Abstract
Phytochemicals play an important role in cancer therapy. Hispolon and 26 of its analogs (9 known and 17 new) were synthesized and evaluated for their antiproliferative activities in a panel of six independent human cancer cell lines using the in vitro cell-based MTT assay. Among the hispolon analogs tested, Compound VA-2, the most potent overall, produced its most significant effect in the colon cancer cell lines HCT-116 (IC50 1.4±1.3 μM) and S1 (IC50 1.8±0.9 μM) compared to its activity in the normal HEK293/pcDNA3.1 cell line (IC50 15.8±3.7 μM; p<0.01 for each comparison). Based on our results, VA-2 was about 9- to 11-times more potent in colon cancer cells and 2- to 3-times more potent in prostate cancer cells compared to HEK293/pcDNA3.1 cells. Morphological analysis of VA-2 showed significant reduction of cell number, while the cells’ sizes were also markedly increased and were obvious at 68 h of treatment with 1 μM in HCT-116 (colon) and PC-3 (prostate) cancer cells. A known analog, Compound VA-4, prepared by simple modifications on the aromatic functional groups of hispolon, inhibited prostate and colon cancer cell lines with IC50 values < 10 μM. In addition, hispolon isoxazole and pyrazole analogs, VA-7 and VA-15 (known), respectively, have shown significant activity with the mean IC50 values in the range 3.3 to 10.7 μM in all the cancer cell lines tested. Activity varied among the analogs in which aromatic functional groups and β-diketone functional groups are modified. But the activity of analogs VA-16 to VA-27 was completely lost when the side chain double-bond was hydrogenated indicating the crucial role of this functionality for anticancer activity. Furthermore, many of the compounds synthesized were not substrates for the ABCB1-transporter, the most common cause of multidrug resistance in anti-cancer drugs, suggesting they may be more effective anticancer agents.
1. Introduction
Cancer is the second leading cause of death in the United States and is a major public health concern worldwide. According to the National Center for Health Statistics, a total of 1,665,540 new cancer cases and 585,720 cancer deaths are projected to occur in the United States in 2014 [1]. Depending on the cancer type, chemotherapy remains an important treatment option. Although, recent advances in biomedical research have expanded our understanding of cancer biology and have led to an increase in anticancer agents in recent years, the treatments are many a time ineffective and patients often relapse. Multidrug resistance, toxicity and mutation of targets remain the major causes of chemotherapy failure [2, 3]. Therefore, there is an ongoing need to develop novel potent chemotherapeutic agents that can circumvent these causes of chemotherapeutic failure. Over the past few years, many anti-cancer drugs have been identified from natural products and few of them are now either in advanced clinical trials or have already been approved for therapeutic use [4].
Hispolon, a polyphenolic compound, was first isolated from the fruit bodies of a fungus and subsequently from tropical mushrooms [5, 6]. Several studies have demonstrated for the antioxidant [7], anti-inflammatory [8, 9], anti-estrogenic activity [10] and anti-cancer properties [11-15] of hispolon. The anti-proliferative activity of hispolon has most especially attracted the attention of many researchers in cancer chemotherapy and chemoprevention. Experimental evidence have revealed that hispolon exhibits its anticancer activity through inhibition of cell growth, induction of cell cycle arrest and suppression of metastasis in various types of cancer cells [5, 10, 16]. In addition, some of the studies have demonstrated that hispolon is non-toxic to most normal cells and its anticancer activity might be related to induced apoptosis [5]. In the present study, we have synthesized several known and new hispolon analogs and evaluated their anticancer activities in order to identify a potential lead compound. This paper reports: (i) the design and synthesis of hispolon and 26 of its analogs; (ii) the evaluation of their anticancer activities in human breast, prostate and colon cancer cell lines in comparison to normal non-cancer cell lines using the in vitro cell-based MTT cytotoxicity assay; and (iii) the effects of the most potent compound on mitochondrial membrane potential (MMP) and apoptosis in the colon cancer cell line (H-116) by flow cytometry.
2. Results and Discussion
2.1. Chemistry
Hispolon (VA-1), a natural polyphenol, is structurally similar to curcumin, but lacks one aryl moiety. In our previous study, we found that hispolon (VA-1), hispolon methyl ether (VA-2) and dehydroxy hispolon (VA-3) were active against prostate (PC-3), colon (HCT-116) and breast (MCF-7) cancer cell lines [15]. However, only their relative percent cell viabilities were reported as the cancer cell lines were treated with each compound at only 10 μM for 72 h, hence, IC50 values could not be obtained. Therefore, in our ongoing effort to identify new lead compounds with anticancer potential, we synthesized (Scheme 1) a series of known and new hispolon analogs and evaluated their antiproliferative activities in a variety of cancer cell lines. Hispolon, Compound VA-1, was synthesized from vanillin as previously reported [16]. Similarly, the known analogs of hispolon, including: Compounds VA-2 [16], VA-3 [15], VA-4 [17], VA-6 and VA-7 [18], VA-11 [19], VA-14 [20], VA-16 [21], and VA-17 [22] were synthesized using methods that were reported previously. Simple modification of the aromatic substituent in hispolon (VA-1) resulted in analogs VA-2, VA-3, VA-4 and VA-5. Hydrogenation of compounds VA-2 to VA-5 yielded compounds VA-16, VA-17, VA-18 and VA-19, respectively. The experimental details of the synthesis of the isoxazole (compounds VA-6, VA-7, VA-8, VA-9, VA-10, VA-20, VA-21, VA-22 and VA-23) and pyrazole (compounds VA-11, VA-12, VA-13, VA-14, VA-15, VA-24, VA-25, VA-26, and VA-27) analogs of VA-2 to VA-5 and VA-16 to VA-19, respectively, are described in the experimental section (Section 4.0 below, Scheme 1). The structures of all the compounds synthesized were analyzed using 1H and 13C NMR, mass spectral data and by comparison with those of melting points and spectral data reported in the literature.
Scheme 1.
Synthetic scheme of hispolon analogs.
2.2. MTT based cell cytotoxicity assay
Phytochemicals isolated from natural sources have historically proven beneficial in combating a number of human ailments. This has led to ongoing global interest in the biomedical community to screen natural products as chemo adjuvants for cancer treatment. As a part of our research program into identifying new anti-cancer candidates, hispolon analogs were synthesized and evaluated for their cytotoxic effects in 6 human cancer cell lines, including colon carcinoma (HCT-116 and S1), prostatic cancer (DU-145 and PC-3), and breast carcinoma (MDA-MB-231 and MCF-7) in comparison to their effects in normal non-cancer canine kidney (MDCK), mouse embryonic fibroblast (NIH/3T3), and human primary embryonic kidney (HEK293/pcDNA3.1) cell lines using the in vitro MTT assay. All the compounds were tested at concentrations ranging from 0.1 to 100 μM. The mean±SD 50% minimum inhibitory concentration (IC50) values obtained for all the analogs in all cancer and non-cancer cell lines are summarized in Table 1.
Table 1.
Mean±SD IC50 values indicating the in vitro activity of novel hispolon analogs against multiple cancer cell lines
| IC50 ± SD (μM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| VA series | CANCER CELLS | NON-CANCER CELLS | |||||||
|
| |||||||||
| Prostate | Colon | Breast | Kidney | Mouse fibroblast | Embryonic kidney | ||||
|
| |||||||||
| PC3 | DU-145 | HCT-116 | S1 | MCF-7 | MDAMB-231 | MDCK | NIH/3T3 | HEK 293/pcDNA.3.1 | |
| VA-1 | 12.9 ± 7.1 | 28.6 ± 11.1 | 5.2 ± 3.9 | 8.4 ± 3.2 | 7.9 ± 4.6 | 32.2 ± 14.4 | 36.2 ± 12.4 | 51.2 ± 16.4 | 7.3 ± 3.3 |
| VA-2 | 6.3 ± 3.1 * | 5.2 ± 3.1 * | 1.4 ± 1.3 ** | 1.8 ± 0.9 ** | 7.4 ± 6.2 | 9.7 ± 5.0 | 13.1 ± 4.6 | 18.9 ± 6.5 | 15.8 ± 3.7 |
| VA-3 | 20.2 ± 4.2 | 8.82 ± 4.9 | 36.5 ± 12.3 | 10.6 ± 3.8 | 19.7 ± 11.8 | 6.8 ± 3.4 | 30.6 ± 8.6 | 18.9 ± 4.6 | 10.3 ± 4.2 |
| VA-4 | 8.9 ± 6.2 | 8.22 ± 3.3 | 4.7 ± 2.6 | 9.3 ± 3.7 | 10.6 ± 4.0 | 43.1 ± 20.5 | 9.9 ± 6.0 | 18.1 ± 4.8 | 8.2 ± 4.3 |
| VA-5 | 15.8 ± 11.2 | 9.11 ± 8.2 | 11.9 ± 5.0 | 44.6 ± 11.8 | 15.8 ± 7.1 | 25.0 ± 12.4 | 41.6 ± 26.6 | 48.2 ± 20.0 | 18.7 ± 2.8 |
| VA-6 | 20.9 ± 5.2 | 28.9 ± 19.1 | 33.4 ± 15.8 | 50.5 ± 17.8 | 20.9 ± 8.8 | 34.6 ± 13.7 | 29.8 ± 12.0 | >100 ± NA | 36.2 ± 7.0 |
| VA-7 | 3.3 ± 2.2 ** | 10.5 ± 2.5 | 5.3 ± 3.2 * | 7.9 ± 4.5 | 6.3 ± 3.3 | 8.9 ± 3.2 | 11.6 ± 6.2 | 26.2 ± 14.4 | 13.0 ± 7.8 |
| VA-8 | 50.2 ± 14.1 | 67.2 ± 8.9 | 67.9 ± 22.3 | 44.2 ± 5.5 | 50.2 ± 13.5 | 55.5 ± 14.9 | 58.3 ± 23.7 | 88.5 ± 36.2 | 50.2 ± 8.9 |
| VA-9 | >100 ± NA | >100 ± NA | 87.4 ± 36.3 | >100 ± NA | >100 ± NA | 79.9 ± 28.2 | 44.7 ± 11.0 | 93.2 ± 16.4 | 63.2 ± 19.9 |
| VA-10 | >100 ± NA | >100 ± NA | 22.4 ± 4.0 | 76.4 ± 15.9 | >100 ± NA | 69.3 ± 18.6 | 70.2 ± 27.5 | >100 ± NA | 58.3 ± 12.5 |
| VA-11 | 16.5 ± 11.2 | 32.2 ± 13.9 | 78.8 ± 28.5 | 29.4 ± 14.1 | 16.5 ± 9.5 | 67.0 ± 36.4 | 35.4 ± 15.6 | 66.3 ± 24.8 | 47.7 ± 16.2 |
| VA-12 | 23.2 ± 8.3 | 33.1 ± 17.1 | 41.2 ± 27.4 | 55.3 ± 22.0 | 23.2 ± 9.9 | 56.7 ± 17.3 | >100 ± NA | 88.4 ± 23.8 | 29.7 ± 18.0 |
| VA-13 | 48.1 ± 14.8 | 45.8 ± 12.0 | 38.8 ± 14.0 | 32.9 ± 12.3 | 48.1 ± 17.2 | 23.2 ± 13.1 | 80.0 ± 12.2 | 59.2 ± 32.2 | 77.4 ± 23.8 |
| VA-14 | 12.6 ± 7.0 | 18.5 ± 11.0 | 51.3 ± 13.8 | 22.4 ± 11.5 | 12.6 ± 5.4 | 38.8 ± 12.0 | 54.1 ± 14.0 | 68.4 ± 14.4 | 23.2 ± 10.6 |
| VA-15 | 8.9 ± 4.1 | 8.4 ± 6.7 | 9.4 ± 3.7 | 8.4 ± 7.1 | 9.7 ± 4.5 | 10.7 ± 5.2 | 16.5 ± 7.9 | 19.9 ± 5.3 | 12.9 ± 5.6 |
| VA-16 | >100 ± NA | 87.9 ± 12.1 | 80.8 ± 14.4 | 98.2 ± 4.1 | >100 ± NA | >100 ± NA | 72.5 ± 33.6 | 97.3 ± 16.6 | 95.3 ± 21.2 |
2.2.1 Hispolon Analogs
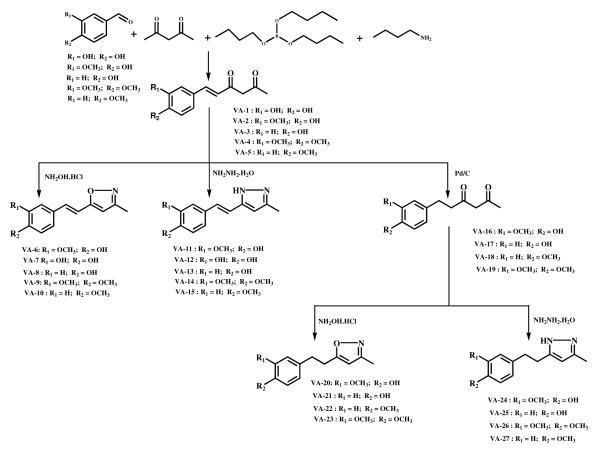
Hispolon (VA-1) showed marked activity in the colon (HCT-116 and S1) and breast (MCF-7) cancer cell lines with the observed IC50 values less than 10 μM (Table 1), however, its activities in these cancer cell lines were not selective as it also showed similar (non-significantly different) activity in the non-cancer normal control HEK293/pcDNA3.1 cell line. The mean±SD IC50 values for hispolon (VA-1) in the PC-3, DU-145, HCT-116, S1, MCF-7, and MDA-MB-231 cancer cell lines were 12.9±7.1, 28.6±11.1, 5.2±3.9, 8.4±3.2, 7.9±4.6, and 32.2±14.4 μM, respectively, compared to 36.2±12.4, 51.2±16.4, and 7.3±3.3 μM, in the MDCK, NIH/3T3, and HEK293/pcDNA3.1 non-cancer cell lines, respectively (Table 1). Compound VA-2, the most potent overall, produced its most significant effect in the colon cancer cell lines HCT-116 (IC50 1.4±1.3 μM) and S1 (IC50 1.8±0.9 μM) compared to its activity in the normal HEK293/pcDNA3.1 cell line (IC50 15.8±3.7 μM; p<0.01 for each comparison) (Table 1). In addition, compound VA-2 was also significantly effective in inhibiting cell survival in the prostate cancer cell lines DU-145 (IC50 5.2±3.1 μM) and PC-3 (IC50 6.3±3.1 μM) compared to the HEK293/pcDNA3.1 cell line (IC50 15.8±3.7 μM; p<0.05 for each comparison). Furthermore, compound VA-2 demonstrated a concentration-dependent inhibition of cell survival in the colon and prostate cancer cell lines (Figure 1). These results indicate compound VA-2 was about 9- to 11-times more potent in colon cancer cells and 2- to 3-times more potent in prostate cancer cells compared to HEK293/pcDNA3.1 cells (Table 1, Figure 1). Compared to hispolon (VA-1), the results revealed compound VA-2 was 3.7 to 4.7 times more potent in the colon cancer cell lines, 2 to 5.5 times more potent in the prostate cancer cell lines, and only half as potent in inhibiting cell survival in the normal control HEK293/pcDNA3.1 cell line. In contrast and as expected, no significant activity was observed either in the cell survival growth curve in the colon (HCT-116 and S1) and prostate (PC-3 and DU-145) cancer cell lines or normal HEK293/pcDNA3.1 cell line after treatment with the inactive control compound VA-16, that was synthesized by the hydrogenation of VA-2, at concentrations of up to 100 μM (Table 1, Figure 1C).
Figure 1.
The cytotoxic effects of hispolon analogs as determined by the in vitro MTT assay shown as concentration-response curves: (A) of Compound VA-2 in colon cancer cells S1 and HCT-116 compared with human embryonic kidney cells HEK293/pcDNA3.1; while the inset shows the comparison of mean±SD IC50 values of Compound VA-2 in colon cancer cells versus the HEK293/pcDNA3.1 cells; (B) of Compound VA-2 in prostate cancer cells DU-145 and PC-3 compared with human embryonic kidney cells HEK293/pcDNA3.1; while the inset shows the comparison of the mean±SD IC50 values of Compound VA-2 in prostate cancer cells versus the HEK293/pcDNA3.1 cells; (C) of the inactive control - Compound VA-16 (an analog that was synthesized by the hydrogenation of VA-2) in prostate cancer cells DU-145 and PC-3 and colon cancer cells S1 and HCT-116 compared with human embryonic kidney cells HEK293/pcDNA3.1; while the inset shows the comparison of the mean±SD IC50 values of Compound VA-16 in prostate cancer cells and colon cancer cells versus the HEK293/pcDNA3.1 cells. The mean±SD values were calculated from the results of at least three experiments each performed in triplicate. IC50 = the concentration of the drug candidate required to inhibit growth by 50%.
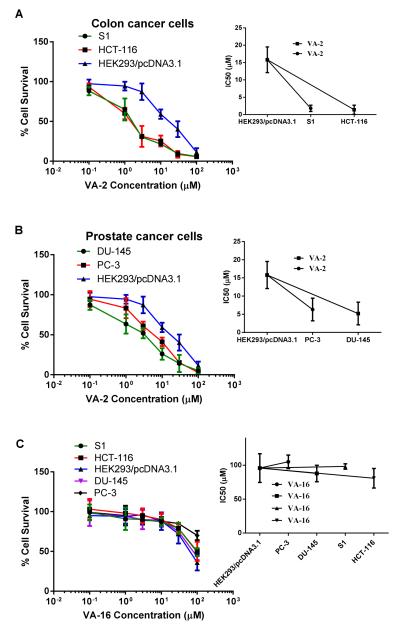
Morphological analysis showed marked reduction of cell number, while the cells’ sizes were also markedly increased and were obvious at 68 h of treatment with 1 μM concentration of compound VA-2 in HCT-116 (colon) and PC-3 (prostate) cancer cells, a concentration below the IC50 values (Figure 2). In contrast, there was no significant change in the morphological characteristics of both HCT-116 (colon) and PC-3 (prostate) cancer cells, when treated with the inactive control compound VA-16, that was synthesized by the hydrogenation of VA-2, at concentrations up to 100 μM (Figure 2). Among the known hispolon analogs prepared by simple modifications of the aromatic functional groups of hispolon, VA-4 inhibited prostate, colon and MCF-7 breast cancer cell lines with IC50 values < 10.6 μM. The IC50 values for VA-4 in the PC-3, DU-145, HCT-116, S1, and MCF-7cancer cell lines were 8.9±6.2, 8.2±3.3, 4.7±2.6, 9.3±3.7, and 10.6±4.0 μM, respectively. But the cytotoxic effects of VA-4 in the cancer cell lines were not selective as it also inhibited cell survival in the normal canine kidney MDCK (IC50 = 9.9±6.0 μM) and the human primary embryonic kidney HEK293/pcDNA3.1 (IC50 = 8.2±4.3 μM) cell lines indicating that it may be associated with general cytotoxicity.
Figure 2.
Morphological analysis of the cytotoxic effects of active compound VA-2 at concentrations of 0, 1 and 10 μM and inactive analog VA-16 (an analog that was synthesized by the hydrogenation of VA-2) at concentrations 0, 10 and 100 μM in HCT-116 colon cancer cells and PC-3 prostate cancer cells performed using microscopic pictures at 20X magnification. The cells were photographed for each triplicate treatment using an inverted microscope (Olympus, BX53F) with fluorescent lamps and digital cameras at 68 h with and without treatment. The images were compared in double-blind fashion for the different concentrations of synthesized compound treatments at different time points for cell size, cell shape and cell numbers. The arrow heads denote: A – cells with increased size; B – decreased numbers of cells; and C – cellular debris, in the representative panel shown for each treatment. The data was acquired and analyzed using CellSens software.
Among the hispolon isoxazole and pyrazole analogs, compounds VA-7 (known analog) and VA-15 (new analog), respectively, showed marked cytotoxicity with the mean IC50 values in the range 3.3 to 10.7 μM in all the cancer cell lines tested. Furthermore, only the isoxazole analog of hispolon, compound VA-7, showed significant inhibition of cell survival (p < 0.05 for each comparison) in one prostate (PC-3) and one colon (HCT-116) cancer cell lines compared to the normal HEK293/pcDNA3.1 cell line. The IC50 values for compound VA-7 in the prostate, colon and breast cancer cell lines were 3.3±2.2 (PC3), 10.5±2.5 (DU-145), 5.3±3.2 (HCT-116), 7.9±4.5 (S1), 6.3±3.3 (MCF-7) and 8.9±3.2 (MDAMB-231) μM, respectively, compared to 11.6±6.2 (MDCK), 26.2±14.4 (NIH/3T3) and 13.0±7.8 (HEK293/pcDNA3.1) μM in the non-cancer cell lines (Table 1). Compound VA-15 markedly but insignificantly (compared to the control HEK293/pcDNA3.1) inhibited cell survival in the prostate, colon and breast cancer cell lines with IC50 values of 8.9±4.1 (PC3), 8.4±6.7 (DU-145), 9.4±3.7 (HCT-116), 8.4±7.1 (S1), 9.7±4.5 (MCF-7) and 10.7±5.2 (MDAMB-231) μM, respectively, compared to 16.5±7.9 (MDCK), 19.9±5.3 (NIH/3T3), and 12.9±5.6 (HEK293/pcDNA3.1) μM, in the non-cancer cell lines. Interestingly, the hispolon analogs in which the side chain double-bond was hydrogenated (compounds VA-16 to VA-27) completely lost activity in the tested concentration range. None of the analogs showed any activity even at 100 μM. This clearly indicates that the side chain double-bond is key for activity. To be selected as the lead compound, an IC50 value in the submicromolar range (i.e., less than 1 μM) is preferred. In addition, the compound should be selective in its cytotoxicity to specific cancer cells compared to normal non-cancer cells. Also, more appropriate organ specific normal cells should be chosen as controls instead of HEK293/pcDNA3.1, MDCK or NIH/3T3 cell lines, a limitation of this study. Based on the fact that compound VA-2 was the most potent overall, with selectivity in colon and prostate cancer cells, and a concentration-dependent effect in both cancer cell lines, we identified it as the lead compound for further study. Further, to understand the mechanism of the selective cytotoxicity of the active hispolon analogs in cancer cells, more in-depth molecular experiments are needed that must be complemented with in vivo studies.
2.2.2 Structure-Activity Relationship (SAR)
In the first set of experiments, hispolon analogs (VA-2 to VA-5) with simple aromatic functional group modifications were prepared to study their anticancer activity (Scheme 1). Interestingly, methylation of the meta position hydroxyl group in hispolon resulted in compound VA-2 which exhibited more prominant activity than hispolon in prostate, colon and breast cancer cell lines. Similarly, dimethyl ether analog of hispolon (VA-4) also exhibited slightly higher activity than that of hispolon. When the meta position substituent was removed, analogs VA-3 and VA-5 showed slightly decreased activity in most of the cancer cell lines indicating that the substituent (hydroxyl/methoxy) on meta position is contributing to the exhibited activity. In the next set of experiments, isoxazole analogs (VA-6 to VA-10) were prepared by chemical modification of β-diketone moiety in VA-1 to VA-5 and were evaluated for their anticancer activity. Among the isoxazoles tested, VA-7 exhibited slightly higher activity. Whereas, the isoxazole analog, compound VA-6, showed a decrease in activity against the cancer cell lines tested. The activity was significantly decreased further for the isoxazole derivatives VA-8, VA-9 and VA-10. Subsequently, a pyrazole unit was introduced resulting in analogs VA-11 to VA-15, and the analogs were evaluated for their anticancer activity. The Pyrazole analog, VA-15 showed slightly higher activity than the parent analog VA-5. In the third set of experiments, several hispolon analogs (VA-16 to VA-20) were prepared by hydrogenation of the side chain double-bond and tested for their anticancer activity. Notably, all these analogs completely lost their activity even at 100 μM when tested against cancer cell lines. The results indicate that the activity varied among the hispolon analogs when aromatic functional groups and β-diketone moiety were modified. However, hydrogenation of the side chain double-bond in the hispolon analogs abolished the activity completely. This clearly implies that the double bond is very essential for the anticancer activity.
2.2.3 Multidrug Resistance of Synthesized Analogs in ABCB1 Overexpressing Cells
Cancer cells, through a number of different mechanisms, become resistant to numerous anticancer drugs that are even structurally and mechanistically unrelated. This trait, which is known as multidrug resistance (MDR), results in the attenuation of the cytotoxic effects of anticancer drugs and remains a major problem in conventional cancer chemotherapy. One of the most common causes of MDR is the overexpression of ATP-binding cassette (ABC) transporters which actively extrude anticancer drugs. ABCB1 (P-glycoprotein or MDR1), a member of the ABC-transporters family, is significantly expressed in a variety of solid and hematological cancers. ABCB1 functions as a drug efflux pump of a varied number of anticancer drugs resulting in MDR [2, 23]. Ideally, the anticancer drugs that are not substrates for these ABC-transporters are preferred candidates as anticancer agents. We therefore screened all the synthesized compounds to see whether their observed activities will be reversed or attenuated in ABCB1 overexpressing cells (HEK293/ABCB1) versus normal HEK293/pcDNA.3.1 cell lines and in comparison to a positive control antineoplastic drug, paclitaxel, a substrate of the ABCB1 transporter.
As shown in Table 2, the fold resistance, defined as the ratio of IC50 value in the HEK293/ABCB1 cells to the IC50 value in the normal HEK293/pcDNA.3.1 cells for a particular analog, ranged from 0.9 to 1.4 for the hispolon analogs for which IC50 values were less than 100 μM. Anlogs VA-17 to VA-27 did not show any activity when tested at high concentration (100μM) and hence the results were not presented. In contrast, the positive control, paclitaxel, was associated with a significant 9-fold resistance when the HEK293/ABCB1 cells were treated with the antineoplastic drug compared to the normal HEK293/pcDNA.3.1 cells. These results indicate there was no significant resistance produced to any of the compounds tested in ABCB1 overexpressing cells and suggest they may not be substrates for the ABCB1-transporter, the most common cause of MDR. However, these results based only on cell cytotoxicity data need be interpreted with caution as the more relevant and direct ATPase and photo affinity-labeling assay will have to be conducted to confirm this property of the synthesized compounds.
Table 2.
Mean±SD IC50 values and fold resistance for the novel curcumin and hispolon analogs compared to paclitaxel following treatment of HEK293/pcDNA3.1 and HEK293/ABCB1 cell lines.
| IC50 ± SD (μM) | |||
|---|---|---|---|
| VA series | HEK 293/pcDNA.3.1 |
HEK293/ABCB1 | FR |
|
| |||
| VA-1 | 7.3 ± 3.3 | 9.60 ± 5.20 | 1.3 |
| VA-2 | 15.8 ± 3.7 | 18.87 ± 5.50 | 1.2 |
| VA-3 | 10.3 ± 4.2 | 11.60 ± 6.50 | 1.1 |
| VA-4 | 8.2 ± 4.3 | 10.60 ± 8.70 | 1.3 |
| VA-5 | 18.7 ± 2.8 | 16.30 ± 8.20 | 0.9 |
| VA-6 | 36.2 ± 7.0 | 41.30 ± 16.20 | 1.1 |
| VA-7 | 13.0 ± 7.8 | 11.80 ± 12.30 | 0.9 |
| VA-18 | 50.2 ± 8.9 | 51.20 ± 10.33 | 1.0 |
| VA-9 | 63.2 ± 19.9 | 77.80 ± 20.60 | 1.2 |
| VA-10 | 58.3 ± 12.5 | 66.30 ± 15.70 | 1.1 |
| VA-11 | 47.7 ± 16.2 | 45.20 ± 13.50 | 0.9 |
| VA-12 | 29.7 ± 18.0 | 39.40 ± 17.20 | 1.3 |
| VA-13 | 77.4 ± 23.8 | 68.90 ± 13.60 | 0.9 |
| VA-14 | 23.2 ± 10.6 | 33.20 ± 21.50 | 1.4 |
| VA-15 | 12.9 ± 5.6 | 16.50 ± 9.50 | 1.3 |
| VA-16 | 95.3 ± 21.2 | 92.50 ± 8.00 | 1.0 |
| Paclitaxel | 0.04 ± 0.02 | 0.36 ± 0.11 | 9.0 ** |
2.2.4 Mitochondrial Membrane Potential and Apoptosis
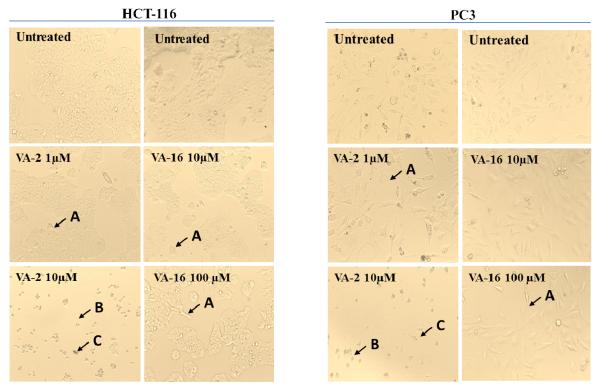
It has been reported that apoptosis is involved in hispolon-induced inhibition of tumor cell growth [5, 13]. The apoptotic signaling cascade is often initiated by mitochondrial dysfunction primarily due to loss of membrane asymmetry and release of cytochrome c and other apoptogenic factors from mitochondria leading to apoptotic cell death. In the process of apoptosis, phosphatidylserine (PS), which is otherwise located on the cytoplasmic surface, is translocated from the inner to the outer leaflet of the plasma membrane. The human vascular anticoagulant, annexin V, is a 35–36 kDa Ca2+-dependent phospholipid-binding protein which binds to the extracellular exposed PS and for which it has a high affinity. By labeling the annexin V with a fluorophore or biotin, this high affinity for binding PS exposed on the outer leaflet of the plasma membrane is used to identify apoptotic cells [25]. The most potent compound (VA-2) was evaluated further for its effects on mitochondrial membrane potential and induction of apoptosis in the HCT-116 colon cancer cell line using flow cytometry. As seen in Figure 3, 63.11% of untreated HCT-116 cells – live cells with intact mitochondrial membrane - are seen in quadrant I (Control or Left Panel). Whereas 27.56% of these cells had changes in mitochondrial membrane potential and started undergoing apoptosis, probably due to stress induced while preparing the samples. Treatment with 0.5 μM of compound VA-2 for 4 h produced significant reduction (p < 0.01) in mitochondrial membrane potential (84.9%) as seen in quadrant II (Middle Panel) of Figure 3. At the higher 5 μM concentration, compound VA-2 treatment clearly showed 2 distinct population of cells (Right Panel of Figure 3) with 11.7% of cells already apoptosed (Quadrant IV) and 72.7% of cells with significant reduction (p <0.01) or loss of their mitochondrial membrane potential (Quadrant II), suggesting a pre-apoptotic condition.
Figure 3.
Human colon cancer cells HCT-116 were treated in complete medium without (Control/Left Panel) and with Compound VA-2 at concentrations of 0.5 and 5 μM for 4 h. Cells were reacted with the reagents of the Mitochondrial Membrane Potential/Annexin V Apoptosis Kit with MitoTracker Red and Alexa Fluor 488 annexin V, followed by flow cytometric analysis – measuring the fluorescence at 530 nm and 585 nm wavelengths.
Conclusions
In conclusion, hispolon and its analogs were synthesized and their cytotoxicities were evaluated in human prostate, colon and breast cancer cell lines in comparison to non-cancer canine kidney, mouse embryonic fibroblast and human primary embryonic kidney cell lines using the MTT assay. Among the 26 hispolon analogs synthesized and evaluated, compound VA-2 was the most active, showing significant concentration-dependent cytotoxicity and selectivity in the colon followed by prostate cancer cell lines, with the observed mean IC50 values in the range 1.4 to 6.3 μM. Compared to hispolon, compound VA-2 was 3.7 to 4.7 times and 2 to 5.5 times more potent in the colon and prostate cancer cell lines, respectively. In addition, compound VA-2 induced a significant concentration-dependent reduction in mitochondrial membrane potential resulting in pre-apoptosis and apoptosis in the HCT-116 colon cancer cell line. Hispolon analogs activity varied significantly when the aromatic functional groups and β-diketone functional groups are modified. As a result VA-2, hispolon analogs VA-4, VA-7 and VA-15 also showed prominent activity on these cell lines. Notably, the activity was completely lost when the side chain double-bond was hydrogenated. This clearly infers that the double bond functionality is very crucial for the anticancer activity. Many of the synthesized compounds were not substrates for the ABCB1-transporter, suggesting they may be associated with significantly less drug resistance. Further, to clearly understand the mechanism of their selective cytotoxicity of associated with the active hispolon analogs identified in the current study, more in-depth molecular experiments are needed that must be complemented with in vivo studies.
3. Experimental section
3.1. General
All the solvents were procured from Avra Laboratories (Hyderabad, India).
3.2. Synthesis of hispolon analogs with aromatic substituent modification
Compounds, VA-1 to VA-4 were synthesized using methods reported previously in the literature [15-17].
3.2.1 (E)-6-(4-methoxyphenyl)hex-5-ene-2,4-dione (VA-5)
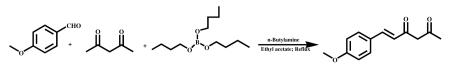
A mixture of tributylborate (50.7g, 220 mmol) and acetylacetone (36.7g, 367 mmol) was taken into a round bottomed flask equipped with a mechanical stirrer and the reaction mass was stirred at 60°C for 30 min. To the complex formed, was added anisaldehyde (10g, 73 mmol) in ethyl acetate (40 ml) and the reaction temperature was raised to 85°C. To the above reaction mixture n-butyl amine (5.3g, 73 mmol) was added drop wise and stirred for 90 min at 85 °C. The reaction mass was cooled to 55°C, acidified with 1N HCl (100 ml) and stirred for 1 h. The product was extracted with ethyl acetate (2 × 100 ml) and the ethyl acetate extract was concentrated under vacuum. The residue was adsorbed over silica gel (100-200#) and purified by column chromatography using n-Hexane and ethyl acetate (85:15) as eluants to obtain a yellow solid (5.5g, 37%).mp- 97.8-99.5°C, HPLC Purity-99%. 1HNMR (400Mz,,CDCl3) ppm: 5.5 (s,1H), 7.6 (s,1H). 7.5 (d,2H), 6.8 (d,2H), 6.3 (d,1H), 5.6 (s,1H), 3.8 (s,3H), 2.2 (s,3H)
3.2.2. Synthesis of hispolon isoxazoles
Compounds VA-6 and VA-7 were synthesized using methods reported previously in the literature [18].
3.2.2.1 (E)4-(2-methylisoxazol-5-yl)vinyl)Phenol (VA-8)
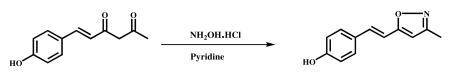
Hydroxylamine hydrochloride (1.66 g, 24.5 mmol) and pyridine (1.97 ml, 24.50 mmol) were added to stirred solution of dehydroxy Hispolon (1.0 g, 4.90 mmol) in ethanol (10 ml) and refluxed for 6 h. The reaction mixture was monitored over TLC and was cooled to ambient temperature. The solvent was evaporated in vacuum to obtain the crude product. It was poured into ice cold water and stirred for 1 h. The precipitated material was filtered, dried and recrystallized from DCM and MeOH. Light brown color solid was obtained (600 mg, 61% ). HPLC Purity-99.4%,m.p −176.2-177.8°C; 1HNMR (400Mz, CDCl3) δ: 7.42 (m, 2H), 7.39 (d, 1H, J=16Hz), 6.84 (d, 1H, J=16Hz), 6.86 ((m, 2H), 6.0(s, 1H), 5.6 (s, 1H), 2.3 (s, 3H); 13CNMR (400Mz, CDCl3): δ ppm 128.8 (C-1′), 128.6 (C-2′), 115.9 (C-3′),1 68 ( C-4′ ) , 115.9 (C-5′), 128.6 (C-6′ ), 134.1 (C-7′), 115.9 (C-8′ ), 168.6 (C -5), 101.1 (C-4), 160.4 (C-3), 10.1 (C-3a); ESI-MS m/z 202.08 [M+H]+.
3.2.2.2 (E)-5-(3,4-dimethoxystyryl)-3-methyl isoxazole (VA-9)
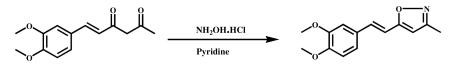
Hydroxylamine hydrochloride (2.741 g, 40.32 mmol) and pyridine (3.25 ml, 40.322 mmol) were added to stirred solution of (E)-6-(3,4-dimethoxypheny)hex-5-ene-2,4-dione (2.0 g, 8.0 mmol) in ethanol (20 ml) and refluxed for 6 h. After completion of the reaction which was monitored by TLC, the mixture was cooled to ambient temperature and the solvent was evaporated in vacuum to obtain the crude product. It was poured into ice cold water and stirred for 1 h. The precipitated material was filtered, dried and recrystallized from DCM and MeOH. A half-white solid was obtained (1.2 g, 61% ). HPLC Purity-98.8%, m.p-94.3-96.1°C; 1HNMR (400Mz, CDCl3) δ:7.26(s,1H), 7.06 (m, 1H), 6.88 (d, 1H) 7.2 (d,1H, J=16Hz), 6.76 (d, 1H, J=16Hz), 6.0 (s, 1H), 3.93 (s, 6H), 2.31 (s, 3H); 13CNMR (400Mz, CDCl3): δ ppm 128.76 (C-1′), 111.3 (C-2′), 149.3 (C-3′), 150.1 ( C-4′ ) , 109.0 (C-5′), 121.09 (C-6′), 134.3 (C-7′), 121.0 (C-8′ ), 56.0 (3′-OCH3), 56.0 (4′-OCH3), 168.5 (C-5), 101.7 (C-4), 160.1 (C-3), 11.6 (C-3a); ESI-MS m/z 246.1 [M+H]+.
3.2.2.3 (E)-5-(4-methoxystyryl)-3-methylisoxazole (VA-10)
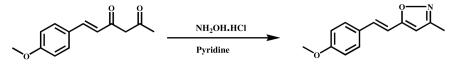
Hydroxylamine hydrochloride (3.1 g, 45.87 mmol) and pyridine (3.7 ml, 45.87 mmol) were added to stirred solution of (E)-6-(4-methoxypheny)hex-5-ene-2,4-dione (2.0 g, 9.1743 mmol) in ethanol (20 ml) and refluxed for 6 h. The reaction mixture was cooled to ambient temperature and the solvent was evaporated in vacuum to obtain the crude product. It was poured into ice cold water and stirred for 1 h. The precipitated material was filtered, dried and recrystallized from DCM and MeOH. A half-white colour solid was obtained (1.3 g, 61%); HPLC purity was 98.5%, m.p -111.2-113.30C. 1HNMR (400Mz, CDCl3) δ: 7.45 (m,2H), 7.22 (d,1H,J=16Hz), 6.75 (d,1H,J=16Hz), 6.9 (m,2H), 6.0 (s,1H), 3.8 (s,3H), 2.3 (s,3H). 13C NMR (400Mz, CDCl3): δ ppm 127.4 (C-1′), 127.4 (C-2′), 111.4 (C-3′), 160.1 ( C-4′), 111.4 (C-5′), 127.4 (C-6′), 128.6 (C-7′), 114.4 (C-8′ ), 168.8 (C-3), 101.1 (C-4), 160.4 (C-5), 11.70 (C-3a); ESI-MS m/z 216.10 [M+H]+.
3.2.3 Synthesis of hispolon pyrazoles
Compounds VA-11 and VA-14 were synthesized using methods reported previously in the literature [19, 20].
3.2.3.1 (E)-4-(2-(3-methyl-1H-Pyrazol-5-yl)vinyl)benzene-1,2-diol (VA-12)
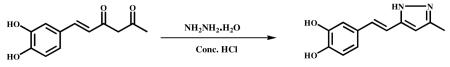
Hydrazine hydrate (0.5 g, 11.36 mmol) and Conc. HCl (catalytic) were added to a stirred solution of hispolon (1.0 g, 4.5 mmol) in ethanol (10 ml) and held at reflux for 6 h. The reaction mixture was cooled to ambient temperature and the solvent was evaporated in vacuum to obtain the crude product. It was poured into ice cold water and stirred for 1 h. Precipitated material was filtered, dried and recrystallized from DCM and MeOH. A light brown colour solid was obtained (900 mg, 90%). HPLC purity-99.5%, m.p. – 199.3-202 °C; 1HNMR (400Mz, DMSO) δ; 12.38 (s, 1H), 9.0 (s, 2H), 6.9 (m, 2H), 6.7 (m, 3H), 6.2 (s, 1H), 2.1 (s, 3H); 13CNMR (500Mz, DMSO+CDCl3) : δ ppm 129.3 (C-1′), 112.7 (C-2′), 142.9 (C-3′), 142.9 ( C-4′ ) , 112.7 (C-5′), 118.6 (C-6′) ,129.4 (C-7′), 115.3 (C-8′ ), 145.4 (C-3), 100.9 (C-4), 145.1 (C-5), 11.7 (C-3a); ESI-MS m/z 217.09 [M+H]+.
3.2.3.2 (E)-4-(2-(3-methyl-1H-Pyrazol-5yl)vinyl)Phenol (VA-13)
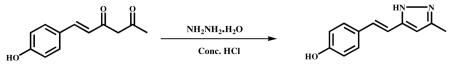
Hydrazine hydrate (0.6 g, 12.2 mmol) and conc. HCl (catalytic) were added to a stirred solution of dehydroxy hispolon (1.0 g, 4.9 mmol) in ethanol (10 ml) and refluxed for 6 h. After completion of the reaction (monitored by TLC), the mixture was cooled to ambient temperature, and the solvent was evaporated in vacuum to obtain a crude product. It was poured into ice cold water and stirred well for 1 h. Precipitated material was filtered, dried and recrystallized from DCM and MeOH. A light brown colour pure solid was obtained (720 mg, 79%). HPLC Purity-99.16%, m.p −197.4-201.3 °C. 1HNMR (400Mz , DMSO-d6) δ ppm : 12.37 (s, 1H), 9.5 (s, 1H), 7.3 (d, 2H), 6.8 (d, 1H), 6.7 (m, 1H), 6.6 ((d, 2H), 6.1 (s, 1H), 2.2 (s, 3H).13CNMR (500Mz, DMSO-d6) : δ ppm 128.4 (C-1′), 127.6 (C-2′), 115.3 (C-3′), 157.3 (C-4′), 115.3 (C-5′), 127.6 (C-6′ ), 129.3 (C-7′), 116.0 (C-8′ ), 157.4 (C-3), 101.2 (C-4), 157.3 (C-5), 12.0 (C-3a); ESI-MS m/z 201.1 [M+H]+.
3.2.3.3 (E)-5-(4-methoxystyryl)-3-methyl-1H-Pyrazole (VA-15)
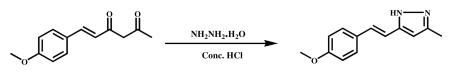
Hydrazine hydrate (1.1 g, 22.93 mmol) and Conc. HCl (catalytic) were added to stirred solution of (E)-6-(4-methoxypheny) hex-5-ene-2,4-dione (2.0 g, 9.1 mmol) in ethanol (20 ml) and refluxed for 6 h. After completion of the reaction (monitored by TLC), the mixture was cooled to ambient temperature, and the solvent was evaporated in vacuum to obtain a crude product. It was poured into ice cold water and stirred well for 1 h. Precipitated material was filtered, dried and recrystallized from DCM and MeOH. A Light brown solid was obtained (1.2 g, 61%). HPLC purity-99.6%, mp-136.4-137.8 °C.1HNMR (400 MHz, DMSO-d6) δ ppm 2.53 (s, 1H), 7.47 (d, 2H), 6.9 (m, 4H), 6.2 (s, 1H), 3.7 (s, 3H), 2.2 (s, 3H); 13C NMR (500Mz, CDCl3): δ ppm 128.2 (C-1′), 127.2 (C-2′), 113.8 (C-3′), 158.0 (C-4′), 113 3 (C-5′), 127.2 (C -6′ ), 129.5 (C-7′), 113.8 (C-8′ ), 55.0 (4′-OCH3), 158.7 (C-3), 101.03 (C-4), 158.6 (C-5), 10.2 (C-3a); IR (cm-1) : 3269, 2996, 2936, 2837, 1603, 1568, 1511, 1464, 1452, 1324, 1284, 1026, 996, 938, 861, 748, 685; ESI-MS m/z 215.11 [M+H]+.
3.2.4 Synthesis of dihydrohispolon analogs
Compounds VA-16 to VA-17 were synthesized using methods reported previously in the literature [21, 22].
3.2.4.1 6-(4-methoxyphenyl)hexane-2,4-dione (VA-18)
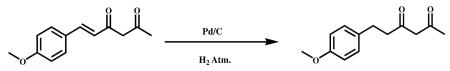
To a solution of (E)-6-(4- methoxy phenyl)hex-5-ene-2,4-dione (10.00 g, 45.87 mmol) in 100 ml of ethyl acetate was added 10 percent palladium/carbon (150 mg) under nitrogen. After the vessel was purged with hydrogen, the reaction mixture was stirred under 4 atm (2×4) of hydrogen at room temperature for 2 h. After the vessel was purged with nitrogen, the reaction mixture was filtered to remove 10 percent palladium/carbon and the filtrate was concentrated in vacuum, and the residue was purified by silica gel column chromatography (hexane/ethyl acetate = 85:15) to obtain the title compound as a colorless oil (9.0 g, 89.12%). HPLC Purity 97.87 %. 1H NMR (400Mz, CDCl3) : δ ppm 7.2 (d,2H), 6.8 (d,2H), 5.5 (s,2H), 3.8(s,3H), 2.8 (t,2H), 2.4 (t,2H), 2.0 (s,3H).
3.2.4.2 6-(3,4-dimethoxyphenyl)hexane-2,4-dione (VA-19)
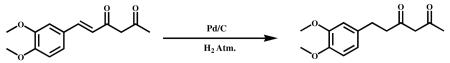
To a solution of (E)-6-(3,4- di methoxy phenyl)hex-5-ene-2,4-dione (8.0 g, 32.25 mmol) in 80 ml of ethyl acetate was added 10 percent palladium/carbon (100 mg ) under nitrogen. After the vessel was purged with hydrogen, the reaction mixture was stirred under 4 atm (2×4) of hydrogen at room temperature for 2 h. After the vessel was purged with nitrogen, the reaction mixture was filtered to remove 10 percent palladium/carbon. The filtrate was concentrated in vacuum, and the residue was purified by silica gel column chromatography (hexane/ethyl acetate = 80:20) to obtain the title compound as a colorless oil (6.8 g, 84.36%). HPLC Purity 99.678%.1H NMR (400Mz, , CDCl3) : δ ppm 6.8 (d,1H), 6.7 (d,2H), 5.3 (s,2H), 3.8 (s,6H), 2.8 (t,2H), 2.6 (t,2H), 2.1 (s,3H).
3.2.4.3 2-methoxy-4-(2-(3-methylisoxazol-5-yl)ethylphenol (VA-20)

Hydroxylamine hydrochloride (0.72 g, 10 mmol) and Conc. HCl (catalytic) were added to stirred solution of 6-(4-methoxy-3-methoxy pheny)hexane-2,4-dione in ethanol (10 ml) and refluxed for 6 h. After completion of the reaction (monitored by TLC), the mixture was cooled to ambient temperature and the solvent was evaporated in vacuum to obtain the crude product. It was poured into ice cold water and DCM (50 ml), and stirred well for 1 h. The DCM layer was extracted and dried over sodium sulphate, and concentrated under vacuum, and recrystallized from DCM and hexane. A half-white colour solid was obtained (0.91 g, 92.17%), HPLC Purity-99.63%, m.p-69.3-72.1 °C. 1H NMR (400Mz, , CDCl3): δ ppm 6.8 (d,1H), 6.7 (d,2H), 5.3(s, 2H), 3.8 (s,6H), 2.8 (t,2H), 2.6 (t,2H), 2.1 (s,3H).
3.2.4.4 4-(2-(3-methylisoxazol-5-yl)ethylphenol (VA-21)
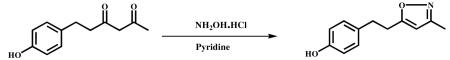
Hydroxylamine hydrochloride (0.8 g, 11.87 mmol) and Conc. HCl (catalytic) were added to stirred solution of 6-(4-hydroxy pheny)hexane-2,4-dione (1.0 g, 4.85 mmol) in ethanol (10 ml) and refluxed for 6 h. After completion of the reaction (monitored by TLC), the mixture was cooled to ambient temperature and the solvent was evaporated in vacuum to obtain the crude product. It was poured into ice cold water and DCM (50ml), and stirred well for 1 h. The DCM layer was extracted and dried over sodium sulphate, and concentrated under vacuum. The compound was recrystallized from DCM and hexane. A half-white colour solid was obtained (0.85 g, 85.8%). HPLC Purity-99.56%, m.p −94.3 – 97.7 °C. 1 H NMR (400Mz,CDCl3) ) : δ ppm 7.02 (d,2H), 6.79-6.75 (t,2H), 5.77 (s,1H), 2.99 (m,4H), 2.37-2.24 (s,3H). 13C NMR (500Mz DMSO-d6) : δ ppm 132.3 (C-1′), 129.5 (C-2′), 115.6 (C-3′), 154.8 ( C-4′ ) , 115.6 (C-5′), 129.5 (C-6′ ), 33.6 (C-7′), 28.8 (C-8′ ), 163.8 (C-3), 102.2 (C-4), 172.7 (C-5), 12.4 and 11.5 (C-3a). ESI-MS m/z 204.2 [M+H]+.
3.2.4.5 5-(4-methoxyphenethyl)-3-methylisoxazole (VA-22)

Hydroxylamine hydrochloride (0.77 g, 11.0 mmol) and Conc. HCl (catalytic) were added to stirred solution of 6-(4-methoxypheny)hexane-2,4-dione (1.0 g, 4.5 mmol) in ethanol (10 ml) and refluxed for 6 h. After completion of the reaction (monitored by TLC), the mixture was cooled to ambient temperature and the solvent was evaporated in vacuum to obtain the crude product. It was poured into ice cold water and DCM (50 ml), and stirred well for 1 h. The DCM layer was extracted and dried over sodium sulphate, and concentrated under vacuum. A colorless liquid was obtained (0.9 g, 91%). HPLC purity −99.65%. 1 H NMR (400Mz, CDCl3): δ ppm 7.2 (d,2H), 6.8 (d,2H), 5.7 (s,1H), 3.7 (s,3H), 2.8 (m,4H), 2.2 (s,3H). 13C NMR (500Mz, CDCl3) : δ ppm 132.2 (C-1′), 129.2 (C-2′), 111.2 (C-3′), 159.6 C-4′), 113.8 (C-5′), 129.1 (C-6′), 33.4 (C-7′), 28.6 (C-8′), 55.8 (31-OCH3), 172.2 (C-3), 101.3 (C-4), 172.2 (C-5), 11.3 (C-3a). ESI-MS m/z 218.2 [M+H]+.
3.2.4.6 5-(3,4-diemthoxyphenethyl)-3-methylisoxazole (VA-23)
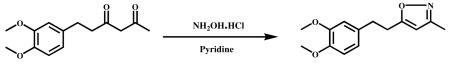
Hydroxylamine hydrochloride (0.68 g, 9.7 mmol) and Conc. HCl (catalytic) were added to stirred solution of 6-(3,4-dimethoxypheny)hexane-2,4-dione (1.0 g, 4.16 mmol) in ethanol (10 ml) and refluxed for 6 h. After completion of the reaction (monitored by TLC), the mixture was cooled to ambient temperature and the solvent was evaporated in vacuum to obtain the crude product. It was poured into ice cold water and DCM (50ml), and stirred well for 1 h. The DCM layer was extracted and dried over sodium sulphate, and concentrated under vacuum. A colorless liquid was obtained (0.85 g, 86% ). HPLC Purity-99.79 %. 1 H NMR (400Mz, CDCl3 ): δ ppm 6.8 (m,3H), 5.8 (s,1H), 3.8 (s,6H), 2.8 (s,4H), 2.2 (s,3H). 13C NMR (400Mz, CDCl3): δ ppm 132.6 (C-1′), 111.6 (C-2′), 159.5 (C-3′), 148.6 ( C-4′ ) , 113.7 (C- 5′), 129.1 (C-6′), 33.8 (C-7′), 28.4 (C-8′ ), 55.7 (3′-OCH3) , 55.7 (4′-OCH3) , 172.1 (C-3), 101.8 (C-4),168.8 (C-5), 12.0 (C-3a).ESI-MS m/z 248.1[M+H]+.
3.2.4.7 2-methoxy-4-(2-(3-methyl-1H-pyrazol-5-yl)ethyl)phenol (VA-24)
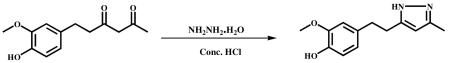
Hydrazine hydrate (0.52 g, 10.59 mmol) and Conc. HCl (catalytic) were added to stirred solution of 6-(4-methoxy-3-methoxy pheny)hexane-2,4-dione (1.0 g, 4.237 mmol) in ethanol (10 ml) and refluxed for 6 h. After completion of the reaction (monitored by TLC), the mixture was cooled to ambient temperature, and the solvent was evaporated in vacuum to obtain the crude product. It was poured into ice cold water and stirred for 1 h. The precipitated material was filtered, dried and recrystallized from water and MeOH. A half-white color solid was obtained HPLC Purity 99% (0.85 g, 85%). m.p −87.2 – 89.8 °C. 1 H NMR (400Mz,CDCl3) : δppm 6.81-6.7 (d,1H), 6.66-6.45 (d,2H), 5.83 (s,1H), 3.79 (s,3H), 2.88-2.84 (m,4H), 2.26 (s,3H). 13C NMR (500Mz, CDCl3): δ ppm 133.8 (C-1′), 111.3 (C-2′), 44.2 (C-3′), 1 44.1 ( C-4′), 111.3 (C-5′), 129.5 (C-6′), 35.5 (C-7′), 29.4 (C-8′ ), 56.0 (3′-OCH3), 148.9 (C-3), 103.5 (C-4), 146.27 (C-5), 12.3 (C-3a). ESI-MS m/z 234.1[M+H]+.
3.2.4.8 4-(2-(3-methyl-1H-pyrazol-5-yl)ethyl)phenol (VA-25)
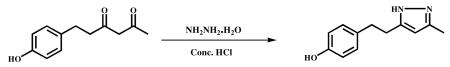
Hydrazine hydrate (0.6 g, 12.13 mmol) and Conc. HCl (catalytic) were added to stirred solution of 6-(4-hydroxypheny)hexane-2,4-dione (1.0 g,4.8 mmol) in ethanol (10 ml) and refluxed for 6 h. After completion of the reaction (monitored by TLC), the mixture was cooled to ambient temperature and the solvent was evaporated in vacuum to obtain the crude product. It was poured into ice cold water and stirred for 1 h. The precipitated material was filtered, dried and recrystallized from water and MeOH. A half-white color solid was obtained (0.86 g, 87%). HPLC purity −99.1%. m.p - 133.4 – 134.6 °C. 1H NMR (400Mz, CDCl3) ) : 6.95 (d,2H), 6.66-6.64 (d,2H), 5.78 (s,1H), 2.79 (s,4H), 2.1 (s,3H). 13C NMR (400Mz, CDCl3):δ ppm 128.4 (C-1′), 127.6 (C-2′), 115.3 (C-3′), 157.3 ( C-4′ ) , 115.3 (C-5′), 127.6 (C-6′ ), 34.3(C-7′), 28.3 (C-8′ ), 157.4 (C-3), 101.2 (C-4), 157.3 (C-5), 12.0 (C-3a). ESI-MS m/z 203 [M+H]+.
3.2.4.9 5-(3,4-dimethoxyphenethyl)-3-methyl-1H-pyrazole (VA-26)
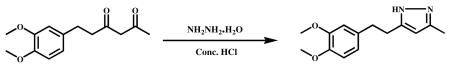
Hydrazine hydrate (0.5 g, 10 mmol) and Conc. HCl (catalytic)) were added to stirred solution of 6-(3,4-dimethoxypheny)hexane--2,4-dione (1.0 g, 4 mmol) in ethanol (10 ml) and refluxed for 6 h. After completion of the reaction (monitored by TLC), the mixture was cooled to ambient temperature and the solvent was evaporated in vacuum to obtain the crude product. It was poured into ice cold water and stirred for 1 h. The precipitated material was filtered, dried and recrystallized from water and MeOH. An off-white color solid was obtained (0.8 g, 90.2%). HPLC purity-98.55%, m.p −70.5-72.2°C. 1H NMR (400Mz, CDCl3) : δ ppm 6.78-6.76 (d,1H), 6.73-6.69 (m,2H), 5.83 (s,1H), 3.84 (s,3H), 3.82 (s,3H), 2.92-2.86 (s,4H),2.26(s,3H). 13C NMR (400Mz, CDCl3):δppm 134.1 (C-1′), 111.8 (C-2′), 147.4 (C-3′), 147.4 ( C-4′ ) , 111.8 (C-5′), 120.3 (C-6′ ), 35.5 (C-7′), 29.3 (C-8′ ), 56.0 (3′-OCH3) , 55.9 (4′-OCH3) ,148.9 (C-3), 103.4 (C-4), 148.8(C-5), 12.3 (C-3a). ESI-MS m/z 247.15 [M+H]+.
3.2.4.10 5-(4-methoxyphenethyl)-3-methyl-1H-pyrazole (VA-27)
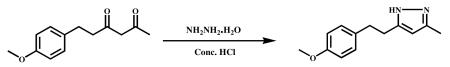
Hydrazine hydrate (0.5 g, 10 mmol) and Conc. HCl (catalytic) were added to stirred solution of 6-(4-methoxypheny)hexane-2,4-dione (1.0 g, 4.5 mmol) in ethanol (10 mL) and refluxed for 6 h. After completion of the reaction (monitored by TLC), the mixture was cooled to ambient temperature and the solvent was evaporated in vacuum to obtain a crude product. It was poured into ice cold water and stirred for 1 h. The precipitated material was filtered, dried and recrystallized from MeOH and water. A half-white solid was obtained (0.8 g, 90%). HPLC purity-99.734%, m.p.-85.2-87.7 °C. 1H NMR (400Mz, CDCl3) ) : δppm 10.24 (s,1H), 7.1 (d,2H), 6.82 (d,2H), 5.84 (s,1H), 3.78 (s,3H), 2.28 (s,3H). 13C NMR (500Mz, CDCl3): δ ppm 133.6 (C-1′), 129.3 (C-2′), 113.9 (C-3′), 147.2 ( C-4′), 113.9 (C-5′), 129.3 (C-6′), 34.9 (C-7′), 29.2 (C-8′ ), 55.3 (3′-OCH3), 158.0 (C-3), 103.3 (C-4), 148.6 (C-5), 12.3 (C-3a). ESI-MS m/z 217.2 [M+H]+.
3.3. Cell lines and culture
The human cancer cell lines including, the colon cancer cell lines HCT-116 and S1 (a clone of LS174T cells), prostatic cancer cell lines DU-145 and PC-3, and the breast cancer cell lines MDA-MB-231 and MCF-7; and the non-cancer cell lines - canine kidney MDCK, mouse fibroblast NIH/3T3, human primary embryonic kidney cell line HEK293/pcDNA3.1 (control, non-cancer cell line transfected with empty vector), the HEK293/ABCB1 cell line, a P-glycoprotein overexpressing cell line, were all obtained from Dr. Gary Kruh’s Laboratory (University of Illinois, Chicago, IL). The cancer and non-cancer cell lines were grown as adherent monolayers in flasks with Dulbecco’s Modified Eagle Medium (DMEM) culture medium supplemented with 10% fetal bovine serum in a humidified incubator containing of 5% CO2 at 37°C.
3.4. Cell cytotoxicity using MTT assay
The MTT (3-(4,5-dimethylthiazole-2yl)-2,5-biphenyltetrazolium bromide) assay [26] was used to determine the cytotoxicity of all the compounds on HCT-116, S1, DU-145, PC-3, MDA-MB-231, and MCF-7 cancer cell lines; and of the non-cancer MDCK, mouse fibroblast NIH/3T3, human primary embryonic kidney HEK293/pcDNA3.1 (control, non-cancer cell line transfected with empty vector) and HEK293/ABCB1 cell lines. Briefly, the cells were harvested with trypsin and resuspended in a final concentration of 5×103 cells/well. Cells were seeded evenly into 96-well multiplates (at 180 μl/well). Different concentrations of compounds, from 0.1 to 100 μM for each compound, were added (20 μl/well) into designated wells. After 72 h of incubation, 20 μl of MTT solution (4 mg/ml) was added to each well, and the plate was further incubated for 4 h, allowing viable cells to convert the yellow-colored MTT into dark-blue formazan crystals. Subsequently, the medium was discarded, and 100 μl of dimethylsulfoxide (DMSO) was added into each well to dissolve the formazan crystals. The absorbance was determined at 570 nm wavelength by an OPSYS microplate Reader from DYNEX Technologies, Inc. (Chantilly, VA, USA). Each mean±SD concentration was calculated from the results of at least three experiments, each also performed in triplicate. The IC50 values (drug candidate concentration required to inhibit growth by 50%) were calculated from survival curves using the Bliss method. The cells were photographed for each triplicate treatment using an inverted microscope (Olympus, BX53F) with fluorescent lamps and digital cameras at 68 h with and without treatment. The images were compared in double-blind fashion for the different concentrations of synthesized compound treatments at different time points for cell size, shape and numbers. A representative figure is shown for each treatment. The data was acquired and analyzed using CellSens software.
3.5. Flow cytometry analysis
The effects of compound VA-2 on mitochondrial membrane potential and apoptosis were measured in colon cancer HCT-116 cells using MitoTracker Red and Alexa Fluor 488 annexin V kit for flow cytometry (Molecular Probes Inc., Invitrogen, Eugene, OR). Briefly, apoptosis was induced by exposing HCT-116 cells (seeded in 6 well plate) with and without Compound VA-2 at 0.5 and 5 μM concentrations for 4 h. Equal amounts of cells were harvested and to each mL of cells, 4 μL of 10 μM Mito Tracker Red working solution was added and stained for 30 minutes at 37°C in an atmosphere of 5% CO2. The cells were washed with PBS and resuspended in 100 μL of 1X annexin-binding buffer to which 5 μL of Alexa Fluor 488 annexin V was added. Cells were incubated for another 15 min, following which 400 μL of 1X annexin-binding buffer was added and mixed gently. The stained cells were analyzed by flow cytometry, measuring the fluorescence emission at 530 nm and 585 nm wavelengths.
3. 6. Statistics
Unless otherwise indicated, all experiments were repeated at least three times, and the differences between the mean effects produced by the drug candidates in the cancer cell lines compared to the mean effect in the control non-cancer cell lines or no-treatment controls were determined using the Student’s t-test (GraphPad PRISM version 5.04). Results are presented as means ±standard deviations (SD). Statistical significance was determined at the p < 0.05 level.
Acknowledgements
Our profound gratitude to Ms. Ciembra Rice (TU) for technical assistance. This study was supported by Tuskegee RCMI Core grant number (G12MD007585-23); MSM/TU/UAB Comprehensive Cancer Center Partnership [NCI] (U54 CA118623) grant to Dr. Tiwari. We also gratefully acknowledge the support provided by the Directors of Natsol Laboratories Private Limited.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Authors declare no conflict of interest.
REFERENCES
- [1].Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- [2].Tiwari AK, Sodani K, Dai CL, Ashby CR, Jr., Chen ZS. Revisiting the ABCs of multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12:570–594. doi: 10.2174/138920111795164048. [DOI] [PubMed] [Google Scholar]
- [3].Chen ZS, Tiwari AK. Multidrug resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic diseases. FEBS J. 2011;278:3226–3245. doi: 10.1111/j.1742-4658.2011.08235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Newman DJ, Cragg GM. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar Drugs. 2014;12:255–278. doi: 10.3390/md12010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen W, Zhao Z, Li L, Wu B, Chen SF, Zhou H, Wang Y, Li YQ. Hispolon induces apoptosis in human gastric cancer cells through a ROS-mediated mitochondrial pathway. Free Radic Biol Med. 2008;45:60–72. doi: 10.1016/j.freeradbiomed.2008.03.013. [DOI] [PubMed] [Google Scholar]
- [6].Mo S, Wang S, Zhou G, Yang Y, Li Y, Chen X, Shi J. Phelligridins C-F: cytotoxic pyrano[4,3-c][2]benzopyran-1,6-dione and furo[3,2-c]pyran-4-one derivatives from the fungus Phellinus igniarius. Journal of natural products. 2004;67:823–828. doi: 10.1021/np030505d. [DOI] [PubMed] [Google Scholar]
- [7].Ali NA, Ludtke J, Pilgrim H, Lindequist U. Inhibition of chemiluminescence response of human mononuclear cells and suppression of mitogen-induced proliferation of spleen lymphocytes of mice by hispolon and hispidin. Die Pharmazie. 1996;51:667–670. [PubMed] [Google Scholar]
- [8].Yang LY, Shen SC, Cheng KT, Subbaraju GV, Chien CC, Chen YC. Hispolon inhibition of inflammatory apoptosis through reduction of iNOS/NO production via HO-1 induction in macrophages. Journal of ethnopharmacology. 2014;156:61–72. doi: 10.1016/j.jep.2014.07.054. [DOI] [PubMed] [Google Scholar]
- [9].Huang GJ, Deng JS, Chiu CS, Liao JC, Hsieh WT, Sheu MJ, Wu CH. Hispolon Protects against Acute Liver Damage in the Rat by Inhibiting Lipid Peroxidation, Proinflammatory Cytokine, and Oxidative Stress and Downregulating the Expressions of iNOS, COX-2, and MMP-9. Evidence-based complementary and alternative medicine : eCAM. 2012;2012:480714. doi: 10.1155/2012/480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang J, Hu F, Luo Y, Luo H, Huang N, Cheng F, Deng Z, Deng W, Zou K. Estrogenic and anti-estrogenic activities of hispolon from Phellinus lonicerinus (Bond.) Bond. et sing. Fitoterapia. 2014;95:93–101. doi: 10.1016/j.fitote.2014.03.007. [DOI] [PubMed] [Google Scholar]
- [11].Chen T, Wong YS. Selenocystine induces S-phase arrest and apoptosis in human breast adenocarcinoma MCF-7 cells by modulating ERK and Akt phosphorylation. Journal of agricultural and food chemistry. 2008;56:10574–10581. doi: 10.1021/jf802125t. [DOI] [PubMed] [Google Scholar]
- [12].Lu TL, Huang GJ, Lu TJ, Wu JB, Wu CH, Yang TC, Iizuka A, Chen YF. Hispolon from Phellinus linteus has antiproliferative effects via MDM2-recruited ERK1/2 activity in breast and bladder cancer cells. Food Chem Toxicol. 2009;47:2013–2021. doi: 10.1016/j.fct.2009.05.023. [DOI] [PubMed] [Google Scholar]
- [13].Hsieh MJ, Chien SY, Chou YE, Chen CJ, Chen J, Chen MK. Hispolon from Phellinus linteus possesses mediate caspases activation and induces human nasopharyngeal carcinomas cells apoptosis through ERK1/2, JNK1/2 and p38 MAPK pathway. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2014;21:1746–1752. doi: 10.1016/j.phymed.2014.07.013. [DOI] [PubMed] [Google Scholar]
- [14].Wu Q, Kang Y, Zhang H, Wang H, Liu Y, Wang J. The anticancer effects of hispolon on lung cancer cells. Biochemical and biophysical research communications. 2014;453:385–391. doi: 10.1016/j.bbrc.2014.09.098. [DOI] [PubMed] [Google Scholar]
- [15].Ravindran J, Subbaraju GV, Ramani MV, Sung B, Aggarwal BB. Bisdemethylcurcumin and structurally related hispolon analogues of curcumin exhibit enhanced prooxidant, anti-proliferative and anti-inflammatory activities in vitro. Biochem Pharmacol. 2010;79:1658–1666. doi: 10.1016/j.bcp.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Venkateswarlu S, Ramachandra MS, Sethuramu K, Subbaraju GV. Synthesis and antioxidant activity of hispolon, a yellow pigment from Inonotus hispidius. Indian Journal of Chemistry. 2002;41:875–877. [Google Scholar]
- [17].Lin L, Shi Q, Nyarko AK, Bastow KF, Wu CC, Su CY, Shih CC, Lee KH. Antitumor agents. 250. Design and synthesis of new curcumin analogues as potential anti-prostate cancer agents. Journal of medicinal chemistry. 2006;49:3963–3972. doi: 10.1021/jm051043z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Flynn DL, Belliotti TR, Boctor AM, Connor DT, Kostlan CR, Nies DE, Ortwine DF, Schrier DJ, Sircar JC. Styrylpyrazoles, styrylisoxazoles, and styrylisothiazoles. Novel 5-lipoxygenase and cyclooxygenase inhibitors. Journal of medicinal chemistry. 1991;34:518–525. doi: 10.1021/jm00106a006. [DOI] [PubMed] [Google Scholar]
- [19].Claramunt RM, Bouissane L, Cabildo MP, Cornago MP, Elguero J, Radziwon A, Medina C. Synthesis and biological evaluation of curcuminoid pyrazoles as new therapeutic agents in inflammatory bowel disease: effect on matrix metalloproteinases. Bioorganic & medicinal chemistry. 2009;17:1290–1296. doi: 10.1016/j.bmc.2008.12.029. [DOI] [PubMed] [Google Scholar]
- [20].Bouissane L, Cabildo P, Cornago P, Claramunt RM. Curcuminoid pyrazoles: Synthesis and structural properties. International Conference on synthetic Organic Chemistry. 2006 [Google Scholar]
- [21].Feng JY, Liu ZQ. Feruloylacetone as the model compound of half-curcumin: synthesis and antioxidant properties. European journal of medicinal chemistry. 2011;46:1198–1206. doi: 10.1016/j.ejmech.2011.01.039. [DOI] [PubMed] [Google Scholar]
- [22].Ley JP, Paetz S, Blings M, Hoffmann-Lucke P, Bertram HJ, Krammer GE. Structural analogues of homoeriodictyol as flavor modifiers. Part III: short chain gingerdione derivatives. Journal of agricultural and food chemistry. 2008;56:6656–6664. doi: 10.1021/jf8006536. [DOI] [PubMed] [Google Scholar]
- [23].Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- [24].Cao A, Li Q, Yin P, Dong Y, Shi H, Wang L, Ji G, Xie J, Wu D. Curcumin induces apoptosis in human gastric carcinoma AGS cells and colon carcinoma HT-29 cells through mitochondrial dysfunction and endoplasmic reticulum stress. Apoptosis : an international journal on programmed cell death. 2013;18:1391–1402. doi: 10.1007/s10495-013-0871-1. [DOI] [PubMed] [Google Scholar]
- [25].Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- [26].Carmichael GG. Application of the MTT-hydroquinone reaction in the study of hard tissue. Journal of anatomy. 1970;106:194. [PubMed] [Google Scholar]