Abstract
AIM: To evaluate the outcome of transarterial chemoemb-olization (TACE) in patients with unresectable hepatocellular carcinoma (HCC) <5 cm in diameter eligible for radiofrequency ablation (RFA).
METHODS: The treatment-related mortality, morbidity, long-term survival, and prognostic factors of HCC patients who had TACE and fulfilled the present inclusion criteria for RFA were evaluated.
RESULTS: Of the 748 patients treated with TACE between January 1990 and December 2002, 114 patients were also eligible for RFA. The treatment-related mortality and morbidity were 1% and 19%, respectively. Survival at 1, 3, and 5 years was 80%, 43%, and 23%, respectively. Older age and a high albumin level were associated with a better survival, whereas a high α-fetoprotein level (AFP) and the size of the largest tumor >3 cm in diameter were adverse prognostic factors in multivariate analysis.
CONCLUSION: The morbidity, mortality, and survival data after TACE for small HCCs eligible for RFA are comparable to those reported after RFA in the literature. Our data suggest the need for a randomized comparison of the two treatment modalities for small HCCs.
Keywords: Hepatocellular carcinoma, Radiofrequency ablation, Transarterial chemoembolization
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common human cancers with poor prognosis if left untreated, and only a small proportion is eligible for intentional curative surgical resection[1,2]. Transarterial chemoembolization (TACE) has been used as a palliative treatment for patients with inoperable disease and those with recurrence after resection. TACE is also used as a (neo-)adjuvant treatment before or after surgical resection, and before liver transplantation[3-6]. Hence, TACE is one of the most frequently performed treatment modalities for HCC. However, this treatment is still controversial and unsupported by evidence of early randomized controlled trials[7-9]. Renewed interest in this form of treatment that has emerged as evidence from recent randomized trials is in favor of this treatment[10,11]. A systematic review has convincingly shown a significant survival benefit for treatment with chemoembolization compared to no treatment[12]. Moreover, in some patients, TACE has even shown a similar survival compared to certain patients who underwent hepatic resection[13].
Local ablative, either open or percutaneous, techniques are considered alternatives for hepatic resection, if HCC is located either bilobar or multifocal rendering the disease unresectable, or if the patient has poor liver function reserve. Radiofrequency ablation (RFA) for HCC is a relatively new and popular local ablative technique. However, the lack of sufficient long-term follow-up data to support the treatment is well recognized. Strict criteria for RFA have not yet been clearly defined, but in general RFA is considered suitable if there are less than four lesions and the largest lesion is 5 cm or less in diameter[4,14,15]. RFA may not be suitable for tumors with portal vein, hepatic vein or vena cava involvement and in patients with severe cirrhosis[16,17]. Given these criteria and the reported promising results of RFA, some patients who might have been given TACE treatment prior to the advent of RFA may now be treated with RFA as the preferred alternative. However, TACE is a better documented treatment that is still used in many centers for HCC even if the tumor is eligible for RFA. Currently there are no studies in the literature directly comparing the results of the two treatment modalities. Such comparative studies may not be available in the near future because in most centers, RFA has been employed for a short duration with no long-term follow-up data, and not many centers have abundant experience in both treatment modalities. The results of TACE treatment for unresectable HCCs <5 cm in diameter eligible for RFA are also unknown because previous studies on TACE for HCC usually included a heterogeneous group of patients, including those with large tumors and vascular invasion.
This study that applied the currently accepted RFA inclusion criteria to our vast experience with TACE in patients with HCC reports the outcome of these patients and the factors associated with poor prognosis of TACE treatment in this particular group.
MATERIALS AND METHODS
A prospective database, which contained the data of all patients with surgical and non-surgical treatments for HCC at the Department of Surgery, Queen Mary Hospital, Hong Kong, was used. All patients with newly diagnosed HCC who had TACE as their initial treatment between January 1990 and December 2002 and had HCC with their largest nodule being <5 cm in diameter were identified and retrospectively analyzed. Other criteria included the number of tumor nodules less than four, absence of severe cirrhosis reflected by a Child’s C grading, and absence of portal vein (intrahepatic or main trunk), hepatic vein or inferior vena cava involvement.
Patients who had a diagnosis of HCC, confirmed either by biopsy or needle cytology, by two diagnostic imaging modalities (contrast computed tomography (CT) scan, magnetic resonance imaging or arteriography) showed typical HCC nodules with arterial hypervascularization or one diagnostic a persistently raised serum α-fetoprotein level (AFP) >400 ng/mL according to the Barcelona-2000 EASL conference criteria[18]. We selected patients for TACE treatment if they had inoperable bilobar disease or unilobar disease in combination with sufficient hepatic function reflected by a bilirubin level <50 μmol/L and no metastatic disease.
Our technique of TACE has been described elsew-here[19,20]. Superselective cannulation of the artery supplying the tumor was performed whenever possible. An emulsion of cisplatin (1 mg/mL) and lipiodol (Lipiodol Ultrafluide; Laboratoire Guerbet, Aulnay-Sous-Bois, France) at a volume ratio of 1:1 was injected up to a maximum of 60 mL. Embolization was performed with 1-mm2 particles of gelatin-sponge (Spongostan’, Johnson and Johnson Ltd, Skipton, UK) mixed with 40 mg of gentamycin. Repetition of TACE treatment was tailored to response of tumor and tolerance of liver rather than a fixed regimen. TACE was repeated every 8-12 wk if there was tumor response (≥50% decrease in tumor size according to World Health Organization criteria) or if the tumor was static in size (<50% decrease or <25% increase in tumor size). Treatment of TACE was terminated if there was no evidence of tumor on reassessment CT (i.e. complete response), disease progression (≥25% increase in tumor size or appearance of new lesions), evidence of liver failure (rise of bilirubin >50 μmol/L, uncontrollable ascites or hepatic encephalopathy), severe life-threatening complications such as liver abscess, or extrahepatic metastasis.
Patient demographics, laboratory data, and tumor characteristics were used for analysis. The Child-Pugh classification (A or B) was used to categorize the patients according to their status of liver cirrhosis and the new TNM classification according to the American Joint Committee on Cancer (AJCC)[21] was used for staging.
Continuous, normally distributed data were expressed as mean±SD, and other continuous data were expressed as medians with their interquartile range. Survival was analyzed by Kaplan-Meier survival curves. Prognostic variables for recurrence and survival were studied with univariate and multivariate Cox proportional hazards regression. Variables were categorized according to their quartile distribution wherever possible. This regression yields a hazard ratio, which may be interpreted as a relative risk within the average follow-up period. We used sex, age, laboratory data (bilirubin, albumin, AFP, platelet count) and tumor characteristics (bilobar disease, size of largest tumor, number of tumors, TNM stage) in a univariate Cox proportional hazard analysis. Next, variables for which univariate test had a P value less than 0.25 and biologically important variables (sex and age) were included in a multivariate analysis with survival as the outcome[22]. For model building, we applied backward stepwise elimination of variables. All reported P values were two-tailed. P<0.05 was considered statistically significant.
RESULTS
In the period between January 1990 and December 2002, a total of 2 850 patients were seen at our outpatient clinic for HCC and included in our database. Of these, 748 patients with newly diagnosed HCC underwent TACE as their initial treatment, and 200 patients had the largest tumor nodule <5 cm in diameter. This group included 15 patients who were considered unresectable during laparotomy and subsequently given TACE as the primary treatment. Patients who had more than three nodules (n = 60) subsequently undergoing hepatic resection (n = 14), and those who had portal vein (intrahepatic or main portal vein, n = 2) were excluded from this study. There were no patients with hepatic vein or inferior vena cava involvement. Of the remaining 124 patients, another 5 patients were excluded because of Child’s C cirrhosis and an additional 5 patients did not fulfill the criteria of HCC diagnosis used in this study. Hence, this study involved 114 patients and all analyses were performed in this group of patients.
The diagnosis of 17 patients was confirmed by either histology (n = 2) or cytology (n = 15). In 93 patients, confirmation of HCC was done by CT and hepatic arteriogram, in 2 patients by magnetic resonance imaging and hepatic arteriogram, and in the remaining 2 patients by an ultrasound of the liver and a consistently increased AFP combined with a hepatic arteriogram.
Patient characteristics are shown in Table 1. One patient died as a direct result of TACE treatment, the treatment-related mortality was 1%. This patient had four previous TACE sessions and developed both a liver abscess and deterioration of liver function after his 5th TACE treatment. He subsequently developed sepsis and pneumonia and died of respiratory failure. The overall treatment morbidity was 18%, the patient who died had two complications. Other complications as a result of TACE were hematoma at the puncture site in the groin (n = 1, 1%) and liver failure, defined as elevated bilirubin >50 μmol/L, development of uncontrolled ascites or hepatic encephalopathy (n = 20, 17%).
Table 1.
Characteristics of 114 patients eligible for RFA but received TACE treatment
| Characteristics | Median | Interquartile range |
| Age (yr) | 63 | 55-71 |
| Gender: male/female (n) | 87/27 | 76/24 |
| Child’s grading (n, %) | ||
| A | 90 | 79 |
| B | 24 | 21 |
| HBsAg status (n, %) | ||
| Positive | 82 | 71 |
| Negative | 32 | 28 |
| TNM stage (n, %) | ||
| Stage I | 72 | 63 |
| Stage II | 39 | 34 |
| Stage IIIA | 1 | 1 |
| Stage IIIC | 2 | 2 |
| Number of tumor nodules (n, %) | 1 | 1-2 |
| 1 | 76 | 67 |
| 2 | 32 | 28 |
| 3 | 6 | 5 |
| Size of largest tumor nodule (cm) | 3 | 2.1-4.0 |
| Tumor distribution (n, %) | ||
| Unilobar | 88 | 77 |
| Bilobar | 26 | 23 |
| Platelet count (×109/L) | 98 | 72-128 |
| Albumin (g/L) | 37 | 33-41 |
| Total bilirubin (μmol/L) | 17 | 12-24 |
| Prothrombin time (s) | 13.2 | 11.9-14.6 |
| INR | 1.1 | 1.0-1.2 |
| AFP (ng/mL) | 80 | 15-373 |
| Number of TACE procedures | 5 | 3-8 |
TACE: transarterial chemoembolization; HBsAg: hepatitis B surface antigen; INR: international normalized ratio; values are median with their interquartile range, unless denoted otherwise.
Seventy-four patients died during our observation period and the reasons for their death are given in Table 2. One patient was lost to follow-up (1%). The 1-, 3-, and 5-year survival rates were 80%, 43%, and 23% respectively (Figure 1). The median survival was 31 months (95%CI: 24-38 mo).
Table 2.
Causes of death in 74 patients after TACE treatment
| Causes | n | % |
| Malignant cachexia | 45 | 61 |
| Bleeding | 3 | 4 |
| Hepatic failure | 9 | 12 |
| Respiratory failure | 1 | 1 |
| Multiorgan failure | 2 | 3 |
| Sepsis | 4 | 5 |
| Other | 10 | 14 |
| Total (n) | 74 |
TACE: transarterial chemoembolization.
Figure 1.
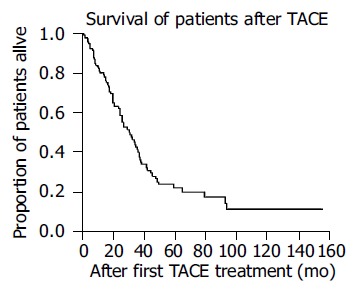
Kaplan-Meier curve for survival of patients undergoing TACE as treatment.
Univariate and multivariate analyses are shown in Tables 3 and 4, respectively. Higher age and a high albumin level (above 41 g/L) were associated with a better survival whereas AFP (above 81.50 ng/mL) and the size of the largest tumor >3 cm in diameter were the risk factors for death in the multivariate analysis.
Table 3.
Univariate analysis for prognostic factors of death
| Variable | Hazard ratio | 95%CI | P |
| Age (per yr) | 1.01 | 0.99-1.03 | 0.347 |
| Female sex | 0.91 | 0.51-1.63 | 0.747 |
| Bilirubin (mmol/L)1 | |||
| 5.00-11.75 | |||
| 11.75-17.00 | 1.16 | 0.62-2.17 | 0.648 |
| 17.00-24.00 | 1.67 | 0.88-3.18 | 0.116 |
| 24.00-83.00 | 1.66 | 0.85-3.25 | 0.14 |
| Albumin (g/L)1 | |||
| 24-33 | |||
| 33-37 | 0.72 | 0.38-1.37 | 0.31 |
| 37-41 | 0.92 | 0.50-1.69 | 0.786 |
| 41-50 | 0.4 | 0.20-0.81 | 0.011 |
| AFP (ng/mL)1 | |||
| 2-14.75 | |||
| 14.75-81.50 | 1.42 | 0.71-2.86 | 0.32 |
| 81.50-366 | 2.3 | 1.16-4.57 | 0.017 |
| 366-11 470 | 1.99 | 1.03-3.83 | 0.041 |
| Platelet count (×109/L)1 | |||
| 36-72 | |||
| 72-99 | 1 | 0.54-1.85 | 0.992 |
| 99-128 | 1.26 | 0.66-2.39 | 0.486 |
| 128-295 | 0.81 | 0.42-1.59 | 0.539 |
| Bilobar disease | 1.06 | 0.62-1.80 | 0.841 |
| Largest tumor >3 cm | 1.4 | 0.87-2.24 | 0.163 |
| Child’s B grade2 | 1.67 | 0.97-2.88 | 0.065 |
| AJCC TNM stage3 | |||
| Stage I | |||
| Stage II | 1.08 | 0.67-1.73 | 0.763 |
| Stage IIIA | 0 | 0.00->100 | 0.971 |
| Stage IIIC | 1.73 | 0.24-12.65 | 0.591 |
CI: confidence interval; AJCC: American Joint Committee on Cancer.
Hazard ratio of bilirubin, albumin, AFP, and platelet count is given relative to the lowest quartile.
Hazard ratio of Child’s B grade is given relative to Child’s A grade.
Hazard ratio of AJCC TNM stage is given relative to stage I.
Table 4.
Multivariate analysis for prognostic factors of death
| Variable | Hazard ratio | 95%CI | P |
| Age (per yr) | 0.98 | 0.95-1.00 | 0.044 |
| Albumin (g/L)1 | |||
| 24-33 | |||
| 33-37 | 0.83 | 0.42-1.63 | 0.586 |
| 37-41 | 1.14 | 0.61-2.13 | 0.685 |
| 41-50 | 0.21 | 0.09-0.49 | <0.0005 |
| AFP (ng/mL)1 | |||
| 2-14.75 | |||
| 14.75-81.50 | 1.57 | 0.77-3.17 | 0.209 |
| 81.50-366 | 4.61 | 2.13-9.98 | <0.0005 |
| 366-11 470 | 3.72 | 1.77-7.85 | 0.001 |
| Largest tumor >3 cm2 | 1.64 | 1.00–2.69 | 0.049 |
CI: confidence interval.
Hazard ratio of albumin and AFP is given relative to the lowest quartile.
Hazard ratio is given relative to the size of the largest tumor smaller than 3 cm in diameter.
DISCUSSION
We evaluated the morbidity, mortality and long-term survival, and identified several independent risk factors for death in patients who underwent TACE treatment with patient and tumor characteristics that fulfilled all our present criteria for RFA treatment. Although there are numerous reports on the results of TACE for inoperable HCC, many included patients with large or advanced HCC with vascular invasion for which the outcome is expectedly worse[3,5,6,19]. Such reports may give the impression that results after TACE are worse than those after RFA, because most studies of RFA on HCCs focused on small HCCs and hence reported more favorable results. To our knowledge, the survival and prognostic factors in this specific group of patients with HCC who were eligible for RFA but underwent TACE treatment have not been reported before. As many patients who were considered to have unresectable HCC <5 cm in diameter received TACE treatment before the RFA era, this knowledge may be of specific interest to those who are rethinking the concept of TACE and RFA and wish to refine their indications for both TACE and RFA treatments. With the availability of RFA, patients with small HCCs <5 cm in diameter previously treated by TACE are now treated by RFA instead as the preferred treatment in some centers, although there is no direct evidence to show that RFA is superior to TACE in terms of treatment safety and long-term survival benefit. The exact place of local ablative techniques and TACE in the overall management of HCC still needs to be determined. Offering a strict order of treatment options to the patient, i.e. first hepatic resection, then local ablative techniques and then TACE, may not be fully justified. Indeed, percutaneous ethanol injection appears to acquire similar results as resection for small HCC in terms of treatment efficacy, recurrence, and survival rates[23]. In addition, Bronowicki et al[24], found similar survival after surgery and TACE, while the patients who underwent surgery were younger and had a better staging, and, just recently, TACE has been shown to be as effective as hepatic resection in certain patients[13]. Moreover, evidence from randomized controlled trials comparing even two local ablative techniques is hardly available, not to mention trials comparing two different treatment modalities such as TACE and RFA. Therefore, one must be careful not to jump to conclusions[25]. In patients who do not want surgery, which is still necessary in a large proportion of cases for RFA because of tumor location at the superior portion of the liver or close to the adjacent viscera[26], TACE is still one of the best options. Furthermore, some centers may not have the availability and/or equal expertise of all treatment options and may stick to the use of TACE as the preferred treatment for all unresectable HCC.
Given the potentially curative intent of RFA, many clinicians consider RFA as the treatment of choice for unresectable small HCC, although long-term survival results of RFA are scarce[27]. In addition, complications and treatment mortality after RFA are generally expected to be fewer than those after TACE, and RFA may result in less deterioration of liver function. The treatment mortality in this study (1%), however, appears comparable to that of RFA[28]. A recent literature review suggests that the treatment morbidity of RFA in the early reports is probably underes-timated[28]. The treatment morbidity of TACE for small HCC in this study was 18%, which is comparable to the complication rates of 13-20% after RFA for HCC reported in some of the more recent studies[28,29]. The complication rate is 14% in our preliminary experience with RFA in 86 patients with HCC between May 2001 and December 2002[30]. The 1-year survival rate of the HCC patients treated by TACE in the current study was 80%, which is comparable to the 1-year survival observed after RFA for HCC in our preliminary experience[30]. The 3-year survival rate of 43% is also comparable with that of 33-50% in recent reports of more long-term survival results of RFA for HCC[31,32]. In this study, the 5-year survival after TACE for HCCs <5 cm in diameter that were eligible for RFA was 23%. Data on 5-year survival rate after RFA for HCC are scarce. In a recent study, Buscarini et al[33], reported a 5-year survival rate of 33% after RFA for HCCs ≤3.5 cm in diameter, which appears comparable to that after TACE for tumors of a similar size in our study.
In this study, we did not directly compare our TACE results to similar patients who underwent RFA treatment in our institution. We have started RFA only 4 years before, like many others, and long-term follow-up is unavailable yet. Furthermore, most patients in this study were treated with TACE in the era long before the availability of RFA. Naturally, a future comparison between similar patients undergoing TACE and RFA within the same time-frame will be made. Ultimately, however, a randomized controlled trial may be needed. This study may serve as a reference to compare present and future RFA series, as in many centers that perform RFA, TACE is either losing ground or experience with TACE is not so large in the first place. Our results of TACE in patients with HCC <5 cm in diameter suggest that the safety of TACE and survival results may be comparable to those of RFA, which is an important message for clinicians treating HCCs. This highlights the need for randomized studies to compare the two treatment modalities rather than simply considering RFA as the preferred treatment based on perceived benefits.
RFA is still an evolving technique and it is therefore understandable that there is a large diversity in indications for RFA. Our inclusion criteria for RFA are consistent with the reported criteria[14-17,27]. A largest tumor size ≤ 5 cm in diameter, the number of tumors less than four and tumors not invading or abutting major hepatic veins or portal vein branches have all been reported as the inclusion criteria for RFA. In our database of HCC patients treated with TACE, 114 of 748 patients who underwent TACE treatment (15%) fulfilled the criteria for RFA treatment. We found that a high albumin concentration was associated with better survival. We believe that this reflects the better liver function of these patients compared to those with low albumin concentrations. Indeed, it is reasonable to speculate that patients with poor liver function may do better with local ablative techniques, such as RFA, because these techniques result in less liver damage than TACE. Child’s B grade liver function did not appear to be an independent predictor in the multivariate analysis because it possibly interacted with albumin concentration. A higher age results in a risk reduction of 2% per year. Others have found that age is an adverse prognostic factor[34,35]. We cannot explain this interesting observation but we propose that it might be related to selection or, partially a more malignant behavior of HCC in younger patients. In any case, age may not be a clinically important factor that influences the clinician’s decision to treat the patient with RFA or TACE. Both a high AFP, which reflects tumor volume and perhaps also tumor aggressiveness, and tumor size are associated with poorer survival. These risk factors have been identified previously in other prognostic studies of TACE[35-38]. Patients with tumors measuring between 3 and 5 cm performed worse compared to those with smaller tumors when put on TACE treatment. Perhaps this particular group may do better when treated by local ablative techniques such as RFA. Advances in RFA technology have allowed ablation of larger HCC. RFA may effectively treat tumors up to 5 cm in diameter by using a clustered probe or by inflow occlusion with the Pringle maneuver[4,30].
This is the first study that investigated the outcomes of TACE for a group of patients with HCC <5 cm in diameter who would now be treated with RFA instead in many centers. TACE for small HCC has been reported before but focused mainly on the technical aspects, and those studies did not specifically look at patients who were also eligible for RFA treatment. Moreover, these are all Japanese studies with mainly hepatitis C patients[39-41]. Our results compare favorably with the results of Nakao et al[39] (34% 3-year survival), and are similar to the results of Yamada et al[40] (47% 3-year survival). Takayasu et al[41] have reported a 77% 3-year survival in their patients with HCC <5 cm in diameter. However, the mean tumor size was only 1.9 cm in their study.
In conclusion, this study provides the morbidity, mortality and long-term survival results of a group of patients who were otherwise eligible for RFA treatment but received TACE. Our data, based on a retrospective analysis, suggest that the immediate and at least the short-term and intermediate-term survival results of TACE for small HCC may be comparable to those of RFA reported in the literature. The data may serve as a useful reference for comparison to RFA treatment when long-term survival of the latter treatment becomes available. This study highlights the need for a prospective comparative study of the two treatment modalities rather than simply replacing TACE with RFA for small inoperable HCCs as the treatment of choice, as what many centers are doing with the availability of this new technology. The identification of the prognostic factors may help in better selection of patients for TACE or RFA treatment.
Footnotes
Supported by the Sun CY Research Foundation for Hepatobiliary and Pancreatic Surgery of the University of Hong Kong, China
Science Editor Wang XL and Li WZ Language Editor Elsevier HK
References
- 1.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Akriviadis EA, Llovet JM, Efremidis SC, Shouval D, Canelo R, Ringe B, Meyers WC. Hepatocellular carcinoma. Br J Surg. 1998;85:1319–1331. doi: 10.1046/j.1365-2168.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 3.Poon RT, Ngan H, Lo CM, Liu CL, Fan ST, Wong J. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol. 2000;73:109–114. doi: 10.1002/(sici)1096-9098(200002)73:2<109::aid-jso10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466–486. doi: 10.1097/00000658-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasugai H, Kojima J, Tatsuta M, Okuda S, Sasaki Y, Imaoka S, Fujita M, Ishiguro S. Treatment of hepatocellular carcinoma by transcatheter arterial embolization combined with intraarterial infusion of a mixture of cisplatin and ethiodized oil. Gastroenterology. 1989;97:965–971. doi: 10.1016/0016-5085(89)91505-9. [DOI] [PubMed] [Google Scholar]
- 6.Carr BI. Hepatic artery chemoembolization for advanced stage HCC: experience of 650 patients. Hepatogastroenterology. 2002;49:79–86. [PubMed] [Google Scholar]
- 7.Miller DL, Lotze MT. A plea for a standard standard. Radiology. 1993;188:19–20. doi: 10.1148/radiology.188.1.8390068. [DOI] [PubMed] [Google Scholar]
- 8.Trevisani F, De Notariis S, Rossi C, Bernardi M. Randomized control trials on chemoembolization for hepatocellular carcinoma: is there room for new studies? J Clin Gastroenterol. 2001;32:383–389. doi: 10.1097/00004836-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 11.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 13.Lee HS, Kim KM, Yoon JH, Lee TR, Suh KS, Lee KU, Chung JW, Park JH, Kim CY. Therapeutic efficacy of transcatheter arterial chemoembolization as compared with hepatic resection in hepatocellular carcinoma patients with compensated liver function in a hepatitis B virus-endemic area: a prospective cohort study. J Clin Oncol. 2002;20:4459–4465. doi: 10.1200/JCO.2002.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Allgaier HP, Deibert P, Zuber I, Olschewski M, Blum HE. Percutaneous radiofrequency interstitial thermal ablation of small hepatocellular carcinoma. Lancet. 1999;353:1676–1677. doi: 10.1016/S0140-6736(99)00368-2. [DOI] [PubMed] [Google Scholar]
- 15.McGhana JP, Dodd GD. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001;176:3–16. doi: 10.2214/ajr.176.1.1760003. [DOI] [PubMed] [Google Scholar]
- 16.Curley SA. Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol. 2003;10:338–347. doi: 10.1245/aso.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 19.Ngan H, Lai CL, Fan ST, Lai EC, Yuen WK, Tso WK. Treatment of inoperable hepatocellular carcinoma by transcatheter arterial chemoembolization using an emulsion of cisplatin in iodized oil and gelfoam. Clin Radiol. 1993;47:315–320. doi: 10.1016/s0009-9260(05)81446-1. [DOI] [PubMed] [Google Scholar]
- 20.O'Suilleabhain CB, Poon RT, Yong JL, Ooi GC, Tso WK, Fan ST. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg. 2003;90:325–331. doi: 10.1002/bjs.4045. [DOI] [PubMed] [Google Scholar]
- 21.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M, editors . AJCC cancer staging manual, 6th ed. Springer-Verlag; 2002. [Google Scholar]
- 22.Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- 23.Castells A, Bruix J, Bru C, Fuster J, Vilana R, Navasa M, Ayuso C, Boix L, Visa J, Rodés J. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology. 1993;18:1121–1126. [PubMed] [Google Scholar]
- 24.Bronowicki JP, Boudjema K, Chone L, Nisand G, Bazin C, Pflumio F, Uhl G, Wenger JJ, Jaeck D, Boissel P, et al. Comparison of resection, liver transplantation and transcatheter oily chemoembolization in the treatment of hepatocellular carcinoma. J Hepatol. 1996;24:293–300. doi: 10.1016/s0168-8278(96)80007-9. [DOI] [PubMed] [Google Scholar]
- 25.Boyle MJ. Percutaneous ablation of liver tumors. Arch Surg. 2003;138:809; author reply 809–810. doi: 10.1001/archsurg.138.7.809-a. [DOI] [PubMed] [Google Scholar]
- 26.Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng KK, Lam CM, Poon RT, Ai V, Tso WK, Fan ST. Thermal ablative therapy for malignant liver tumors: a critical appraisal. J Gastroenterol Hepatol. 2003;18:616–629. doi: 10.1046/j.1440-1746.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- 28.Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 29.Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST. Effectiveness of radiofrequency ablation for hepatocellular carcinomas larger than 3 cm in diameter. Arch Surg. 2004;139:281–287. doi: 10.1001/archsurg.139.3.281. [DOI] [PubMed] [Google Scholar]
- 31.Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102–107. doi: 10.1097/01.sla.0000129672.51886.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu HX, Xie XY, Lu MD, Chen JW, Yin XY, Xu ZF, Liu GJ. Ultrasound-guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol. 2004;59:53–61. doi: 10.1016/j.crad.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Buscarini L, Buscarini E, Di Stasi M, Vallisa D, Quaretti P, Rocca A. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11:914–921. doi: 10.1007/s003300000659. [DOI] [PubMed] [Google Scholar]
- 34.Mondazzi L, Bottelli R, Brambilla G, Rampoldi A, Rezakovic I, Zavaglia C, Alberti A, Idèo G. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology. 1994;19:1115–1123. [PubMed] [Google Scholar]
- 35.Ikeda M, Okada S, Yamamoto S, Sato T, Ueno H, Okusaka T, Kuriyama H, Takayasu K, Furukawa H, Iwata R. Prognostic factors in patients with hepatocellular carcinoma treated by transcatheter arterial embolization. Jpn J Clin Oncol. 2002;32:455–460. doi: 10.1093/jjco/hyf097. [DOI] [PubMed] [Google Scholar]
- 36.Lladó L, Virgili J, Figueras J, Valls C, Dominguez J, Rafecas A, Torras J, Fabregat J, Guardiola J, Jaurrieta E. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50–57. doi: 10.1002/(sici)1097-0142(20000101)88:1<50::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 37.Savastano S, Miotto D, Casarrubea G, Teso S, Chiesura-Corona M, Feltrin GP. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with Child's grade A or B cirrhosis: a multivariate analysis of prognostic factors. J Clin Gastroenterol. 1999;28:334–340. doi: 10.1097/00004836-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Akashi Y, Koreeda C, Enomoto S, Uchiyama S, Mizuno T, Shiozaki Y, Sameshima Y, Inoue K. Prognosis of unresectable hepatocellular carcinoma: an evaluation based on multivariate analysis of 90 cases. Hepatology. 1991;14:262–268. [PubMed] [Google Scholar]
- 39.Nakao N, Kamino K, Miura K, Takayasu Y, Ohnishi M, Miura T. Transcatheter arterial embolization in hepatocellular carcinoma: a long-term follow-up. Radiat Med. 1992;10:13–18. [PubMed] [Google Scholar]
- 40.Yamada R, Kishi K, Sonomura T, Tsuda M, Nomura S, Satoh M. Transcatheter arterial embolization in unresectable hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1990;13:135–139. doi: 10.1007/BF02575464. [DOI] [PubMed] [Google Scholar]
- 41.Takayasu K, Muramatsu Y, Maeda T, Iwata R, Furukawa H, Muramatsu Y, Moriyama N, Okusaka T, Okada S, Ueno H. Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates. AJR Am J Roentgenol. 2001;176:681–688. doi: 10.2214/ajr.176.3.1760681. [DOI] [PubMed] [Google Scholar]


