Abstract
The transforming growth factor beta (TGFβ) superfamily regulates a broad range of cellular processes including proliferation, cell-fate specification, differentiation, and migration. Molecular mechanisms underlying this high degree of pleiotropy and cell-type specificity are not well understood. The TGFβ family is composed of two branches: 1) TGFβs, activins, and nodals which signal through SMAD2/3, and 2) bone morphogenetic proteins (BMPs) which signal through SMAD1/5/8. SMADs have weak DNA-binding affinity and rely on coactivators and corepressors to specify their transcriptional outputs. This report reveals that p53 and ΔNp63α act as transcriptional partners for SMAD proteins and thereby influence cellular responses to TGFβ and BMPs. Suppression of p53 or overexpression of Np63α synergistically enhance BMP induced transcription. Mechanistically, p53 and ΔNp63α physically interact with SMAD1/5/8 proteins and co-occupy the promoter region of inhibitor of differentation (ID2), a pro-survival BMP target gene. Demonstrating further convergence of these pathways, TGFβ induced canonical BMP regulated transcription in a ΔNp63α- and p53-dependent manner. Furthermore, bioinformatic analyses revealed that SMAD2/3 and ΔNp63α co-regulate a significant number of transcripts involved in the regulation of epithelial-mesenchyme transition (EMT). Thus, p53 and ΔNp63α are transcriptional partners for a subset of TGFβ and BMP regulated SMAD target genes in the mammary epithelium. Collectively, these results establish an integrated gene network of SMADs, p53 and ΔNp63α that contribute to EMT and metastasis.
Implications
This study identifies aberrant BMP activation as a result of p53 mutation or Δ Np63α expression.
Keywords: BMP, TGFβ, ΔNp63α, p53, Epithelial to Mesenchymal Transition
Introduction
The transforming growth factor-β (TGFβ) superfamily is composed of TGFβs, bone morphogenetic proteins (BMPs), growth differentiation factors (GDFs), the anti-mullerian hormone, activin, inhibin and nodal (1). These ligands signal through heterotetrameric complexes of Type I and Type II serine/threonine kinase transmembrane receptors. Type II receptors are constitutively active and phosphorylate Type1 receptors resulting in their activation. Once activated, the Type I receptors phosphorylate receptor-regulated SMADs (R-SMADs). BMPs bind to BMP-specific Type I receptors; ALK2, ALK3, and ALK6, and signal through the R-SMADs; SMAD1, SMAD5, and SMAD8. Traditionally, it has been thought that TGFβs activate the TGFβ-specific Type I receptor, ALK5, which in turn phosphorylates the R-SMADs: SMAD2 and SMAD3. However, this view has been challenged by recent studies which demonstrate that TGFβ can induce phosphorylation of SMAD1/5/8 in specific contexts (2, 3). Once R-SMADs are phosphorylated, they interact with SMAD4, a common partner SMAD for all TGFβ superfamily ligands. This R-SMAD/SMAD4 complex translocates into the nucleus, and together with co-activators and co-repressors, exerts effects on gene transcription (1).
The TGFβ family plays diverse roles in regulating epithelial cells, including controlling cell fate decisions, proliferation, migration and differentiation. TGFβ exerts paradoxical effects during carcinogenesis-- acting as a tumor suppressor in the early stages of tumor progression and switching to a pro-metastatic signal during later stages of tumorigenesis (4). Notably, TGFβ has a well characterized role in activating and maintaining epithelial to mesenchymal transition (EMT) in both physiological and pathological contexts (5). EMT is a process that involves the reversible transdifferentiation of epithelial cells to mesenchymal cells via activation of a network of transcription factors, microRNAs and cytoskeletal components (6). The rapid and reversible switch to a mesenchymal phenotype renders cells more migratory and invasive. Recent reports have implicated EMT in tumor progression, chemotherapeutic resistance, the expansion of tumor stem cells, and the initiation of a metastatic cascade (6). Together these studies suggest that EMT may be targeted for therapeutic benefit.
One of the most frequent genetic alterations in human cancer is mutation of the tumor suppressor gene TP53. Patients with Li-Fraumeni syndrome, a disorder associated with germline mutations in p53, exhibit a high incidence of breast tumors and sarcomas (7). Abundant evidence indicates that loss of function (LOF) p53 mutations can fundamentally alter the biological outcomes of diverse cellular signaling pathways. For example, a spectrum of cancer-associated p53 LOF mutations convert TGFβ signaling outputs from tumor suppressive to tumor promoting. In many cellular contexts, p53 gain of function (GOF) mutations are required for TGF-β induced migration and invasion (8). Additionally, the TGFβ effector proteins, SMAD2/3, physically interact with GOF mutant p53 and ΔNp63 (the predominant isoform of the p53 family member TP63) to exert a pro-metastatic phenotype. TP63, which is rarely mutated in human cancer, plays a critical role in epithelial specification and the preservation of stem cell regenerative capacity in stratified epithelial tissues (9). Additionally, ΔNp63α has been shown to possess oncogenic and pro-survival activity in triple negative breast cancers and squamous cell carcinomas of the head and neck (10, 11). The high degree of homology between the DNA-binding domain of ΔNp63α and that of p53 enables ΔNp63α isoforms to bind to p53-response elements (12). Studies have demonstrated that ΔNp63α isoforms are able to act as dominant negative suppressors of p53-dependent transcription (12).
Previously we reported that Np63α induces BMP signaling via activation of BMP7 expression (13). Furthermore, inhibition of BMP signaling was sufficient to prevent tumorigenesis in an allograft mouse model of breast cancer (13). These results, coupled to reports that BMP7 is overexpressed in >70% of breast cancers, raised questions regarding the molecular basis of BMP signaling activation in breast tumorigenesis. Here we present data indicating that changes in either p53 or ΔNp63α status alter cellular responsiveness to BMP signals. We further investigate the involvement of BMPs and ΔNp63α in the crosstalk between p53 and TGFβ signaling, and elucidate the functional importance of this network in cancer cells. Our data indicate that ΔNp63α is a co-activator of BMP signaling and that wild-type p53 is a co-repressor. We report that both ΔNp63α and p53 directly bind to canonical p53-binding elements located within the promoter of the pro-survival BMP target gene, Inhibitor of Differentiation 2 (ID2). Additionally, we demonstrate that ΔNp63α and P53 physically interact with BMP specific SMAD effector proteins.
Unexpectedly, we observed increased expression of canonical BMP target genes following TGFβ treatment. Full activation of these target genes by TGFβ required ΔNp63α activity and was synergistically enhanced with p53 loss. We also report that the master EMT initiator, Snail, is co-regulated by TGFβ, ΔNp63α, and p53 in mammary epithelial cells. Using computational tools, we investigated the activity of 29 distinct transcriptional programs during TGFβ-induced EMT and found that the p63-driven transcriptional program was the most active. Additionally, the transcriptional targets of p53 and SMAD proteins were significantly enriched in genes upregulated during EMT, suggesting that these transcriptional programs are also active during EMT. Finally, we found that ΔNp63α shares a significant number of target genes with TGFβ-specific SMADs and that BMP signaling components are significantly enriched among ΔNp63α target genes. Collectively, our results support a model in which the p53 family complexes with TGFβ and BMP effector SMADs to influence transcriptional and cellular outputs. We hypothesize that the differential expression of these co-regulatory molecules contributes to the pleiotropic nature of these signaling pathways.
Materials and Methods
Cell culture and Reagents
Immortalized mammary epithelial cells (IMECs) and IMEC-sh-p53 cells were derived as previously described (14, 15). IMECs were cultured in MEGM complete media (Lonza CC-3051) with 50 μg/ml puromycin, Bovine Pituitary Extract (BPE), and 100 units/ml Penicillin/Streptomycin. Treatments with TGFβ1 and A83-01 were performed without BPE and with the addition of 0.1%BSA. H1299 cells were cultured according to ATCC guidelines. Cells were serum starved for 3 hours prior to all TGFβ treatments. Recombinant TGFβ1 (Miltenyi) was used at 500pM. A83-01 (Tocris) was used at 2uM for 1 hr prior to TGFβ1 treatment, or as indicated. LDN193189 (Stemgent Technologies) was used at 1uM, unless otherwise indicated. Recombinant BMP7 (R&D systems) was used at 50 ng/ml.
Quantitative RT-PCR
Total cellular RNA was prepared using i-Script RT-qPCR sample preparation reagent (BioRad) according to the manufacturer’s protocol. cDNA was prepared using the iScript cDNA synthesis kit (BioRad). Quantitative PCR was performed with iQ-SYBR Green Super mix (BioRad). Relative changes in gene expression were obtained using the 2−ΔΔCT method normalizing to the GAPDH housekeeping gene.
Western Blot Analysis
Cells were lysed in NETN lysis buffer (100 nM Tris-Cl [pH 7.8], 1 mM EDTA, 100 mM NaCl, and 0.1% Triton X-100) with protease and phosphatase inhibitors (Roche). Protein concentration was determined by Lowry protein assay. Protein samples were separated by SDS-PAGE and transferred onto PVDF membranes. Membranes were incubated with antibodies directed against phospho-SMAD1/5/8 (Cell Signaling), total SMAD1/5/8 (Cell Signaling), p63 (4A4 clone; Lab Vision), p53 (Clone DO-7; Thermo Scientific), and β-Actin (Cell Signaling) primary antibodies. Horseradish peroxidase (HRP) conjugated secondary antibodies were used. Blots were visualized by enhanced chemi-luminescence (Millipore).
Colony Formation Assay
Cell lines were plated at 500–1,000 cells per well in standard six-well plates. Cells were treated with 1uM LDN193189, 50ng/ml rHBMP7, or vehicle control every 48 hours for 10–14 days. Colonies were fixed in 80% methanol and stained with 0.1% crystal violet solution.
Adenovirus generation
ΔNp63α and GFP were sub-cloned from pcDNA3.1 into pShuttleCMV plasmids and recombined using the AdEasy Adenoviral production system. Viral titers were generated by amplification in HEK-293Ad cells. Adenovirus stocks were diluted 1:1000 to infect cell lines.
Retrovirus generation
pLPC-ΔNp63α and pLPC-empty vector retroviral expression plasmids were a gift from Dr. Lief Ellisen. The pBMN-ID2-Flag retroviral expression plasmid was a gift from Dr. Mark Israel. Viral titers were generated by amplification in Platinum-A retroviral packaging cells (Cell BioLabs). To infect cells, normal growth media supplemented with 4ug/ml polybrene was added to cells in a 1:1 ratio with viral supernatant for 24 hours.
Chromatin immunoprecipitation (ChIP)/re-ChIP
Cells were crosslinked with 1% formaldehyde for 10 min at 37°C. Cells were lysed in 1% SDS, 10mM EDTA, 50mM Tris-HCl pH 8.1, and protease inhibitors. Lysates were sonicated using a Bioruptor for three 5 min cycles (30 sec on/30sec off). Prior to IP, lysates (~5 million cells) were diluted 1:10 in dilution buffer (1% Triton, 2mM EDTA, 150mM NaCl, 20nM Tris-HCl pH 8.1). Protein A/G Dynabeads (Life Technologies) were incubated with 2.5ug of indicated antibodies (p63: 4A4 clone; Lab Vision, and p53: Clone DO-7; Thermo Scientific) and rotated overnight at 4°C before adding dilute chromatin. IP reactions were carried out overnight rotating at 4°C. To control for non-specific binding, lysates were also immunoprecipitated with mouse IgG. Crosslinking was reversed by incubating lysates in 0.1M NaHCO3 + 1% SDS overnight at 65°C. DNA was isolated using a QIAgen PCR purification kit. Sequences of PCR primers appear in the Supplemental Data.
For the Re-ChIP, beads from the first cycle of p53 or p63 ChIP were incubated with 1μM dithiothreitol (DTT) at 37°C for 30 min to elute immune complexes. The elution was diluted 1:20 in dilution buffer (1% Triton, 2mM EDTA, 150mM NaCl, 20nM Tris-HCl pH 8.1) and re-immunoprecipitated with SMAD1/5/8 antibodies (Cell Signaling) overnight at 4°C. Crosslinking was reversed by incubating lysates in 0.1M NaHCO3 + 1% SDS overnight at 65°C. DNA was isolated using a QIAgen PCR purification kit.
Immunofluorescence
Cells were fixed in Cytorich Red at RT for 30 min and subsequently permeabilised in 0.1% Triton-X-100/PBS for 20 min at RT. Cells were then blocked in 5% goat serum/PBS for 20 min at 37°C and incubated with anti-Vimentin primary antibody (1:100, Lab Vision) in 1% goat serum/PBS at 37°C for 45 min. Cells were washed in PBS/0.1% Tween-20 and incubated with Alexa-Fluor-conjugated secondary antibody in 5% goat serum/PBS (anti-mouse-Alexa Fluor 555, 1:500, Invitrogen) for 15 min at 37°C. Cells were washed in PBS, and Phalloidin-Alexa Fluor 488 (Life Technologies) was added to cells at a 1:1000 dilution for 20 min at room temperature. Cells were washed in PBS/0.1% Tween-20, mounted in Vectashield mounting media with DAPI (Vector Laboratories) and imaged by fluorescent microscopy.
Co-immunoprecipitation
H1299 cells were transfected with plasmids expressing Np63α, wt p53 or GOF mutant p53 (R175H and R273H). Co-immunoprecipitation was carried out using the Pierce Co-immunoprecipitation Kit according to manufacturer’s protocol (Thermo Scientific). Briefly, cells were lysed in the provided lysis buffer. Lysates were pre-cleared by incubating with control agarose resin for 30 min rotating at 4°C. Cleared lysates were incubated with 2ug of antibody (per 5 million cells) or an isotype-matched IgG control rotating overnight at 4°C. Antibody/lysate complexes were incubated with Coupling Resin for 4hr rotating at 4°C. The co-IP was eluted according to manufacturer’s protocol.
Dual Luciferase Assay
Luciferase experiments were carried out using the Dual-Luciferase reporter assay system (Promega) according to the manufacturer’s protocol. H1299 cells were transfected with 50 ng of the CAGA-luciferase reporter containing 12 repeats of the core SMAD binding motif (CAGA) upstream of a minimal promoter that drives luciferase expression. They were co-tranfected with 5 ng of Renilla reniformis luciferase under a thymidine kinase promoter (rRL-tk) and 500ng of the indicated expression plasmids. Transfections were carried out using LipofectAMINE according to the manufacturer’s protocol. Forty-eight hours post-transfection, cells were treated with 500ρM TGF-β for 1 hour and then harvested.
Transcription Factor Target Gene Enrichment Analysis
EMT time course data was downloaded from the GEO database (GSE17708). In this previously published dataset, Sartor, et al treated human A549 lung adenocarcinoma cells with 5ng/ml TGFβ for 0, 0.5, 1, 2, 4, 8, 16, 24, and 72 hr to induce EMT (16). Each time point was performed in triplicate. Gene expression was profiled using Affymetrix HG_U133_plus_2 arrays with 54675 probe-sets, applying standard techniques (16). Gene expression data was normalized and compared to identify differentially expressed genes between each time point after EMT induction and time 0. Upregulated genes were defined as genes that increased in expression by >1.5 fold, and conversely, down-regulated genes were defined as genes that decreased in expression by >1.5 fold. Target genes for 29 human transcription factors were downloaded from the CHEA database (17), which were identified based on ChIP-CHIP, ChIP-Seq, or ChIP-PET data.
To calculate enrichment in up-regulated genes, we calculated the number of target and non-target genes in up-regulated genes (denoted as P1 and P0), and the number of target and non-target genes in all other genes (genes that are not up-regulated) (denoted as C1 and C0). Then, we calculated the enrichment ratio as [P1/(P1+P0)]/[C1/(C1+C0)]. A ratio > 1 indicates enrichment of target genes of a transcription factor in up-regulated genes; a ratio <1 indicates depletion of target genes of a transcription factor in up-regulated genes. Similarly, we examined the enrichment of transcription factor target genes in down-regulated genes at each time point. The significance of enrichment is calculated by using the Fisher’s Exact Test.
Overlapping analysis between gene sets
The overlapping between TP63 and SMAD3 target genes was examined and the significance of overlap was calculated using the Fisher’s Exact Test. Similarly, the enrichment of TP63 targets in BMP pathway genes was analyzed. TP63 targets were defined based on Vigano et al. (18) and Perez et al. (19), which were downloaded from the CHEA database (17) and MsigDB database (20) respectively. SMAD3 targets were defined based on Koinuma et al. (21) data downloaded from the CHEA database. The BMP pathway gene set was defined by MSigDB.
Statistical Analysis
Quantitative data is displayed as mean values of triplicate points. Error bars represent the standard error of the mean (SEM). P-values <0.05 are considered significant.
Results
P53 family members regulate canonical BMP signaling
Previously we reported a regulatory relationship between TP63 and BMP signaling in which ΔNp63α, the predominant TP63 gene product, promotes expression of BMP7 in the mammary epithelium (13). Other studies indicate that suppression of BMP7 inhibits proliferation of p53-deficient, but not p53 wild-type, breast cancer cell lines (22). Together these reports suggest that expression and activity of p53 family members influences BMP signaling. We observed that shRNA-mediated suppression of p53 in an hTERT-immortalized mammary epithelial cell (IMEC) line sharply increased phosphorylation of SMAD1/5/8, indicating enhanced BMP signaling (Figure 1A). Consistent with the opposing activities of p53 and ΔNp63α, siRNA-mediated suppression of ΔNp63α reduced P-SMAD1/5/8 levels (Figure 1B), and ΔNp63α overexpression elevated P-SMAD1/5/8 (Figure 1C). These results predict that p53 and ΔNp63α will differentially regulate canonical BMP target genes.
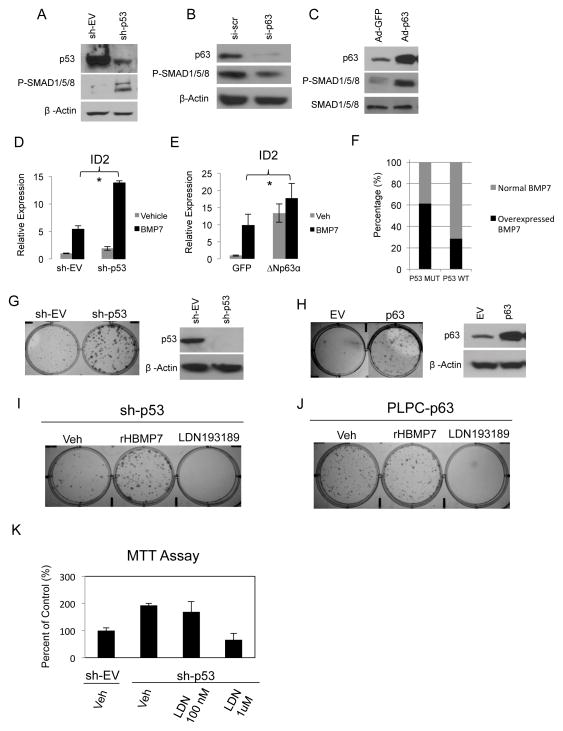
Figure 1. p53 family members regulate canonical BMP signaling.
A. Western blot analysis of P-SMAD1/5/8 and p53 in IMECs stably transfected with p53 shRNA or an empty vector shRNA expression plasmid. β-Actin is used as a loading control. B. Western blots were prepared with extracts from IMECs transiently transfected with siRNA directed against ΔNp63α or a scrambled control. Blots were probed with antibodies against P-SMAD1/5/8 and p63. β-Actin is used as a loading control. C. Western blots from IMECs infected with adenoviral ΔNp63α or GFP as a control. Blots were probed with antibodies against P-SMAD1/5/8 and p63. Total SMAD1/5/8 is used as a loading control. D. Quantitative PCR analysis of ID2 gene expression in IMECs with stable knockdown of p53 by sh-RNA E. Quantitative PCR analysis of ID2 expression in IMECs with ΔNp63α overexpression by adenoviral infection. Data is presented as mean values of triplicate points ± S.E. Asterisks represent p-values <0.05. F. BMP7 overexpression or gene amplification among breast cancer cells lines stratified by p53 status in the Cancer Cell Line Encyclopedia. G. Colony formation assay in IMECs with stable p53 knockdown and H. Np63α retroviral overexpression (confirmed by western blot) treated with vehicle control or LDN193189. Cells were stained with crystal violet 14 days after plating at 500 cells/well in 6-well plates. I. Colony formation assay in IMECs with stable p53 knockdown or J. Np63α retroviral overexpression, treated with vehicle control, 50 ng/ml rHBMP7 or 1uM LDN19189. Cells were stained with crystal violet 14 days after plating at 500 cells/well in 6-well plates. K. MTT proliferation assay of IMEC-sh-EV and IMEC-sh-p53 cells treated with vehicle control and the indicated concentrations of LDN193189. Data is presented as mean values of triplicate points ± S.E.
Inhibitor of Differentiation genes, ID1, ID2, ID3 and ID4, are established transcriptional targets of BMP signaling that coordinate cellular responses to BMP activation primarily by maintaining proliferative capacity and governing cell fate decisions (23). In cancer, ID proteins inhibit differentiation and promote survival and metastatic colonization (24). ID2 has been shown to be highly expressed in several tumor types (25) and behaves as an oncogene by inhibiting anti-proliferative activity of the retinoblastoma protein, RB (26). Previous studies indicated that p53 represses ID2 expression in neural progenitor cells as a mechanism to suppress proliferation of this cell population (27). To determine the effects of p53 loss on BMP induced transcription, we measured the induction of ID2 mRNA levels in response to BMP stimulation in IMECs and IMECs with stable p53 knockdown. Results indicated that suppression of p53 significantly enhanced induction of ID2 by BMP7 (Figure 1D). Stimulation of IMECs with recombinant BMP7 in the presence of overexpressed Np63α also synergistically enhanced ID2 transcription (Figure 1E). Furthermore, analysis of breast cancer cell lines in the Cancer Cell Line Encyclopedia (CCLE) revealed that BMP7 mRNA overexpression or amplification is a significantly more common event in p53 mutant (62%) vs. p53 wild-type (28%) breast cancer cells (Figure 1F). Together these results indicate that loss of p53 or gain of ΔNp63α leads to increased sensitivity to BMP signaling, suggesting that tumors with these genetic lesions may be more responsive to BMP signaling inhibitors.
Enhanced BMP signaling mediates increased clonogenicity resulting from p53 inactivation and Np63α overexpression
The previous results indicate that BMP signaling is negatively regulated by p53 and positively regulated by ΔNp63α. TP53 is a negative regulator of self-renewal in hematopoietic, neural, and mammary stem/progenitor cells (28–30), whereas ΔNp63α is required for prolonged proliferative capacity and self-renewal of epithelial stem cell populations. This suggested the possibility that enhanced BMP signaling mediates the activities of p53 and Np63α on stem cell populations. ShRNA-mediated suppression of p53 or ectopic expression of ΔNp63α lead to a marked increase in clonogenic capacity (Figure 1G,H, respectively). Under both conditions, the increased clonogenicity was sensitive to LDN193189, a potent and selective inhibitor of BMP Type 1 receptor kinases. These results indicate that BMP signaling mediates the enhanced clonogenic capacity that is the result of p53 loss or ΔNp63α gain (Figure 1I,J). Additionally, the increased proliferation associated with p53 loss is suppressed by LDN193189 (Figure 1K), indicating that BMP signaling contributes to the increased proliferation of cells which loose p53. This data suggests that tumors with impaired p53 will display increased sensitivity to anti-BMP therapeutics. This is consistent with our previous report that basal breast cancers, which have the highest rates of p53 loss of function mutations, also have the highest levels of BMP signaling activity (31).
P53 and ΔNp63α directly bind to the promoter region of the BMP target gene, ID2
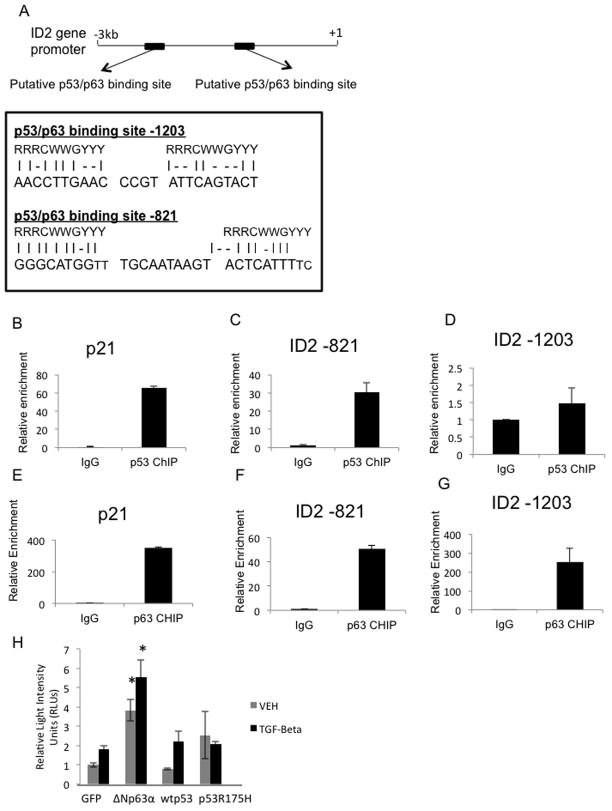
The previous results indicated that p53 is able to repress BMP induced transcription and that ΔNp63α is able to enhance BMP transcriptional activity. We therefore sought to determine if p53 and ΔNp63α regulate BMP signaling via direct transcriptional regulation of BMP targets. Analysis of a region of the ID2 gene, spanning from 3kb upstream of the transcription start site to 1 kb downstream, identified two putative p53/p63 binding elements (Figure 2A). Chromatin immunoprecipitation (ChIP) studies indicated that one of these elements, ID2-821, is directly bound by both p53 and ΔNp63α (Figure 2C, F). ΔNp63α, but not p53, binds a second site in the ID2 promoter -1203 bp upstream of the transcription start site (Figure 2D, G). Studies using the promoter of CDKN1A (p21Cip/WAF) (Figure 2B+E) serve as a positive control as both p53 and ΔNp63α have been reported to directly bind to the p21 promoter (32). The ID2 site at -821 was previously shown to be bound by ΔNp63α in human keratinocytes (33). Together these data demonstrate that p53 and ΔNp63α occupy regulatory regions within the ID2 gene, suggesting a mechanism by which p53 family members regulate transcription of ID2 and possibly other BMP targets.
Figure 2. P53 and ΔNp63α directly bind to the promoter region of the BMP target gene, ID2.
A. Schematic summarizing the identification of p53/p63 binding elements in the ID2 gene promoter. ChIP analysis of p53 (B–D) and ΔNp63α (E–G) binding at p21 (positive control) and ID2 promoters in IMECs. H. Dual luciferase assay performed in H1299 cells co-transfected with a CAGA-luciferase reporter. Renilla luciferase, and the indicated expression plasmids, followed by treatment with vehicle or TGFβ for 1 hour.
P53 and ΔNp63α physically interact with BMP specific SMADs
It has previously been shown that p53 co-activates a subset of TGFβ target genes by physically interacting with SMAD2/3 (34, 35). The high degree of conservation between TGFβ-specific and BMP-specific SMADS coupled to the differential regulation of BMP signaling by p53 and ΔNp63α suggested that p53, and possibly ΔNp63α, regulate BMP SMAD activity via direct physical interactions. Immunoprecipitation of ectopic p53 or ΔNp63α resulted in co-precipitation of endogenous SMAD1/5/8 (Figure 3A,B). Interestingly, two different p53 gain-of-function (GOF) mutants (R175H and R273H) were also able to co-immunoprecipitate SMAD1/5/8 (Figure 3C,D) suggesting that these mutants retain the ability to regulate BMP transcriptional activity. As SMAD proteins are known to be weak transcription factors that rely on co-activators to induce transcription, data presented here support a model in which p53 and ΔNp63α physically interact with BMP-specific SMADs and co-regulate their transcriptional activity (Figure 3F). To test if ΔNp63α, p53 and SMAD1/5/8 co-occupy elements within the ID2 promoter, we performed anti-p63 and anti-p53 ChIP followed by elution and re-ChIP with antibodies directed against SMAD1/5/8. This analysis failed to enrich the ID2-821 and ID2-2013 p63/p53 binding elements (Figure 3E), suggesting that SMAD1/5/8 proteins do not co-occupy these elements. In order to search for other candidate enhancers, we analyzed publicly available ChIP-seq data for binding of p53-family members and SMAD proteins within the ID2 gene. This analysis (Supplemental Table 1) identified a 259 bp region located 3056 bp upstream of exon 1 of ID2 that was enriched by p63-ChIP in keratinocytes (36), SMAD2/3 in an endoderm progenitor model (37), and SMAD3 and SMAD4 in hES cells (38). To determine if this element is co-occupied by ΔNp63α, p53, and SMAD1/5/8 we performed ChIP-reChIP experiments in which p63 or p53-bound chromatin was eluted and subsequently re-immunoprecipitated with antibodies directed against SMAD1/5/8. Q-PCR based analysis indicated that this region was significantly enriched by p63 or p53-ChIP. Additionally, this region could be further enriched by SMAD1/5/8 re-ChIP (Figure 3E). This result suggests that this site is co-occupied by ΔNp63α, p53 and SMAD1/5/8 in IMEC cells.
Figure 3. P53 and ΔNp63α physically interact with BMP specific SMADs.
A–D. Co-immunoprecipitation of H1299 cells transfected with plasmids expressing ΔNp63α wt p53 or GOF mutant p53 (R175H and R273H). Cell lysates were immunoprecipitated with anti-p63 or p53 antibodies and immunoblotted with the indicated antibodies. Input lanes represent 5% of the total cell lysate. E. ChIP and reChIP analyses in IMEC cells across the ID2 promoter. P53 and P63 antibodies were used for the first immunoprecipitation, and SMAD1/5/8 directed antibodies were used for the re-ChIP. F. Proposed model of interaction between SMADs, p53 and p63.
TGF-β, ΔNp63α, and p53 co-regulate canonical BMP induced transcription
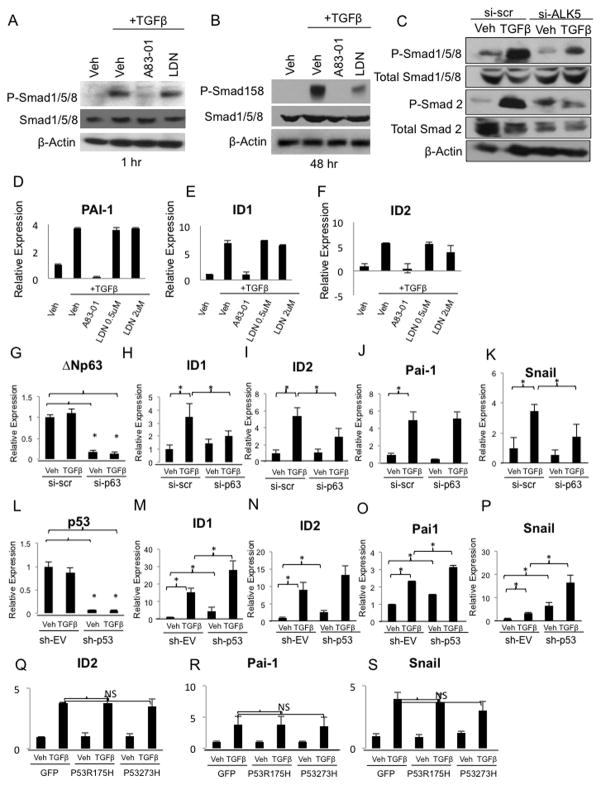
Recent studies have challenged the traditional view that TGFβ signals exclusively through SMAD2/3 while BMPs signal through SMAD1/5/8. TGFβ has been reported to phosphorylate and activate SMAD1/5/8 in endothelial and epithelial cell populations via ALK1 and ALK5 receptor complexes, respectively (2, 39). Consistent with these reports, we found that TGFβ induces the rapid phosphorylation of SMAD1/5/8 as early as one hour after treatment (Figure 4A). This induction is sensitive to A83-01, an ALK5 (TGFβ type I receptor) inhibitor, but not LDN193189, indicating that the phosphorylation of SMAD1/5/8 by TGFβ is carried out by ALK5. Analysis at 48 hours indicated that TGFβ-induced phosphorylation of SMAD1/5/8 was stable and persistent and had become partially sensitive to LDN193189 (Figure 4B). Additionally, siRNA-mediated suppression of ALK5 dramatically reduced phosphorylation of SMAD1/5/8 in response to TGFβ, further demonstrating that TGFβ-induced phosphorylation of SMAD1/5/8 is mediated by an ALK5-containing receptor complex (Figure 4C). TGFβ also induced expression of canonical BMP target genes, ID1 and ID2 (Figure 4E,F) as well as the canonical TGFβ target gene, Pai-1 (Figure 4D). These inductions were sensitive to A83-01, and resistant to LDN193189 (Figure 4E–D) demonstrating that TGFβ directly induces phosphorylation of SMAD1/5/8, and subsequently induces canonical BMP transcriptional responses, through ALK5 kinase activity.
Figure 4. TGF-β, ΔNp63α, and p53 co-regulate canonical BMP induced transcription.
A. Western blot analysis of MCF10As pretreated with vehicle, 2uM A83-01 or 1uM LDN-193189 for 10min, followed by treatment with vehicle or 500pM TGFβ for 1 hr and B. 48hrs. Blots were probed with P-SMAD1/5/8 antibody. Total SMAD1/5/8 and β-Actin are used as loading controls. C. MCF10A cells transfected with ALK5 siRNA or a scrambled control siRNA and treated with vehicle or TGFβ. Blots were probed with P-SMAD1/5/8, total SMAD1/5/8, P-SMAD2 and total SMAD 2 antibodies. β-Actin is used as a loading control. D–F. Quantitative PCR analysis of Pai-1, ID1, and ID2 expression in MCF10As pre-treated with A83-01 or LDN193189 for 1 hour before treatment with vehicle or 500pM TGFβ for 48 hours. QPCR analysis of ΔNp63, p53, ID1, ID2, Pai-1, and Snail levels in MCF10As cells G–K transiently transfected with ΔNp63α siRNA or scrambled control siRNA for 72 hours followed by treatment with vehicle or TGFβ for 1 hour, or L–P stably transfected with p53 or empty vector shRNA and treated with vehicle or TGFβ for 1 hour. Asterisks represent p-values <0.05. Q–S. QPCR analysis of ID2, Pai-1 and Snail levels in MCF10A cells transiently transfected with GOF mutant p53 plasmids (R175H and R273H) followed by treatment with vehicle or TGFβ for 1 hour.
Based upon our model in which ΔNp63α and p53 act as transcriptional partners for SMADs, we investigated if ΔNp63α and p53 influenced TGFβ induced transcription of canonical BMP targets. SiRNA-mediated suppression of ΔNp63α significantly reduced TGFβ-dependent induction of ID1 and ID2, demonstrating that ΔNp63α is required for full activation of these genes by TGFβ (Figure 4G–I). We also tested the influence of ΔNp63α on the transcriptional activation of two well-characterized TGFβ target genes, Pai-1 and Snail. Similarly, we found that ΔNp63α was required for full activation of the pro-EMT regulator, Snail, by TGFβ (Figure 4K). As Snail induction alone is sufficient to induce EMT, this data suggests that ΔNp63α may play a critical role in TGFβ induced EMT (Figure 4K) (40). In contrast, ΔNp63α suppression did not alter TGFβ induction of Pai-1, suggesting that ΔNp63α co-regulates only a subset of Smad target genes (Figure 4J). In these assays, the suppression of ΔNp63α is transient, and under these conditions, growth and survival of the cells is not impacted. Conversely, stable sh-RNA knock down of wt p53 synergistically enhanced TGFβ-mediated induction of Pai-1, ID1, and Snail, and a similar trend was observed for ID2 (Figure 4L–P). This data suggests that p53 is a co-repressor and ΔNp63α is a co-activator of TGFβ mediated transcription of a subset of target genes. In contrast, gain-of-function mutants of p53 (R175H and R273H) did not alter TGF-β-mediated induction of Pai-1, ID2, or Snail (Figure 4Q–S), suggesting that the ability of p53 to co-regulate a subset of TGFβ target genes may contribute to its tumor suppressor activity.
To further investigate the role of p53 and ΔNp63α as co-regulators of Smad signaling, we performed Dual Luciferase assays using the CAGA-luciferase reporter containing 12 repeats of the core SMAD binding motif (CAGA) upstream of a minimal promoter that drives luciferase expression. As expected, we found that TGF-β induces the expression of this reporter, and that Np63α enhances the activity of this reporter both endogenously and under TGFβ stimulation (Figure 2H). This supports our data that Np63α is a co-activator to TGFβ mediated SMAD signaling. We found that both wt p53 and GOF mutant p53 do not enhance the activity of this reporter both in the presence or absence TGFβ treatment (Figure 2H).
P63 and SMAD transcriptional networks are robustly active during epithelial to mesenchymal transition
Epithelial to Mesenchymal Transition (EMT) is a critical mechanism for mediating tumor metastasis. It was first described as a developmental process responsible for remodeling during gastrulation, cardiac morphogenesis, and neural crest formation (41). EMT is defined as a reversible change in cell adhesion proteins, resulting in changes in cell polarity and cytoskeletal structures. It is typically marked by decreased levels of epithelial markers including E-cadherin, MUC1, and laminin-1, and a corresponding increase in mesenchymal markers, including vimentin, N-cadherin, and fibronectin (41). Blocking E-cadherin in transformed cells has been shown to increase their invasive and metastatic potential by decreasing cell-cell adhesion in vivo (42). The transcription factors Twist1, Zeb1, Snail and Slug have been identified as master initiators of EMT. Under certain conditions, overexpression of any one of these transcription factors is sufficient to induce EMT in a variety of cell types (40). Having discovered that Snail is co-regulated by ΔNp63α, p53 and TGFβ, we investigated the role of crosstalk between p53 and TGFβ family members in regulating the EMT state. For these studies, we used MCF10A cells, an immortalized but non-transformed mammary epithelial cell line, due to the ability of these cells to undergo EMT when stimulated with TGFβ. We treated MCF10A cells with TGFβ and observed changes in morphology characteristic of EMT after 72 hours of treatment; a loss of epithelial/cuboidal-like shape and a switch to a more mesenchymal/fibroblast-like morphology (Figure 5A). Conversely, cells took on a cuboidal epithelial morphology when treated with the TGFβR1 (ALK5) kinase inhibitor, A83-01 (Fig. 5A). Staining of actin filaments with FITC-phalloidin highlights the mesenchymal morphology seen after TGFβ treatment (Figure 5B) and enhanced expression of the mesenchymal marker Vimentin is also observed (Figure 5B). Q-PCR based analysis demonstrates induction of pro-EMT effectors as well as EMT markers in response to TGFβ (Supplemental Figure S1). To investigate the function of p63, p53, and SMADs in regulating EMT, we performed transcriptional network analysis of gene expression data present within the GEO database (GSE17708) of A549 lung cancer cells treated with TGFβ to induce EMT over a 72 hour time course (16). Expression levels of genes were compared between each time point after EMT induction and T=0. Genes with >1.5 fold increase in expression were defined as up-regulated genes, and genes with >1.5 fold decrease in expression were defined as down-regulated genes.
Figure 5. ΔNp63α and SMAD transcriptional networks are robustly active during epithelial to mesenchymal transition.
A. Phase-contrast images of MCF10As treated with vehicle, 500pM TGFβ, or 2uM A83-01 for 72 hours. B. Immunofluorescent analysis of Phalloidin (green) and Vimentin (red) in MCF10A cells treated with vehicle or 500pM TGFβ for 72 hours. The nuclei are stained with DAPI. C. Schematic summarizing the experimental design. Raw gene expression data from A549 cells treated with a 72hr time course of TGFβ was downloaded from the GEO database. Expression levels of genes were compared between each time point after TGFβ treatment and time point t=0. Transcription factor target genes were identified by ChIP-CHIP, ChIP-Seq, and ChIP-PET data downloaded from the CHEA database. D–H. p53, p63, SMAD4, SMAD2, SMAD3 target gene enrichment in genes differentially expressed during EMT. I. Overlap of ΔNp63α and SMAD3 target genes downloaded from the CHEA data base (see methods). J–L. QPCR-based validation of the predicted shared SMAD3 and ΔNp63α target genes. Analysis of CDKN1A, CK14, and ITGA3 gene expression levels in H1299 cells infected with GFP control or Np63α followed by treatment with Vehicle or TGFβ for one hour. Asterisks represent p-values <0.05.
We mined publicly available human ChIP-Seq, ChIP-CHIP, and ChIP-PET data in the CHEA database (17) to obtain lists of target genes for 29 transcription factors. We then integrated this data by performing statistical analyses to determine if targets of a transcription factor were overrepresented in the differentially expressed genes during EMT. A diagram of the experimental design is displayed in Figure 5C. At each time point, the overlap between up and down-regulated genes and transcription factor target genes was examined (see methods for details). SMAD proteins and p53 family members (Figure 5D–H) were identified as being highly active during TGFβ induced EMT, as evidenced by their transcriptional targets being enriched in upregulated genes. Strikingly, TP63 was the most active transcription factor of the 29 transcription factors for which data was available in the CHEA database (Supplemental Figure S2). These results suggest that p63-directed transcriptional networks contribute to EMT.
To investigate global co-regulation of ΔNp63α and SMAD3 target genes, we analyzed their target gene sets identified from ChIP-CHIP data by Vigano et al and Koinuma et al, respectively (18, 21). We found that ΔNp63α shares a significant number of target genes (17 out of 40) with SMAD3 (Figure 5I). Additionally, a significant number of these shared genes were enriched during TGFβ induced EMT indicating that a subset of ΔNp63α and SMAD3 co-regulated genes are important for maintaining EMT (Supplemental Figure S3). We validated the predicted SMAD3 and ΔNp63α shared target genes by real time PCR analysis. We were able to confirm that Cytokeratin14, CDKN1A, and ITGA3 are shared TGFβ and Np63α direct target genes, thereby validating our computational analyses (Figure 5J–L). Due to lack of human SMAD1/5/8 data available in the CHEA database, we utilized BMP (PID_BMPPATHWAY) and ΔNp63α (PEREZ_TP63_TARGETS) gene sets from the Molecular Signatures Database (MSigDB). Gene sets in MSigDB are compiled based on pathway databases and previously published reports. We discovered a significant enrichment of p63 targets in the BMP pathway genes (Supplemental Figure S4). This data indicates the presence of a complex network of SMAD proteins, p53, and ΔNp63α that regulates EMT, and further supports the hypothesis that ΔNp63α acts as a co-activator to a subset of BMP and TGFβ target genes.
Discussion
BMP signaling is activated in multiple tumor types and has been implicated in a diverse range of both tumor suppressive and oncogenic activities (43, 44). BMPs, like TGFβ, exert context-dependent effects on cancer cell populations (44). Here, we report that p53 loss of function mutations enhance cellular responsiveness to BMP signaling. Our results suggest that p53 family member status may predict sensitivity to anti-BMP therapeutics.
SMAD proteins have weak DNA binding affinity and require the association of other transcription factors to increase DNA binding and transcriptional activity. CBP/p300(45) and Runx (46) have been shown to behave as co-activators for R-SMADs in certain cellular contexts; however, co-activators of BMP-mediated transcription involved in many cellular responses are unknown. Our results reveal that ΔNp63α and p53 act as transcriptional partners for SMAD1/5/8. We present for the first time that ID2 is a direct positively regulated Np63α target gene, and a direct negatively regulated p53 target gene in mammary epithelial cells. We propose that p53 and ΔNp63α physically interact with BMP or TGFβ specific SMADs and co-occupy the regulatory regions of target genes to exert synergistic or antagonistic effects on transcription. Future studies are needed to elucidate the role of other p53 family members in regulating SMAD-directed transcription and cellular outputs. The transcriptional responses will likely depend on the presence and abundance of each protein in the complex. It is unclear if these proteins compete with each other for binding sites or if they cooperate together. The balance between these proteins and their affinities for DNA binding remains to be determined. For example, it will be important to discern if TGFβ-regulated SMADs are displaced from p53/p63 complexes in favor of BMP-specific SMADs in the context of high BMP ligand stimulation, and vice versa.
During development, EMT is essential for the maintenance of epithelial plasticity allowing for the massive alterations in cell polarity and migration that occur during gastrulation and organogenesis (6, 47). During cancer progression, this developmental program is aberrantly reactivated leading to an aggressive phenotype marked by decreased proliferation, increased motility, acquisition of stem-like characteristics, and acquired resistance to conventional and targeted therapies (48). Here, we determine the role of p63, p53 and SMAD proteins in regulating the transcriptional changes observed during epithelial to mesenchymal transition. BMPs have been shown to both promote and reverse EMT depending on the particular cell type and context (49, 50). We report that during EMT, TGFβ can directly induce phosphorylation of SMAD1/5/8 through the ALK5 receptor and turn on expression of canonical BMP target genes in a ΔNp63α and p53 dependent manner. Finally, we show that the transcriptional targets of p53, SMADs, and ΔNp63α are significantly enriched in differentially expressed genes during EMT. This is the first report showing ΔNp63α transcriptional outputs contribute significantly to the gene expression changes observed during EMT. The subset of ΔNp63α transcriptional targets responsible for regulating EMT remains to be elucidated. Future work will shed light on the presence and activity of this crosstalk in other cellular outputs influencing cancer progression.
Supplementary Material
Acknowledgments
Grant Support
This work was supported in part by grants from the National Cancer Institute (5RO1CA108539-05) (J.D.R), the United States Department of Defense Breast Cancer Research Program (BC095560) (J.D.R), a Prouty Pilot Award from the Friends of the Norris Cotton Cancer Center (J.D.R), The Center for Integrative Biomedical Sciences at Dartmouth College (C.C. and J.D.R.), and the COBRE NIH/NIGMS 8 P20 GM03534 Quantitative Biology Research Institute (C.C. and J.D.R.). C.C. was supported by the American Cancer Society Research Grant, #IRG-82-003-27 and by the start-up funding package provided by the Geisel School of Medicine at Dartmouth College.
Footnotes
The authors declare that no conflicts of interest exist.
References
- 1.Wakefield LM, Hill CS. Beyond TGFbeta: roles of other TGFbeta superfamily members in cancer. Nature reviews Cancer. 2013;13:328–41. doi: 10.1038/nrc3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daly AC, Randall RA, Hill CS. Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Molecular and cellular biology. 2008;28:6889–902. doi: 10.1128/MCB.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, et al. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Molecular cell. 2003;12:817–28. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 4.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nature genetics. 2001;29:117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 5.Savagner P. Epithelial-mesenchymal transition: the concept. Annales de pathologie. 2009;29(Spec No 1):S59–60. doi: 10.1016/j.annpat.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 6.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature reviews Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 7.Li FP, Fraumeni JF, Jr, Mulvihill JJ, Blattner WA, Dreyfus MG, Tucker MA, et al. A cancer family syndrome in twenty-four kindreds. Cancer research. 1988;48:5358–62. [PubMed] [Google Scholar]
- 8.Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 9.Senoo M, Pinto F, Crum CP, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–36. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 10.Leong CO, Vidnovic N, DeYoung MP, Sgroi D, Ellisen LW. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. The Journal of clinical investigation. 2007;117:1370–80. doi: 10.1172/JCI30866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, et al. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Molecular cell. 1998;2:305–16. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 13.Balboni AL, Hutchinson JA, DeCastro AJ, Cherukuri P, Liby K, Sporn MB, et al. DeltaNp63alpha-mediated activation of bone morphogenetic protein signaling governs stem cell activity and plasticity in normal and malignant mammary epithelial cells. Cancer research. 2013;73:1020–30. doi: 10.1158/0008-5472.CAN-12-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiRenzo J, Signoretti S, Nakamura N, Rivera-Gonzalez R, Sellers W, Loda M, et al. Growth factor requirements and basal phenotype of an immortalized mammary epithelial cell line. Cancer research. 2002;62:89–98. [PubMed] [Google Scholar]
- 15.Harmes DC, Bresnick E, Lubin EA, Watson JK, Heim KE, Curtin JC, et al. Positive and negative regulation of deltaN-p63 promoter activity by p53 and deltaN-p63-alpha contributes to differential regulation of p53 target genes. Oncogene. 2003;22:7607–16. doi: 10.1038/sj.onc.1207129. [DOI] [PubMed] [Google Scholar]
- 16.Sartor MA, Mahavisno V, Keshamouni VG, Cavalcoli J, Wright Z, Karnovsky A, et al. ConceptGen: a gene set enrichment and gene set relation mapping tool. Bioinformatics. 2010;26:456–63. doi: 10.1093/bioinformatics/btp683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lachmann A, Xu H, Krishnan J, Berger SI, Mazloom AR, Ma’ayan A. ChEA: transcription factor regulation inferred from integrating genome-wide ChIP-X experiments. Bioinformatics. 2010;26:2438–44. doi: 10.1093/bioinformatics/btq466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigano MA, Mantovani R. Hitting the numbers: the emerging network of p63 targets. Cell Cycle. 2007;6:233–9. doi: 10.4161/cc.6.3.3802. [DOI] [PubMed] [Google Scholar]
- 19.Perez CA, Ott J, Mays DJ, Pietenpol JA. p63 consensus DNA-binding site: identification, analysis and application into a p63MH algorithm. Oncogene. 2007;26:7363–70. doi: 10.1038/sj.onc.1210561. [DOI] [PubMed] [Google Scholar]
- 20.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–40. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koinuma D, Tsutsumi S, Kamimura N, Taniguchi H, Miyazawa K, Sunamura M, et al. Chromatin immunoprecipitation on microarray analysis of Smad2/3 binding sites reveals roles of ETS1 and TFAP2A in transforming growth factor beta signaling. Molecular and cellular biology. 2009;29:172–86. doi: 10.1128/MCB.01038-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan W, Chen X. Targeted repression of bone morphogenetic protein 7, a novel target of the p53 family, triggers proliferative defect in p53-deficient breast cancer cells. Cancer research. 2007;67:9117–24. doi: 10.1158/0008-5472.CAN-07-0996. [DOI] [PubMed] [Google Scholar]
- 23.Miyazono K, Miyazawa K. Id: a target of BMP signaling. Science’s STKE : signal transduction knowledge environment. 2002;2002:pe40. doi: 10.1126/stke.2002.151.pe40. [DOI] [PubMed] [Google Scholar]
- 24.Gupta GP, Perk J, Acharyya S, de Candia P, Mittal V, Todorova-Manova K, et al. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19506–11. doi: 10.1073/pnas.0709185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson JW, Deed RW, Inoue T, Balzi M, Becciolini A, Faraoni P, et al. Expression of Id helix-loop-helix proteins in colorectal adenocarcinoma correlates with p53 expression and mitotic index. Cancer research. 2001;61:8803–10. [PubMed] [Google Scholar]
- 26.Lasorella A, Noseda M, Beyna M, Yokota Y, Iavarone A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature. 2000;407:592–8. doi: 10.1038/35036504. [DOI] [PubMed] [Google Scholar]
- 27.Paolella BR, Havrda MC, Mantani A, Wray CM, Zhang Z, Israel MA. p53 directly represses Id2 to inhibit the proliferation of neural progenitor cells. Stem cells. 2011;29:1090–101. doi: 10.1002/stem.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumble M, Moore L, Chambers SM, Geiger H, Van Zant G, Goodell MA, et al. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood. 2007;109:1736–42. doi: 10.1182/blood-2006-03-010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–9. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- 30.Tao L, Roberts AL, Dunphy KA, Bigelow C, Yan H, Jerry DJ. Repression of mammary stem/progenitor cells by p53 is mediated by Notch and separable from apoptotic activity. Stem cells. 2011;29:119–27. doi: 10.1002/stem.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumay A, Feugeas JP, Wittmer E, Lehmann-Che J, Bertheau P, Espie M, et al. Distinct tumor protein p53 mutants in breast cancer subgroups. International journal of cancer Journal international du cancer. 2013;132:1227–31. doi: 10.1002/ijc.27767. [DOI] [PubMed] [Google Scholar]
- 32.Westfall MD, Mays DJ, Sniezek JC, Pietenpol JA. The Delta Np63 alpha phosphoprotein binds the p21 and 14–3–3 sigma promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Molecular and cellular biology. 2003;23:2264–76. doi: 10.1128/MCB.23.7.2264-2276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu N, Castel D, Debily MA, Vigano MA, Alibert O, Mantovani R, et al. Large scale RNAi screen reveals that the inhibitor of DNA binding 2 (ID2) protein is repressed by p53 family member p63 and functions in human keratinocyte differentiation. J Biol Chem. 2011;286:20870–9. doi: 10.1074/jbc.M110.169433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dupont S, Zacchigna L, Adorno M, Soligo S, Volpin D, Piccolo S, et al. Convergence of p53 and TGF-beta signaling networks. Cancer letters. 2004;213:129–38. doi: 10.1016/j.canlet.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Cordenonsi M, Dupont S, Maretto S, Insinga A, Imbriano C, Piccolo S. Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with Smads. Cell. 2003;113:301–14. doi: 10.1016/s0092-8674(03)00308-8. [DOI] [PubMed] [Google Scholar]
- 36.Kouwenhoven EN, van Heeringen SJ, Tena JJ, Oti M, Dutilh BE, Alonso ME, et al. Genome-wide profiling of p63 DNA-binding sites identifies an element that regulates gene expression during limb development in the 7q21 SHFM1 locus. PLoS genetics. 2010;6:e1001065. doi: 10.1371/journal.pgen.1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SW, Yoon SJ, Chuong E, Oyolu C, Wills AE, Gupta R, et al. Chromatin and transcriptional signatures for Nodal signaling during endoderm formation in hESCs. Developmental biology. 2011;357:492–504. doi: 10.1016/j.ydbio.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Mullen AC, Orlando DA, Newman JJ, Loven J, Kumar RM, Bilodeau S, et al. Master transcription factors determine cell-type-specific responses to TGF-beta signaling. Cell. 2011;147:565–76. doi: 10.1016/j.cell.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu IM, Schilling SH, Knouse KA, Choy L, Derynck R, Wang XF. TGFbeta-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFbeta switch. The EMBO journal. 2009;28:88–98. doi: 10.1038/emboj.2008.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masui T, Ota I, Yook JI, Mikami S, Yane K, Yamanaka T, et al. Snail-induced epithelial-mesenchymal transition promotes cancer stem cell-like phenotype in head and neck cancer cells. International journal of oncology. 2014;44:693–9. doi: 10.3892/ijo.2013.2225. [DOI] [PubMed] [Google Scholar]
- 41.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–3. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 43.Blanco Calvo M, Bolos Fernandez V, Medina Villaamil V, Aparicio Gallego G, Diaz Prado S, Grande Pulido E. Biology of BMP signalling and cancer. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2009;11:126–37. doi: 10.1007/s12094-009-0328-8. [DOI] [PubMed] [Google Scholar]
- 44.Alarmo EL, Kallioniemi A. Bone morphogenetic proteins in breast cancer: dual role in tumourigenesis? Endocrine-related cancer. 2010;17:R123–39. doi: 10.1677/ERC-09-0273. [DOI] [PubMed] [Google Scholar]
- 45.Pearson KL, Hunter T, Janknecht R. Activation of Smad1-mediated transcription by p300/CBP. Biochimica et biophysica acta. 1999;1489:354–64. doi: 10.1016/s0167-4781(99)00166-9. [DOI] [PubMed] [Google Scholar]
- 46.Zaidi SK, Sullivan AJ, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Integration of Runx and Smad regulatory signals at transcriptionally active subnuclear sites. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8048–53. doi: 10.1073/pnas.112664499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Developmental cell. 2008;14:818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeisberg M, Shah AA, Kalluri R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. The Journal of biological chemistry. 2005;280:8094–100. doi: 10.1074/jbc.M413102200. [DOI] [PubMed] [Google Scholar]
- 50.Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, et al. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene. 2008;27:6322–33. doi: 10.1038/onc.2008.232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.