may be the developmental progression of disruptive and delinquent behavior, rather than their presence at any given point in time, that determines risk for later substance use. Temporary delinquency is common in adolescence (24), but early-onset, persistent delinquency occurs in only a minority of the population (25). Although children with ADHD exhibit high rates of delinquency (2,26), it is persistent and escalating delinquency that may carry the greatest risk for problematic substance use in adulthood.
Prospective assessments of the Multimodal Treatment Study of ADHD (MTA) sample provide an opportunity to evaluate developmental progression to early adult substance use. The MTA began as a multi-site, 14-month randomized controlled trial comparing the effectiveness of intensive multicomponent behavioral treatment (Beh), systematic medication management (MedMgt), a combined behavioral and medication protocol (Comb), and referral to usual community care (CC). Children who received study medication (MedMgt, Comb) showed stronger improvements in ADHD symptoms compared to children in other treatments (17). However, group differences dissipated two years after study treatments ended (27), a pattern that persisted through the 8-year follow-up (19). Study-assigned and prospectively-tracked ADHD medication use were also unrelated to substance use at the 8-year follow-up (28). This loss of treatment group differences suggests that heterogeneity in the developmental course of ADHD takes hold at an early age, and a focus on patterns of change (not necessarily treatment-related) may reveal windows of opportunity for intervention to deflect vulnerable trajectories away from addiction endpoints.
In the current study, we tested whether children followed distinct trajectories of ADHD symptoms and delinquency, beginning prior to study randomization and ending in adolescence. Our goal was to identify trajectories of change associated with the highest levels of early adult binge drinking and marijuana use (the most common substance use behaviors in the early twenties (29)), controlling for childhood risk and protective factors previously associated with symptom trajectories (18). Based on that previous analysis, we anticipated three trajectories of ADHD symptoms reaching into adolescence: children whose symptoms diminished and remained at low levels; children whose symptoms declined gradually over time; and children whose symptoms rebounded after reaching low levels during the treatment phase. We anticipated that worsening ADHD symptoms and increasing delinquency would be associated with the highest substance use rates in early adulthood.
Method
Sample and Procedure
The MTA recruited 579 participants, aged 7 to 9.9 (M=8.5, SD=.80), diagnosed in childhood with DSM-IV ADHD combined type. Children with comorbid diagnoses participated, and exclusion criteria were limited to conditions requiring study-incompatible treatments or those that would prevent families’ full participation. At each of six sites, 95-98 children were randomly assigned to Beh, MedMgt, Comb, or CC treatments. Recruitment strategy, detailed inclusion/exclusion criteria, diagnostic procedures, treatment protocol, and sample demographics appear elsewhere (17,30).
The treatment phase included baseline assessments at baseline (prior to treatment randomization), 3 and 9 months following randomization, and at the conclusion of the treatment phase (14 months following randomization; Mage=9.57, SD=.85). Follow-up assessments occurred at 24 months (Mage=10.43, SD=.86), 36 months (Mage=11.72, SD=.92), 6 years (Mage=14.90, SD=1.0), 8 years (Mage=16.80, SD=1.0), and 12 years (Mage=21.05, SD=1.08) following randomization. Participation rates were 97%, 93%, 84%, 78%, 75%, and 74% for the 14-month through 12-year follow-ups, respectively (a 10-year follow-up was also administered but these data were not included). There were no significant differences in baseline characteristics between participants and nonparticipants at the 36-month assessment, but differences were present in subsequent waves (19). Comparisons between participants retained and lost to the 12-year follow-up are shown in Table S1.
Measures
ADHD symptoms
Symptoms of inattention and hyperactivity-impulsivity were measured with combined parent and teacher ratings from the Swanson, Nolan, and Pelham Rating Scale (SNAP (31)). Scores were first rescaled by standardizing these baseline scores (M=0, SD=1) and centering parents’ and teachers’ scores at subsequent assessments around their respective raw baseline means and standard deviations. Rescaled scores within each assessment were then averaged to create composite parent-teacher ratings of inattention and hyperactivity-impulsivity at baseline through 8 years (H. Kraemer, personal communication).
Delinquency
Following procedures developed elsewhere (32,33) and used in prior MTA analyses (3,19), children were assigned a delinquency classification code based on parent- and self-report measures of delinquency and antisocial behavior at baseline, 14, 24, and 36 months, and at 6 and 8 years. Delinquency was coded on a 5-point scale based on the most serious act committed in the preceding 6 months, from 0 (no delinquency) to 4 (serious delinquency). Examples of minor delinquency are theft of less than $5 at home and minor vandalism outside of the home; examples of serious delinquency are breaking/entering and attacking someone with intent to harm.
Substance use outcomes
At the 12-year assessment, substance use was assessed by the self-report Substance Use Questionnaire (2) adapted for the MTA. Outcomes were binge drinking, the frequency of consuming five or more drinks at a time during the past year (applying the SAMHSA sex-invariant definition), and marijuana use, the frequency of using marijuana during the past year. Both items were rated from 0 (not at all) to 11 (several times a day or more). Mean binge drinking was 2.55 (SD = 2.72), and mean marijuana use was 2.90 (SD = 4.01), both corresponding to use at a rate of 6-10 times per year.
Baseline covariates
We selected 13 covariates as control variables that were relevant in previous MTA publications and had minimal conceptual and statistical overlap: children's demographic characteristics (sex, race/ethnicity, family marital status, family income from public assistance/welfare/social security); history of medication use for ADHD prior to study enrollment; original randomized treatment assignment (Beh, MedMgt, Comb, CC); Wechsler Intelligence Scale for Children (WISC-III) full scale IQ; Wechsler Individual Achievement Test (WIAT (34)) mathematics reasoning score; teacher ratings of ODD symptoms with the SNAP (35); self-reported anxiety symptoms (MASC); parent ratings of aggressive conduct based on DSM-IV symptoms of conduct disorder; and parent and teacher ratings of social competence from the Social Skills Rating System (SSRS (36)).
Analytic Strategy
Growth mixture models (GMM) in Mplus 6.1 (37) examined trajectories of ADHD symptoms and delinquency in relation to early adult binge drinking and marijuana use. GMM analyses sort individuals with similar profiles of change into latent trajectory classes, resulting in a distinct trajectory for each class identified in the sample (38,39). Each person has a probability of membership in each class. GMMs included baseline covariates predicting class membership and levels of 12-year substance use. Trajectory classes predicted levels of 12-year binge drinking and marijuana use, producing estimates of different average levels of use in each class. We tested separate models for inattention, hyperactivity-impulsivity, and delinquency, because of the small sample size in relation to the analytical complexity of testing a multivariate GMM. This approach precluded direct tests of covariation between ADHD symptoms and delinquency, but in a supplemental analysis we compared participants’ class memberships across variables.
Data from all 579 participants were used in this study. Although participants lost to follow-up may have been at higher risk for heavy substance use in early adulthood (see Table S1), we included covariates known to predict substance use (satisfying assumptions of missingness at random) and retained all participants using multiple imputation (20 imputations analyzed in Mplus 6.1). Thus, our analyses should produce largely unbiased results (40).
Results
Growth mixture models were implemented in two stages. First, we fit unconditional growth curve models to establish appropriate functional forms of change, and considered polynomial, piecewise, and freed-loading methods for modeling change. Trajectories of inattention and hyperactivity-impulsivity showed sharp symptom declines through the 14-month treatment phase, followed by linear trends. Piecewise models best captured this pattern, with freed loadings from baseline to 14 months, and linear change from 14 months to 8 years. A quadratic trajectory best captured the pattern of change in delinquency. Second, we tested a series of models permitting between 1 and 5 latent classes (Table 1 shows indices of model fit). The best-fitting models were those with the lowest BIC and AIC values (41,42), provided that entropy exceeded .80 (43,44) and that model results were stable across random start values (45). For all measures, 4-class solutions provided the best fit. We investigated possible heterogeneity across study sites, but found that between-site differences were negligible (intraclass correlations ranged from .015 to .052), and unconditional models for inattention, hyperactivity-impulsivity, and delinquency showed no significant random effects of site. Models were thus reduced for parsimony by excluding study site.
Table 1.
Fit Statistics from Competing Models for Inattention, Hyperactivity-impulsivity, and Delinquency
| AIC | BIC | Entropy | |
|---|---|---|---|
| Inattention | |||
| 1-Class Solution | 17975 | 18219 | - |
| 2-Class Solution | 17517 | 17870 | .97 |
| 3-Class Solution | 17454 | 17916 | .87 |
| 4-Class Solution | 17236 | 17808 | .89 |
| 5-Class Solutiona | 17216 | 17897 | .91 |
| Hyperactivity-Impulsivity | |||
| 1-Class Solution | 16605 | 16849 | - |
| 2-Class Solution | 16431 | 16784 | .68 |
| 3-Class Solution | 16041 | 16503 | .82 |
| 4-Class Solution | 15801 | 16373 | .84 |
| 5-Class Solutiona | 15756 | 16436 | .89 |
| Delinquency | |||
| 1-Class Solution | 17645 | 17859 | - |
| 2-Class Solution | 17139 | 17445 | .97 |
| 3-Class Solution | 16986 | 17391 | .86 |
| 4-Class Solution | 16734 | 17240 | .91 |
| 5-Class Solutiona | 16567 | 17173 | .91 |
Note. AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion. Criteria for model selection included lower values for the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC), entropy values greater than .80 (indicating model confidence in assigning cases to latent trajectory classes), and model stability across randomly-assigned starting values. Estimates in bold are the fit statistics of the chosen solution.
Best loglikelihood was not replicated over random starts.
Overall, the final models showed two primary patterns of change that also corresponded with substance use outcomes. First, trajectories of worsening symptoms and delinquency during adolescence predicted higher levels of early adult substance use. On average, these substance use rates were binge drinking about once a month and marijuana use once a week (class 3 in all models) to multiple times a day (class 4 in all models). Second, trajectories of stable or improving symptoms and delinquency predicted lower levels of early adult substance use. On average, these substance use rates were binge drinking up to 7 times per year and marijuana use up to 3 times per year.
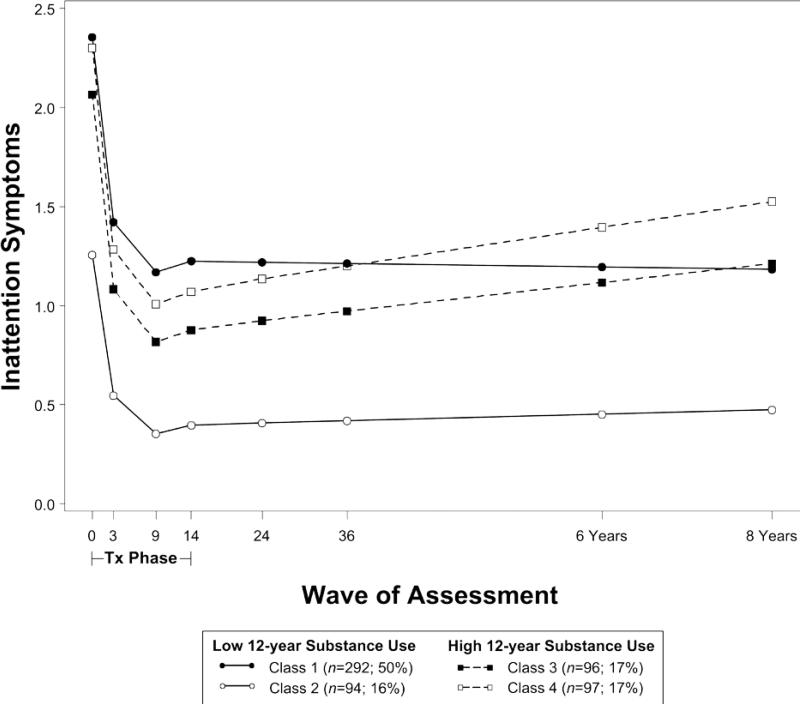
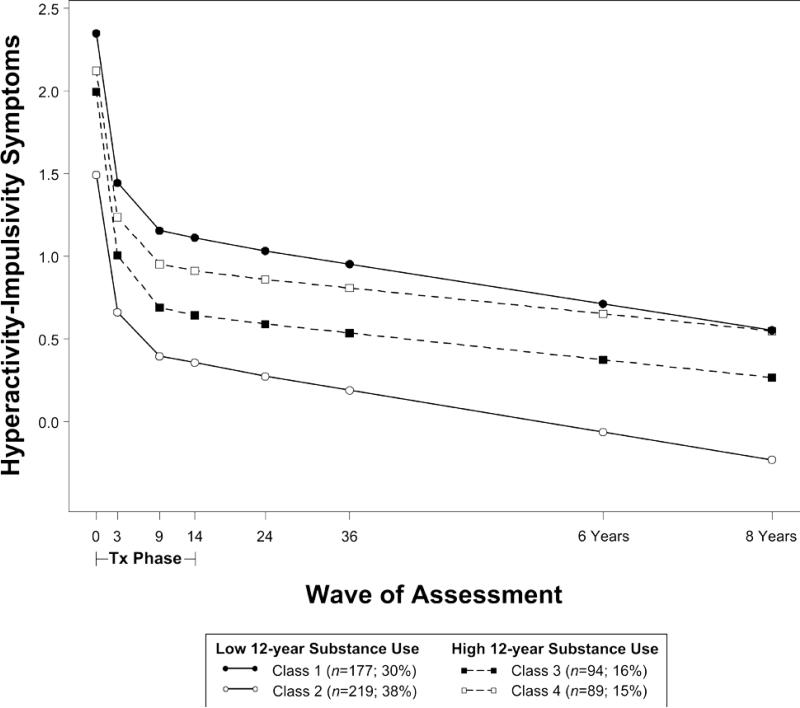
ADHD symptoms improved in all classes during the treatment phase. In the model for inattention (Figure 1; Table 2), symptoms in classes 1 and 2 remained stable in adolescence (class 1: B=-.01, SE=.02; class 2: B=.01, SE=.05), whereas symptoms in classes 3 and 4 worsened (class 3: B=.05, SE=.02; class 4: B=.07, SE=.02). In the model for hyperactivityimpulsivity (Figure 2; Table 3), symptoms in all classes improved even further in adolescence (class 1: B=-.08, SE=.04; class 2: B=-.08, SE=.03), although classes 3 and 4 appeared to improve at slower rates (class 3: B=-.05, SE=.02; class 4: B=-.05, SE=.02). In both models, binge drinking and marijuana use were highest in classes 3 and 4 (predicted mean levels of use reported in Tables 2 and 3).
Figure 1.
Trajectories of inattention symptoms from baseline through 8 years by later substance use risk. Note: Symptom scores are presented in the original metric of the SNAP measure (range = 0 to 3), where scores in each class are centered around the mean of parent and teacher scores at baseline.
Table 2.
Class Differences in Baseline Covariates and Predicted Levels of Substance Use at 12-Year Follow-up for Latent Classes Modeling Inattention Trajectories.
| Inattention | Class 1 | Class 2 | Class 3 | Class 4 | p |
|---|---|---|---|---|---|
| Baseline covariates | |||||
| Sex (% male) | 80 | 69 | 81 | 91 | .002* |
| Ethnicity | |||||
| %African-American | 20 | 11 | 17 | 32 | .003* |
| %Other minority | 17 | 24 | 15 | 25 | .174 |
| Prior medication use (%) | 45 | 34 | 36 | 24 | .003* |
| Family intactness (%married) | 66 | 72 | 67 | 53 | .034 |
| Family income (% on welfare) | 17 | 20 | 14 | 27 | .103 |
| Treatment group | |||||
| % Comb | 22 | 33 | 34 | 18 | |
| % MedMgt | 23 | 23 | 30 | 27 | |
| % Beh | 26 | 18 | 24 | 28 | |
| % CC | 28 | 26 | 12 | 28 | .011 |
| WISC mean full scale IQ (SD) | 98.01 (13.41) | 109.86 (15.23) | 102.90 (15.75) | 99.56 (13.14) | <.001* |
| WIAT mean mathematics (SD) | 95.59 (13.49) | 104.06 (14.09) | 98.95 (12.71) | 97.35 (13.14) | <.001* |
| SNAP mean ODD (SD) | 1.47 (.91) | 1.32 (.88) | 1.46 (.92) | 1.50 (.85) | .467 |
| MASC mean total score (SD) | 2.57 (.53) | 2.43 (.52) | 2.39 (.45) | 2.47 (.58) | .008 |
| Mean aggression conduct (SD) | 1.26 (.25) | 1.16 (.16) | 1.26 (.20) | 1.27 (.24) | .001* |
| Mean social support, parent-rated (SD) | .99 (.22) | 1.14 (.22) | 1.01 (.23) | .97 (.25) | <.001* |
| Mean social support, teacher-rated (SD) | .76 (.25) | 1.03 (.26) | .86 (.28) | .80 (.30) | <.001* |
| Predicted substance use (SE), early adult | |||||
| Binge drinking | 1.65a (.42) | 2.30a,b (.88) | 3.65b (.56) | 3.64b (.60) | |
| Marijuana | .44a (.24) | .35a (.47) | 6.33c (.38) | 10.33b (.30) | |
| Class sample size (%) | 292 (50) | 94 (16) | 96 (17) | 97 (17) | |
Note. Substance use was coded on a 12-point scale, ranging from 0 (not at all) to 4 (once a month) to 6 (once a week) to 9 (once a day) to 11 (several times a day or more). Substance use means designated with different superscripts (a,b,c) are significantly different within the GMM.
p ≤ .003
Bonferroni correction for 15 comparisons. The intraclass correlation for study site showed negligible between-site differences in inattention (ICC=.015).
Figure 2.
Trajectories of hyperactivity-impulsivity symptoms from baseline through 8 years by substance use risk. Note: Symptom scores are presented in the original metric of the SNAP measure (range = 0 to 3), where scores in each class are centered around the mean of parent and teacher scores at baseline.
Table 3.
Class Differences in Baseline Covariates and Predicted Levels of Substance Use at 12-Year Follow-up for Latent Classes Modeling Hyperactivity-Impulsivity Trajectories.
| Hyperactivity-impulsivity | Class 1 | Class 2 | Class 3 | Class 4 | p |
|---|---|---|---|---|---|
| Baseline covariates | |||||
| Sex (% male) | 86 | 72 | 81 | 89 | <.001* |
| Ethnicity | |||||
| %African-American | 29 | 11 | 16 | 28 | <.001* |
| %Other minority | 20 | 20 | 10 | 28 | .017 |
| Prior medication use (%) | 34 | 48 | 36 | 26 | .002* |
| Family intactness (%married) | 55 | 73 | 70 | 58 | .002* |
| Family income (% on welfare) | 37 | 6 | 12 | 21 | <.001* |
| Treatment group | |||||
| % Comb | 17 | 30 | 32 | 21 | |
| % MedMgt | 25 | 23 | 30 | 24 | |
| % Beh | 29 | 20 | 21 | 31 | |
| % CC | 29 | 26 | 17 | 24 | .021 |
| WISC mean full scale IQ (SD) | 99.06 (15.30) | 101.38 (14.83) | 104.65 (14.38) | 100.08 (12.75) | .025 |
| WIAT mean mathematics (SD) | 98.10 (14.04) | 97.12 (13.91) | 101.07 (12.81) | 95.64 (13.07) | .042 |
| SNAP mean ODD (SD) | 2.05 (.74) | .99 (.72) | 1.38 (.90) | 1.46 (.89) | <.001* |
| MASC mean total score (SD) | 2.51 (.53) | 2.52 (.52) | 2.43 (.52) | 2.50 (.56) | .503 |
| Mean aggression conduct (SD) | 1.34 (.28) | 1.15 (.14) | 1.26 (.20) | 1.27 (.24) | <.001* |
| Mean social support, parent-rated (SD) | .98 (.22) | 1.06 (.22) | 1.00 (.26) | .99 (.24) | .002* |
| Mean social support, teacher-rated (SD) | .76 (.29) | .87 (.27) | .88 (.26) | .82 (.29) | <.001* |
| Predicted substance use (SE), early adult | |||||
| Binge drinking | 1.99a (.49) | 1.56a (.41) | 3.62b (.48) | 3.69b (.88) | |
| Marijuana | .47a (.26) | .37a (.21) | 6.32b (.42) | 10.37c (.34) | |
| Class sample size (%) | 177 (30) | 219 (38) | 94 (16) | 89 (15) | |
Note. Substance use was coded on a 12-point scale, ranging from 0 (not at all) to 4 (once a month) to 6 (once a week) to 9 (once a day) to 11 (several times a day or more). Substance use means designated with different superscripts (a,b,c) are significantly different within the GMM.
p ≤ .003
Bonferroni correction for 15 comparisons. The intraclass correlation for study site showed negligible between-site differences in hyperactivity-impulsivity (ICC=.052).
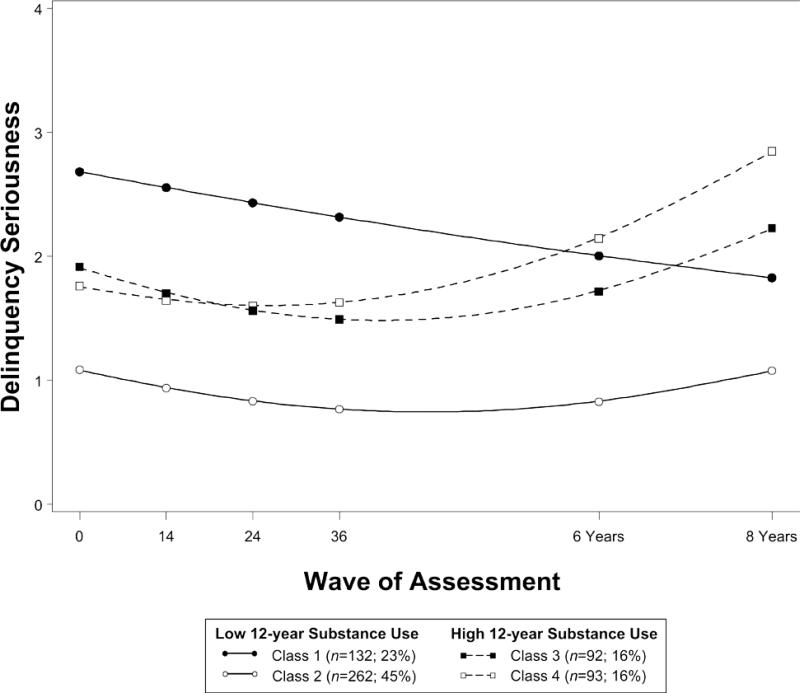
Delinquency initially improved in all classes (Figure 3; Table 4). Thereafter, delinquency in classes 3 and 4 worsened (quadratic acceleration in classes 3 and 4: B=.04, SE=.01), whereas delinquency in class 2 remained at low and stable levels through adolescence (quadratic acceleration: B=.02, SE=.01). The apparent decline in class 1 was not significant (linear trend: B=-.13, SE=.11), indicating higher but stable levels of delinquency through adolescence. Binge drinking and marijuana use were highest in classes 3 and 4 (predicted mean levels of use reported in Table 4).
Figure 3.
Trajectories of delinquency from baseline through 8 years by substance use risk.
Table 4.
Class Differences in Baseline Covariates and Predicted Levels of Substance Use at 12-Year Follow-up for Latent Classes Modeling Delinquency Trajectories.
| Delinquency | Class 1 | Class 2 | Class 3 | Class 4 | p |
|---|---|---|---|---|---|
| Baseline covariates | |||||
| Sex (% male) | 87 | 72 | 84 | 90 | <.001* |
| Ethnicity | |||||
| %African-American | 25 | 15 | 16 | 30 | .004 |
| %Other minority | 20 | 19 | 12 | 26 | .121 |
| Prior medication use (%) | 39 | 42 | 38 | 27 | .076 |
| Family intactness (%married) | 74 | 63 | 66 | 56 | .033 |
| Family income (% on welfare) | 27 | 14 | 16 | 24 | .010 |
| Treatment group | |||||
| % Comb | 19 | 25 | 34 | 26 | |
| % MedMgt | 27 | 23 | 28 | 26 | |
| % Beh | 27 | 26 | 21 | 24 | |
| % CC | 27 | 27 | 17 | 25 | .365 |
| WISC mean full scale IQ (SD) | 97.87(13.75) | 101.87(15.59) | 104.53(13.89) | 99.54(13.18) | .005 |
| WIAT mean mathematics (SD) | 97.05(13.77) | 98.26(14.29) | 98.73(12.27) | 96.85(13.46) | .671 |
| SNAP mean ODD (SD) | 1.87 (.85) | 1.23 (.85) | 1.39 (.91) | 1.56 (.85) | <.001* |
| MASC mean total score (SD) | 2.50 (.53) | 2.53 (.54) | 2.42 (.46) | 2.51 (.58) | .411 |
| Mean aggression conduct (SD) | 1.45 (.28) | 1.13 (.11) | 1.27 (.21) | 1.24 (.22) | <.001* |
| Mean social support, parent-rated (SD) | .92 (.21) | 1.07 (.23) | 1.01 (.23) | 1.02 (.24) | <.001* |
| Mean social support, teacher-rated (SD) | .76 (.27) | .86 (.27) | .86 (.29) | .81 (.28) | .008 |
| Predicted substance use (SE), early adult | |||||
| Binge drinking | 2.13a (.68) | 1.80a (.37) | 3.70b (.49) | 3.69b (.56) | |
| Marijuana | .32a (.23) | .43a (.20) | 6.21c (.39) | 10.35b (.30) | |
| Class sample size (%) | 132 (23) | 262 (45) | 92 (16) | 93 (16) | |
Note. Substance use was coded on a 12-point scale, ranging from 0 (not at all) to 4 (once a month) to 6 (once a week) to 9 (once a day) to 11 (several times a day or more). Substance use means designated with different superscripts (a,b,c) are significantly different within the GMM.
p ≤ .003
Bonferroni correction for 15 comparisons. The intraclass correlation for study site showed negligible between-site differences in delinquency (ICC=.035).
Tables 2 to 4 show differences between classes on baseline covariates from individual t-tests and χ2 tests. When tested simultaneously in the full models, few of these differences remained significant in the presence of other covariates. Children in class 2 (stable/improving ADHD symptoms and delinquency; lower substance use) had lower levels of baseline aggression in all models. Children in class 1 (higher symptoms/delinquency; lower substance use) had higher ODD symptoms at baseline and came from families who received welfare income (hyperactivity-impulsivity model only), and more often came from two-parent families (delinquency model only). Children in class 4 (steepest increases in symptoms/delinquency; highest substance use) were more often boys and had no reported ADHD medication history prior to study entry (hyperactivity-impulsivity model only).
There were few direct effects of baseline covariates on substance use. Binge drinking was more frequent among boys (B=.78, SE=.30 in each model) and children with higher teacher-rated social skills (B=1.05, SE=.53). Binge drinking was less frequent among African-American participants (B=−1.28, SE=.37 for inattention; B=−1.17, SE=.38 for hyperactivity-impulsivity; B=−1.44, SE=.36 for delinquency) and other minority participants (B=-.80, SE=.40 for inattention; B=-.86, SE=.36 for delinquency); all p < .05. No covariates predicted marijuana use.
Supplementary analysis: Convergence across classes
In each of the models for inattention, hyperactivity-impulsivity, and delinquency, participants were assigned to the class for which they had the highest probability of membership. Across all three variables, 83% (n =478 of 579) of participants were consistently assigned to the same type of trajectory class, either worsening or stable/improving (for hyperactivity-impulsivity, less decline). For example, among participants whose most likely class for inattention symptoms was a worsening trajectory, most were also assigned to worsening delinquency classes and to hyperactivity-impulsivity classes with less apparent improvement (i.e., classes 3 and 4). Tables S2 and S3 show two-way correspondence between inattention and delinquency and between hyperactivity-impulsivity and delinquency.
Supplementary analysis: Substance use during adolescence
Because heavy young adult substance use often begins in adolescence, as in the present sample (28), we examined mean differences in participants’ binge drinking and marijuana use at adolescent waves of assessment across trajectory classes (see Tables 5 and 6). In all three models, there was significantly greater binge drinking at the 8-year follow-up assessment in classes 3 and 4 (classes with high use at the 12-year follow-up). For delinquency, class 4 also showed significantly greater binge drinking at the 6-year follow-up. In all three models, there was significantly greater marijuana use in class 4 at the 6- and 8-year follow-up assessments.
Table 5.
Mean (SD) Differences Across Classes in Binge Drinking at Adolescent Waves of Assessment
| Class 1 | Class 2 | Class 3 | Class 4 | N | F | |
|---|---|---|---|---|---|---|
| Inattention | ||||||
| 6 years | 0.24 (.94) | 0.41 (1.40) | 0.41 (1.24) | 0.41 (1.17) | 443 | .88, p=.4493 |
| 8 years | 0.60a (1.58) | 0.75a,b (1.53) | 1.26b (2.03) | 1.29b (2.06) | 421 | 4.35, p=.0049 |
| Hyperactivity-impulsivity | ||||||
| 6 years | 0.24 (.98) | 0.32 (1.19) | 0.33 (1.03) | 0.46 (1.24) | 443 | .58, p=.6264 |
| 8 years | 0.64a,b (1.68) | 0.60b (1.44) | 1.46c (2.13) | 1.27a,c (2.04) | 421 | 5.56, p=.0009 |
| Delinquency | ||||||
| 6 years | 0.33a,b (1.11) | 0.19a (.87) | 0.31a,b (.98) | 0.69b (1.69) | 443 | 3.44, p=.0168 |
| 8 years | 0.80a,b (1.90) | 0.46a (1.23) | 1.39b (2.09) | 1.47b (2.14) | 421 | 8.58, p<.0001 |
Note. All means within rows with different superscripts correspond to significant pairwise differences (Tukey). Higher scores indicate more binge drinking.
Table 6.
Mean (SD) Differences Across Classes in Marijuana Use at Adolescent Waves of Assessment
| Class 1 | Class 2 | Class 3 | Class 4 | N | F | |
|---|---|---|---|---|---|---|
| Inattention | ||||||
| 6 years | 0.42a (1.45) | 0.37a (1.47) | 0.54a (1.69) | 1.54b (2.70) | 443 | 7.88, p<.0001 |
| 8 years | 0.65a (1.81) | 0.68a (1.51) | 1.68b (2.61) | 3.03c (3.46) | 422 | 21.14, p<.0001 |
| Hyperactivity-impulsivity | ||||||
| 6 years | 0.55a (1.74) | 0.37a (1.47) | 0.54a (1.69) | 1.54b (2.70) | 443 | 8.38, p<.0001 |
| 8 years | 0.77a (1.88) | 0.61a (1.73) | 1.70b (2.64) | 3.01c (3.37) | 422 | 20.98, p<.0001 |
| Delinquency | ||||||
| 6 years | 0.52a (1.56) | 0.31a (1.26) | 0.41a (1.44) | 1.81b (2.89) | 443 | 14.20, p<.0001 |
| 8 years | 0.52a (1.49) | 0.53a (1.53) | 1.91b (2.76) | 3.34c (3.44) | 422 | 33.94, p<.0001 |
Note. All means within rows with different superscripts correspond to significant pairwise differences (Tukey). Higher scores indicate more marijuana use.
Discussion
This study is the first to identify distinct developmental pathways of ADHD symptoms and delinquency that forecast early adult substance use outcomes at the age of peak prevalence for alcohol and marijuana use in the United States (29). Our results support the hypothesis that patterns of increasingly severe ADHD symptoms and delinquency through adolescence predict regular binge drinking and marijuana use in early adulthood. At the same time, patterns of improvement and even stability in inattentive symptoms and delinquency (and greater improvement in hyperactivity-impulsivity) were associated with less substance use. Thus, individual differences in patterns of progression may be just as, if not more, important than absolute symptom or delinquency levels at a given point in time. In general, adolescents who were best characterized by a trajectory at high risk for adult substance use for one variable (e.g., inattention) were usually also characterized by high risk trajectories for other variables (e.g., delinquency).
Children who followed worsening trajectories did not necessarily have the highest ADHD symptom and delinquency scores at all times. For instance, classes 1 and 3 for inattention reached similar symptom levels by the 8-year follow-up, but class 1 maintained this level after study treatments ended, whereas class 3 deteriorated. Reaching similar symptom levels via distinct pathways may reflect variability in how children and their families adapt and function in the face of changing situational demands. Adolescence brings on a host of novel, stressful circumstances such as puberty (46), changing schools (47), and reductions in family time (48) relative to time with peers (49). Adolescents who adapt to uncertainty and changing demands show more success in school, fewer externalizing problems, and less emotional instability (50,51). A stable profile of symptoms and behaviors, even at higher levels, may occur when adolescents and their families face fewer demands for adaptation or accommodate them successfully. If so, it is especially encouraging that two-thirds of the current sample followed stable or improving trajectories. Whether these trajectories portend healthy adjustment in other domains, such as educational and vocational functioning, remains to be tested.
Profiles of worsening ADHD symptoms and delinquency may reflect in part a cascading pattern of vulnerability (52) as earlier symptoms escalate to serious behavior problems, experimentation with drugs and alcohol in adolescence, and heavy substance use in adulthood. We observed that inattention started worsening before delinquency began to escalate—an apparent temporal precedence consistent with prior research (11,53,54). It is common to find that ADHD doesn't predict substance use after controlling for conduct disorder (7,55), but our results are consistent with the possibility that conduct problems (including delinquency) emerge as children progress along an externalizing developmental pathway beginning with ADHD and leading to substance use (56). Our supplemental findings of synchronous trajectory membership and higher adolescent substance use among those in higher-risk trajectories provide evidence that developmental vulnerability manifests in multiple domains, suggesting a possible shared predisposition toward worsening ADHD, increasing delinquency, and problematic substance use. Conduct problems, delinquency, and early experimentation with substances are prominent adolescent indicators of an externalizing pathway to substance use (57,58), but bidirectional relations between multiple domains likely propel children toward problematic substance use in early adulthood. Direct tests of these relations may be fruitful goals for future ADHD research.
In all classes, adolescent symptoms of hyperactivity-impulsivity, on average, fell below levels considered clinically significant (59), consistent with the widely observed maturational decrease in hyperactivity (60). In addition, many childhood symptoms are endorsed less frequently in adolescence (12). A limitation of our measurement of impulsivity is that it was restricted to three items and diluted by 6 hyperactivity items (61,62). Our single-item, single time-point measurement of binge drinking and marijuana use is another limitation. It would be valuable to know for whom the heaviest levels of use continue into later adulthood. We also excluded tobacco use from the present study because of its distinct pharmacologic properties (63). Improved and comprehensive measurement of these variables, including replication of the current study findings, would further increase confidence in our results. Indeed, an important caveat to the use of GMM is that replication is essential to confirm that patterns found in single studies accurately represent behavior trends in the population (64).
In an effort to understand how ADHD symptom/delinquency trajectories relate to substance use outcomes net the influence of baseline characteristics, we selected a large, but not exhaustive, set of 13 covariates for use as controls. This strategy piques curiosity about other variables not included here. For example, parents’ knowledge of their adolescents’ activities and whereabouts is more strongly associated with reduced alcohol use for adolescents with ADHD histories (65). In the MTA sample, no protective or predisposing effects of study-assigned treatment or prospectively-tracked medication use have been found with respect to adolescent substance use (28). Other candidate variables identified in comprehensive models of addiction vulnerability (57,58) suggest opportunities for future investigation of mediators and moderators (52).
Overall, the patterns of progression identified in the current study suggest that children follow distinct developmental trajectories, but worsening profiles of inattention and delinquency forecast the highest risk for regular substance use in early adulthood. However, many children maintained the gains accrued during treatment and subsequently reported low levels of early adult substance use. With respect to preventing later substance use, it may be important not to abandon the pursuit of effective treatment for ADHD and its associated difficulties after achieving short-term success, particularly if initial gains are not maintained. If treatment efforts contribute to stability of symptoms and associated risk factors over time, including the impairments and substance-related risks implicated in this population (52), it may be worthwhile to evaluate options for ongoing assistance or “booster” sessions to maintain gains accrued during initial treatment. Targeted follow-up interventions that align with sensitive periods such as the transition to middle school may help to buttress families’ treatment efforts during challenging phases of development. Such interventions should be invoked when signs of worsening symptoms and behavior begin to appear. Parents, clinics, and practitioners should view ADHD as a potentially chronic condition and consider periodic but regular monitoring to detect signs of vulnerability to worsening symptoms over time that may indicate a need for further intervention.
Supplementary Material
Acknowledgements
Appreciation is extended to Dr. Patrick Curran, University of North Carolina at Chapel Hill, for his feedback and guidance regarding technical aspects of growth mixture modeling used in this article.
The Multimodal Treatment Study of Children with ADHD (MTA) was a National Institute of Mental Health (NIMH) cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and finally under a National Institute on Drug Abuse (NIDA) contract. Collaborators from NIMH: Benedetto Vitiello, M.D. (Child & Adolescent Treatment and Preventive Interventions Research Branch), Joanne B. Severe, M.S. (Clinical Trials Operations and Biostatistics Unit, Division of Services and Intervention Research), Peter S. Jensen, M.D. (currently at REACH Institute and Mayo Clinic), L. Eugene Arnold, M.D., M.Ed. (currently at Ohio State University), Kimberly Hoagwood, Ph.D. (currently at Columbia); previous contributors from NIMH to the early phases: John Richters, Ph.D. (currently at National Institute of Nursing Research); Donald Vereen, M.D. (currently at NIDA). Principal investigators and co-investigators from the sites are: University of California, Berkeley/San Francisco: Stephen P. Hinshaw, Ph.D. (Berkeley), Glen R. Elliott, Ph.D., M.D. (San Francisco); Duke University: Karen C. Wells, Ph.D., Jeffery N. Epstein, Ph.D. (currently at Cincinnati Children's Hospital Medical Center), Desiree W. Murray, Ph.D.; previous Duke contributors to early phases: C. Keith Conners, Ph.D. (former PI); John March, M.D., M.P.H.; University of California, Irvine: James Swanson, Ph.D., Timothy Wigal, Ph.D.; previous contributor from UCLA to the early phases: Dennis P. Cantwell, M.D. (deceased); New York University: Howard B. Abikoff, Ph.D.; Montreal Children's Hospital/ McGill University: Lily Hechtman, M.D.; New York State Psychiatric Institute/Columbia University/Mount Sinai Medical Center: Laurence L. Greenhill, M.D. (Columbia), Jeffrey H. Newcorn, M.D. (Mount Sinai School of Medicine). University of Pittsburgh: Brooke Molina, Ph.D., Betsy Hoza, Ph.D. (currently at University of Vermont), William E. Pelham, Ph.D. (PI for early phases, currently at Florida International University). Follow-up phase statistical collaborators: Robert D. Gibbons, Ph.D. (University of Illinois, Chicago); Sue Marcus, Ph.D. (Mt. Sinai College of Medicine); Kwan Hur, Ph.D. (University of Illinois, Chicago). Original study statistical and design consultant: Helena C. Kraemer, Ph.D. (Stanford University). Collaborator from the Office of Special Education Programs/US Department of Education: Thomas Hanley, Ed.D. Collaborator from Office of Juvenile Justice and Delinquency Prevention/Department of Justice: Karen Stern, Ph.D.
James M. Swanson acknowledges research support, advisory board membership, speaker's bureau membership, and/or consulting for Alza, Richwood, Shire, Celgene, Novartis, Celltech, Gliatech, Cephalon, Watson, CIBA, UCB, Janssen, McNeil, and Lilly. Katherine A. Belendiuk owns an investment in Shire Pharmaceuticals. L. Eugene Arnold received research funding from Curemark, Forest, Lilly, and Shire; advisory board honoraria from Biomarin, Novartis, Noven, Roche, Seaside Therapeutics, and Shire; consulting fees from Tris Pharma, Pfizer, and Gowlings; and travel support from Noven. Howard B. Abikoff receives royalty income from Multi-Health Systems, School Specialty Inc., and Guilford Press. Lily Hechtman received research funds, has served on advisory boards, and has been a speaker for Eli Lilly, Jannsen, Ortho, Purdue, and Shire. Laurence L. Greenhill has received grant support from Shire Pharmaceuticals and from Rhodes Pharmaceuticals, and is a member of the Scientific Advisory Board of BioBDX LLC. Jeffrey H. Newcorn has received research support from Shire. He has served as an advisor and/or consultant to Alcobra, BioBehavioral Diagnostics, Enzymotec, GencoSciences, Ironshore, Neurovance, Sunovion, and Shire. Timothy Wigal has received research support, consulting honoraria, and/or has participated in the speakers’ bureau for Eli Lilly, Noven, Rhodes, Otuska, and Shire pharmaceutical companies.
The opinions and assertions contained in this report are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Health and Human Services, the National Institutes of Health, or the National Institute of Mental Health.
Footnotes
Declarations of Interest
The remaining authors have no conflicts to disclose.
References
- 1.King SM, Iacono WG, McGue M. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction. 2004 Dec;99(12):1548–59. doi: 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- 2.Molina BSG, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of Abnormal Psychology. 2003;112(3):497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- 3.Molina BSG, Flory K, Hinshaw SP, GREINER AR, Arnold LE, Swanson JM, et al. Delinquent behavior and emerging substance use in the MTA at 36 months: Prevalence, course, and treatment effects. Journal of the American Academy of Child & Adolescent Psychiatry. 2007 Aug;46(8):1028–40. doi: 10.1097/chi.0b013e3180686d96. [DOI] [PubMed] [Google Scholar]
- 4.Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, et al. Young adult outcome of attention deficit hyperactivity disorder: A controlled 10-year follow-up study. Psychological Medicine. 2006;36(2) doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- 5.Gittelman R, Mannuzza S, Shenker R, Bonagura N. Hyperactive boys almost grown up: 1. Psychiatric status. Archives of General Psychiatry. 1985;42:937–47. doi: 10.1001/archpsyc.1985.01790330017002. [DOI] [PubMed] [Google Scholar]
- 6.Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. Journal of Learning Disabilities. 1998;31(6):533–44. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- 7.Flory K, Lynam DR. The relation between attention deficit hyperactivity disorder and substance abuse: What role does conduct disorder play? Clin Child Fam Psychol Rev. 2003;6(1):1–18. doi: 10.1023/a:1022260221570. [DOI] [PubMed] [Google Scholar]
- 8.August GJ, Winters KC, Realmuto GM, Fahnhorst T, Botzet A, Lee S. Prospective study of adolescent drug use among community samples of ADHD and non-ADHD participants. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(7):824–32. doi: 10.1097/01.chi.0000219831.16226.f8. [DOI] [PubMed] [Google Scholar]
- 9.Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: Comparative meta-analyses. Journal of the American Academic of Child & Adolescent Psychiatry. 2011;50(1):9–921. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clinical Psychology Review. 2011 Apr;31(3):328–41. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. Journal of the American Academic of Child & Adolescent Psychiatry. 1990;29(4):546–57. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Sibley MH, Pelham WE, Molina BSG, Gnagy EM, Waschbusch DA, Garefino AC, et al. Diagnosing ADHD in adolescence. Journal of Consulting and Clinical Psychology. 2012;80(1):139–50. doi: 10.1037/a0026577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barkley RA, Murphy KR, Fischer M. ADHD in adults: What the science says. Guilford Press; New York: 2008. [Google Scholar]
- 15.Malone PS, Van Eck K, Flory K, Lamis DA. A mixture-model approach to linking ADHD to adolescent onset of illicit drug use. Developmental Psychology. 2010;46(6):1543–55. doi: 10.1037/a0020549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina BSG, Pelham WE, Cheong J, Marshal MP, Gnagy EM, Curran PJ. Childhood attention-deficit/hyperactivity disorder (ADHD) and growth in adolescent alcohol use: The roles of functional impairments, ADHD symptom persistence, and parental knowledge. Journal of Abnormal Psychology. 2012;121(4):922–35. doi: 10.1037/a0028260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MTA Cooperative Group A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 1999;56(1073-1086) doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 18.Swanson J, Hinshaw S, Arnold L, GIBBONS R, Marcus S, Hur K, et al. Secondary evaluations of MTA 36-month outcomes: propensity score and growth mixture model analyses. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(8):1003–14. doi: 10.1097/CHI.0b013e3180686d63. [DOI] [PubMed] [Google Scholar]
- 19.Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 Years: Prospective Follow-up of Children Treated for Combined-Type ADHD in a Multisite Study. Journal of the American Academy of Child & Adolescent Psychiatry. 2009 May;48(5):484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Klein KL, et al. Psychopathology in females with Attention-Deficit/Hyperactivity Disorder: A controlled, five-year prospective study. Biological Psychiatry. 2006 Nov;60(10):1098–105. doi: 10.1016/j.biopsych.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child Psychol & Psychiat. 2004;45(2):195–211. doi: 10.1111/j.1469-7610.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 22.Jester JM, Nigg JT, Buu A, Puttler LI, Glass JM, Heitzeg MM, et al. Trajectories of childhood aggression and inattention/hyperactivity: Differential effects on substance abuse in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2008 Oct;47(10):1158–65. doi: 10.1097/CHI.0b013e3181825a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, Petty C, et al. Does ADHD predict substance use disorders? A 10-year follow-up study of young adults with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(6):543–53. doi: 10.1016/j.jaac.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100(4):674–701. [PubMed] [Google Scholar]
- 25.Moffitt TE, Caspi A, Harrington H, Milne BJ. Males on the life-course-persistent and adolescence-limited antisocial pathways: Follow-up at age 26 years. Development and Psychopathology. 2002;14:179–207. doi: 10.1017/s0954579402001104. [DOI] [PubMed] [Google Scholar]
- 26.Lee SS, Hinshaw SP. Severity of adolescent delinquency among boys with and without Attention Deficit Hyperactivity Disorder: Predictions from early antisocial behavior and peer status. Journal of Clinical Child & Adolescent Psychology. 2004;33(4):705–16. doi: 10.1207/s15374424jccp3304_6. [DOI] [PubMed] [Google Scholar]
- 27.Jensen P, Arnold L, Swanson J, Vitiello B, Abikoff H, Greenhill L, et al. 3-year follow-up of the NIMH MTA study. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(8):989–1002. doi: 10.1097/CHI.0b013e3180686d48. [DOI] [PubMed] [Google Scholar]
- 28.Molina B, Hinshaw SP, Arnold LE, Swanson JM, Pelham WE, Hechtman L, et al. Adolescent substance use in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(3):250–63. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SAMHSA Center for Behavioral Health Statistics and Quality . Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. pp. 1–156. Report No.: NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. [Google Scholar]
- 30.Arnold LE, Abikoff H, Cantwell DP, Conners CK, Elliott G, Greenhill LL, et al. National Institute of Mental Health Collaborative Multimodal Treatment Study of children with ADHD (the MTA). Archives of General Psychiatry. 1997;54:865–70. doi: 10.1001/archpsyc.1997.01830210113015. [DOI] [PubMed] [Google Scholar]
- 31.Swanson JM. School-based assessments and interventions for ADD students. KC Publications; Irvine, CA: 1992. [Google Scholar]
- 32.Wolfgang ME, Figlio RM, Tracy PE, Singer SI. The National Survey of Crime Severity. U.S. Government Printing Office; Washington, DC: 1985. Report No.: NCJ-96017. [Google Scholar]
- 33.Loeber R, Stouthamer-Loeber M, Van Kammen W, Farrington DP. Initiation, escalation and desistance in juvenile offending and their correlates. Journal of Criminal Law & Criminology. 1991;82(1):1–48. [Google Scholar]
- 34.Wechsler D. Wechsler Individual Achievement Test. Psychological Corporation; San Antonio, TX: 1992. [Google Scholar]
- 35.Atkins MS, Pelham WE, Licht MH. A comparison of objective classroom measures and teacher ratings of Attention Deficit Disorder. Journal of Abnormal Child Psychology. 1985;13(1):155–67. doi: 10.1007/BF00918379. [DOI] [PubMed] [Google Scholar]
- 36.Gresham FN, Elliott SN. Social Skills Rating System: Parent, Teacher, and Child Forms. American Guidance Systems; Circle Pines: 1989. [Google Scholar]
- 37.Muthén L, Muthén B. Mplus. 6 ed. Muthén & Muthén; Los Angeles, CA: 2011. [Google Scholar]
- 38.Muthén B. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of Quantitative Methodology for the Social Sciences. Sage Publications, Inc; Newbury Park, CA: 2004. pp. 345–64. [Google Scholar]
- 39.Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychological Methods. 1999 Aug 27;4(2):139–57. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 40.Enders CK. Applied missing data analysis. Guilford Press; New York: 2010. [Google Scholar]
- 41.Bauer D, Curran PJ. The integration of continuous and discrete latent variable models: Potential problems and promising opportunities. Psychological Methods. 2004;9(1):3–29. doi: 10.1037/1082-989X.9.1.3. [DOI] [PubMed] [Google Scholar]
- 42.Jedidi K, Jagpal HS, DeSarbo WS. Finite-mixture structural equation models for response-based segmentation and unobserved heterogeneity. Marketing Science. 1997;16(1):39–59. [Google Scholar]
- 43.Lubke G, Muthen BO. Performance of Factor Mixture Models as a Function of Model Size, Covariate Effects, and Class-Specific Parameters. Structural Equation Modeling: A Multidisciplinary J. Taylor & Francis Group. 2007 Jan;14(1):26–47. [Google Scholar]
- 44.Ram N, Grimm K. Growth mixture modeling: A method for identifying differences in longitudinal change among unobserved groups. International Journal of Behavioral Development. 2009;33(6):565. doi: 10.1177/0165025409343765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hipp JR, Bauer DJ. Local solutions in the estimation of growth mixture models. Psychological Methods. 2006;11(1):36–53. doi: 10.1037/1082-989X.11.1.36. [DOI] [PubMed] [Google Scholar]
- 46.Graber JA, Brooks-Gunn J. Transitions and turning points: Navigating the passage from childhood through adolescence. Developmental Psychology. 1996 Mar 14;32(4):768–726. [Google Scholar]
- 47.Langberg JM, Epstein JN, Altaye M, Molina BSG, Arnold LE, Vitiello B. The transition to middle school is associated with changes in the developmental trajectory of ADHD symptomatology in young adolescents with ADHD. Journal of Clinical Child & Adolescent Psychology. 2008 Jul 14;37(3):651–63. doi: 10.1080/15374410802148095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larson R, Richards MH. Daily companionship in late childhood and early adolescence: Changing developmental contexts. Child Development. 1991;62(2):284–300. doi: 10.1111/j.1467-8624.1991.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 49.Brown BB. Adolescents' relationships with peers. In: Lerner RM, Steinberg L, editors. Handbook of Adolescent Psychology. 2nd ed. Wiley; New York: 2004. pp. 363–94. [Google Scholar]
- 50.Martin AJ, Nejad HG, Colmar S, Liem GAD. Adaptability: How students’ responses to uncertainty and novelty predict their academic and non-academic outcomes. Journal of Educational Psychology. 2013;105(3):728–46. [Google Scholar]
- 51.Causadias JM, Salvatore JE, Sroufe LA. Early patterns of self-regulation as risk and promotive factors in development: A longitudinal study from childhood to adulthood in a high-risk sample. International Journal of Behavioral Development. 2012 Jul 10;36(4):293–302. doi: 10.1177/0165025412444076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molina BSG, Pelham WE., Jr Attention-Deficit/Hyperactivity Disorder and Risk of Substance Use Disorder: Developmental Considerations, Potential Pathways, and Opportunities for Research. Annual Review of Clinical Psychology. 2014 Mar 28;10(1):607–39. doi: 10.1146/annurev-clinpsy-032813-153722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burke JD, Loeber R, Lahey BB, Rathouz PJ. Developmental transitions among affective and behavioral disorders in adolescent boys. J Child Psychol & Psychiat. 2005 Nov;46(11):1200–10. doi: 10.1111/j.1469-7610.2005.00422.x. [DOI] [PubMed] [Google Scholar]
- 54.Kuperman S, Schlosser SS, Kramer JR, Bucholz KK, Hesselbrock V, Reich T, et al. Risk domains associated with an adolescent alcohol dependence diagnosis. Addiction. 2001;96:629–36. doi: 10.1046/j.1360-0443.2001.96462911.x. [DOI] [PubMed] [Google Scholar]
- 55.Harty SC, Ivanov I, Newcorn JH, Halperin JM. The impact of conduct disorder and stimulant medication on later substance use in an ethnically diverse sample of individuals with Attention-Deficit/Hyperactivity Disorder in childhood. Journal of CHild and Adolescent Psychopharmacology. 2011 Aug;21(4):331–9. doi: 10.1089/cap.2010.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry. Am Med Assoc. Am Med Assoc. 2007;64(10):1145. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- 57.Zucker RA. Alcohol use and the alcohol use disorders: A developmental-biopsychosocial systems formulation covering the life course. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology: Risk, Disorder, and Adaptation. 2nd ed. John Wiley & Sons; Hoboken, NJ: 2006. pp. 620–56. [Google Scholar]
- 58.Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annual Review of Clinical Psychology. Annual Reviews. 2005 Apr;1(1):493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- 59.Bussing R, Fernandez M, Harwood M, Wei Hou, Garvan CW, Eyberg SM, et al. Parent and teacher SNAP-IV ratings of Attention Deficit Hyperactivity Disorder symptoms: Psychometric properties and normative ratings from a school district sample. Assessment. 2008 Feb 29;15(3):317–28. doi: 10.1177/1073191107313888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, et al. Validity of DSM-IV Attention Deficit/Hyperactivity Disorder symptom dimensions and subtypes. Journal of Abnormal Psychology. 2012;121(4):991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Association American Psychiatric . Diagnostic and statistical manual of mental disorders. 4 ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 62.Association American Psychiatric . Diagnostic and statistical manual of mental disorders. 5 ed. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- 63.Rezvani AH, Levin ED. Cognitive effects of nicotine. Biological Psychiatry. 2001 Feb;49(3):258–67. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- 64.Little TD, Card NA, Preacher KJ, McConnell E. Modeling longitudinal data from research on adolescence. In: Lerner RM, Steinberg L, editors. Handbook of Adolescent Psychology. 3rd ed. John Wiley & Sons; Hoboken, NJ: 2009. pp. 15–54. [Google Scholar]
- 65.Walther CAP, Cheong J, Molina BSG, Pelham WE, Wymbs BT, Belendiuk KA, et al. Substance use and delinquency among adolescents with childhood ADHD: The protective role of parenting. Psychology of Addictive Behaviors. 2012;26(3):585–98. doi: 10.1037/a0026818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.