Abstract
The folate receptor (FR) has been widely recognized as an excellent target for the tumor-selective delivery of cytotoxic agents, and four folate-drug conjugates have entered clinical evaluations for the treatment of solid tumors to date. However, most of these conjugates required structural modification of the cytotoxic warheads in order to achieve efficient drug release from the linkers. We designed and constructed a novel folate conjugate of a highly-potent next-generation taxoid, SB-T-1214, by exploiting bioorthogonal Cu-free “click” chemistry. The synthesis was highly convergent and required no HPLC purification to obtain the final folate-taxoid conjugate 1. Conjugate 1 demonstrated highly FR-specific potency (IC50 2.1–3.5 nM) against a panel of cancer cell lines, with a >1,000-fold decrease in cytotoxicity against normal human cells (IC50 >5,000 nM). The remarkable potency and selectivity of conjugate 1 can be attributed to highly FR-specific receptor-mediated endocytosis as well as efficient release of the unmodified cytotoxic warhead using a mechanism-based self-immolative linker.
Keywords: Tumor-targeted, drug conjugate, folic acid, taxoid, anticancer agent
1. Introduction
Cancer is a group of over 200 diseases characterized by the uncontrolled growth and spread of abnormal cells. Left untreated, it results in organ failure and death. Despite the recent advances in our understanding of the molecular processes that lead to its development and progression, cancer remains the second most common cause of death in the U.S. and a leading cause of death worldwide.1 Traditionally, treatment options include the use of chemotherapy, which typically entails the administration of cytotoxic agents that act on the processes of cell division. The rationale for this treatment modality is that rapidly dividing cancer cells will be more susceptible to the cytotoxic effects of the drugs than healthy bystander cells. However, in reality, this is not the case. Commonly used therapies, such as doxorubicin, paclitaxel and cisplatin, cause severe dose-limiting side effects due to their adverse effects on the highly-proliferating cells in certain tissues, including the bone marrow, heart and GI tract. Therefore, there is an urgent need to develop safe and effective alternatives to the existing chemotherapy options.
One strategy that has gained significant attention in recent decades is the use of tumor-targeted drug delivery systems (TTDDS’s). By exploiting the molecular and physiological differences between healthy and cancerous tissues, it is possible to rationally develop a TTDDS that has the capability to deliver cytotoxic agents selectively to cancer cells thereby reducing the off-target side-effects observed with traditional chemotherapy. An effective TTDDS is typically composed of three basic components, i.e., (i) a highly potent cytotoxic drug, (ii) a tumor-recognition moiety that directs the drug conjugate to cancer specific receptors and promotes efficient internalization into cancer cells via receptor-mediated endocytosis (RME), and (iii) a smart linker that is stable in blood plasma yet efficiently releases the drug upon internalization.
The next-generation taxoids developed in our laboratory have demonstrated remarkably enhanced potency against drug-sensitive and drug-resistant cancer cells, as well as tumor xenografts, as compared to paclitaxel and docetaxel. 2 With subnanomolar IC50 values against a broad panel of cancer cell lines, these compounds are ideally suited to be used as high-potency warheads for TTDDS’s.3 Many of these taxoids retain their remarkable potency against multidrug resistant cell lines derived from colon, pancreatic and renal cancers that overexpress the P-glycoprotein efflux pump.2 For example, one such next-generation taxoid, SB-T-1214 has demonstrated profound activity against 3D-spheroids derived from highly metastatic colon cancer stem cells, causing the down-regulation of stem cell-related genes and eventually leading to apoptosis.4 Accordingly, this next-generation taxoid has been incorporated into various TTDDS’s, bearing biotin and omega-3-fatty acid as tumor-targeting modules (TTMs),5–7 as well as single-walled carbon nanotubes and dendrimers as nano-scale vehicles.8–10
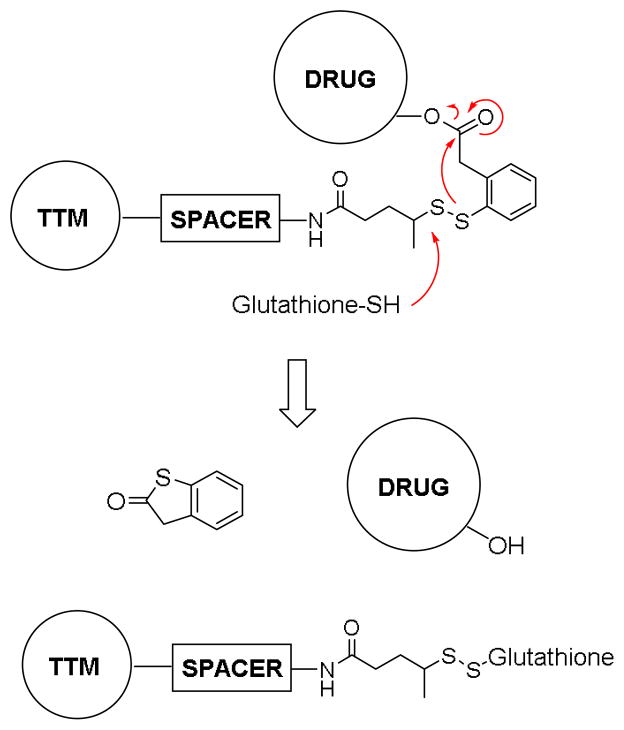
It is essential to use a well-designed mechanism-based linker system for efficacious TTDD. The self-immolative disulfide linkers, which we have been developing, are rapidly cleaved following internalization of drug conjugates, resulting in a cascade drug release via thiolactonization to release the unmodified original drug (Figure 1).3 As the concentration of glutathione is three orders of magnitude greater in tumor cells compared to that in the blood, disulfide linkers are stable in circulation, yet break down during receptor mediated endocytosis (RME).11 This mechanism-based drug release was demonstrated using a fluorinated model system by 19F NMR spectroscopy, 3, 12, 13 and has been validated in vitro by confocal fluorescence microscopy (CFM) and flow cytometry using fluorescent probes.5 Thus, this “smart-linker system” has been successfully integrated into macromolecule- and small molecule-based TTDDS’s.3, 5, 8
Figure 1.
Mechanism of the self-immolative disulfide linker.
Folic acid, also known as vitamin B9, is the precursor for tetrahydrofolate and is necessary for many biological processes required in cell division, such as DNA synthesis and repair.14 Consequently, many tumors exhibit greatly enhanced folate uptake and overexpress the transporters required for its internalization.15 Folic acid–drug conjugates are internalized via the folate receptor (FR), which is highly expressed in some tumors and virtually absent in most healthy tissues.16 Folate-mediated tumor targeting has been demonstrated to be highly effective both in vitro and in vivo.17 To date, four folate-drug conjugates have been evaluated in clinical trials for the treatment of solid tumors and the leading candidate, Vintafolide, has advanced to the phase III clinical trials in patients with platinum resistant ovarian cancer, and recently obtained expedited approval by EU.18–21 Thus, the FR is a widely accepted and validated target for TTDD.
We report here the design, synthesis and biological evaluation of a novel and highly potent folate–taxoid conjugate with extremely high specificity to FR-overexpressing cancer cell lines.
2. Results and Discussion
2.1. Design of Folate-Taxoid Conjugate
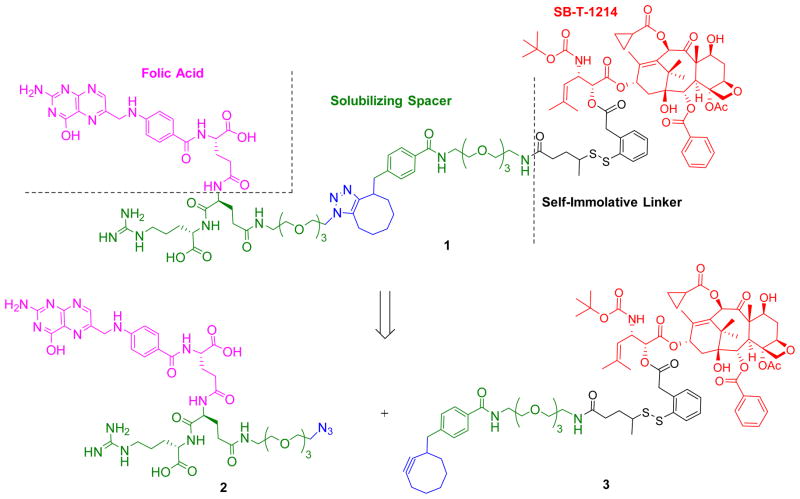
As Figure 2 illustrates, conjugate 1 was designed to include a folic acid moiety for tumor targeting, a PEGylated dipeptide spacer and a self-immolative linker bearing a highly-potent next-generation taxoid, SB-T-1214. Due to the finite number of FRs (1–3 × 106)15, 22 on a given tumor cell and the 8–12 hour receptor recycling rate,23 the use of potent warheads with IC50 values of low nM or below is necessary to achieve meaningful activity in solid tumors.24 Furthermore, the cytotoxic drug should be released inside the cancer cell unmodified to fully retain its activity. Early drug conjugates that did not possess these qualities were found to possess insufficient potency and/or specificity.25–27 Thus, our TTDDS bearing a self-immolative disulfide linker and a highly potent next-generation taxoid is ideally suited for the development of FR-targeted drug conjugates.
Figure 2.
Retrosynthetic analysis for the folic acid conjugate of next-generation taxoid SB-T-1214 (1)
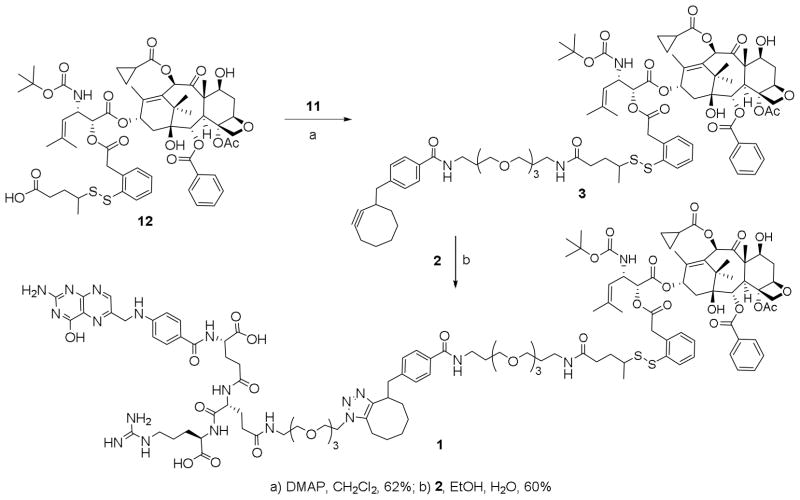
We developed a convergent and scalable route to the novel folic acid–taxoid conjugate 1. As Figure 2 shows, we incorporated a chemically modified Glu-Arg dipeptide unit as a handle for bioorthogonal chemistry, i.e., click reaction, and used solid phase peptide synthesis (SPPS) to construct a water-soluble folyl-dipeptide component 2, bearing an azidoethyl triethylene glycol moiety in the Glu residue. Charged amino acid residues were incorporated into the conjugate to reduce passive diffusion to cells28 and to increase the solubility of the folic acid moiety to facilitate chemical transformations. The use of a cyclooctyne group in the taxoid-linker component 3 enables a Cu-free click reaction29 with component 2 to assemble the conjugate 1 in minimal linear synthetic steps without need for elimination of residual Cu-related impurities.
2.2. Synthesis of Conjugate 1
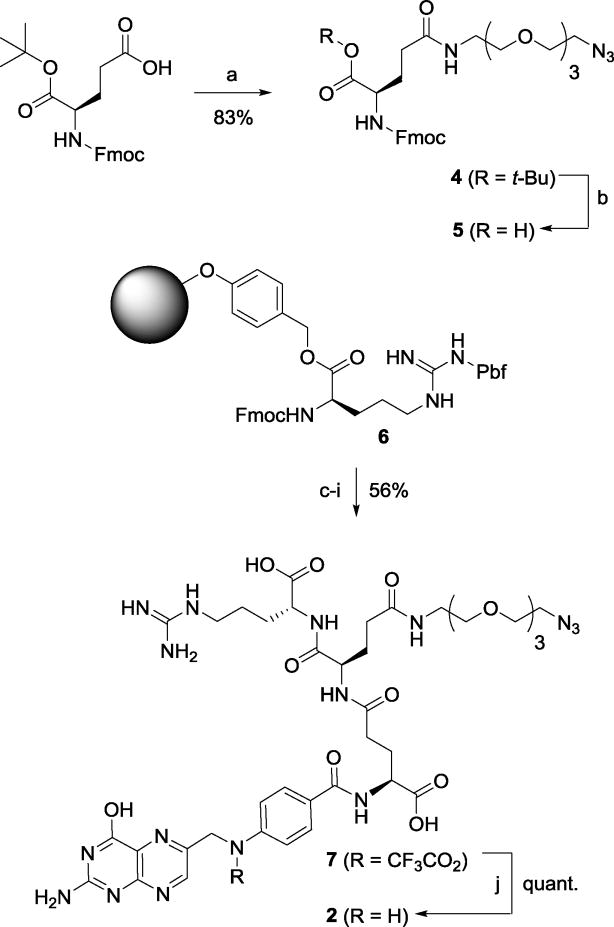
The synthesis of component 2 by means of SPPS is illustrated in Scheme 1. First, the EDC coupling of Fmoc-(S)-Glu-OBut with 11-azido-3,6,9-trioxaundecan-1-amine gave 4 in 83% yield, and the subsequent deprotection of tert-butyl ester gave Fmoc-Glu(ω-N3)-OH (5) in nearly quantitative yield.
Scheme 1.
Synthesis of folyl(dipeptide)-PEG-azide 2
Reagents and Conditions: a) 11-azido-3,6,9-trioxa-11-undecan-1-amine, EDC·HCl, CH2Cl2, 83%; b) TFA, CH2Cl2, 98%; c) piperidine, DMF; d) 5, HOBt, HBTU, DMF; e) piperidine, DMF; f) Fmoc-Glu(OH)-OtBu, HOBt, HBTU, DMF; g) piperidine, DMF; h) N10-TFA-pteroic acid, HOBt, HBTU, DMF; i) TFA, TIPS, H2O, 56%; j) NH4 OH, H2O, quant.
Next, the peptide couplings, starting with Fmoc-(S)-Arg(Pbf)-Wang resin (6), were performed sequentially using HOBt and HBTU as coupling agents and piperidine for Fmoc deprotection. The sequence of couplings was as follows (Fmoc of the Wang-resin bound amino acid or peptide was removed before coupling in each case): (i) 6 with 5 to form Fmoc-Glu(ω-N3)-Arg-O-Wang; (ii) H-Glu(ω-N3)-Arg-O-Wang with Fmoc-(S)-Glu(γ-OH)-α-OBut to afford Fmoc-Glu(α-OBut)-γ-Glu(ω-N3)-Arg-O-Wang. Finally, H-Glu(α-OBut)-γ-Glu(ω-N3)-Arg-O-Wang was coupled with N10-trifluoroacetylpteroic acid to afford γ-(N10-trifluoroacetyl-α-OBut-folyl)-Glu(ω-N3)-Arg-O-Wang, which was treated with TFA/TIPS/H2O to cleave the peptide from the Wang resin to give γ-(N10-trifluoroacetylfolyl)-Glu(ω-N3)-Arg-OH (7) in 56% overall yield from 6. The trifluoroacetyl group of 7 was removed by ammonium hydroxide to afford γ-folyl-Glu(ω-N3)-Arg-OH (2) in quantitative yield.
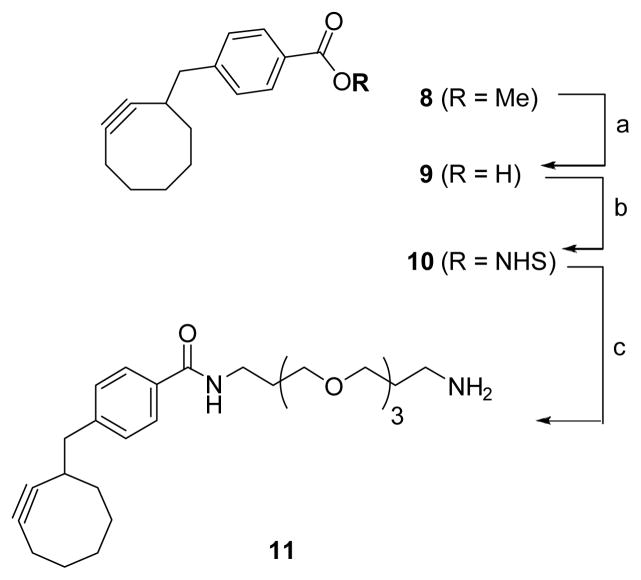
Initial attempts at conjugation were made by using the standard Cu(I)-catalyzed click chemistry. However, the Cu-mediated reaction failed to produce the desired conjugation product, probably due to the interactions between Cu(I) and free folic amide as well as basic amino acid residues. Accordingly, a Cu-free and strain-promoted azide-cyclooctyne [3+2] cycloaddition was employed for the final conjugation.29 Cyclooctynylmethyl-benzoate 8 was prepared using the literature procedure29 with modifications, which gave the desired product 11 in significantly improved overall yield (Scheme 2).29 Hydrolysis of 8, followed by NHS activation provided activated ester 10. Using excess 4,7,10-trioxa-1,13-tridecanediamine, 10 was converted to monosubstituted amino-PEG3-cyclooctyne 11 in good isolated yield.
Scheme 2.
Synthesis of amino-PEG3-cyclooctyne 11
Reagents and Conditions: a) LiOH, H2O, dioxane, 50 °C, 91%; b) NHS, DIC, DMAP, CH2Cl2, 83% c.) 4,7,10-trioxa-1,13-tridecanediamine, CH2Cl2, 73%.
Drug-linker construct 12, bearing SB-T-1214 and the self-immolative disulfide linker was synthesized according to the procedure previously reported by us.30 Cyclooctyne-PEG-amine tether 11 was coupled to the free acid of 12 to afford click-ready taxoid conjugate 3. When equimolar amounts of 2 and 3 were reacted in a 1:1 ethanol:water mixture, drug conjugate 1 was formed and precipitated out of solution during the course of the reaction. In this way, 1 was isolated by centrifugation and filtration in 60% yield, without chromatographic purification (Scheme 3). Thus, this synthetic strategy using Cu-free click chemistry without any protecting groups in the coupling partners avoids any use of HPLC purification, which may be adapted for gram-scale preparation of taxoid-folate conjugate 1
Scheme 3.
Synthesis of folate-taxoid conjugate 1
We assessed the FR-specific anticancer activity of conjugate 1, as compared to free SB-T-1214 and paclitaxel in cell lines with varying degrees of FR expression, i.e., ID8 (murine ovarian, FR+++),31 MX-1 (human breast, FR++), L1210-FR (murine leukemia, FR++)31 and WI-38 (normal human lung fibroblast, FR− 32, 33). Results are summarized in Table 1. As anticipated, free SB-T-1214 was found to be highly potent with single digit nanomolar IC50 values against all cell lines, including normal cell line, WI38. In sharp contrast, conjugate 1 did not show any appreciable cytotoxicity against WI38 (IC50 >5,000 nM), while exhibiting excellent potency, equal to that of SB-T-1214, against ID8 ovarian cancer cell line (FR+++). The result suggests that conjugate 1 was internalized by receptor-mediated endocytosis (RME) and released a free drug (SB-T-1214) inside the cells highly efficiently. The result also indicates that there was sufficient endogenous glutathione (GSH) or other thiols in this cancer cell line.
Table 1.
IC50 values (nM)a of conjugate 1 compared with free SB-T-1214 and paclitaxel against cancer cell lines with varying levels of FR expression
| ID8b (FR+++) | MX-1c (FR++) | L1210-FRd (FR++) | WI-38e (FR−) | |
|---|---|---|---|---|
| Paclitaxelf | 13.8 ± 2.7 | 5.59 ± 2.12 | 27.6 ± 7.5 | 55.7 ± 9.5 |
| SB-T-1214f | 2.82 ± 0.70 | 1.89 ± 0.80 | 2.66 ± 1.33 | 4.89 ± 2.24 |
| 1f | 2.52 ± 1.50 | 3.43 ± 2.23 | 3.51 ± 1.16 | > 5,000 |
| 1g (GSH-OEt) | N.D.i | 2.08 ± 1.30 | 3.35 ± 2.00 | N.D.i |
| 1h (GSH) | N.D.i | 3.42 ± 2.98 | 3.87 ± 2.61 | N.D.i |
IC50: concentration required for 50% growth inhibition;
murine ovarian cancer cell line (FR+++);
human breast cancer cell line (FR++);
murine leukemia cell line (FR++);
human lung fibroblasts (normal) cell line (FR−);
Cells were incubated with each drug for 72 h.;
After cells were treated with 1 for 24 h, GSH-OEt (6 equiv.) was added and then incubated for another 48 h (Total 72 h);
After cells were treated with 1 for 24 h, GSH (6 equiv.) was added, and then incubated for another 48 h (total 72 h).
Not determined.
Conjugate 1 also exhibited high potency against MX-1 (FR++) and L1210FR (FR++) cell lines. Although the IC50 values for conjugate 1 were in the same range as those for SB-T-1214 by taking into account the standard deviations (S.D.s), there was a possibility that the endogenous thiol level was not sufficient in this cell line. If it were the case, free SB-T-1214 would not be released in spite of efficient internalization of 1. In order to examine this possibility, two experiments were performed, i.e., (i) addition of glutathione ethyl ester (GSH-OEt) after 24 h incubation followed by additional 48 h incubation and (ii) addition of GSH at the 24 h period followed by additional 48 h incubation. Thus, the total incubation time was 72 h, which was the same as the experiment without addition of GSH-OEt or GSH. Since GSH-OEt would get into the cancer cells, while GSH would not, the comparison of two results should indicate the extent of internalization and release of free SB-T-1214 outside and inside of the cancer cells.
As Table 1 shows, the IC50 value of conjugate 1 against MX-1 was reduced to 2.08 ± 1.30 nM on addition of GSH-OEt, which was the same as that for free SB-T-1214 within S.D., while the value was unchanged by the addition of GSH. The result may suggest that the endogenous thiol in MX-1 cells was not quite sufficient to release all warheads inside the cells. In the case of L1210FR, there seems to no change in IC50 values by the addition of GSH-OEt or GSH within S.D. Also, the IC50 values for free SB-T-1214 and conjugate 1 are comparable within S.D. Thus, it appears that there was more or less sufficient endogenous thiol to release warheads. The results clearly demonstrate that the internalization of conjugate 1 via RME was excellent for these two FR++ cells.
It should be noted that there was more than three orders of magnitude difference in the IC50 values against FR-overexpressing cancer cells and that against FR− cells, i.e., normal WI38 cells. This demonstrates the extremely high cancer cell selectivity of conjugate 1 through FR-targeting.
It would also be worthy of note that the RPMI cell culture medium used in the MTT assays naturally includes folic acid, which could interfere with the RME of conjugate 1. Nevertheless, the results shown in Table 1 indicate that the internalization of conjugate 1 via FR-RME was not affected by the folic acid in the RPMI medium, which may imply that folic acid necessary for cell growth is predominantly supplied to the cells through folate transporter such as the reduced folate carrier,34 but not via FR-RME.
In conclusion, we have constructed novel taxoid-folate conjugate 1, using a combination of SPPS with synthetically modified amino acids and Cu-free click chemistry. The synthetic route requires no HPLC purification and can therefore be adapted for a gram-scale synthesis of this conjugate. Conjugate 1 exhibited highly FR-specific potency against a panel of cancer cell lines, and a >1,000-fold decrease in cytotoxicity against healthy cells, as compared to the free drug. The remarkable FR-specific potency exhibited by conjugate 1 demonstrates the power of rational drug-conjugate design in the context of developing safe and effective new chemotherapy options. Further biological evaluation of folic acid conjugates of next generation taxoids is actively underway in our laboratory.
3. Experimental
General Methods
1H and 13C NMR spectra were measured on a Varian 300 MHz spectrometer or a Bruker 400 or 500 MHz NMR spectrometer. Melting points were measured on a Thomas-Hoover capillary melting point apparatus and are uncorrected. TLC was performed on Sorbent Technologies aluminum-backed Silica G TLC plates (Sorbent Technologies, 200 μm, 20 × 20 cm), and column chromatography was carried out on silica gel 60 (Merck, 230–400 mesh ASTM). LC/HRMS analysis was performed on an Agilent LC-UV-TOF system with a G6224A TOF mass spectrometer, operating in the m/z range of 100–3200 Da with a resolution of 20,000 at m/z = 1,522 Da. Purity was determined with a Shimadzu L-2010A HPLC HT series HPLC assembly, using a Phenomenex® PFP column (Kinetex, 2.6μ, 100 × 4.6 mm) with aqueous ammonium acetate and acetonitrile as the mobile phase at a flow rate of 0.6 mL/min and a gradient of 40 → 80% acetonitrile for the 0–40 minute period. Low-resolution mass spectra were obtained using flow injection analysis on an Agilent LC-MSD with a single quadrupole mass analyzer operating in the m/z range of 100–1500 Da. High-resolution mass spectra were obtained at the Institute of Chemical Biology & Drug Discovery Mass Spectrometry Laboratory, Stony Brook, NY, or at the Mass Spectrometry Laboratory, University of Illinois at Urbana Champaign, Urbana, IL.
Materials
The chemicals were purchased from Sigma-Aldrich, Fisher Scientific, and VWR International, and used as received or purified before use by standard methods. N10-trfluoroacetylpteroic acid was purchased from Irvine Chemistry Laboratory and used as received. Tetrahydrofuran was freshly distilled under nitrogen from sodium metal and benzophenone. Dichloromethane was distilled under nitrogen from calcium hydride. 3,6,9-Trioxa-11-azido-undecan-1-amine was prepared according to the literature method.35 10-Deacetylbaccatin III was generously provided by Indena, SpA, Italy.
3.1. Fmoc-Glu(ω-N3)-OtBu (4)36
To a solution of Fmoc-(S)-Glu-OtBu (200 mg, 0.47 mmol) and 3,6,9-trioxa-11-azido-undecan-1-amine (120 mg, 0.56 mmol) in CH2Cl2 (10 mL) was added a suspension of EDC•HCl (135 mg, 0.71 mmol) in CH2Cl2 (5 mL). The solution was stirred at room temperature for 4 hrs and the reaction was monitored by TLC. Upon completion of the reaction, water (20 mL) was added to the reaction mixture and extracted with CH2Cl2 (3 × 20 mL). The combined organic layers were washed with brine (3 × 15 mL), dried over MgSO4, filtered and the filtrate was concentrated in vacuo. The crude product was purified by column chromatography on silica gel with increasing amounts of EtOAc in hexanes (hexanes:EtOAc 1:0–1:3) to afford 4 (234 mg, 83%) as a white solid: 1H NMR (400 MHz, CDCl3) δ 1.46 (s, 9H), 1.95 (m, 1H), 2.16–2.27 (m, 3H), 3.34 (m, 2H), 3.44 (m, 2H), 3.55 (m, 2H), 3.63 (m, 10H), 4.21 (m, 2H), 4.39 (m, 2H), 5.69 (d, J = 8.0 Hz, 1H), 6.27 (s, br, 1H), 7.30 (t, J = 7.6 Hz, 2H), 7.39 (t, J = 7.2 Hz, 2H), 7.60 (m, 2H), 7.75 (d, J = 7.6 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 28.01, 28.73, 32.51, 39.33, 47.21, 50.67, 54.11, 60.39, 66.94, 69.75, 70.01, 70.27, 70.53, 70.60, 70.66, 82.37, 119.97, 125.15, 127.08, 127.71, 141.31, 143.75, 143.96, 156.25, 171.16, 171.97. HRMS (ESI) calcd for C32H44N5O8 (M+H)+: 626.3190, found: 626.3188 (Δ −0.3 ppm, −0.2 mDa). All data were in agreement with literature values.36
3.2. Fmoc-Glu(ω-N3)-OH (5)36
A solution of 4 (170 mg, 0.15 mmol) in CH2Cl2 (8 mL) was cooled to 0 °C and TFA (2 mL) was added dropwise. The mixture was stirred at 0 °C for 3 hrs and the reaction progress was monitored by TLC. Upon completion of the reaction, the mixture was diluted with CH2Cl2 (30 mL), washed with H2O (5 × 20 mL) until the pH of the aqueous was no longer acidic. The organic layer was then dried over MgSO4, filtered, and the filtrate was concentrated in vacuo to afford 5 as a yellow oil that was triturated using ether/hexanes to an off-white solid (155 mg, 98%): 1H NMR (400 MHz, CDCl3) δ 2.11 (m, 1H), 2.19 (m, 1H), 2.39 (m, 1H), 2.48 (m, 1H), 3.35 (m, 2H), 3.46 (m, 2H), 3.55 (m, 2H), 3.63 (m, 10H), 4.21 (t, J = 7.2 Hz, 1H), 4.37 (m, 2H), 6.01 (d, J = 6.8 Hz, 1H), 6.69 (s, br, 1H), 7.30 (t, J = 7.2 Hz, 2H), 7.39 (t, J = 7.2 Hz, 2H), 7.59 (m, 2H), 7.75 (d, J = 7.6 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 28.62, 32.30, 39.60, 47.12, 50.61, 53.40, 67.04, 69.45, 69.93, 70.11, 70.40, 70.54, 119.96, 125.14, 125.20, 127.12, 127.73, 141.28, 143.70, 143.93, 156.27, 173.56, 173.62. HRMS (ESI) calcd for C28H36N5O8 (M+H)+: 570.2564, found 570.2560 (Δ −0.7 ppm, −0.4 mDa). All data were in agreement with literature values.36
3.3. N10-TFA-Folyl-Glu(ω-N3)-Arg-OH (7)
Fmoc-Arg(Pbf)-Wang resin (6) (150 mg, 75 μmol) was suspended in DMF (2 mL), shaken 1 hr, and the solution was filtered off. A 20% solution of piperidine in DMF (2 mL) was added to the resin, and the mixture was shaken for 30 min. The solvent was filtered off, and the resin was washed with DMF (2 × 2 mL). A solution of 5 (130 mg, 225 μmol), DIPEA (165 μL), HOBt (35 mg, 225 μmol) and HBTU (85 mg, 225 μmol) in DMF (1.835 mL) was added to the resin, and the reaction mixture was shaken for 4 hrs. The solution was then filtered off and the beads were washed with DMF (3 × 2 mL).
To the resin was added a 20% solution of piperidine in DMF (2 mL), and the mixture was shaken for 30 min. The solution mixture was filtered off, and the resin was washed with DMF (2 × 2 mL). A solution of Fmoc-Glu-OtBu (95 mg, 225 μmol), DIPEA (165 μL), HOBt (35 mg, 225 μmol) and HBTU (85 mg, 225 μmol) in DMF (1.835 mL) was added to the resin, and the reaction mixture was shaken for 4 hrs. The solution was filtered off, and the resin was washed with DMF (3 × 2 mL). At this stage, a qualitative ninhydrin test was performed, and, if free amine was observed, the previous coupling step was repeated.
To the resin was added a 20% solution of piperidine in DMF (2 mL), and the mixture was shaken for 30 min. The solution was filtered off, and the resin was washed with DMF (2 × 2 mL). A small aliquot was dried in vacuo, and a 95:2.5:2.5 mixture of TFA:TIPS:H2O (50 μL) was added to the resin. After 30 min, the solution was siphoned off with a pipette and evaporated by N2 stream before being dissolved in methanol to run FIA analysis for H-Glu(α-OBut)-γ-Glu(ω-N3)-Arg-OH. MS (ESI) calcd for C25H45N10O10 (M+H)+: 633.33, found 633.3.
A solution of N10-TFA-pteroic acid (90 mg, 225 μmol), DIPEA (165 μL), HOBt (35 mg, 225 μmol) and HBTU (85 mg, 150 μmol) in DMF (1.835 mL) was added to the resin, and the reaction mixture was shaken for 4 hrs. The solution was filtered off, and the resin was washed with DMF (3 × 2 mL). A small aliquot was dried in vacuo, and a 95:2.5:2.5 mixture of TFA:TIPS:H2O (50 μL) was added to the resin. After 30 min, the solution was siphoned off with a pipette and evaporated by N2 stream before being dissolved in methanol to run FIA analysis for γ-(N10-trifluoroacetyl-α-OBut-folyl)-Glu(ω-N3)-Arg-OH. MS (ESI) calcd for C40H54F3N16O13 (M+H)+: 1023.40, found 1023.4.
The resin was then transferred into a conical vial, dried in vacuo and a 95:2.5:2.5 mixture of TFA:TIPS:H2O (2 mL) was added. The mixture was shaken lightly for 1 hr and the yellow solution was siphoned off with a pipette, transferred to a round bottom flask, and the solution was concentrated in vacuo. Upon the addition of ether (5 mL), the yellow oil was triturated to a pale yellow solid, which was dissolved in water (20 mL), washed with ether (3 × 10 mL), and lyophilized to afford 7 (43 mg, 56%) as a pale yellow solid: 1H NMR (400 MHz, CD3OD) δ 162–1.80 (m, 4H), 1.83–1.98 (m, 3H), 2.06–2.18 (m, 2H), 2.28–2.40 (m, 1H), 2.36 (t, J = 7.6 Hz, 2H), 2.45 (t, J = 8.4 Hz, 2H), 3.24 (m, 2H), 3.37 (m, 2H), 3.55 (t, J = 5.6 Hz, 2H), 3.60–3.71 (m, 10H), 4.31 (dd, J = 8.8, 5.6 Hz, 1H), 4.44 (m, 1H), 4.61 (dd, J = 9.6, 4.8 Hz, 1H), 5.20 (s, 2H), 7.58 (d, J = 8.4 Hz, 2H), 7.95 (d, J = 8.4 Hz, 2H), 8.73 (s, br, 1H). HRMS (ESI) calcd for C40H54F3N16O13 (M+H)+: 1023.4003, found 1023.4024 (Δ 2.1 ppm).
3.4. Folyl-Glu(ω-N3)-Arg-OH (2)
To a round bottomed flask containing 7 (20 mg, 20 μmol) was added a pH 9 solution of NH4OH in H2O (2 mL). Deprotection of the N10-TFA moiety was monitored by FIA, and after 1 hr the sample was lyophilized to afford 2 (20 mg, 100%) as a pale yellow solid: MS (ESI) calcd for C38H55N16O12 (M+H)+: 927.41, found 927.4. Compound 2, thus obtained, was used directly for conjugation with cyclooctynyl-linker-taxoid 3 via click chemistry.
3.5. 4-(Cyclooct-2-ynylmethyl)benzoic acid (9)29
To a solution of methyl 4-(cyclooct-2-ynylmethyl)benzoate (8)29 (600 mg, 2.34 mmol) in dioxane (25mL) was added water (6 mL) and LiOH (1.10 g, 45 mmol). The suspension was then heated to 50 °C for 4 hrs, and the reaction was monitored by TLC. Upon completion of the reaction, dioxane was removed under vacuum, water (20 mL) was added, and the reaction mixture was acidified to pH 2 with conc. hydrochloric acid. Ethyl acetate (50 mL) was added, and the organic layer was washed with water (2 × 30 mL), brine (3 × 30 mL), dried over MgSO4, filtered, and the filtrate concentrated in vacuo to afford 9 (514 mg, 91%) as a white solid: 1H NMR (500 MHz, CDCl3) δ 1.36–1.47 (m, 2H), 1.58–1.66 (m, 1H), 1.66–1.89 (m, 3H), 1.90–1.96 (m, 1H), 2.72–2.78 (m, 2H), 7.27 (d, J = 8.5 Hz, 2H), 7.95 (d, J = 8.5 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 20.89, 28.49, 29.97, 34.49, 36.49, 40.31, 41.71, 51.98, 94.97, 96.17, 128.10, 128.94, 129.60, 145.69. All data were in agreement with the literature values.29
3.6. N-4-(Cyclooct-2-ynylmethyl)benzoylsuccinimide (10)37
To a solution of 9 (242 mg, 1.00 mmol), NHS (150 mg, 1.30 mmol) and DMAP (160 mg, 1.30 mmol) in CH2Cl2 (10 mL) was added a solution of DIC (150 mg, 1.30 mmol) in CH2Cl2 (5 mL) at 0 °C. The resulting solution was stirred for 2 hrs at room temperature and the reaction was monitored by TLC. Upon completion of the reaction, water was added (10 mL), and the reaction mixture was extracted with CH2Cl2 (3 × 15 mL). The combined organic layer was washed with brine (3 × 20 mL), dried over MgSO4, filtered, and the filtrate concentrated in vacuo. The crude product was purified by column chromatography on silica gel with increasing amounts of EtOAc in hexanes (hexanes: EtOAc 1:0 – 2:3) to afford 10 (282 mg, 83%) as a white solid: m.p. 114–115 °C; 1H NMR (500 MHz, CDCl3) δ 1.45 (m, 2H), 1.66 (m, 1H), 1.72–1.91 (m, 3H), 1.98 (m, 1H), 2.10 (m, 1H), 2.15–2.22 (m, 2H), 2.71–2.82 (m, 3H), 2.93 (s, br, 4H), 7.39 (d, J = 8.0 Hz, 2H), 8.09 (d, J = 8.0 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 20.87, 25.71, 28.50, 29.95, 34.78, 36.45, 40.43, 41.71, 95.40, 95.73, 122.96, 129.52, 130.65, 148.16, 161.86, 169.31. HRMS (ESI) calcd for C20H22NO4 (M+H)+: 340.1549, found 340.1565 (Δ 4.7 ppm). All data were in agreement with literature values.37
3.7. 1-Amino-13-[4-(cyclooct-2-yn-1-ylmethyl)benzamido]-4,7,10-trioxa-1,13-tridecane (11)
A solution of 10 (100 mg, 0.30 mmol) in CH2Cl2 (10 mL) was added dropwise to a solution of 4,7,10-trioxa-1,13-tridecane-diamine (650 mg, 3.0 mmol) in CH2Cl2 (5 mL), and the resulting mixture was stirred at room temperature for 3 hrs. The solution was washed with water (3 × 20 mL) and brine (3 × 15 mL), dried over MgSO4, filtered, and the filtrate was concentrated in vacuo to give a yellow oil with trace white solid of NHS. The oil was dissolved in cold Et2O (20 mL), filtered to remove the precipitated NHS, and the filtrate was concentrated in vacuo to afford 11 as a yellow oil (114 mg, 73%): 1H NMR (500 MHz, CDCl3) δ 1.42 (m, 2H), 1.58 (m, 1H), 1.62 (t, J = 7.0 Hz, 2H), 1.63–2.03 (m, 9H), 2.06 (m, 1H), 2.18 (m, 2H), 2.66 (m, 1H), 2.70–2.81 (m, 4H), 3.45–3.72 (m, 14H), 7.24 (d, J = 7.5 Hz, 2H), 7.35 (S, br, 1H), 7.74 (d, J = 7.5 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 20.90, 28.49, 28.90, 29.97, 33.07, 34.81, 36.61, 38.56, 39.62, 40.13, 41.68, 69.50, 70.09, 70.31, 70.41, 70.49, 70.54, 94.83, 96.36, 127.03, 128.93, 132.76, 143.65, 167.31. MS (ESI) calcd for C26H41N2O4, (M+H)+: 445.30, found: 445.3 (M+H).
3.8. 4-(Cyclooct-2-yn-1-ylmethyl)benzoyl-NH-PEG3-(CH2)2NH-linker-SB-T-1214 (3)
To a solution of 11 (80 mg, 70 μmol), 10 (38 mg, 84 μmol) and DMAP (11 mg, 91 μmol) in in CH2Cl2 (3 mL) was added EDC·HCl (16 mg, 84 mmol) at room temperature. The resulting solution was stirred for 5 hrs, and the reaction was monitored by TLC. Upon completion of the reaction, water was added (10 mL) and the reaction mixture was extracted with CH2Cl2 (3 × 10 mL). The combined organic layer was washed with brine (3 × 10 mL), dried over MgSO4, filtered, and the filtrate was concentrated in vacuo. The crude product was purified by column chromatography on silica gel with increasing amounts of methanol in CH2Cl2 (CH2Cl2:methanol 1:0–20:1) to afford 3 (68 mg, 62%) as a white solid: m.p. 108–111 °C; 1H NMR (500 MHz, CDCl3) δ 0.86–0.99 (m, 2H), 1.06–1.15 (m, 2H), 1.14 (s, 3H), 1.23 (s, 3H), 1.28 (d, 3H), 1.34 (s, 9H), 1.36–1.45 (m, 2H), 1.54–1.61 (m, 2H), 1.66 (s, 3H), 1.70–1.97 (m, 5H), 1.72 (s, 3H), 1.74 (s, 3H), 1.90 (s, 3H), 1.99–2.08 (m, 1H), 2.04 (s, 3H), 2.10–2.20 (m, 3H), 2.24–2.42 (m, 2H), 2.35 (s, 1H), 2.48–2.56 (m, 1H), 2.60–2.75 (m, 3H), 2.88 (q, J = 6.5 Hz, 1H), 3.16–3.32 (m, 2H), 3.45 (m, 2H), 3.50 (m, 2H), 3.55 (dd, J = 11.5, 6.0 Hz, 2H), 3.55–3.75 (m, 8H), 3.79 (d, J = 7.0 Hz, 1H), 3.97 (d, J = 21.0 Hz, 1H), 4.05 (m, 2H), 4.17 (d, J = 8.5 Hz, 1H), 7.29 (d, J = 8.5 Hz, 1H), 4.91 (dd, J = 7.0, 10.0 Hz, 1H), 4.87–5.03 (m, 4H), 5.11 (d, J = 7.0 Hz, 1H), 5.66 (d, 7.0 Hz, 1H), 6.11 (s, br, 1H), 6.18 (t, J = 9.0 Hz, 1H), 6.29 (s, 1H), 7.03 (s, br, 1H), 7.20–7.35 (m, 3H), 7.22 (d, J = 8.0 Hz, 2H), 7.47 (t, J = 7.5 Hz, 2H), 7.60 (t, J = 7.5 Hz, 1H), 7.71 (d, J = 8.0 Hz, 2H), 7.78 (m, 1H), 8.11 (d, J = 7.5 Hz, 2H); 13C NMR (125 MHz, CD3OD) δ 9.17, 9.37, 9.59, 11.47, 13.04, 14.16, 14.84, 18.54, 20.67, 20.72, 20.91, 22.22, 22.47, 22.68, 28.26, 28.51, 29.04, 29.09, 29.99, 31.34, 31.62, 33.53, 33.67, 34.69, 34.83, 35.51, 36.66, 37.63, 37.67, 38.75, 38.79, 40.15, 41.74, 43.21, 45.68, 46.27, 46.42, 58.47, 69.76, 70.04, 70.29, 70.45, 70.51, 71.84, 72.12, 75.08, 75.20, 75.46, 76.42, 79.26, 80.98, 84.52, 94.91, 96.35, 127.02, 127.79. 127.86, 128.32, 128.66, 129.01, 129.30, 130.20, 130.52, 130.58, 131.01, 131.08, 132.64, 133.64, 137.59, 143.84, 167.00, 167.27, 168.19, 172.06, 175.07, 204.13. HRMS (ESI) calcd for C84H112N3O21S2 (M+H)+: 1562.7230, found: 1562.7192 (Δ −2.4 ppm). HPLC: t = 20.82 min.
Folate-taxoid conjugate (1)
To a solution of folyl-ω-azide 2 (12 mg, 13 μmol) in water (1 mL) was added a solution of cyclooctynyl-linker-taxoid 3 (20 mg, 13 μmol) in ethanol (1 mL), and the resulting yellow solution was stirred at room temperature in the dark for 2 days. During the course of the reaction, a yellow precipitate formed and the solution became increasingly colorless. The resulting suspension was centrifuged, the supernatant decanted and the residual solid was washed with EtOH/H2O (1:1) (3 × 3 mL) followed by Et2O (3 × 3 mL). After suspension of the resulting solid in water (1 mL) and lyophilization, folate-taxoid conjugate 1 (19.4 mg, 60%) was obtained as a yellow solid: 1H NMR (DMSO-d6, 500 MHz) δ 0.82–0.98 (m, 2H), 0.99–1.11 (m, 4 h), 1.06 (s, 3H), 1.12–1.37 (m, 4H), 1.20 (d, J = 6.5 Hz, 3H), 1.24 (S, 3H), 1.39 (s, 9H), 1.43–1.90 (m, 14H), 1.53 (s, 3H), 1.61 (s, 3H), 1.67 (s, 3H), 1.72 (s, 3H), 1.82 (s, 3H), 1.92–2.12 (m, 6H), 2.13–2.30 (m, 4H), 2.31–2.41 (m, 2H), 2.34 (s, 3H), 2.66 (m, 1H), 2.72–2.94 (m, 3H), 2.95–3.21 (m, 8H), 3.40–3.52 (m, 24H, overlaps with H2O), 5.58 (d, J = 7.0 Hz, 1H), 3.76 (m, 1H), 5.89–4.10 (m, 4H), 4.11–4.31 (m, 3H), 4.37 (m, 1H), 4.49 (s, 2H), 7.72 (m, 1H), 4.83 (d, J = 8.0 Hz, 1H), 4.89–5.10 (m, 3H), 5.17 (m, 1H), 5.49 (d, J = 7.0 Hz, 1H), 6.01 (t, 1H), 6.33 (s, 1H), 6.65 (d, J = 6.5 Hz, 2H), 6.95 (m, 2H), 7.22–7.37 (m, 5H), 7.37 (t, J = 7.5 Hz, 1H), 7.55 (t, J = 7.5 Hz, 2H), 7.58–7.72 (m, 4H), 7.75 (t, J = 7.5 Hz, 1H), 7.77 (s, br, 1H), 7.89 (s, br, 1H), 8.02 (d, J = 7.5 Hz, 2H), 8.07 (s, br, 1H), 8.32–8.45 (m, 2H) 8.41–8.49 (m, 1H), 8.65 (s, 1H); 13C NMR (125 MHz, DMSO-d6) δ 8.76, 8.84, 10.24, 13.15, 14.21, 18.36, 20.38, 20.47, 20.76, 21.51, 21.91, 22.99, 23.69, 24.80, 25.13, 25.96, 26.08, 26.81, 27.73, 27.92, 28.23, 28.39, 29.19, 29.50, 29.81, 29.88, 30.03, 31.28, 31.64, 32.06, 32.39, 32.95, 33.06, 35.24, 35.41, 35.89, 36.26, 36.30, 36.40, 37.05, 37.09, 38.27, 38.29, 43.49, 45.96, 46.11, 46.40, 46.69, 47.42, 47.69, 49.74, 52.45, 53.03, 53.49, 57.95, 68.52, 68.76, 69.52, 69.82, 70.01, 70.03, 70.14, 70.25, 70.34, 70.88, 71.02, 74.99, 75.12, 75.78, 77.21, 78.59, 80.87, 84.06, 111.75, 120.72, 121.90, 122.44, 127.39, 127.90, 128.40, 128.84, 129.12, 129.36, 129.99, 130.40, 131.66, 132.64, 132.95, 132.96, 133.30, 133.33, 133.89, 134.57, 136.25, 136.45, 137.31, 137.36, 139.99, 142.79, 143.35, 143.73, 144.87, 145.55, 149.05, 151.03, 154.36, 155.40, 157.58, 165.60, 166.53, 166.58, 169.27, 170.07, 170.68, 171.64, 171.94, 172.16, 172.66, 203.03. HRMS (ESI) calcd for C122H167N19O33S22+ (M+2H)2+: 1245.5719, found: 1245.5696 (Δ −1.8 ppm). HPLC: t = 5.53 min.
3.9. Cell Culture
All cell lines were obtained from ATCC unless otherwise noted. Cells were cultured in RPMI-1640 cell culture medium (Gibco) or DMEM culture medium (Gibco), both supplemented with 5% (v/v) heat-inactivated fetal bovine serum (FBS), 5% (v/v) NuSerum, and 1% (v/v) penicillin and streptomycin (PenStrep) at 37 °C in a humidified atmosphere with 5% CO2. L1210FR cells (a gift from Dr. Gregory Russell-Jones, Access Pharmaceuticals Pty Ltd., Australia) were grown as a suspension in supplemented RPMI-1640. ID8, MX-1 and WI-38 (ATCC) cells were cultured as monolayers on 100 mm tissue culture dishes in a supplemented RPMI-1640 cell culture medium. Cells were harvested, collected by centrifugation at 850 rpm for 5 min, and re-suspended in fresh culture medium. Cell cultures were routinely divided by treatment with trypsin (TrypLE, Gibco) as necessary every 2–4 days and collected by centrifugation at 850 rpm for 5 min, and resuspended in fresh cell culture medium containing varying cell densities for subsequent biological experiments and analysis.
3.10. In Vitro Cytotoxicity Assays
The cytotoxicities of paclitaxel, SB-T-1214 and 1 were evaluated in single-agent administrations and in time-dependent equimolar combinations in vitro against various cancer and non-cancer cell lines by means of a quantitative colorimetric assay using tetrazolium salt-based analysis (“MTT assay”38; MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma Chemical Co.). The inhibitory activity of each compound is represented by the IC50 value, which is defined as the concentration required for inhibiting 50% of the cell growth. Cells were harvested, collected, and resuspended in 100 μL cell culture medium at a concentrations ranging from 0.5–1.5 × 104 cells per well over a 96-well plate. For adhesive cell types, cells were allowed to descend to the bottom of the wells overnight, before fresh medium was added to each well upon removal of the old medium. In DMSO stock solutions, compounds were each diluted to a series of concentrations in cell culture media to prepare test solutions. After removing the old medium, these test solutions were added to the wells in the 96-well plate to give final concentrations ranging from 0.5 to 5,000 nM (100 μL), and the cells were subsequently cultured for 72 h. For the leukemia cell lines, cells were harvested, collected, and resuspended in the test solutions ranging from 0.5 to 5,000 nM (100 μL) at 0.5 to 0.8 × 104 cells per well over a 96-well plate and subsequently incubated for 72 h. After removing the test solution medium, the fresh solution of MTT in PBS (40 μL of 0.5 mg MTT / mL) was added to the wells, and the cells were incubated at 37 °C for 3 h. The MTT solution was then removed, and the resulting insoluble violet formazan crystals were dissolved in 0.1 N HCl in isopropanol with 10% Triton X-100 (40 μL) to give a violet solution. The spectrophotometric absorbance measurement of each well in the 96-well plate was run at 570 nm using a Labsystems Multiskan Ascent microplate reader. The IC50 values and their standard errors were calculated from the viability-concentration curve using Four Parameter Logistic Model of Sigmaplot. The concentration of DMSO per well was ≤1% in all cases.
Supplementary Material
Acknowledgments
This research was funded by the National Cancer Institute (CA 103314 to I.O.). The authors acknowledge the technical service and advice of Dr. Anne Savitt and Ms. Rebecca Rowehl, Cell Culture and Hybridoma facility, Stony Brook University, for their valuable help with cell culture preparations
Abbreviations
- ATCC
American Type Culture Collection
- CFM
confocal microscopy
- DIC
N,N′-diisopropylcarbodiimide
- DIPEA
N,N-diisopropylethylamine
- DMAP
4-(dimethylamino)pyridine
- DMEM
Dulbecco’s modified eagle’s medium
- DMF
N,N-dimethylformamide
- DMSO
Dimethyl sulfoxide
- EDC
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
- EU
European Union
- FBS
fetal bovine serum
- ESI
electrospray ionization
- FIA
flow injection analysis
- Fmoc
9-fluorenylmethoxycarbonyl
- FR
folate receptor
- GI
gastrointestinal
- GSH
L-glutathione reduced
- GSH-OEt
L-glutathione reduced ethyl ester
- HBTU
N,N,N′,N′-tetramethyl-O-(1H-benzotriazol-1-yl)uranium hexafluorophosphate
- HOBt
1-hydroxybenzotriazole hydrate
- HPLC
high-perfomance liquid chromatography
- LC
liquid chromatography
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MS
mass spectrometry
- NHS
N-hydroxysuccinimide
- NMR
nuclear magnetic resonance
- Pbf
2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl
- PBS
phosphate-buffered saline
- PEG
polyethylene glycol
- RME
receptor-mediated endocytosis
- RPMI
Roswell Park Memorial Institute medium
- SPPS
solid phase peptide synthesis
- TFA
trifluoroacetate/trifluoroacetic acid
- TIPS
triisopropylsilane
- TLC
thin-layer chromatography
- TOF
time-of-flight
- TTDDS
tumor-targeted drug delivery system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Siegel R, Ward E, Brawley O, Jemal A. CA: Cancer J Clin. 2011;61:212. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Ojima I, Chen J, Sun L, Borella C, Wang T, Miller M, Lin S, Geng X, Kuznetsova L, Qu C, Gallager D, Zhao X, Zanardi I, Xia S, Horwitz S, Clair JMS, Guerriero J, Bar-Sagi D, Veith J, Pera P, Bernacki R. J Med Chem. 2008;51:3203. doi: 10.1021/jm800086e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ojima I. Acc Chem Res. 2008;41:108. doi: 10.1021/ar700093f. [DOI] [PubMed] [Google Scholar]
- 4.Botchkina G, Zuniga E, Das M, Wang Y, Wang H, Zhu S, Savitt A, Rowehl R, Leyfman Y, Ju J, Shroyer K, Ojima I. Mol Cancer. 2010;9:192. doi: 10.1186/1476-4598-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S, Zhao X, Chen J, Chen J, Kuznetsova L, Wong S, Ojima I. Bioconjugate Chem. 2010;21:979. doi: 10.1021/bc9005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuznetsova L, Chen J, Sun L, Wu X, Pepe A, Veith J, Pera P, Bernacki R, Ojima I. Bioorg Med Chem Lett. 2006;16:974. doi: 10.1016/j.bmcl.2005.10.089. [DOI] [PubMed] [Google Scholar]
- 7.Ojima I, Zuniga E, Berger W, Seitz J. Future Med Chem. 2012;4:33. doi: 10.4155/fmc.11.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Chen S, Zhao X, Kuznetsova L, Wong S, Ojima I. J Am Chem Soc. 2008;130:16778. doi: 10.1021/ja805570f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojima I. Pure & Appl Chem. 2011;83:1685. [Google Scholar]
- 10.Ojima I. J Org Chem. 2013;78:6358. doi: 10.1021/jo400301u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito G, Swanson J, Lee KD. Adv Drug Delivery Rev. 2003;55:199. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 12.Ojima I. ChemBioChem. 2004;5:28. doi: 10.1002/cbic.200300844. [DOI] [PubMed] [Google Scholar]
- 13.Seitz J, Vineberg J, Wei L, Khan J, Lichtenthal B, Lin C-F, Ojima I. J Fluor Chem. 2015;169 doi: 10.1016/j.jfluchem.2014.08.006. published online August 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner C. In: Folate in health and disease. Bailey L, editor. CRC Press; 1995. p. 23. [Google Scholar]
- 15.Weitman S, Lark R, Coney L, Fort D, Frasca V, Zurawski V, Kamen B. Cancer Res. 1992;52:3396. [PubMed] [Google Scholar]
- 16.Xia W, Low P. J Med Chem. 2010;53:6811. doi: 10.1021/jm100509v. [DOI] [PubMed] [Google Scholar]
- 17.Lee R, Low P. J Biol Chem. 1994;269:3198. [PubMed] [Google Scholar]
- 18.Nauman R, Coleman R, Burger R, Herzog T, Morris R, Sausville E, Kutarska E, Ghamande S, Gabrail N, Pasquale SD, Nowara E, Gilbert L, Caton J, Gersh R, Teneriello M, Harb W, Konstantinopoulos P, Symanowski J, Lovejoy C, Messmann R. Am Soc Clin Oncol Ann Meeting. 2010:abstract 5045. [Google Scholar]
- 19.Sharma S, Sausville E, LoRusso P, Vogelzang N, Samlowski W, Carter J, Forman K, Bever S, Messmann R. Am Soc Clin Oncol Ann Meeting. 2010:abstract 3082. [Google Scholar]
- 20.Harb W, Conley B, LoRusso P, Sausville E, Heath E, Chandana S, Hamm M, Carter J, Perez W, Messman R. Am Soc Clin Oncol Ann Meeting. 2010:abstract 3088. [Google Scholar]
- 21.Peethambaramoss P, Hartmann L, Goss G, Jonker D, Plummer R. Am Soc Clin Oncol Ann Meeting. 2010:abstract e13005. [Google Scholar]
- 22.Parker N, Turk M, Westrick E, Lewis J, Low P, Leamon C. Anal Biochem. 2005;338:284. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Paulos C, Reddy J, Leamon C, Turk M, Low P. Mol Pharmacol. 2004;66:1406. doi: 10.1124/mol.104.003723. [DOI] [PubMed] [Google Scholar]
- 24.Vlahov I, Leamon C. Bioconj Chem. 2012;23:1357. doi: 10.1021/bc2005522. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg G, Borch R. J Med Chem. 2001;44:69. doi: 10.1021/jm000306g. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Lu J, Low P, Fuchs P. Bioorg Med Chem Lett. 2002;10:2397. doi: 10.1016/s0968-0896(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 27.Aronov O, Horowitz A, Gabizon A, Gibson D. Bioconjugate Chem. 2003;14:563. doi: 10.1021/bc025642l. [DOI] [PubMed] [Google Scholar]
- 28.Artursson P, Palm K, Luthman K. Adv Drug Delivery Rev. 2001;46:27. doi: 10.1016/s0169-409x(00)00128-9. [DOI] [PubMed] [Google Scholar]
- 29.Agard N, Baskin J, Prescher J, Lo A, Bertozzi C. ACS Chem Biol. 2006;1:644. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 30.Bannerjee P, Zuniga E, Ojima I, Carrico I. Bioorg Med Chem Lett. 2011;21:4985. doi: 10.1016/j.bmcl.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell-Jones G, McTavish K, McEwan J, Rice J, Nowotnik D. J Inorg Biochem. 2004;98:1625. doi: 10.1016/j.jinorgbio.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Dixit V, Bossche JVd, Sherman D, Thompson D, Andres R. Bioconjugate Chem. 2006;17:603. doi: 10.1021/bc050335b. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida T, Oide N, Sakamoto T, Yotsumoto S, Negishi Y, Tsuchiya S, Aramaki Y. J Control Release. 2006;111:325. doi: 10.1016/j.jconrel.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Leamon CP, Reddy JA. Adv Drug Deliv Rev. 2004;56:1127. doi: 10.1016/j.addr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Schwabacher A, Lane J, Sciesher M, Leigh K, Johnson C. J Org Chem. 1998;63:1727. [Google Scholar]
- 36.Willibald J, Halrder J, Sparrer K, Conzelmann KK, Carell T. J Am Chem Soc. 2012;134:12330. doi: 10.1021/ja303251f. [DOI] [PubMed] [Google Scholar]
- 37.Shelbourne M, Chen X, Brown T, Afaf A. Chem Comm. 2011;47:6257. doi: 10.1039/c1cc10743g. [DOI] [PubMed] [Google Scholar]
- 38.Mosmann T. J Immunol method. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.