Abstract
Background
CFTR plays a key role in maintenance of lung fluid homeostasis. Cigarette smoke decreases CFTR expression in the lung but neither the mechanisms leading to CFTR loss, nor potential ways to prevent its loss have been identified to date.
Methods
The molecular mechanisms leading to down-regulation of CFTR by cigarette smoke were determined using pharmacologic inhibitors and silencing RNAs.
Results
Using human bronchial epithelial cells, here we show that cigarette smoke induces degradation of CFTR that is attenuated by the lysosomal inhibitors, but not proteasome inhibitors. Cigarette smoke can activate multiple signaling pathways in airway epithelial cells, including the MEK/Erk1/2 MAPK pathway regulating cell survival. Interestingly, pharmacological inhibition of the MEK/Erk1/2 MAPK pathway prevented the loss of plasma membrane CFTR upon cigarette smoke exposure. Similarly, decreased expression of Erk1/2 using silencing RNAs prevented the suppression of CFTR protein by cigarette smoke. Conversely, specific inhibitors of the JNK or p38 MAPK pathways had no effect on CFTR decrease after cigarette smoke exposure. In addition, inhibition of the MEK/Erk1/2 MAPK pathway prevented the reduction of the airway surface liquid observed upon cigarette smoke exposure of primary human airway epithelial cells. Finally, addition of the antioxidant NAC inhibited activation of Erk1/2 by cigarette smoke and precluded the cigarette smoke-induced decrease of CFTR.
Conclusions
These results show that the MEK/Erk1/2 MAPK pathway regulates plasma membrane CFTR in human airway cells.
General Significance
The MEK/Erk1/2 MAPK pathway should be considered as a target for strategies to maintain/restore CFTR expression in the lung of smokers.
Keywords: CFTR, cigarette smoke, airway epithelial cells, MAPK pathway
BACKGROUND
The cystic fibrosis transmembrane conductance regulator (CFTR) is a chloride channel that plays a critical role in the lung by regulating airway fluid homeostasis allowing cilia to beat and clear pathogens [1]. Absence of functional CFTR leads to cystic fibrosis a genetic disease associated with impaired mucus clearance, and chronic infection and inflammation [2]. These past few years there has been a lot of interest in the negative regulation of CFTR by pollutants such as cigarette smoke, cadmium, and arsenic [3–6]. We and others have shown that CFTR expression is reduced in the lung of patients who developed chronic obstructive pulmonary disease (COPD) after years of cigarette smoking [7–9]. These findings suggest that suppression of CFTR could contribute to the development of chronic bronchitis seen in COPD which is characterized by mucus secretion, infection and inflammation similarly to what is observed in the lungs of patients with Cystic Fibrosis.
Suppression of CFTR can occur via degradation by two main pathways: the ubiquitin-proteasome pathway and the lysosomal pathway [10–12]. Plasma membrane CFTR is rapidly endocytosed and undergoes rapid and efficient recycling back to the plasma membrane in human airway epithelial cells, with more than 75% of endocytosed wild-type CFTR recycling back to the plasma membrane [13–15]. The plasma membrane stability of CFTR depends on its biosynthetic processing and post-maturational trafficking, which involves endocytic uptake followed by recycling to the plasma membrane or degradation in the lysosomes [16, 17]. The E3 ubiquitin ligase c-Cbl has been shown to facilitate CFTR endocytosis and ubiquitination with subsequent lysosomal degradation [18]. The molecular mechanism by which cigarette smoke alters expression of the CFTR ion channel is still unknown. We have previously shown that using a heterologous expression system, cigarette smoke exposure causes rapid internalization of CFTR. During this internalization, CFTR does not co-localize with lysosomes [6] but is instead internalized into an aggresome-like pathway in a calcium-dependent manner [6, 19].
Cigarette smoke activates several mitogen-activated protein kinase (MAPK) pathways including the MEK/Erk1/2 MAPK pathway [20]. Activation of this latter MAPK pathway results in cell survival and proliferation [21]. It was recently shown that the MEK/Erk1/2 MAPK pathway can regulate the expression of the epithelial sodium channel ENaC by regulating its interaction with the E3 ubiquitin ligase Nedd4-2 leading to lysosomal degradation of ENaC [22, 23]. Whether the MEK/Erk1/2 MAPK pathway also regulates the expression of plasma membrane CFTR is unknown.
Herein, we conducted this study to determine the underlying mechanisms by which cigarette smoke decreases CFTR abundance in human bronchial epithelial cells and determine the role of the MEK/Erk1/2 MAPK pathway in this process. We also evaluated whether the antioxidant N-acetyl-cysteine (NAC) could prevent the cigarette smoke-induced suppression of CFTR.
METHODS
Cell Culture and Reagents
The human bronchial epithelial cell line 16HBE14o-, an immortalized human bronchial epithelial cell line, was cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing L-glutamine, 10% FBS and penicillin (100 U/ml) and streptomycin (100 µg/ml). The tissue culture plates were coated using human fibronectin (1 mg/ml), collagen I bovine (3 mg/ml), and bovine serum albumin (1 mg/ml). All the cells used in the experiments were between passages 25 and 50, and were grown and maintained at 37 °C in a 5% CO2 humidified incubator. Primary human bronchial epithelial cells (HBECs) were isolated from excess donor tissue obtained at the time of lung transplantation under a protocol approved by UNC Medical School IRB. Primary HBECs were cultured as previously described and studied when fully differentiated [6, 24]. Lactacystin, UO126, UO124, SB203580, and SP600125 were from Calbiochem (La Jolla, CA). PD98059 was purchased from Cell Signaling Technology. The proteasome inhibitor MG132, and the lysosomal inhibitors, leupeptin and chloroquine, were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Transfection
16HBE14o- cells were transfected with Erk1 and Erk2 small interfering RNAs (siRNA), cbl siRNA (Ambion), or negative control #1 siRNA (Ambion) using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. Forty eight hours after transfection, cells were treated with or without 10% cigarette smoke extract (CSE) prepared from Camel cigarettes (R.J. Reynolds). The cells were then lysed in PBS with 1% Triton X-100 containing a cocktail of protease inhibitors (Roche Diagnostics, IN) for protein analysis.
Cell Surface Biotinylation
The 16HBE14o- cells were rinsed with ice-cold phosphate-buffered saline (PBS) containing 0.1mM CaCl2 and 1mM MgCl2 to eliminate the proteins present in the media. Cell surface proteins were labeled with 1 mg/ml EZ-Link NHS-SS Biotin (Pierce) for 30 min at 4 °C. Biotinylation was stopped by removing the biotin solution and incubating the cells with PBS containing 1% bovine serum albumin for 10 min at 4 °C to quench any residual NHS-SS biotin. At the end of the experiment, the cells were lysed with PBS-1% Triton X-100 and protease inhibitors (Roche). Biotinylated proteins were incubated with streptavidin beads overnight at 4 °C. After extensive washings, bound proteins were subjected to Western blot analysis. Biotinylated CFTR was detected using a C-CFTR monoclonal antibody (24-1; R&D Systems).
Immunoblotting
Cells were lysed in PBS containing 1% Triton X-100 and a cocktail of protease inhibitors (Roche). Western blotting was performed as previously described [4]. In brief, 20 µg of the protein were separated with SDS-PAGE in 4–15% polyacrylamide gel and then transferred to polyvinylidenedifluoride (PVDF) membranes (Bio-Rad, Hercules, CA). The membranes were blocked with 5% non-fat milk in PBS-Tween 20 and immunoblotted with primary antibodies against CFTR antibody (24-1, R&D Systems), phosphor-Erk1/2 (Cell Signaling), Erk1/2 (Cell Signaling), or β-actin (Santa Cruz Biotechnology) followed by treatment with appropriate HRP-conjugated secondary antibody (Pierce, Rockford, IL, USA). The signals were detected with enhanced chemiluminescence (Super Signal West Pico; Thermo Scientific) followed by exposure to X-ray films. The protein bands on the X-ray film were scanned, and band density was measured using ImageJ software (NIH).
ASL height measurements
To measure ASL height, PBS (20 µl) containing 2 mg/ml rhodamine-dextran (10 kDa; Invitrogen, USA) ± 10 µM MEK inhibitor was added to cultures at the start of the experiment for 10 mins. To measure ASL secretion, excess fluid was aspirated with a Pasteur pipette to bring ASL height down to ~ 7 µm, as described by Tarran et al. [25]. Before CS exposure, 10 µM MEK inhibitor was added basolateraly. In all cases, five predetermined points (one central, four 2 mm from the edge of the culture) were XZ scanned using a confocal microscope (Leica SP5; glycerol 63× immersion lens) as described [26]. Cultures were returned to the incubator between time points. For all studies, PFC was added mucosally during imaging to prevent evaporation of the ASL.
Cigarette smoke extract (CSE) preparation and whole Cigarette Smoke (CS) exposure
CSE (100%) was prepared as previously described and used to treat 16HBE14o- cells [27]. Primary HBECs were exposed to whole cigarette smoke (CS) after being placed in a specially built smoke exposure chamber that exposes apical but not basolateral surfaces [6, 28]. CS was then generated according to the International Organization of Standardization (ISO) standards (35 ml draw over 2 s) using a LC1 smoke engine (Borgwaldt, Richmond, Virginia, USA) and applied to the cultures at a rate of 1 puff every 30 s until the cigarette is smoked (~ 5 min; ~ 12 puffs). For ASL experiments cells are treated with CS for about 5 min. This maneuver has previously been shown to drive removal of CFTR from the plasma membrane without inducing gross cellular toxicity [6, 28].
Confocal Microscopy
16HBE14o- cells were fixed in ice-cold 100% methanol for 20 min at −20°C. The slides were then incubated in 1% bovine serum albumin (BSA)/PBS for 10 min, followed by incubation at 37 °C for 1 hr with primary antibody against CFTR (24-1; R&D Systems) and the lysosomal marker LAMP-1 (Cell Signaling Technology). After several washings, the slides were incubated at 37 °C for 45 min with appropriate Alexa Fluor® 488- and Alexa Fluor® 594-conjugated secondary antibody. Coverslips were mounted onto slides with Vectashield mounting medium containing DAPI (Vector Laboratories) prior to being imaged on a Leica DMIRE2 inverted confocal microscope using a 63× objective lens.
Statistical Analysis
Data are expressed as mean ± standard error (SE) of at least three independent experiments. The results of the experiments were analyzed by unpaired t tests. In all cases, a p value of <0.05 was considered as statistically significant.
RESULTS
Effect of lysosomal and proteasome inhibitors on cigarette smoke extract (CSE)-induced decrease of CFTR protein in human airway epithelial cells
Several studies have recently shown that CSE decreases the expression of CFTR in human airway epithelial cells [3, 6, 27]. Here we used the normal human bronchial epithelia cell line 16HBE14o- that endogenously expresses the ion channel CFTR. The main two pathways leading to CFTR degradation are the proteasomal and lysosomal pathways [5, 11, 29]. In order to investigate whether the underlying pathway involves either lysosomes or the proteasome, 16HBE14o- cells were treated with CSE in presence of the lysosomal or proteasome inhibitors. As expected, CSE reduced the expression of CFTR (Figure 1). It has to be noted that only mature CFTR (Band C) is seen on the blots. This result is in agreement with previous report showing that CFTR biogenesis is very efficient (close to 100%) in cells endogenously expressing CFTR such as 16HBE14o- [30]. The lysosomal inhibitors chloroquine (Figure 1A) and leupeptin (Figure 1B) both significantly prevented the CSE-induced decrease of CFTR, but they both had no effect on steady state level of CFTR. As previously described [6] the proteasomal inhibitor MG132 did not prevent CFTR diminution after CSE exposure (Figure 1A). However MG132 alone decreased CFTR expression. We therefore used another proteasomal inhibitor lactacystin (Figure 1C) which had no effect on steady-state levels of CFTR. Again, this inhibitor could not preclude the loss of CFTR induced by CSE exposure. Taken together our data show that cigarette smoke induces lysosomal degradation of CFTR.
Figure 1. Effect of the lysosomal inhibitors leupeptin and chloroquine and the proteasomal inhibitor lactacystin on the expression of CFTR after exposure to cigarette smoke extract.
16HBE14o- cells were treated with 10% cigarette smoke extract (CSE) with or without the lysosomal inhibitor leupeptin (LP, 50 µg/ml) or chloroquine (CQ, 10 µM), or the proteasome inhibitor lactacystin (LC, 5 µM) for 48 hrs. CFTR protein was detected by immunoblotting as described in Methods. CTRL, Control. N=4. *, p < 0.05; **, p < 0.001; NS, not significant.
Role of MAPK pathways in CSE-induced suppression of CFTR
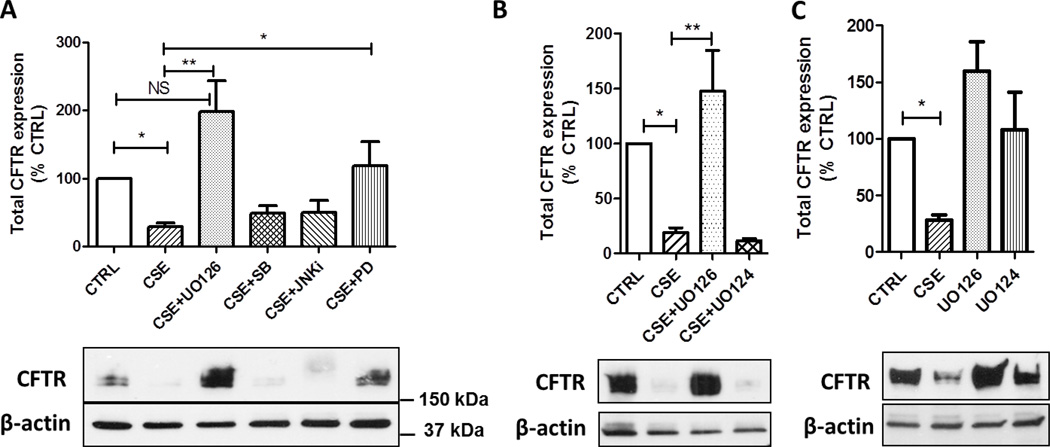
Cigarette smoke contains over 3,000 chemicals including reactive oxygen species (ROS) that can act on various pathways in the cell. Accordingly, CSE can stimulate multiple signaling pathways including mitogen-activated protein kinase (MAPK) pathways. We therefore investigated whether the main classical MAPK pathways (i.e. p38, JNK, and MEK) contribute to the decrease in the expression of CFTR protein after CSE exposure. As shown in Figure 2A, inhibition of the MEK/Erk1/2 MAPK pathway using two specific inhibitors, UO126 and PD98059, prevented the loss of CFTR induced by CSE. These results were further confirmed using UO124, the inactive form of UO126 which has no inhibitory property on MEK, and had no protective effect on CFTR after exposure to cigarette smoke (Figure 2B). UO124 alone had no effect on the expression of CFTR (p > 0.05). Although UO126 alone has a trend to increase the expression of CFTR when compared with the control group, this increase failed to reach significance (p = 0.063, Supplemental Figure 1). Conversely, inhibition of the p38 or JNK MAPK pathways had no effect on the suppression of CFTR after exposure of human bronchial epithelial cells 16HBE14o- to CSE (Figure 2A).
Figure 2. Role of MAPK inhibitors on CFTR expression after cigarette smoke exposure.
16HBE14o- cells were treated with 10% CSE with or without the MEK/Erk1/2 inhibitors UO126 (10 µM) or PD98059 (PD, 20 µM), the p38 inhibitor SB203580 (SB, 20 µM), the JNK inhibitor SP600125 (JNKi, 20 µM), or UO124 (10 µM) for 48 hrs. CFTR protein was detected by immunoblotting. CTRL, Control. N=4. *, p < 0.05; **, p < 0.001; NS, not significant.
To further confirm the role of Erk1/2 in down-regulation of CFTR by CSE, the expression of Erk1/2 was decreased using silencing RNAs targeting Erk1 and Erk2. As shown in Figure 3, the expression of Erk1 and Erk2 was decreased by about 50%. No difference in the expression of CFTR was observed between the control and Erk siRNA groups in absence of CSE treatment. Addition of CSE reduced the expression of CFTR in the control group but reduced expression of Erk1/2 significantly prevented the loss of CFTR protein. To confirm that Erk silencing was sufficient to impair downstream phosphorylation of Erk targets, phosphorylation of Elk was detected. As observed in Fig. S1, addition of CSE increased phosphorylation of Elk which was prevented in cells treated with Erk siRNAs.
Figure 3. Decreased expression of Erk1 and 2 prevents the CSE-induced suppression of CFTR.
16HBE14o- cells were incubated with Erk1 and 2 siRNAs or control siRNA. Forty eight hours later 16BE14o- cells were incubated with 10% CSE for 24 hrs. CFTR and Erk 1 and 2 proteins were detected by immunoblotting. β-actin was detected to confirm equal loading between samples. CTRL, Control. N=4.*, p < 0.05; **, p < 0.001.
Inhibition of the MEK/Erk1/2 MAPK pathway prevents the loss of CFTR from the plasma membrane of human airway epithelial cells after CSE exposure
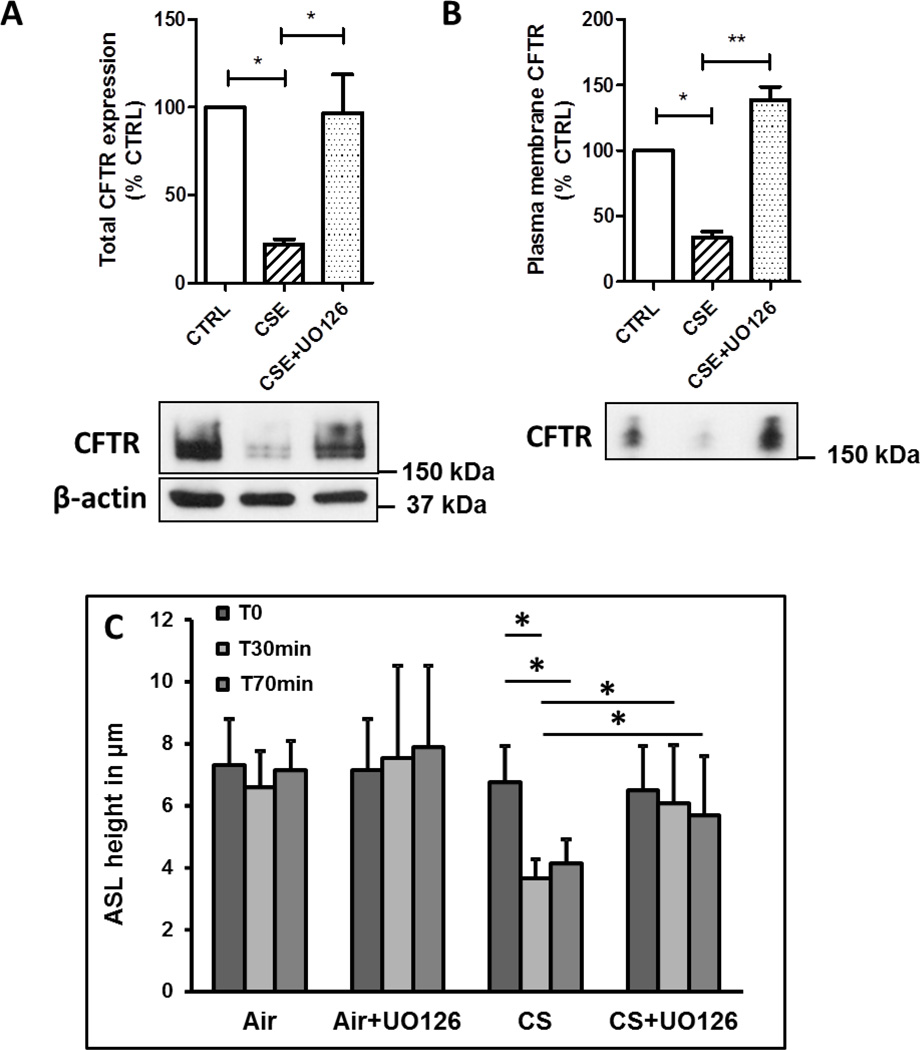
CFTR has to be present at the plasma membrane of bronchial epithelial cells to exert its role as a chloride channel and regulate the airway surface hydration [1]. We therefore wanted to determine whether inhibition of the MEK/Erk1/2 MAPK pathway would have any protective effect on plasma membrane CFTR after exposure to CSE. The human bronchial epithelial cells 16HBE14o- were incubated simultaneously with CSE and the MEK/Erk1/2 inhibitor UO126 and CFTR present at the plasma membrane was assessed using cell surface biotinylation. Not only inhibition of the MEK/Erk1/2 MAPK pathway prevented decrease of CFTR protein (Figure 4A), it also prevented the loss of CFTR from the plasma membrane of airway epithelial cells (Figure 4B).
Figure 4. Inhibition of the MEK/Erk1/2 MAPK pathway prevents loss of plasma membrane CFTR after cigarette smoke exposure.
(A) and (B) 16HBE14o- cells were treated with 10% Camel cigarette smoke extract (CSE) with or without UO126 (10 µM) for 48 hrs. CFTR expression (total (A) or plasma membrane (B)) was detected as described in Methods. CTRL, Control. N=4. (C) Primary human bronchial epithelial cells were pretreated with 10 µM UO126 and then exposed to air or cigarette smoke (CS) as described in Methods section. ASL was measured at the indicated time. N=6 from two normal donors. *, p < 0.05; **, p < 0.001
Inhibition of the MEK MAPK pathway prevents cigarette smoke-induced decrease of airway surface liquid (ASL)
CFTR is present at the plasma membrane of bronchial epithelial cells and regulates hydration of the airway surface liquid layer [1]. As seen in cystic fibrosis, absence of functional CFTR results in impaired mucociliary clearance due to reduced ASL. We recently showed that cigarette smoke decreases expression of membrane CFTR in primary human bronchial epithelial cells resulting in impaired ASL [27]. As shown above (Figure 4B), inhibition of the MEK MAPK pathway prevented the decrease of plasma membrane CFTR. Accordingly, inhibition of the MEK pathway using the specific inhibitor UO126 prevented the reduction in the height of the ASL observed upon exposure to cigarette smoke (Figure 4C).
Role of the E3 ligase c-Cbl on CFTR expression after exposure to cigarette smoke
Ubiquitination of CFTR can lead to proteasomal or lysosomal degradation. The E3 ubiquitin ligase c-Cbl has been shown to be linked to lysosomal degradation of CFTR in airway epithelial cells [18, 31]. To determine whether c-Cbl is involved in the CSE-induced degradation of CFTR, we used c-Cbl siRNA to decrease c-Cbl abundance. As shown in Figure 5, transfection of c-Cbl siRNA reduced c-Cbl expression by ~77%. The CFTR expression was comparable between the control and c-Cbl siRNA groups in absence of CSE treatment. Conversely, addition of CSE significantly decreased the expression of CFTR in the control group, whereas suppression of c-Cbl expression using siRNA partly prevented the CSE-induced down-regulation of CFTR. These data indicate that the E3 ligase c-Cbl contributes to suppression of CFTR upon cigarette smoke exposure.
Figure 5. Effect of the E3 ligase c-Cbl on CFTR expression after exposure to CSE.
16HBE14o- cells were transfected with c-Cbl or control siRNA for 48 hrs, followed by treatment with 10% Camel cigarette smoke extract (CSE) for 24 hrs. CFTR and c-Cbl were detected by immunoblotting. β-actin was detected to confirm equal loading between samples. CTRL, Control. N=4. *, p < 0.05; **, p < 0.001.
Inhibition of the MEK/Erk1/2 pathway prevents lysosomal degradation of CFTR
Lysosome-associated membrane protein 1 (LAMP-1) is a marker of the lysosomes and was used to determine the intracellular localization of CFTR. As shown in Figure 6 and S2, using confocal microscopy the CFTR signal was reduced after exposure to CSE as expected. No co-localization of CFTR with LAMP-1 was observed in 16HBE14o- cells in those conditions and could be due to CFTR degradation preventing its detection with the antibody used. However, inhibition of lysosmal degradation using chloroquine (CQ) allowed detection of CFTR in the lysosomes when the cells were exposed to CSE. The co-localization of CFTR with LAMP-1 was reduced in presence of the MEK/Erk1/2 inhibitor UO126. These results suggest that CSE induces lysosomal degradation of CFTR in human bronchial epithelial cells via activation of the MEK/Erk1/2 MAPK pathway.
Figure 6. Effect of CSE on intracellular localization of CFTR.
Representative confocal microscopic images of CFTR (Alexa488, green color) and LAMP-1 (Alexa594, red color)-stained cells. 16HBE14o- cells were treated with 10% CSE with or without the lysosomal inhibitor chloroquine (CQ, 20 µg/ml) and the MEK/Erk1/2 MAPK inhibitor UO126 (10 µM) for 24 hrs. Representative images showing the co-localization of CFTR and LAMP-1 are shown. CTRL: Control. Original magnification 630×.
The antioxidant N-acetylcysteine (NAC) prevents the CSE-induced loss of CFTR
Cigarette smoke contains many chemical compounds as well as reactive oxygen species (ROS) that can trigger activation of signaling pathways such as MEK/Erk1/2 [20]. N-acetylcysteine (NAC) is an antioxidant that can inhibit ROS directly via the redox potential of its thiol or indirectly by increasing intracellular glutathione levels. We therefore investigated whether NAC could prevent the loss of CFTR after exposure to CSE. Cells were treated with NAC and CSE simultaneously and CFTR expression was analyzed 24 hours later. As observed in Figure 7A, addition of 10 mM NAC prevented the loss of CFTR due to CSE exposure. NAC alone had no effect on CFTR expression (Figure 7A). Since the data presented above show that CSE alters the expression of the CFTR protein via activation of the MEK/Erk1/2 MAPK pathway, we investigated whether NAC prevented the loss of CFTR after CSE exposure by blocking activation of Erk1/2 (phosphorylation). CSE induced activation of Erk1/2 as shown by detection of phosphorylated Erk1/2 (Figure 7B) which was inhibited in the presence of 10 mM NAC. A lower concentration of 2 mM NAC had little effect on CSE-induced decrease of CFTR or phosphorylation of Erk1/2 (Figures 7A and 7C).
Figure 7. The antioxidant N-Acetylcysteine (NAC) prevents suppression of CFTR upon cigarette smoke exposure.
(A) 16HBE14o- cells were treated with 10% CSE with or without 0.5, 2, or 10 mM NAC. CFTR protein was detected by immunoblotting. N=4. *, p < 0.05; **, p < 0.001. (B) 16HBE14o- cells were treated with 10% CSE for the indicated times. PhosphoErk1/2 and total Erk1/2 were detected by immunoblotting. N=4. (C) 16HBE14o- cells were treated with 10% CSE with or without NAC (2 or 10 mM) for the indicated time. Phospho-Erk1/2 was detected by immunoblotting. Blots are representative of at least three experiments.
DISCUSSION
In this study we investigated the molecular mechanism by which cigarette smoke suppresses expression of CFTR in human bronchial epithelial cells. Our results revealed that cigarette smoke negatively regulates CFTR via activation of the MEK/Erk1/2 MAPK pathway. We found that cigarette smoke leads to internalization of the CFTR ion channel, and inhibition of the MEK/Erk1/2 MAPK pathway prevented the cigarette smoke-induced loss of CFTR as well as decreased of airway surface liquid (ASL). In addition we show that the antioxidant NAC prevented the loss of CFTR by inhibiting Erk1/2 phosphorylation.
Dr. Welsh’s laboratory was the first to report that cigarette smoke inhibits chloride ion transport across tracheal epithelium [32]. Several reports have shown that cigarette smoke inhibits the expression and function of the ion channels CFTR and ENaC [6, 8, 33, 34] and recently, a cigarette smoke-induced increase in intracellular calcium has been implicated in CFTR internalization[19]. However, key questions remain regarding the molecular pathway leading to CFTR deregulation. In this study, we used normal bronchial epithelial cell line 16HBE14o- which endogenously expresses CFTR since many studies used cells derived from cancer or heterologous systems with cells overexpressing CFTR. Consistent with a previous study where Bafilomycin A1 prevented CFTR inhibition, here we show that the lysosomal inhibitors, chloroquine and leupeptin, prevented the loss of CFTR, whereas inhibition of the proteasome had no effect. However, CFTR could not be detected in the lysosomes using immunohistochemistry in presence of CSE and could be due to CFTR degradation preventing its detection. Absence of co-localization of CFTR and LAMP-1 were previously reported [6] even though lysosomes have been shown to contribute to CFTR degradation [10]. Conversely, addition of the lysosmal inhibitor chloroquine, which prevents acidification of the lysosomes, allowed co-localization of CFTR with the lysosomal marker LAMP-1 (see Figure 6). Bafilomycin A1 inhibits smoke-induced calcium release and also prevents CFTR diminution [19]. Thus, sorting out effects caused by altered lysosomal calcium release versus inhibition of lysosomal degradation are hard to differentiate and additional studies will be required. Interestingly, the heavy metal arsenic has been shown to trigger lysosomal degradation of the CFTR ion channel in airway epithelial cells [18, 31]. Taken together, these results suggest that targeting the lysosomes would prevent CFTR degradation upon exposure to pollutants.
Lysosmal degradation of membrane proteins is generally associated with monoubiquitination. C-Cbl is an E3 ligase previously reported to facilitate the lysosomal degradation of CD5, gp130, as well as CFTR [18, 31, 35, 36]. However, decreasing the expression of c-Cbl using silencing RNA prevented the CSE-induced suppression of CFTR suggesting that c-Cbl contributes to regulation of CFTR in human bronchial epithelial cells.
The epithelial sodium channel ENaC which interacts with CFTR in bronchial epithelial cells [37, 38] is degraded by the lysosomes after activation of the MEK/Erk1/2 MAPK pathway by interacting with the E3 ligase Nedd4-2 [39–41]. Here we show that pharmacological inhibition of MEK using UO126 or PD98059, or genetic inhibition of Erk1/2 using siRNAs prevented the cigarette smoke-induced suppression of CFTR. Most importantly, inhibition of the MEK/Erk1/2 MAPK pathway prevented the loss of CFTR from the plasma membrane of the human bronchial epithelial cells 16HBE and most importantly prevented the cigarette smoke-induced decreased of ASL. This is an important finding since CFTR needs to be present at the apical membrane of airway epithelial cells to play its role as a chloride channel to maintain fluid homeostasis in the lung. Activation of the Erk1/2 pathway by the pollutant cadmium was reported to increase CFTR activity in kidney cells [42]. In this latter study, CFTR activity was measured 5 minutes after exposure to cadmium. It is therefore possible that activation of Erk1/2 has distinct effects depending of the type of cells studied (renal versus airway epithelial cells) and/or the time after MEK/Erk1/2 activation (short-term versus long-term). Since both CFTR and ENaC are downregulated following activation of the MEK/Erk1/2 MAPK pathway, it might be an unrecognized pathway to regulate plasma membrane ion channels.
About 20% of smokers develop chronic obstructive pulmonary disease (COPD) but over 90–95% of patients with COPD were smokers (http://www.goldcopd.com). Some reports indicate that as many as 50% of smokers develop COPD if an advanced age is reached [43]. Cigarette smoke contains over 3,000 chemicals as well as reactive oxygen species that can lead to activation of several signaling pathways including the MEK/Erk1/2 MAPK pathway [20]. For example our team has recently showed that cadmium, a toxic heavy metal present in cigarette smoke, induces secretion of the pro-inflammatory cytokine IL-8 via an Erk1/2-dependent pathway when added to human bronchial epithelial cells [44]. Interestingly, sustained activation of Erk1/2 has been found in mice and rats exposed to cigarette smoke [45, 46]. Most importantly, analysis of phospho-Erk1/2 revealed that patients with COPD (emphysema) have elevated Erk activation when compared to healthy control subjects [20, 45]. Based on our results we predict that sustained activation of Erk in the lung will contribute to suppression of CFTR expression.
NAC has been used in patients with COPD with mixed success [47, 48]. Recently it was shown that higher doses might be required to obtain beneficial effects [49]. Several studies reported that long-term high dose of NAC treatment may reduce the risk of exacerbations and improve lung function (FEV1) [50, 51]. The doses used in our study are within the range of doses used in clinical practice (4–10 mM daily) [50]. We observed that 2 mM NAC had very little inhibitory effect on activation of Erk1/2 MAPK pathway, whereas 10 mM prevented activation of Erk1/2 and consequently loss of CFTR protein (see Figures 7A and C). It is important to note that the cells were not pre-treated with NAC so the protective effect of NAC is not due to increased levels of glutathione but rather by acting directly as an antioxidant. In addition, the inhibition of Erk1/2 activation was seen only after 5–10 minutes (see Figure 7). Interestingly, Varelogianni et al. reported that NAC increases chloride efflux via activation of the CFTR chloride channel in human bronchial epithelial cells expressing the CFTR mutant deltaF508 [52]. This latter mutation is the most common mutation leading to cystic fibrosis (CF). Since CF cells have higher Erk1/2 activation when compared to control non-CF cells [53] it is possible that NAC could inhibit the MEK/Erk1/2 pathway in CF cells resulting in rescue of deltaF508-CFTR. Accordingly, a recent study identified kinase inhibitors, including inhibitors of the Ras/Raf/MEK/Erk1/2 pathway as potent correctors of deltaF508-CFTR.
GENERAL SIGNIFICANCE
Due to the role of CFTR in the bronchial epithelium and its potential role in chronic bronchitis seen in COPD patients, our data suggest that NAC would benefit COPD patients with chronic bronchitis by inhibiting activation of the MEK/Erk1/2 pathway resulting in stabilization of plasma membrane CFTR.
Supplementary Material
Highlights.
-
-
The Erk pathway contributes to degradation of CFTR in cells exposed to CSE
-
-
Inhibition of the Erk pathway prevents loss of membrane CFTR and impairment of ASL
-
-
High doses of the NAC inhibits activation of Erk pathway
-
-
High doses of NAC precludes cigarette smoke-induced decrease of CFTR
-
-
We report that targeting Erk pathway improves stability of CFTR in bronchial cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. Journal of internal medicine. 2007;261:5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 2.Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. The European respiratory journal. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 3.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 4.Rennolds J, Butler S, Maloney K, Boyaka PN, Davis IC, Knoell DL, Parinandi NL, Cormet-Boyaka E. Cadmium regulates the expression of the CFTR chloride channel in human airway epithelial cells. Toxicological sciences : an official journal of the Society of Toxicology. 2010;116:349–358. doi: 10.1093/toxsci/kfq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanton CR, Thibodeau R, Lankowski A, Shaw JR, Hamilton JW, Stanton BA. Arsenic inhibits CFTR-mediated chloride secretion by killifish (Fundulus heteroclitus) opercular membrane. Cell Physiol Biochem. 2006;17:269–278. doi: 10.1159/000094139. [DOI] [PubMed] [Google Scholar]
- 6.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, Bennett WD, Riordan JR, Boucher RC, Tarran R. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan F, Nuovo GJ, Crawford M, Boyaka PN, Kirkby S, Nana-Sinkam SP, Cormet-Boyaka E. MiR-101 and miR-144 regulate the expression of the CFTR chloride channel in the lung. PloS one. 2012;7:e50837. doi: 10.1371/journal.pone.0050837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, Gaggar A, Steele C, Tang LP, Liu B, Rowe SM. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144:498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, Tidwell S, Tang LP, Liu B, Fortenberry JA, Jones CW, Boydston JA, Clancy JP, Bowen LE, Accurso FJ, Blalock JE, Dransfield MT, Rowe SM. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med. 2013;188:1321–1330. doi: 10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma M, Pampinella F, Nemes C, Benharouga M, So J, Du K, Bache KG, Papsin B, Zerangue N, Stenmark H, Lukacs GL. Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J Cell Biol. 2004;164:923–933. doi: 10.1083/jcb.200312018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell. 1995;83:121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- 12.Matsumura Y, Sakai J, Skach WR. Endoplasmic reticulum protein quality control is determined by cooperative interactions between Hsp/c70 protein and the CHIP E3 ligase. The Journal of biological chemistry. 2013;288:31069–31079. doi: 10.1074/jbc.M113.479345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bomberger JM, Barnaby RL, Stanton BA. The deubiquitinating enzyme USP10 regulates the postendocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. The Journal of biological chemistry. 2009;284:18778–18789. doi: 10.1074/jbc.M109.001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swiatecka-Urban A, Brown A, Moreau-Marquis S, Renuka J, Coutermarsh B, Barnaby R, Karlson KH, Flotte TR, Fukuda M, Langford GM, Stanton BA. The short apical membrane half-life of rescued {Delta}F508-cystic fibrosis transmembrane conductance regulator (CFTR) results from accelerated endocytosis of {Delta}F508-CFTR in polarized human airway epithelial cells. The Journal of biological chemistry. 2005;280:36762–36772. doi: 10.1074/jbc.M508944200. [DOI] [PubMed] [Google Scholar]
- 15.Swiatecka-Urban A, Talebian L, Kanno E, Moreau-Marquis S, Coutermarsh B, Hansen K, Karlson KH, Barnaby R, Cheney RE, Langford GM, Fukuda M, Stanton BA. Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. The Journal of biological chemistry. 2007;282:23725–23736. doi: 10.1074/jbc.M608531200. [DOI] [PubMed] [Google Scholar]
- 16.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nature reviews. Molecular cell biology. 2006;7:426–436. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 17.Riordan JR. CFTR function and prospects for therapy. Annual review of biochemistry. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 18.Ye S, Cihil K, Stolz DB, Pilewski JM, Stanton BA, Swiatecka-Urban A. c-Cbl facilitates endocytosis and lysosomal degradation of cystic fibrosis transmembrane conductance regulator in human airway epithelial cells. The Journal of biological chemistry. 2010;285:27008–27018. doi: 10.1074/jbc.M110.139881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen JE, Sheridan JT, Polk W, Davies CM, Tarran R. Cigarette smoke-induced Ca2+ release leads to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. The Journal of biological chemistry. 2014;289:7671–7681. doi: 10.1074/jbc.M113.545137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercer BA, D'Armiento JM. Emerging role of MAP kinase pathways as therapeutic targets in COPD. International journal of chronic obstructive pulmonary disease. 2006;1:137–150. doi: 10.2147/copd.2006.1.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roskoski R., Jr ERK1/2 MAP kinases: structure, function, and regulation. Pharmacological research : the official journal of the Italian Pharmacological Society. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Booth RE, Stockand JD. Targeted degradation of ENaC in response to PKC activation of the ERK1/2 cascade. American journal of physiology. Renal physiology. 2003;284:F938–F947. doi: 10.1152/ajprenal.00373.2002. [DOI] [PubMed] [Google Scholar]
- 23.Falin RA, Cotton CU. Acute downregulation of ENaC by EGF involves the PY motif and putative ERK phosphorylation site. The Journal of general physiology. 2007;130:313–328. doi: 10.1085/jgp.200709775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 25.Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. The Journal of general physiology. 2006;127:591–604. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol. 2006;68:543–561. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 27.Hassan F, Xu X, Nuovo G, Killilea DW, Tyrrell J, Da Tan C, Tarran R, Diaz P, Jee J, Knoell D, Boyaka PN, Cormet-Boyaka E. Accumulation of metals in GOLD4 COPD lungs is associated with decreased CFTR levels. Respiratory research. 2014;15:69. doi: 10.1186/1465-9921-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clunes LA, Bridges A, Alexis N, Tarran R. In vivo versus in vitro airway surface liquid nicotine levels following cigarette smoke exposure. J Anal Toxicol. 2008;32:201–207. doi: 10.1093/jat/32.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumura Y, David LL, Skach WR. Role of Hsc70 binding cycle in CFTR folding and endoplasmic reticulum-associated degradation. Molecular biology of the cell. 2011;22:2797–2809. doi: 10.1091/mbc.E11-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varga K, Jurkuvenaite A, Wakefield J, Hong JS, Guimbellot JS, Venglarik CJ, Niraj A, Mazur M, Sorscher EJ, Collawn JF, Bebok Z. Efficient intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. The Journal of biological chemistry. 2004;279:22578–22584. doi: 10.1074/jbc.M401522200. [DOI] [PubMed] [Google Scholar]
- 31.Bomberger JM, Coutermarsh BA, Barnaby RL, Stanton BA. Arsenic promotes ubiquitinylation and lysosomal degradation of cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels in human airway epithelial cells. The Journal of biological chemistry. 2012;287:17130–17139. doi: 10.1074/jbc.M111.338855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welsh MJ. Cigarette smoke inhibition of ion transport in canine tracheal epithelium. J Clin Invest. 1983;71:1614–1623. doi: 10.1172/JCI110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H, Ferro TJ, Chu S. Cigarette smoke condensate inhibits ENaC alpha-subunit expression in lung epithelial cells. The European respiratory journal. 2007;30:633–642. doi: 10.1183/09031936.00014107. [DOI] [PubMed] [Google Scholar]
- 34.Bodas M, Min T, Vij N. Critical role of CFTR-dependent lipid rafts in cigarette smoke-induced lung epithelial injury. American journal of physiology. Lung cellular and molecular physiology. 2011;300:L811–L820. doi: 10.1152/ajplung.00408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka Y, Tanaka N, Saeki Y, Tanaka K, Murakami M, Hirano T, Ishii N, Sugamura K. c-Cbldependent monoubiquitination and lysosomal degradation of gp130. Molecular and cellular biology. 2008;28:4805–4818. doi: 10.1128/MCB.01784-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roa NS, Ordonez-Rueda D, Chavez-Rios JR, Raman C, Garcia-Zepeda EA, Lozano F, Soldevila G. The carboxy-terminal region of CD5 is required for c-CBL mediated TCR signaling downmodulation in thymocytes. Biochemical and biophysical research communications. 2013;432:52–59. doi: 10.1016/j.bbrc.2013.01.086. [DOI] [PubMed] [Google Scholar]
- 37.Livraghi-Butrico A, Kelly EJ, Wilkinson KJ, Rogers TD, Gilmore RC, Harkema JR, Randell SH, Boucher RC, O'Neal WK, Grubb BR. Loss of Cftr function exacerbates the phenotype of Na(+) hyperabsorption in murine airways. American journal of physiology. Lung cellular and molecular physiology. 2013;304:L469–L480. doi: 10.1152/ajplung.00150.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berdiev BK, Qadri YJ, Benos DJ. Assessment of the CFTR and ENaC association. Molecular bioSystems. 2009;5:123–127. doi: 10.1039/b810471a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou R, Patel SV, Snyder PM. Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. The Journal of biological chemistry. 2007;282:20207–20212. doi: 10.1074/jbc.M611329200. [DOI] [PubMed] [Google Scholar]
- 40.Soundararajan R, Melters D, Shih IC, Wang J, Pearce D. Epithelial sodium channel regulated by differential composition of a signaling complex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7804–7809. doi: 10.1073/pnas.0809892106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi H, Asher C, Chigaev A, Yung Y, Reuveny E, Seger R, Garty H. Interactions of beta and gamma ENaC with Nedd4 can be facilitated by an ERK-mediated phosphorylation. The Journal of biological chemistry. 2002;277:13539–13547. doi: 10.1074/jbc.M111717200. [DOI] [PubMed] [Google Scholar]
- 42.L'Hoste S, Chargui A, Belfodil R, Duranton C, Rubera I, Mograbi B, Poujeol C, Tauc M, Poujeol P. CFTR mediates cadmium-induced apoptosis through modulation of ROS level in mouse proximal tubule cells. Free Radic Biol Med. 2009;46:1017–1031. doi: 10.1016/j.freeradbiomed.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Teramoto S. 1. COPD pathogenesis from the viewpoint of risk factors. Internal medicine. 2007;46:77–79. doi: 10.2169/internalmedicine.46.1775. [DOI] [PubMed] [Google Scholar]
- 44.Cormet-Boyaka E, Jolivette K, Bonnegarde A, Rennolds J, Hassan F, Mehta P, Tridandapani S, Webster-Marketon J, Boyaka PN. An NF-kappaB-independent and Erk1/2-dependent mechanism controls CXCL8/IL-8 responses of airway epithelial cells to cadmium. Toxicological sciences : an official journal of the Society of Toxicology. 2012;125:418–429. doi: 10.1093/toxsci/kfr310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mercer BA, Kolesnikova N, Sonett J, D'Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. The Journal of biological chemistry. 2004;279:17690–17696. doi: 10.1074/jbc.M313842200. [DOI] [PubMed] [Google Scholar]
- 46.Chang WC, Lee YC, Liu CL, Hsu JD, Wang HC, Chen CC, Wang CJ. Increased expression of iNOS and c-fos via regulation of protein tyrosine phosphorylation and MEK1/ERK2 proteins in terminal bronchiole lesions in the lungs of rats exposed to cigarette smoke. Archives of toxicology. 2001;75:28–35. doi: 10.1007/s002040000168. [DOI] [PubMed] [Google Scholar]
- 47.Aylward M, Maddock J, Dewland P. Clinical evaluation of acetylcysteine in the treatment of patients with chronic obstructive bronchitis: a balanced double-blind trial with placebo control. European journal of respiratory diseases. Supplement. 1980;111:81–89. [PubMed] [Google Scholar]
- 48.Long-term oral acetylcysteine in chronic bronchitis. a double-blind controlled study. European journal of respiratory diseases. Supplement. 1980;111:93–108. [PubMed] [Google Scholar]
- 49.Shen Y, Cai W, Lei S, Zhang Z. Effect of High/Low Dose N-Acetylcysteine on Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-analysis. Copd. 2013 doi: 10.3109/15412555.2013.858315. [DOI] [PubMed] [Google Scholar]
- 50.Zuin R, Palamidese A, Negrin R, Catozzo L, Scarda A, Balbinot M. High-dose N-acetylcysteine in patients with exacerbations of chronic obstructive pulmonary disease. Clinical drug investigation. 2005;25:401–408. doi: 10.2165/00044011-200525060-00005. [DOI] [PubMed] [Google Scholar]
- 51.Tse HN, Raiteri L, Wong KY, Ng LY, Yee KS, Tseng CZ. Benefits of high-dose N-acetylcysteine to exacerbation-prone patients with COPD. Chest. 2014;146:611–623. doi: 10.1378/chest.13-2784. [DOI] [PubMed] [Google Scholar]
- 52.Varelogianni G, Oliynyk I, Roomans GM, Johannesson M. The effect of N-acetylcysteine on chloride efflux from airway epithelial cells. Cell biology international. 2010;34:245–252. doi: 10.1042/CBI20090007. [DOI] [PubMed] [Google Scholar]
- 53.Verhaeghe C, Remouchamps C, Hennuy B, Vanderplasschen A, Chariot A, Tabruyn SP, Oury C, Bours V. Role of IKK and ERK pathways in intrinsic inflammation of cystic fibrosis airways. Biochemical pharmacology. 2007;73:1982–1994. doi: 10.1016/j.bcp.2007.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.