Abstract
Chronic Hepatitis C virus (HCV) infection pre-disposes patients to develop liver failure after acetaminophen (APAP) overdose. Mechanisms involved in this were explored using transgenic mice expressing the HCV structural proteins core, E1 and E2. Treatment of C57BL/6J mice with 200 mg/kg body weight APAP resulted in significant liver injury at 6 h as indicated by elevated ALT levels, focal centrilobular necrosis and nuclear DNA fragmentation. HCV transgenic mice showed a variable response, with approximately half the animals showing exacerbation of all parameters of liver injury while the other half was protected. HCV transgenic mice with higher liver injury had lower liver glutathione levels, elevated mitochondrial oxidative stress, and enhanced release of apoptosis inducing factor (AIF) from the mitochondria. This was accompanied by induction of a higher ER stress response and induction of autophagy. Transgenic animals showing protection against liver injury had a robust recovery of liver glutathione content at 6 hours when compared to wild type animals, accompanied by reduction of mitochondrial oxidative stress and AIF release. This was accompanied by an elevation in glutathione S transferase mRNA levels and activity, which suggests that an efficient clearance of the reactive intermediate may contribute to the protection against APAP hepatotoxicity in these mice. These results demonstrate that while HCV infection could exacerbate APAP induced liver injury due to induction and amplification of mitochondrial oxidant stress it could also protect against injury by activation of APAP scavenging mechanisms.
Keywords: HCV, mitochondria, drug hepatotoxicity, acetaminophen, oxidant stress
INTRODUCTION
Acetaminophen (APAP) is one of the most common drugs used worldwide and is safe at therapeutic doses. APAP metabolism generates a reactive intermediate, N-acetyl-p-quinone imine (NAPQI), which is effectively scavenged by the glutathione system in the liver after therapeutic doses of the drug. However, it is well recognized that an overdose of APAP can result in severe liver injury in both experimental animals and man (Larson, 2007; McGill et al., 2012). The injury process after an overdose is initiated by generation of large amounts of NAPQI, which overwhelm the scavenging capability of glutathione (Mitchell et al., 1973). NAPQI generation results in the subsequent formation of NAPQI-protein adducts, especially on mitochondrial proteins, which has been shown to be a critical feature of APAP induced hepatotoxicity (Nelson, 1990; McGill and Jaeschke, 2013). Mitochondrial protein adduct formation triggers a cascade of events, including mitochondrial oxidant stress (Jaeschke, 1990; Knight et al., 2001) and activation of the c-jun N-terminal kinase (JNK) (Hanawa et al., 2008), which further amplify the mitochondrial oxidant stress (Saito et al., 2010a), resulting in induction of the mitochondrial permeability transition (MPT) pore opening (Kon et al., 2004; Masubuchi et al., 2005; Reid et al., 2005; Ramachandran et al., 2011). This in turn facilitates the release of mitochondrial intermembrane proteins such as apoptosis inducing factor (AIF) and endonuclease G, which translocate to the nucleus and induce DNA fragmentation, resulting in the subsequent hepatocyte necrosis ((Bajt et al., 2006; 2008; 2011). Thus, mitochondrial dysfunction is central to the pathophysiology of APAP-induced cell death in animals (Jaeschke et al., 2012) and humans (McGill et al., 2012).
Acetaminophen overdose is the leading cause of liver failure in the Western world (Larson et al., 2005) and recent epidemiological studies examining predisposition to APAP-induced liver failure in humans demonstrate that patients with Hepatitis C virus (HCV) infection have a significant predisposition to develop liver failure after APAP overdose (Nguyen et al., 2008). HCV is a hepatotropic pathogen of significant importance to public health, chronically infecting an estimated 130 million people worldwide (Ploss and Rice, 2009). Chronic HCV infection leads to the development of cirrhosis and hepatocellular carcinoma, making HCV infection one of the most common causes of liver transplantation (Brown, 2005). Understanding the molecular basis for the increased susceptibility of HCV infected patients to APAP overdose thus has significant clinical importance.
HCV infection has been shown to have significant consequences on organelle function in the hepatocyte. Transgenic mice expressing HCV proteins have been shown to have impaired function of the mitochondrial respiratory chain, resulting in overproduction of ROS, and also compromised antioxidant systems (Moriya et al., 2001; Okuda et al., 2002). In addition, the HCV core protein has been shown to localize to the endoplasmic reticulum (ER) and induce ER stress in cells (Benali-Furet et al., 2005; Wang and Weinman, 2006) and transgenic mice expressing the HCV structural proteins core, E1 and E2 demonstrate impair mitochondrial respiratory function (Korenaga et al 2005). In light of the fact that mitochondrial function is compromised after HCV infection and is a critical determinant of APAP-induced hepatocyte injury, we explored the role of this organelle in amplifying APAP-induced injury in HCV transgenic mice expressing the HCV structural proteins in the liver.
MATERIALS and METHODS
Animals
The SL-139 strain of HCV structural protein expressing mice, which are on a C57Bl/6 background, along with control C57Bl/6 mice, were used in this study (Korenaga et al., 2005). Since HCV infection is usually chronic, older mice (18 ± 2.5 weeks, mean ± SEM) were used for these studies. All animals were housed in an environmentally controlled room with 12 h light/dark cycle and allowed free access to food and water. The experimental protocol was approved by the Institutional Animal Care and Use Committees of the University of Kansas Medical Center and followed the criteria of the National Research Council for the care and use of laboratory animals in research. All chemicals were purchased from Sigma Chemical Co. (St Louis, MO, USA) unless stated otherwise.
Experimental Design
Mice were injected intra-peritoneally with 200 mg/kg APAP (dissolved in warm saline) after overnight fasting. The animals were killed 20 min or 6 h after APAP treatment and blood was withdrawn from the vena cava into a heparinized syringe for measurement of alanine aminotransferase (ALT) activities (Kinetic Test Kit 68-B, Biotron Diagnostics, Inc., Hernet, CA, USA). The liver was removed and was rinsed in saline; liver sections were fixed in 10% phosphate-buffered formalin for histological analyses. A portion of the liver was used for mitochondrial isolation and the remaining liver was snap-frozen in liquid nitrogen and stored at −80 °C.
Histology and immunohistochemistry
Formalin-fixed tissue samples were embedded in paraffin and 4 μm sections were cut. Replicate sections were stained with hematoxylin and eosin (H&E) for evaluation of necrosis (Gujral et al., 2002). For the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay, sections of liver were stained with the In Situ Cell Death Detection Kit, AP (Roche Diagnostics, Indianapolis, IN) as described in the manufacturer's instructions (Gujral et al., 2002).
Measurement of GSH and glutathione S transferase activity
Total soluble GSH was measured in the liver homogenate with a modified method of Tietze as described in detail (Knight et al., 2001). Briefly, the frozen tissue was homogenized at 0 °C in 3% sulfosalicylic acid containing 0.1 mM EDTA. The samples were assayed using dithionitrobenzoic acid. All data are expressed in GSH-equivalents. Glutathione S-Transferase activity was measured by the increase in absorbance at 340nm on conjugation of 1-chloro-2,4-dinitrobenzene (CDNB) with reduced glutathione as described (Habig et al., 1974).
Isolation of subcellular fractions and western blotting
Mitochondria and cytosolic fractions were isolated as described (Cover et al., 2005). Briefly, the liver was homogenized in ice cold isolation buffer (pH7.4) containing 220 mM mannitol, 70 mM sucrose, 2.5 mM HEPES, 10 mM EDTA, 1 mM EGTA, and 0.1% bovine serum albumin. Mitochondria were isolated by differential centrifugation (20,000 xg) and washed with 2 ml of isolation buffer. The 20,000 xg supernatant was used to evaluate the release of mitochondrial factors such as apoptosis-inducing factor (AIF) by western blotting. Western blotting was carried out as described in detail (Bajt et al., 2000) using the following antibodies: a rabbit anti-AIF antibody (Abcam, Cambridge, MA), a rabbit anti-Cyp2E1 polyclonal antibody (Abcam, Cambridge, MA), a mouse anti-Bcl-xl monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), a rabbit anti-LC-3 polyclonal antibody (MBL, Naka-ku Nagoya, Japan), a mouse anti-GADD 153/CHOP monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), a rat anti-Grp 78 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and a mouse monoclonal anti-metallothionein antibody (DAKO Corp., Carpinteria, CA). A horseradish peroxidase-coupled anti-rabbit IgG (Santa Cruz) was used as secondary antibody. Proteins were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech. Inc., Piscataway, NJ).
Redox western blotting for Thioredoxin-2
For detection of oxidized and reduced forms of thioredoxin 2, mitochondria were resuspended in 20 mM Tris, pH 8.0, and 50 μg of the mitochondrial fraction were incubated with AMS (4-acetoamido-4-maleimidylstilbene-2,2-disulfonic acid, Molecular Probes) at a final concentration of 15 mM for 3 h and subsequently analyzed by 16% SDS-PAGE and Western blot using an anti-Thioredoxin 2 antibody (Abcam).
RNA analysis
Mouse Stress and Toxicity PathwayFinder (Cat no# PAMM-003E-4) PCR array was purchased from SA Biosciences (Qiagen), and was used according to the manufacturer's instructions. For real time PCR analysis, expression of B-cell lymphoma-extra large (Bcl-xl) and GST m3 mRNA was quantified using qRT-PCR analysis as previously described (Williams, et al., 2010) using the following primers – BCL-XL Fwd: GCTGGGACACTTTTGTGGAT, Rev: TGTCTGGTCACTTCCGACTG. GST-mu-3 Fwd: CACCCGCATACAGCTCATGAT, Rev: TTCTCAGGGATGGCCTTCAA. Briefly, total RNA was reversed transcribed with M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA) and oligo-dT primers (ABI Primer Express software, Foster City, CA). The SYBR green PCR Master Mix (Applied Biosystems, Foster City, CA) was used for real-time PCR analysis. The relative differences in expression between groups were expressed using cycle time (Ct) values. Ct values for the gene was first normalized with that of β-actin in the same sample, and then relative differences between groups were expressed as relative increases setting control as 1.
Statistics
The Shapiro-Wilk test was used to assess normality. For normally distributed data, one-way analysis of variance (ANOVA) was performed to test for significance, with Student-Newman-Keuls post-hoc comparison between groups. For non-normally distributed data, ANOVA was performed on ranks, followed by Dunn's multiple comparisons. For all tests, P<0.05 was considered significant.
RESULTS
HCV core protein expression and APAP-induced liver injury
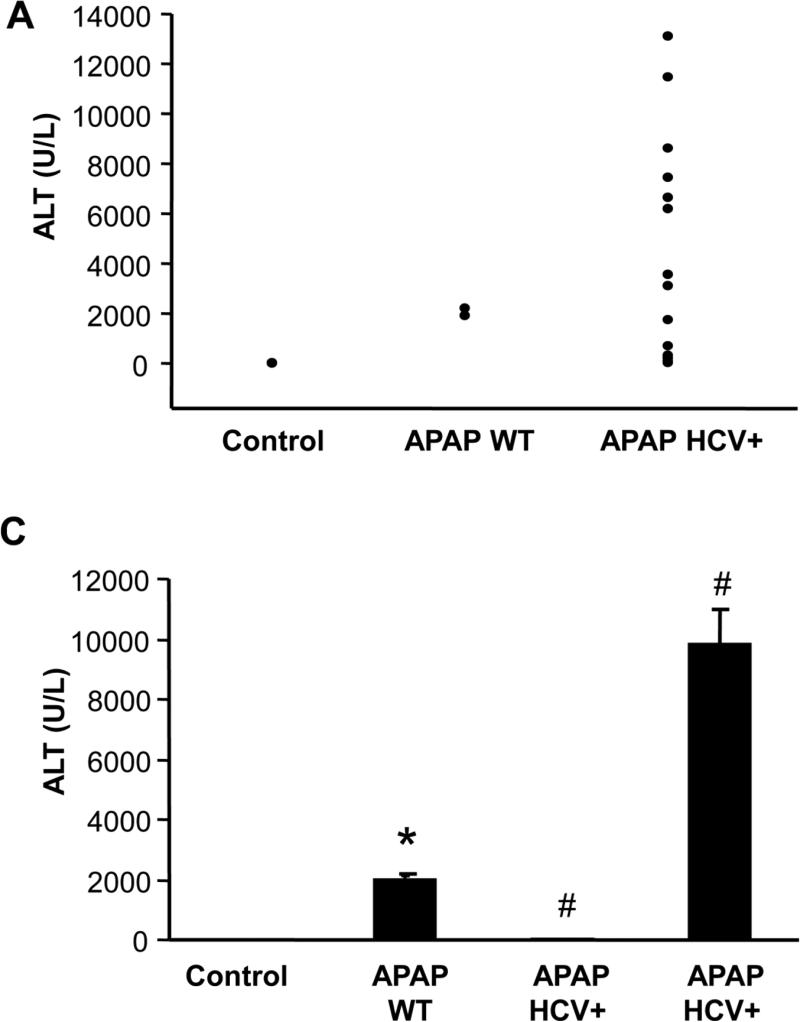
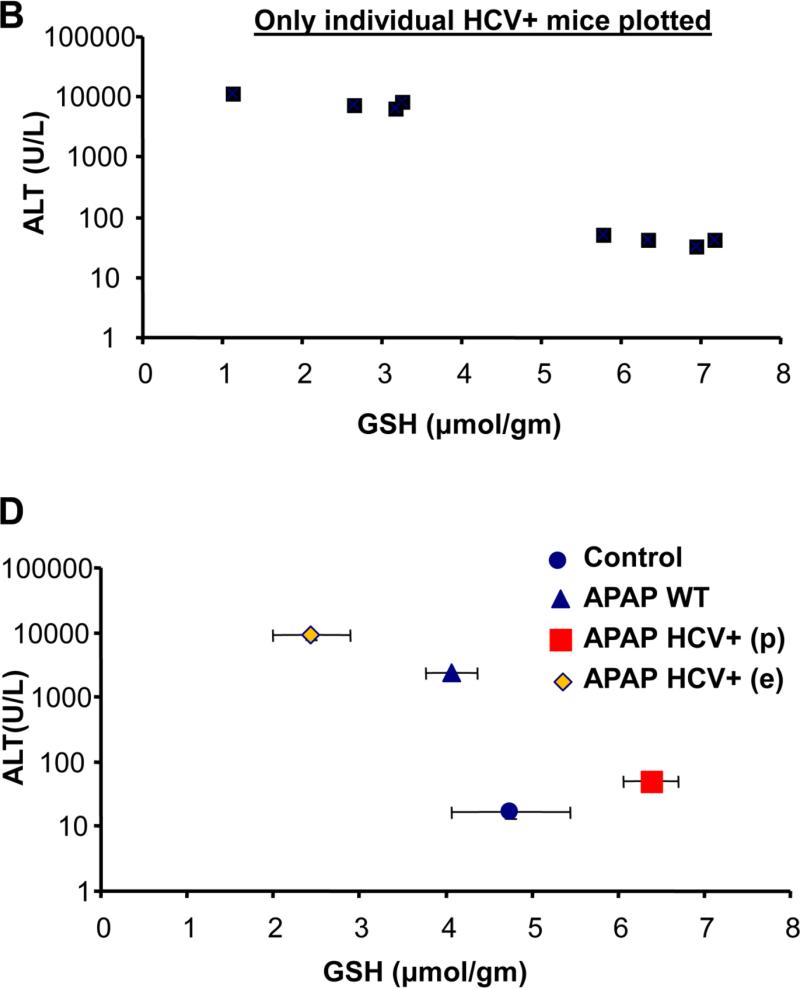
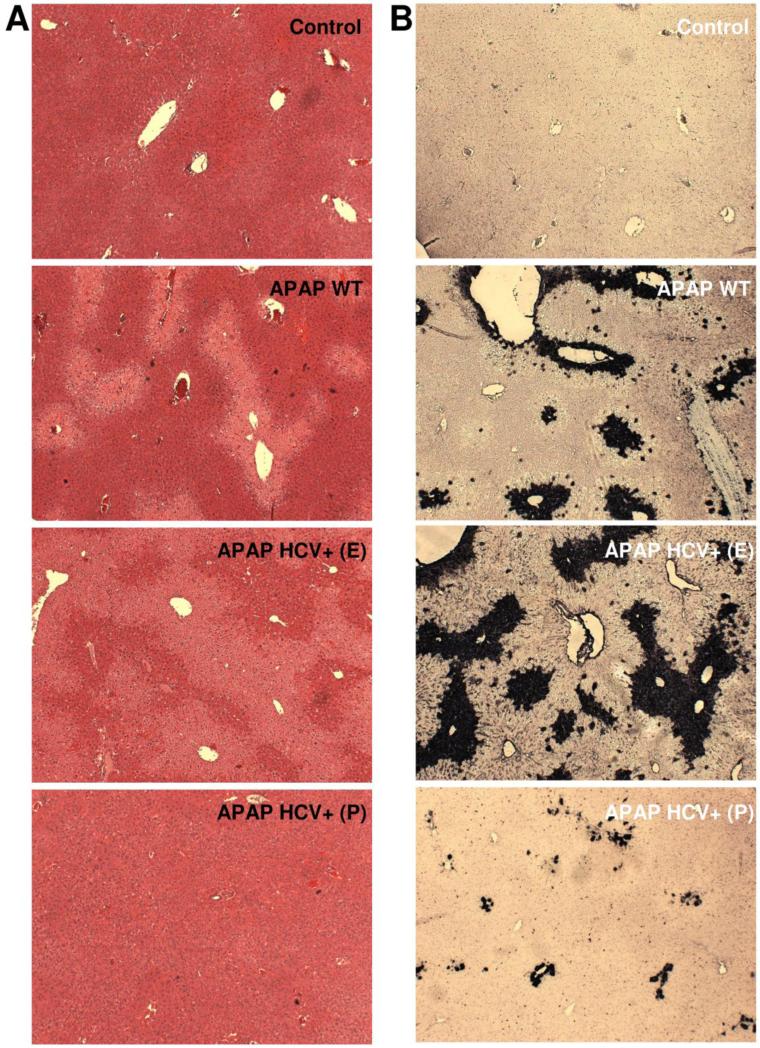
To evaluate the potential role of hepatocellular HCV protein expression on APAP hepatotoxicity, a moderate overdose of 200 mg/kg was selected. APAP induced significant liver injury at 6 h as indicated by the increase in plasma ALT activities in wild type mice (Figure 1A). Interestingly however, HCV protein expressing mice showed a wide range of ALT activities. Some of the values are clearly below WT levels and some are substantially above (Figure 1A). If the ALT value of each individual HCV core protein expressing animal was plotted against their hepatic GSH level, the animals separated into 2 distinct groups (Figure 1B). The difference to untreated controls and APAP WT animals is also obvious (Figure 1C, D). The results from the ALT activities were confirmed by both histology (Figure 2A) and TUNEL staining (Figure 2B), which showed significantly lower cell necrosis and DNA fragmentation in HCV protein expressing animals that had lower plasma ALT activities. In contrast, animals showing exacerbated injury had much higher cell necrosis and DNA fragmentation than wild type animals (Figure 2 A,B).
Figure 1.
(A) ALT levels in plasma from individual wild type (WT) and HCV transgenic mice 6 hours after APAP treatment (200mg/kg) (n=3 for Control and APAP WT and 12 for HCV core protein transgenic mice). (B) ALT values of individual HCV transgenic mice were plotted against their respective liver GSH content. (C) ALT data represented as mean ± SE for wild type and HCV transgenic mice showing protection (p) and exacerbation (e) of liver injury after APAP overdose. *P<0.05 (compared to WT Control; #P<0.05 (compared to WT APAP). (D) Mean of ALT values are plotted against mean ± SE of GSH values of the 4 different groups.
Figure 2.
Representative liver sections stained with H&E (A) and the TUNEL assay (B) in wild type controls and HCV transgenic mice 6 hours after treatment with APAP (200 mg/kg). HCV transgenic mice with exacerbated injury (HCV+E) or protection (HCV+P) are indicated.
HCV protein expression and APAP metabolic activation
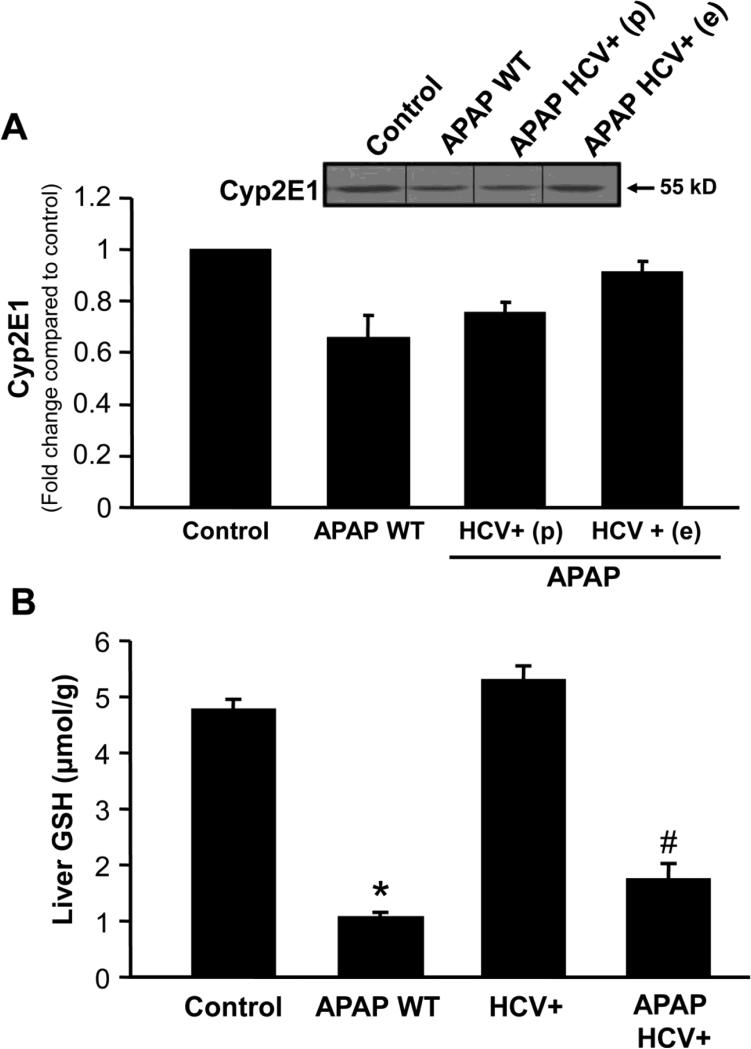
A recent study suggested that down-regulation of cytochrome P450 activity, especially Cyp2e1, by viral infection could result in modulation of APAP toxicity (Getachew et al., 2010). However, cytochrome P450 protein levels were similar in both HCV transgenic mice which were protected as well as those which showed aggravated damage (Figure 3A). Liver glutathione levels were also similarly depleted in all HCV transgenic animals tested 20 minutes after APAP treatment (Figure 3B), confirming that changes in metabolism of APAP were not contributing to these effects. These effects could also not be explained by alterations in levels of HCV core protein being expressed within liver mitochondria (data not shown).
Figure 3.
(A) Cyp 2E1 protein levels in wild type and HCV transgenic mice 6 hours after APAP treatment (200 mg/kg). The lower histogram illustrates densitometric data from western blots. Values are mean ± SE of ≥3 mice per group. (B) Liver glutathione content in wild type and HCV transgenic mice 20 minutes after APAP treatment (200mg/kg). Values are mean ± SE of at least 3 mice per group. *P<0.05 (compared to WT Control; #P<0.05 (compared to WT APAP).
Mitochondrial oxidant stress and dysfunction in HCV core protein expressing mice
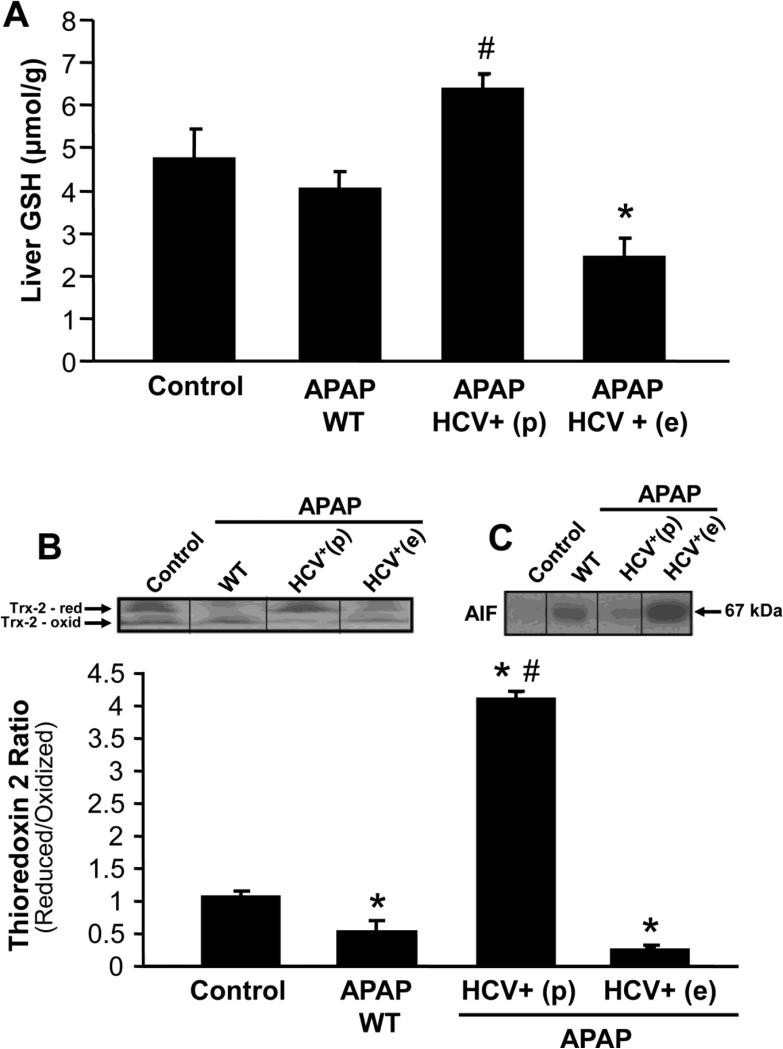
Further experiments were then carried out to elucidate potential mechanisms behind the aggravated injury as well as protection against APAP-hepatotoxicity in HCV transgenic animals. Measurement of liver glutathione levels 6 hours after APAP administration indicated that in wild type animals GSH levels had completely recovered (Figure 4A). However, in HCV transgenic mice with aggravated injury, GSH levels were significantly lower than both APAP treated wild type as well as untreated control animals (Figure 4A). Mitochondrial oxidative stress has been implicated in APAP-induced hepatotoxicity and thioredoxin-2 (Trx-2) is a mitochondrial anti-oxidant enzyme whose oxidation is an indication of mitochondrial oxidative stress (Zhang et al., 2007). Redox western blotting of Trx-2 showed that APAP treatment resulted in a significant decrease in the ratio of reduced-to-oxidized protein indicative of mitochondrial oxidative stress in wild type and HCV transgenic mice with higher injury (Figure 4B). In contrast, the protected HCV transgenic mice showed a drastically enhanced reduced-to-oxidized ratio of Trx-2 (Figure 4B). APAP-induced mitochondrial oxidative stress induces translocation of apoptosis-inducing factor (AIF) from the mitochondria to the nucleus and subsequent DNA fragmentation and cell necrosis (Bajt et al., 2006; 2011). Treatment of wild type animals with APAP resulted in in AIF release from mitochondria into the cytosol (Figure 4C). However, this was further enhanced in HCV transgenic mice with higher injury and prevented in the protected animals (Figure 4C).
Figure 4.
(A) Liver glutathione content in wild type and HCV transgenic mice 6 hours after APAP treatment (200 mg/kg). Values are mean ± SE of 3-5 mice per group. (B) Representative redox western blot for mitochondrial thioredoxin 2, 6 hours after APAP treatment, and its densitometric quantitation. Values are mean ± SE of 3-5 mice per group. (C) Western blot for apoptosis-inducing factor (AIF) in the cytosol 6 hours after APAP treatment in wild type control and HCV transgenic mice. *P<0.05 (compared to WT Control; #P<0.05 (compared to WT APAP).
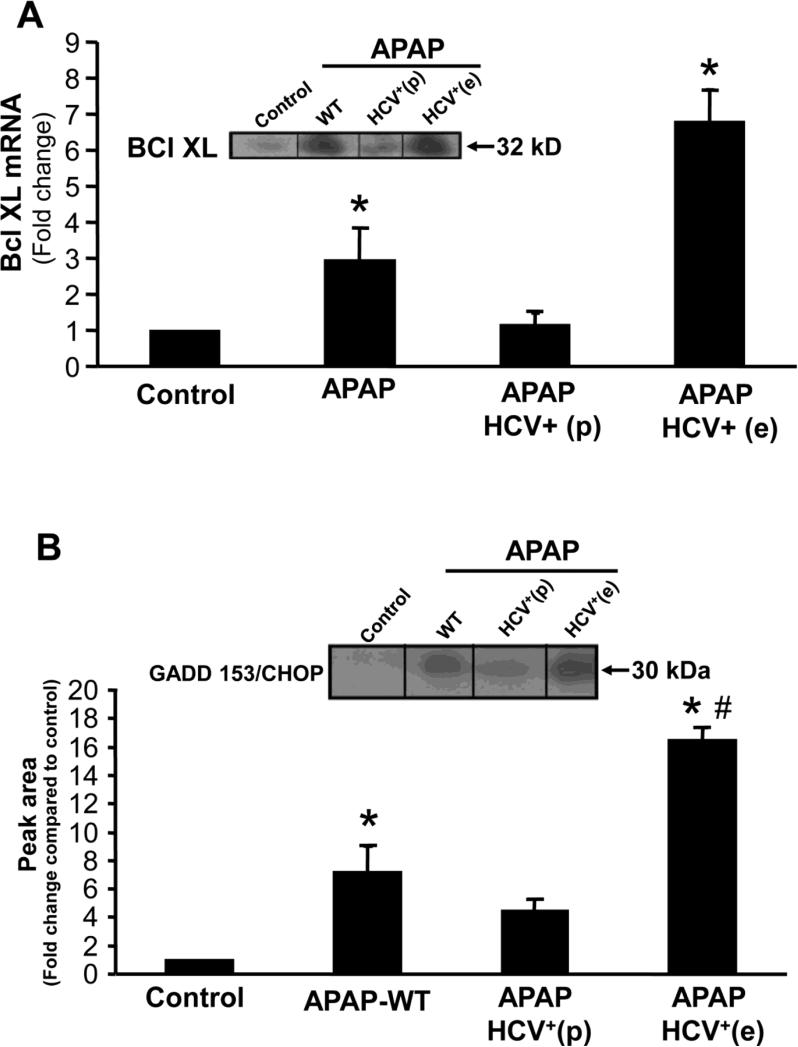
To evaluate changes in gene expression which could result in modulation of the response to APAP, a PCR array was initially carried out to examine mRNA levels of 84 genes in the samples. Paradoxically, HCV transgenic mice which had aggravation of injury showed elevated mRNA levels of Bcl-xL (Figure 5A) an anti-apoptotic member of the Bcl-2 family. This increase was confirmed by western blotting of mitochondrial proteins from these animals (Figure 5A). In contrast, in the protected animals Bcl-xL mRNA and protein induction was prevented (Figure 5A).
Figure 5.
(A) Quantitation of Bcl-xL mRNA and a representative western blot (insert) of Bcl-xL protein in wild type controls and HCV transgenic mice, 6 hours after APAP (200 mg/kg). Values are mean ± SE of ≥ 3 mice per group. (B) A representative western blot and densitometric quantitation of GADD153/CHOP protein in wild type and HCV transgenic mice 6 hours after APAP (200 mg/kg). Values are mean ± SE of ≥3 mice per group. *P<0.05 (compared to control), #P<0.05 (compared to APAP-WT)
ER stress and autophagy in HCV core protein overexpressing mice
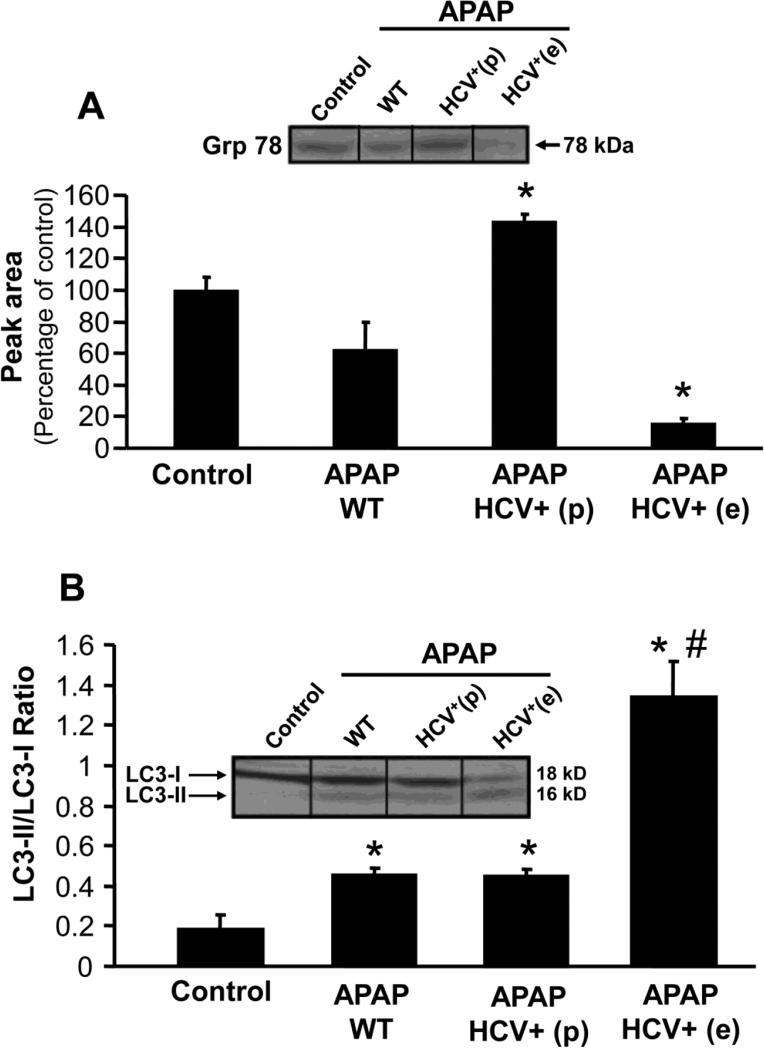
Cells overexpressing Bcl-xL and subjected to ER stress die by necrosis subsequent to induction of autophagy (Ullman et al., 2008). Thus, ER stress and autophagy was investigated in HCV transgenic mice. Treatment of wild type mice with APAP resulted in an upregulation of the ER stress marker GADD153/CHOP (Figure 5B). However, this was significantly more elevated in HCV transgenic mice which showed exacerbated liver injury and attenuated in animals that were protected (Figure 5B). This increase in GADD153/CHOP protein was accompanied by a decrease in Grp78 levels, again to a much greater extent in the HCV transgenic mice with higher injury when compared to wild type animals (Figure 6A). Again, the loss of Grp-78 was prevented in the HCV transgenic mice (Figure 6A). Further experiments were then carried out to evaluate the role of autophagy in the aggravated response to APAP in HCV transgenic mice.
Figure 6.
(A) A representative western blot and densitometric quantitation of Grp78 protein in wild type and HCV transgenic mice 6 hours after APAP (200 mg/kg). Values are mean ± SE of ≥3 mice per group. *P<0.05 (compared to control). (B) Quantitation of LC3-I and LC3-II protein in liver from wild type and HCV transgenic mice 6 hours after APAP, with a representative western blot (insert). Values are mean ± SE of ≥3 mice per group. *P<0.05 (compared to control), #P<0.05 when compared to APAP-WT)
Conversion of the microtubule-associated protein 1A/1B-light chain 3 (LC3) – I to II has been used as a marker of autophagy in general (Tanida et al., 2008) and for enhanced autophagy after APAP overdose (Ni et al., 2012a). A significant increase in the ratio of LC3 II/I was seen in wild type animals exposed to APAP compared to controls (Figure 6B); HCV transgenic mice with higher injury showed a much pronounced elevation in the ratio indicative of higher induction of autophagy or suppression of LC3II degradation in lysosomes.
The data so far indicate that in HCV transgenic mice with aggravated injury compared to wild type animals, there is an increased Bcl-xL expression in mitochondria, higher induction of the ER stress response and enhanced autophagy. In contrast, in the animals that showed protection, there was increased recovery of hepatic GSH levels (Figure 4A) not only compared to HCV core protein overexpressing animals with higher injury but also compared to APAP-treated wild type animals. In addition, there was a more reduced-to-oxidized ratio for mitochondrial Trx-2 (Figure 4B), less mitochondrial AIF release (Figure 4C), less mitochondrial Bcl-xL expression (Figure 5A), less induction of the ER stress gene GADD153/Chop (Figure 5B) and reduced loss of Grp78 (Figure 6A). However, the ratio of LC II/I in HCV transgenic mice protected against injury was similar to that seen in wild type mice (Figure 6B).
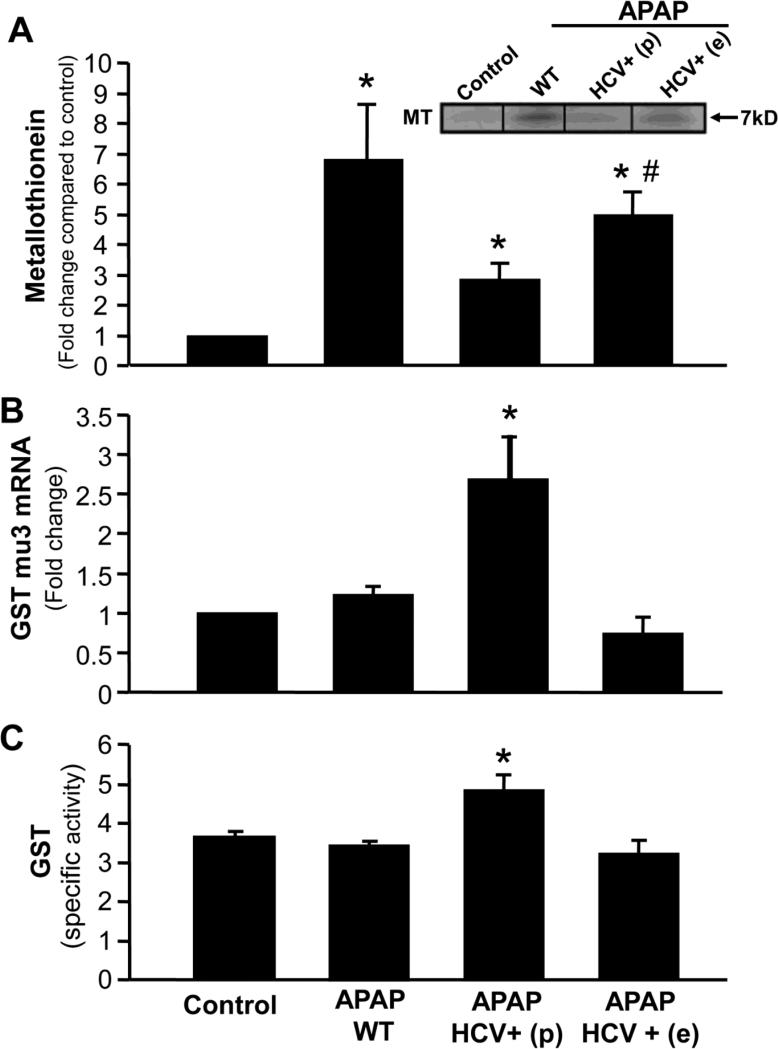
We have earlier demonstrated that metallothionein induction can influence APAP hepatotoxicity presumably by scavenging NAPQI after GSH is exhausted (Saito et al., 2010b). To determine if this could play a role in protection against APAP hepatotoxicity, metallothionein protein levels were measured. APAP treatment resulted in an increase in metallothionein levels in wild type animals as well as HCV transgenic mice, though levels were not as high as in the wild type animals (Figure 7A). The PCR array for 84 genes carried out to elucidate global alterations in protein expression induced by APAP in the HCV transgenic mice also indicated that HCV transgenic animals which were protected against APAP hepatotoxicity, showed an elevation of mRNA for glutathione S transferase (GST) mu-3 (Figure 7B). This elevation in gene expression had a functional effect, where activity of GST was significantly elevated in the HCV transgenic animals when compared to wild type animals and controls (Figure 7C).
Figure 7.
(A) Quantitation of metallothionein protein in liver from wild type and HCV transgenic mice 6 hours after APAP (200 mg/kg), with a representative western blot (insert). Values are mean ± SE of ≥3 mice per group. *P<0.05 (compared to control), #P<0.05 (compared to APAP-WT). (B) Quantitation of GST mu3 mRNA levels in liver from wild type and HCV transgenic mice 6 hours after APAP (200 mg/kg). Values are mean ± SE of at least 3 mice per group. (C) Glutathione S Transferase activity in liver homogenates from wild type and HCV transgenic mice 6 hours after APAP. Values are mean ± SE of ≥3 mice per group. *P<0.05 (compared to APAP control)
DISCUSSION
Effect of HCV core protein on APAP hepatotoxicity
The hypothesis that HCV core protein over-expressing mice would be more susceptible to APAP induced hepatotoxicity was rather straightforward based on the earlier data showing mitochondrial dysfunction in HCV transgenic mice (Korenaga M. et al., 2005), the critical role of mitochondria in APAP induced liver injury (Jaeschke et al., 2012) and epidemiological data indicating increased susceptibility of HCV infected humans to APAP-induced liver failure (Nguyen et al., 2008). However, the data from the initial experiments in transgenic mice were rather intriguing, since a significant variation in response to APAP induced liver damage was evident among the animals. In this scenario, it could be argued that these variations could be due to the animal-to-animal variation seen during experimentation, and the average response (namely no effect) would be the best interpretation. Consistent with this conclusion, a recently published study conducted in parallel with ours observed no difference in liver injury between wild type and HCV transgenic mice 24 h after oral doses of 300-500 mg/kg APAP in fed animals (Uehara et al., 2013). However, when using the same dose as we did (200 mg/kg) a moderate aggravation of injury was observed in fasted animals (Uehara et al., 2013). Since the authors also observed an increased mitochondrial stress, even in animals without enhanced injury, it appears that the dose or use of fed versus fasted mice may not explain the different results. However, the fact that our mice were slightly older, i.e., longer exposed to the chronic stress of the HCV core protein-mediated mitochondrial dysfunction, may have contributed to the increased susceptibility and adaptation.
As we have recently demonstrated using conditional Atg5-deficient animals, which have impaired autophagy, animals exposed to chronic stress can be completely resistant to APAP-induced liver injury (Ni et al., 2012b). Likewise, conditional Atg7-deficient mice do not show any stress during the first few weeks but still respond to APAP with apoptotic cell death rather than necrosis (Igusa et al., 2012; Jaeschke and Ding, 2012). Thus, it is feasible that the chronic, low level mitochondrial stress of HCV core protein overexpression leads to adaptive responses, which make animals more resistant to the subsequent insults, i.e. APAP overdose. The fact that not all animals show this adaptation would indicate that this mild stress does not trigger a uniform response in all mice. Unfortunately, the nature of the response (protection versus aggravation of injury) is only obvious after challenging the animals, which makes it impossible to predict which animals fall into which category. As a result, we were unable to find a difference in untreated animals that could explain the subsequent response. Even a PCR array looking at 84 different genes revealed only a few minor differences. More experiments are necessary to elucidate these differences.
Metabolic activation of APAP in HCV transgenic animals
A major concern with any modulation of APAP-induced liver injury is the question whether there is a difference in metabolic activation. In particular, a recent study demonstrated a 90% down-regulation of Cyp2e1 protein, the dominant Cyp responsible for NAPQI formation in mice (Lee et al., 1996), in animals that were infected with a replication-deficient adenovirus to produce acute viral hepatitis. These virus-infected animals were highly resistant to APAP toxicity (Getachew et al., 2010). However, there was no significant difference in Cyp2e1 protein expression between our experimental groups. In addition, the depletion of hepatic GSH levels during the first 20 min after APAP overdose, generally considered an indirect but sensitive parameter for NAPQI formation in vivo (Jaeschke 1990; Jaeschke et al., 2011), which correlates with protein adducts (Xie et al., 2013; McGill et al., 2013), did not show any significant difference between the groups. Thus, it is highly unlikely that a difference in metabolic activation can explain the difference in the injury response in HCV transgenic mice.
Mitochondrial dysfunction in HCV transgenic animals
APAP overdose causes mitochondrial oxidant stress as reflected by increased GSSG levels (Jaeschke, 1990), Mitosox Red staining (Yan et al., 2010), increased susceptibility of MnSOD-deficient mice (Fujimoto et al., 2009; Ramachandran et al., 2011b), selective nitrotyrosine adduct formation in mitochondria (Cover et al., 2005), and inactivation of MnSOD by nitration (Agarwal et al., 2011). In addition, the shift to the more oxidized form of mitochondrial Trx-2, as shown in the current study, further supports this hypothesis. Indirect evidence for mitochondrial dysfunction is provided by the release of intermembrane proteins such as endonuclease G and AIF (Bajt et al., 2006, 2011), which trigger nuclear DNA fragmentation as documented by the TUNEL assay and other parameters of DNA damage (Bajt et al., 2006, 2011). AIF is initially released through a Bax pore and later, after the MPT, due to matrix swelling and rupture of the outer membrane (Bajt et al., 2008). It is also well documented that mitochondrial dysfunction triggers autophagy to remove the damaged organelles and impair the propagation of cell death signaling (Ni et al., 2012a). The current findings support these concepts. Trx-2 oxidation indicates a mitochondrial oxidant stress, AIF release and TUNEL-positive cells indicate mitochondrial dysfunction and DNA damage, increase in LC3II compared to LC3I reflects an increase in autophagy and the induction of Bcl-xL suggests a response to limit Bax pore formation. In addition, the induction of GADD153/CHOP and reduction of GRP78 confirms an ER stress during APAP overdose (Nagy et al., 2007; Uzi et al., 2013). It has been shown that the modulation of almost all of these events or processes can affect APAP-induced cell death. Not surprising, the mitochondrial oxidant stress (Trx-2) and dysfunction (AIF release, DNA damage), and the evidence of ER stress (GADD153/CHOP induction, Grp78 decline) was exacerbated in the more susceptible HCV transgenic animals and attenuated in the resistant mice. Along with the higher injury, the recovery of hepatic GSH levels was delayed compared to the resistant animals. However, Bcl-xL and MT protein induction, generally considered protective effects, were higher in the more severely injured HCV transgenic animals compared to the resistant mice. Likewise, there appears to be a higher degree of autophagy activation in the animals with higher injury. These findings would suggest that these responses are consequences of the variation in injury rather than the cause of it. Furthermore, mitochondrial AIF release and nuclear DNA damage are consequences of the mitochondrial oxidant stress and dysfunction (Cover et al., 2005). This would suggest that the most upstream event that controls most other downstream effects and is significantly different is the mitochondrial oxidant stress. For animals with aggravated injury, this means that the HCV core protein enhances the susceptibility of the mitochondria to a secondary insult.
However, in the resistant animals it suggested that a part of the transgenic animals developed an adaptation, which made it more difficult to trigger a mitochondrial oxidant stress with APAP overdose. Analysis of mRNA and enzyme activity indicated that glutathione S transferase mu-3 (GSTm3), was significantly induced in these animals compared to wild type animals. GSTm3 can catalyze conjugation of electrophiles, e.g. NAPQI, with GSH (Hayes and Pulford, 1995) and could thus be protective by attenuating the upstream effects of the injury process (Hayes and McLellan, 1999). It has also been shown that mouse GSTm 1-1 physically interacts with apoptosis signal-regulating kinase 1 (ASK1) and functions as an endogenous inhibitor of ASK1 inside cells, repressing ASK1-mediated signals (Cho et al., 2001). Since APAP induced activation of ASK1 (Nakagawa et al., 2008) and c-jun N-terminal kinase (JNK), and amplification of the mitochondrial oxidant stress by mitochondrial JNK translocation (Hanawa et al., 2008; Saito et al., 2010a) play an important role in the cascade of APAP hepatotoxicity, this could be an additional mechanism for protection in these mice.
In summary, chronic overexpression of HCV core protein leads to increased susceptibility to APAP-induced liver injury in some animals by enhanced mitochondrial oxidant stress and amplification of downstream events. However, an as yet undefined chronic stress response appears to trigger an adaptation mechanism, which is responsible for the lower susceptibility of some of the animals. Although there may be multiple protective mechanisms, activation of GSTs is likely an important component. Thus, our data reproduced in an animal model the epidemiological studies showing the increased susceptibility to APAP-induced liver injury in HCV infected patients and also demonstrated that the chronic stress by HCV core protein overexpression can lead to cellular adaptations rendering the animals more resistant to APAP overdose. A better understanding of the nature of the protection response and what differentiates the exacerbation and protection groups could lead to new ways to treat patients with oxidant-related liver diseases.
ACKNOWLEDGEMENTS
This investigation was supported in part by the National Institutes of Health grants R01 DK070195 and R01 AA12916 to H.J., and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) from the National Institutes of Health and grant AA012863 from the National Institute on Alcoholism and Alcohol Abuse and a grant from the Hubert and Richard Hanlon Trust (SW).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
REFERENCES
- Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, Hinson JA. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther. 2011;337:110–116. doi: 10.1124/jpet.110.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. Apoptosis-induced factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 2011;122:598–605. doi: 10.1093/toxsci/kfr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RS. Hepatitis C and liver transplantation. Nature. 2005;436:973–978. doi: 10.1038/nature04083. [DOI] [PubMed] [Google Scholar]
- Benali-Furet NL, Chami M, Houel L, De Giorgi F, Vernejoul F, Lagorce D, Buscail L, Bartenschlager R, Ichas F, Rizzuto R, Paterlini-Bréchot P. Hepatitis C virus core triggers apoptosis in liver cells by inducing ER stress and ER calcium depletion. Oncogene. 2005;24:4921–4933. doi: 10.1038/sj.onc.1208673. [DOI] [PubMed] [Google Scholar]
- Chang ML, Chen JC, Chang MY, Yeh CT, Lin WP, Liang CK, Huang SF, Dang KN, Chiu CT, Lin DY. Acute expression of hepatitis C core protein in adult mouse liver: Mitochondrial stress and apoptosis. Scand J Gastroenterol. 2008;43:747–755. doi: 10.1080/00365520701875987. [DOI] [PubMed] [Google Scholar]
- Cho SG, Lee YH, Park HS, Ryoo K, Kang KW, Park J, Eom SJ, Kim MJ, Chang TS, Choi SY, Shim J, Kim Y, Dong MS, Lee MJ, Kim SG, Ichijo H, Choi EJ. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem. 2001;276:12749–12755. doi: 10.1074/jbc.M005561200. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Kumagai K, Ito K, Arakawa S, Ando Y, Oda S, Yamoto T, Manabe S. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol Pathol. 2009;37:193–200. doi: 10.1177/0192623308329282. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Getachew Y, James L, Lee W, Thiele D, Miller B. Susceptibility to acetaminophen (APAP) toxicity unexpectedly is decreased during acute viral hepatitis in mice. Biochem Pharmacol. 2010;79:1363–1371. doi: 10.1016/j.bcp.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-Transferases: The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Igusa Y, Yamashina S, Izumi K, Inami Y, Fukada H, Komatsu M, Tanaka K, Ikejima K, Watanabe S. Loss of autophagy promotes murine acetaminophen hepatotoxicity. J Gastroenterol. 2012;47:433–443. doi: 10.1007/s00535-011-0500-0. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- Jaeschke H, Ding WX. Autophagy and acetaminophen hepatotoxicity: how useful are Atg7-deficient mice? J Gastroenterol. 2012;47:845–846. doi: 10.1007/s00535-012-0606-z. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity--a clinically relevant model to test the efficacy of natural products. Life Sci. 2011;88:737–745. doi: 10.1016/j.lfs.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, Weinman SA. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005;280:37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525–548. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM, Acute Liver Failure Study Group Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42:110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30:2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269:240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- Moriya K, Nakagawa K, Santa T, Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Miyazawa T, Ishibashi K, Horie T, Imai K, Todoroki T, Kimura S, Koike K. Oxidative stress in the absence of inflammation in a mouse model for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res. 2001;61:4365–4370. [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Nagy G, Kardon T, Wunderlich L, Szarka A, Kiss A, Schaff Z, Bánhegyi G, Mandl J. Acetaminophen induces ER dependent signaling in mouse liver. Arch Biochem Biophys. 2007;459:273–279. doi: 10.1016/j.abb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- Nguyen GC, Sam J, Thuluvath PJ. Hepatitis C is a predictor of acute liver injury among hospitalizations for acetaminophen overdose in the United States: a nationwide analysis. Hepatology. 2008;48:1336–1341. doi: 10.1002/hep.22536. [DOI] [PubMed] [Google Scholar]
- Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012a;55:222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U, Jaeschke H, Ding WX. Liver-specific loss of Atg5 causes persistent activation of Nrf2 and protects against acetaminophen-induced liver injury. Toxicol Sci. 2012b;127:438–450. doi: 10.1093/toxsci/kfs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AD, Carlson BA, Li F, Bonzo JA, Yoo MH, Krausz KW, Conrad M, Chen C, Gonzalez FJ, Hatfield DL. Disruption of thioredoxin reductase 1 protects mice from acute acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. Chem Res Toxicol. 2013;26:1088–1096. doi: 10.1021/tx4001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- Ploss A, Rice CM. Towards a small animal model for hepatitis C. EMBO Rep. 2009;10:1220–1227. doi: 10.1038/embor.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Lebosfky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress. Free Rad Res. 2011a;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2011b;251:226–233. doi: 10.1016/j.taap.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther. 2005;312:509–516. doi: 10.1124/jpet.104.075945. [DOI] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. C-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010a;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Yan HM, Artigues A, Villar MT, Farhood A, Jaeschke H. Mechanism of protection by metallothionein against acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010b;242:182–190. doi: 10.1016/j.taap.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Ueno T, Kominami E. LC3 and Autophagy. In: Deretic V, editor. Autophagosome and Phagosome-Methods in Molecular Biology series. Humana Press; Totowa, NJ: 2008. pp. 77–88. [DOI] [PubMed] [Google Scholar]
- Uehara T, Kosyk O, Jeannot E, Bradford BU, Tech K, Macdonald JM, Boorman GA, Chatterjee S, Mason RP, Melnyk SB, Tryndyak VP, Pogribny IP, Rusyn I. Acetaminophen-induced acute liver injury in HCV transgenic mice. Toxicol Appl Pharmacol. 2013;266:224–232. doi: 10.1016/j.taap.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman E, Fan Y, Stawowczyk M, Chen HM, Yue Z, Zong WX. Autophagy promotes necrosis in apoptosis-deficient cells in response to ER stress. Cell Death Differ. 2008;15:422–425. doi: 10.1038/sj.cdd.4402234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzi D, Barda L, Scaiewicz V, Mills M, Mueller T, Gonzalez-Rodriguez A, Valverde AM, Iwawaki T, Nahmias Y, Xavier R, Chung RT, Tirosh B, Shibolet O. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J Hepatol. 2013;59:495–503. doi: 10.1016/j.jhep.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Wang T, Weinman SA. Causes and consequences of mitochondrial reactive oxygen species generation in hepatitis C. J Gastroenterol Hepatol. 2006;21(Suppl 3):S34–S37. doi: 10.1111/j.1440-1746.2006.04591.x. [DOI] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010;30:1280–1292. doi: 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, McGill MR, Farhood A, Jaeschke H. Fas receptor-deficient lpr mice are protected against acetaminophen hepatotoxicity due to higher glutathione synthesis and enhanced detoxification of oxidant stress. Food Chem Toxicol. 2013;58:228–235. doi: 10.1016/j.fct.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Williams CD, McGill MR, Lebofsky M, Ramachandran A, Jaeschke H. Purinergic receptor antagonist A438079 protects against acetaminophen-induced liver injury by inhibiting p450 isoenzymes, not by inflammasome activation. Toxicol Sci. 2013;131:325–335. doi: 10.1093/toxsci/kfs283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Go YM, Jones DP. Mitochondrial thioredoxin-2/peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Arch Biochem Biophys. 2007;465:119–126. doi: 10.1016/j.abb.2007.05.001. [DOI] [PubMed] [Google Scholar]