Abstract
AIM: The origin of putative liver cells from distinct bone marrow stem cells, e.g. hematopoietic stem cells or multipotent adult progenitor cells was found in recent in vitro studies. Cell culture experiments revealed a key role of growth factors for the induction of liver-specific genes in stem cell cultures. We investigated the potential of rat mesenchymal stem cells (MSC) from bone marrow to differentiate into hepatocytic cells in vitro. Furthermore, we assessed the influence of cocultured liver cells on induction of liver-specific gene expression.
METHODS: Mesenchymal stem cells were marked with green fluorescent protein (GFP) by retroviral gene transduction. Clonal marked MSC were either cultured under liver stimulating conditions using fibronectin-coated culture dishes and medium supplemented with SCF, HGF, EGF, and FGF-4 alone, or in presence of freshly isolated rat liver cells. Cells in cocultures were harvested and GFP+ or GFP- cells were separated using fluorescence activated cell sorting. RT-PCR analysis for the stem cell marker Thy1 and the hepatocytic markers CK-18, albumin, CK-19, and AFP was performed in the different cell populations.
RESULTS: Under the specified culture conditions, rat MSC cocultured with liver cells expressed albumin-, CK-18, CK-19, and AFP-RNA over 3 weeks, whereas MSC cultured alone did not show liver specific gene expression.
CONCLUSION: The results indicate that (1) rat MSC from bone marrow can differentiate towards hepatocytic lineage in vitro, and (2) that the microenvironment plays a decisive role for the induction of hepatic differentiation of rMSC.
Keywords: Mesenchymal stem cells, Liver-specific differentiation, Coculture
INTRODUCTION
The existence of putative liver stem cells in the bone marrow was first demonstrated by Petersen et al[1], who showed that bone marrow cells transplanted into lethally irradiated mice engrafted in the recipient’s liver differentiated into liver stem cells (oval cells) or mature liver cells (hepatocytes). These in vivo results were confirmed by mouse experiments[2] and in patients who received a bone marrow transplantation or peripheral blood stem cell transplantation for hematological disorders[3-5]. Furthermore, Lagasse et al[6], found liver-specific gene expression and function in FACS-sorted mouse hematopoietic stem cells (HSC, KTLS cells: c-kithigh, thy1+/-, linneg, sca-1+) after transplantation into FAH-deficient mice. Recent studies in the same animal model indicated that cells observed in the recipients’ liver after stem cell transplantation bearing donor markers and liver specific markers were rather a product of cell fusion than of a real “transdifferentiation”[7,8]. However, variable data concerning cellular fusion vs transdifferentiation as the mechanism of liver-like differentiated cells derived from bone marrow were found in other animal models: Engraftment of human albumin producing cells in livers of NOD/SCID recipient mice was observed after xenogeneic transplantation of human hematopoietic or umbilical cord blood stem cells. However, fusion events were not ruled out[9]. In contrast to these results, two studies using similar settings found liver specific differentiation of the transplanted cells occurring without any evidence of cell fusion events after transplantation of human sorted CD34+ or unsorted mononuclear cord blood cells into NOD/SCID mice[10,11]. These results were supported by a recent report of Jang et al[12], showing conversion of HSC into viable hepatocytes in vitro and in vivo, notably without any cell fusion.
Several in vitro studies suggested the differentiation potential of various types of bone marrow cells/stem cells towards hepatocytic cells under appropriate culture conditions. Oh et al[13], found an expression of the liver specific genes alpha-fetoprotein (AFP) and albumin in cultures of unsorted rat bone marrow cells after 21 d. The liver specific gene expression was induced by hepatocyte growth factor (HGF) and was mediated by the expression of its receptor c-met. The expression of liver specific genes (albumin, cytokeratins (CK)) in cultured human CD34-positive hematopoietic stem cells or mononuclear cord blood cells was also demonstrated to be induced by HGF in culture[14,15].
From cultures of mature hepatocytes it is known that important stimuli for an adequate maintenance of cellular function in vitro are (1) the addition of growth hormones and cytokines to the culture medium[16,17], (2) coating culture dishes with extracellular matrix (ECM) molecules[18,19], and (3) coculturing with other cell types[20,21]. In stem cell cultures, Miyazaki et al[22] showed an induction of the liver specific genes, albumin, tryptophan-2, 3-dioxygenase and tyrosine amino-transferase of rat bone marrow cells indicating a maturation towards hepatocytes when cultured in a hepatocyte growth medium supplemented with HGF and epidermal growth factor (EGF). Avital et al[23] demonstrated that β2-microglobulin-negative Thy1-positive stem cells residing in rat bone marrow expressed the liver marker albumin. In cocultures of these cells with hepatocytes (separated by a PTFE-membrane), cells were shown to adopt metabolic activity after 7 d in culture. An important influence of hepatocytes on the differentiation of stem cell enriched bone marrow was also highlighted by Okumoto et al, Here, immuno-selected bone marrow stem cells cultured in the presence of HGF and fetal bovine serum (FBS) expressed the markers, hepatic nuclear factor 1 (HNF1-α) and CK-8 only after 7 d. In cocultures with hepatocytes seperated by a semipermeable membrane, the stem cells additionally expressed the liver specific markers- AFP and albumin[24]. This suggested that cocultured hepatocytes have a stimulatory effect for the differentiation of stem cells toward liver cells, which was mediated by soluble factors. For the culture of adult hepatocytes, it has been shown that cell-to-cell or cell-to-matrix contacts also play an important role for the control of liver specific differentiation[25]. Thus, an optimal in vitro environment for the induction of liver specific differentiation in stem cells should be achieved by cocultures with liver cells.
Besides the HSCs, bone marrow contains nonhematopoietic, i.e. mesenchymal stem cells (MSC) reported to differentiate into cell lineages of all three germ layers[26-29]. One distinct adherent growing cell population derived from bone marrow was described by Schwartz et al[30]. The authors isolated CD45- and GlycophorinA-depleted multipotent adult progenitor cells (MAPC) from rat, mouse, or human plastic-adherent bone marrow cells that expressed liver specific markers after 14 d in culture with FGF and HGF, e.g. albumin and CK-18. However, the relation between MSC and MAPC remains unclear. MAPCs were separated differently from bone marrow in comparison to MSCs and appear after long periods of time in vitro using low serum concentrations. For cloned MSCs, differentiation in hepatic cells has not been shown yet. Additionally, little evidence for cell fusion in MSC cultures has been published so far. Recently, coculture of MSCs with heatshocked airway epithelial cells revealed the differentiation into epithelial cells[31]. Cell fusion in this setting was a rather frequent than a rare event with a frequency of ≈10-2 and has to be expected in other experimental settings as well.
In this study, we investigated the potential of rat mesenchymal stem cells derived from adult bone marrow to differentiate into hepatic lineage cells in vitro. Furthermore, we assessed the impact of coculture with adult liver cells permitting cell-cell contacts for the initiation of liver specific gene expression.
MATERIALS AND METHODS
An outline of the experimental design is shown in Figure 1.
Figure 1.
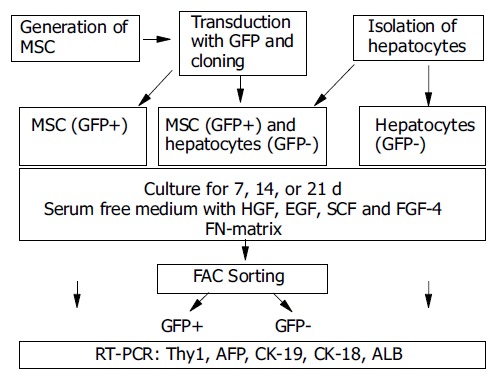
Experimental design. rMSC were harvested from rat bone marrow and were transduced with GFP and cloned. Cloned GFP+ rMSC were expanded and either seeded with hepatocytes in cocultures or alone. Controls included hepatocytes cultured alone. Cells were cultured for 7, 14, or 21 d. Cocultured cells were sorted by FACS into GFP+ (rMSC-derived) or GFP- (hepatocyte-derived) cells. Gene expression analysis for stem cell marker or liver specific genes was performed by RT-PCR.
Isolation, transduction, and cloning of rat mesenchymal stem cells
Bone marrow was harvested from Lewis.1WR2 rats by flushing femurs and tibiae with icecold IMDM (Gibco BRL, Karlsruhe, Germany) and 10% fetal calf serum (FCS, Gibco). Cells were centrifugated at 400 r/min for 10 min at 4 °C. The pellet was suspended in DMEM/Hams-F12 medium (1:1; Biochrom, Berlin, Germany) supplemented with 20% preselected fetal bovine serum (FBS, Biochrom), 2 mol/L L-glutamine (Gibco), 100 U/mL penicillin, and 100 mg/mL streptomycin (both Gibco). Cells of one femur and one tibia were seeded in tissue culture flasks (A = 75 cm2, Greiner, Frickenhausen, Germany). Non-adherent cells were removed after 3 d by washing the cultures with PBS (Gibco). Plastic adherent cells were grown to near confluency, and were passaged twice. Retroviral gene transduction with ectropic-packaged GFP was performed as described elsewhere[32]. In brief, cells were removed from cultures with Trypsin/EDTA (Gibco), and 1103 MSC/well were seeded in six-well culture plates. 2106 virus particles containing the ectropic vector SFα11-GFP[33] and 4 µg/mL protamine sulfate (Gibco) were added, and the culture plates were centrifuged for 1 h with 1 000 r/min at room temperature. Transduction efficiency was 25.5%, as determined by FACS analysis (BD FACScan with CellQuest software, BD Heidelberg, Germany). After transfection, 0.3 cells were seeded per well in 96 well plates for cloning. The clones were checked for GFP expression with a fluorescence microscope. GFP-positive clones were expanded and the clone with the highest proliferation capacity was chosen for further work (IG3 clone). Clonal GFP-marked rMSC were stored frozen at -196 °C until culture for cell differentiation.
Hepatocyte isolation
Hepatocytes were isolated from Sprague-Dawley rats by two step collagenase perfusion described by Seglen[34] and performed in our laboratory[35]. Briefly, donor animals received 250 U heparin (Liquemin, Hoffmann La-Roche, Mannheim, Germany) prior to cell isolation. After cannulation of the portal vein, the liver was perfused with a calcium-free buffer solution [1 000 mL distilled water, 8.3 g NaCl, 0.5 g KCl, 2.38 g HEPES (Sigma, Seelzen, Germany); pH 7.4; flow 30 mL/min] at 37.0 °C for 7 min. Then, the liver was perfused with a collagenase solution [1 000 mL distilled water, 8.3 g NaCl, 0.5 g KCl, 2.38 g HEPES, 0.7 g CaCl2·2H2O, 7.5 mg trypsin-inhibitor (ICN, Eschwege, Germany) and 500 mg collagenase (Collagenase H, Boehringer Mannheim, Mannheim, Germany); pH 7.35; flow 30 mL/min] at 37 °C for 8-11 min. The perfused liver was resected, and the cells were released by gentle shaking and collected in 20 mL Williams medium E without L-glutamine (Gibco). The cell suspension was filtered using a 200 µm nylon mesh and washed twice with Williams medium E (centrifugation at 50 r/min; 4 °C for 3 min). Cells were purified by PercollR (density 1.13 g/mL; Sigma) gradient centrifugation (400 r/min; 4 °C for 12 min) and washed twice in Williams medium E.
Coculture of rMSC with rat hepatocytes
GFP-transduced and cloned rMSC from passage (P) nine or later (≥P 9) were cocultured with freshly isolated rat hepatocytes. As controls, rMSC and rat hepatocytes cultured alone were included. Cultures were analyzed at d 0, 7, 14, and 21. Cells were seeded into culture wells coated with 4 µg/well fibronectin (FN, Sigma) in 24-well plates (Greiner). The culture medium used for the differentiation in cocultures consisted of Stem Span serum-free essential medium (SFEM, Stem Cell Technologies, St. Katherinen, Germany) supplemented with 100 mg/mL penicillin/streptomycin (Gibco), 2.5 nmol/L dexamethasone (Sigma), 100 ng/mL human recombinant stem cell factor (SCF), 20 ng/mL hepatocyte growth factor (HGF, both Immunotools, Friesoythe, Germany), 50 ng/mL epidermal growth factor (EGF), and 10 ng/mL fibroblast growth factor-4 (FGF-4, both R&D, Wiesbaden, Germany).
9×104 rMSC were seeded per well for MSC-controls, or 6×104 hepatocytes per well for hepatocyte controls. For cocultures of rMSC with rat hepatocytes 9×104 rMSC per well were preseeded in 24 well-plates for 2-3 h. Then, 6×104 hepatocytes per well were added to the cultures. Medium was changed twice a week.
Separation of GFP-positive and -negative cells in cocultures by fluorescence activated cell sorting (FACS)
After the culture period, cells from cocultures were trypsinized, counted with trypan blue, and resuspended in 3 mL PBS. To get single cell suspension, cells were filtered through a 35 µm filter (BD, Heidelberg, Germany). Cells were sorted using the FACS AriaTM Cell Sorter (BD) into GFP-positive (GFP+) or GFP-negative (GFP-) cells, focusing on the highest possible purity of GFP+ cells.
RNA extraction from cells, reverse transcription and polymerase chain reaction (RT-PCR)
RNA was extracted using the Invisorb Spin Cell-RNATM Mini-kit (Invitek, Berlin, Germany) according to the manufacturer’s instructions. RNA was stored at -80 °C. Reverse transcription (RT) of extracted RNA was performed using the bulk first-strand c-DNA synthesis kit (Amersham, Freiburg, Germany). The cDNA was stored at -20 °C. For the semiquantitative PCR reaction, 5 µL cDNA-template was mixed with 2.5 µL 10 PCR-buffer, 0.5 µL 10 mmol/L dNTPs, 0.5 µL of each primer (50 ng/µL), and 0.5 µL polymerase (Ampli-Taq., Gibco) in a total volume of 25 µL for each probe. PCR was carried out in a programmable Biometra Uno-Thermobloc (Biometra, Göttingen, Germany) using the primers and conditions is shown in Table 1. Negative controls were performed for each set of primers. Samples were analyzed on 1% agarose gels. The size of the PCR-fragments was estimated using a 100-base-pair ladder (Gibco BRL).
Table 1.
RT-PCR analysis
| Primer name | Sequence | PCR conditions | Fragment length | Refer-ence |
| GAPDH | S: 5’-CCT TCA TTG ACC TCA ACT AC -3’ | 60 °C; 30× | 593 bp | 14 |
| A: 5’-GGA AGG CCA TGC CAG TGA GC-3’ | ||||
| Thy1 | S: 5’-CGC TTT ATC AAG GTC CTT ACT C-3’ | 52 °C; 29× | 343 bp | 39 |
| A: 5’-GCG TTT TGA GAT ATT TGA AGG T-3’ | ||||
| CK-18 | S: 5’-GGA CCT CAG CAA GAT CAT GGC-3 | 60 °C; 30× | 518 bp | |
| A: 5’-CCA CGA TCT TAC GGG TAG TTG-3 | ||||
| CK-19 | S: 5’-ACC ATG CAG AAC CTG AAC GAT-3’ | 60 °C; 30× | 261 bp | 30 |
| A: 5’-CAC CTC CAG CTC GCC ATT AG-3’ | ||||
| AFP I. | S I: 5’-AAC AGC AGA GTG CTG CAA AC-3 | 55 °C; 35× | 13 | |
| A I: 5’-AGG TTT CGT CCC TCA GAA AG-3’ | ||||
| AFP II. (nested) | S II: 5’-CAC CAT CGA GCT CGC CTA TT-3 | 60 °C; 30× | 619 bp | 13 |
| A II: 5’-TGA TGC AGA GCC TCC TGT TG-3’ | ||||
| Albumin | S: 5’-ATA CAC CCA GAA AGC ACC TC-3’ | 60 °C; 30× | 416 bp | 13 |
| A: 5’-CAC GAA TTG TGC GAA TGT CAC-3’ |
RESULTS
Culture morphology
rMSC cultured alone attached to the culture substratum within 3 h and grew to confluency within 3 d as long, spindle-shaped cells. Within 3 wk, they adopted a polygonal cell morphology (Figure 2A). Freshly isolated hepatocytes were round in shape and showed a high nuclear to cytoplasm ratio. A minimal proportion of cells attached within the 1st day to the culture substratum. In the first week of culture, the cells tend to form clumps of rounded cells. After 3 wk, only few viable cells were found in the cultures of hepatocytes alone (Figure 2B). In mixed cultures, mainly mesenchymal cells attached to the culture substratum, whereas hepatocytes attached to the mesenchymal cell layer forming clusters (Figure 2C). Hepatocytes were smaller in size compared to mesenchymal stem cells. From the second week on wards big polygonal shaped diploid (Figure 2D) cells were observed in the attached cell layer. As revealed by morphological studies, attached and adjacent cells were still viable after 21 d in the cocultures.
Figure 2.
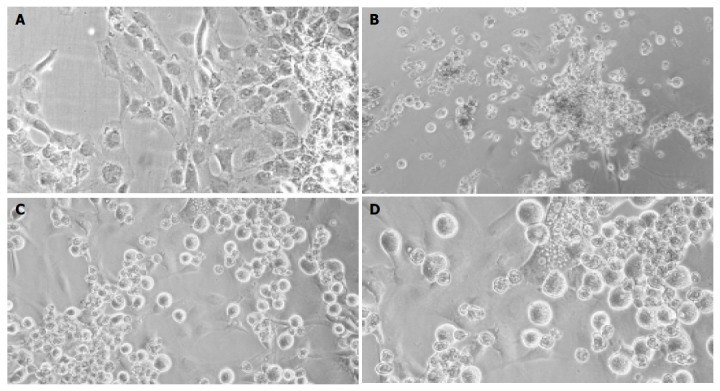
Light-microscopic pictures of cultured cells. Cultured rMSC (A), hepatocyte controls (B), or cocultures of rMSC with hepatocytes (C + enlargement D) after 3 wk. Cultured rMSC grew adherent and adopted a polygonal cell morphology after weeks in culture (A). Hepatocytes formed clumps of rounded cells, and few viable cells were observed after 3 wk in culture (B). In mixed cultures, mainly MSC attached to the substratum, whereas hepatocytes grew over the MSC-layer forming clusters (C). Binucleated cells were found within the attached layer of the cultured MSC beginning with 1 wk in culture. Shown is one example at wk 2 out of three experiments. Original magnification ×200.
Gene-expression of GFP+ rMSC
GFP-transduced rat MSC (≥P9) showed no expression of the liver specific genes, CK-18, CK-19, albumin, or AFP, as detected by RT-PCR (Figure 3, lanes M). In contrast, fetal liver cells as positive control cells revealed a constant expression of all markers analyzed (Figure 3, lanes C).
Figure 3.
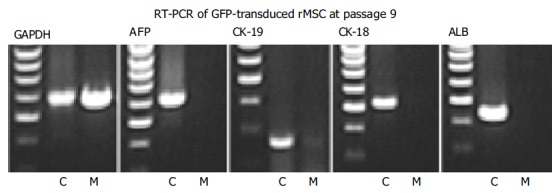
RT-PCR analysis of cloned GFP+ rMSC. rMSC (M) and liver control cells (C) were investigated before differentiation. GAPDH showed a strong signal in both liver controls and rMSC, whereas no liver specific genes (AFP, CK-19, CK-18, and albumin ALB) were expressed in GFP+rMSC (results from one out of three experiments).
FAC sorting of cocultured cells
The sorting strategy aimed at maximal purity of GFP+ cells to exclude contamination with hepatocyte-derived RNA used in the RT-PCR. A representative example for FACS-sorting 2 wk after coculture from 1 out of 3 experiments is shown in Figure 4. First, the viable cells were gated according to forward and side scatter properties (Figure 4A). Two populations were seen, which varied in size and granulation. GFP-expression of this gated cells were detected in two populations, differing in granulation (Figure 4B) and were gated as P2. From these P2-cells, the contaminating GFP-cells were sorted out (Figure 4C). All non-P1, non-P2 and non-GFP cells were collected as GFP-cells.
Figure 4.
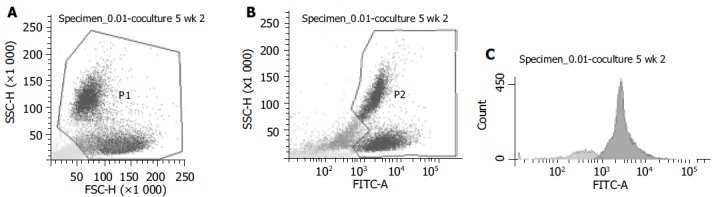
FACS-pictograms. One example out of three experiments from sorting of GFP+ and GFP- cells of cocultures at wk 2 is shown. A: Viable cells were gated as P1; B: P1 cells were gated in GFP- or GFP+ (P2); C: For highest purification of GFP+ cells, P2 was analyzed and only GFP+ cells were sorted in the sample tube for PCR-analysis (dark gray peak). 80.8% of cells from P2 were GFP+(sorted into the GFP+ test tube) and 19.2% were GFP-.
Comparing the number of seeded cells set as 100%, recovered cell numbers were decreased at all three time points in two experiments (Figure 5). In one experiment, cocultured cells proliferated between wk 1 and 2 leading to a non-significant increase at d 14 in the mean of all experiments. An identical picture was observed for sorted GFP+ and GFP- cells: in 2 wk a slight increase in the number of GFP-cells and the maintenance of GFP+ counts could be observed. It is important to note that the GFP-cell population contained not only hepatocytes but also all dead/dying cells as well as conflicting events.
Figure 5.
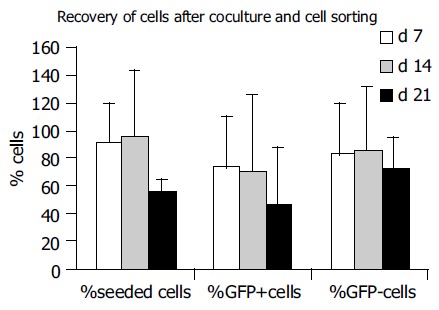
Recovery of cultured cells in cocultures. Recovery of seeded cells decreased from 91.6±28.8 % in the 1st wk towards 56.7±8.6 % in the 3rd wk (data from 3 experiments). Recovery of GFP+ cells decreased from 72.2 ± 38.5% to 46.3±41.6%, and recovery of GFP-cells from 81.9±38.2 % to 71.5±24.3 % from the 1st to the 3rd wk in culture, respectively.
Gene-expression of Thy1 in GFP+ rMSC cocultured with hepatocytes
GFP+ and GFP-cells from cocultures showed a stable Thy1 gene-expression (Figure 6A) over the whole observation period (lanes GFP-or GFP+ cocultures). Also, in cultures of rMSC, a stable Thy1 expression was observed (lane rMSC), whereas hepatocytes showed no Thy1 expression (Figure 6A, lane rHep).
Figure 6.
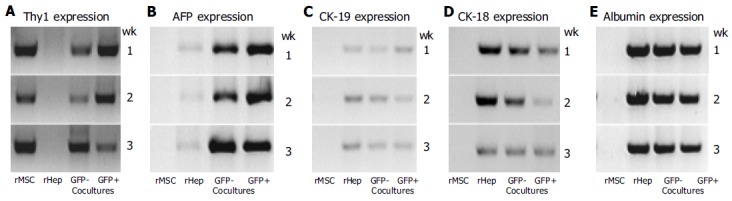
Gene expression profile. Cultured rMSC, hepatocytes (rHep), and GFP+ or GFP- cells of the cocultures after 1-3 wk were investigated for their expression of the stem cell marker Thy1 (A) and of the liver specific markers AFP (B), CK-19 (C), CK-18 (D), or albumin (E). Results from one out of three experiments are shown. rMSC (lane rMSC), GFP- (lane GFP- cocultures) or GFP+ (lane GFP+ cocultures) cells showed a stable Thy1 gene expression over the whole observation period, whereas hepatocytes (lane rHep) showed no signal for Thy1 gene expression in the RT-PCR analysis (A). In cultures of hepatocytes, GFP- or GFP+ cells show, the expression of the studied liver markers was observed, whereas in cultured rMSC none of the studied liver specific genes was observed at all time points (B-E). Cultured hepatocytes expressed AFP and CK-19 weakly (B and C), and CK-18 (D) and albumin (E) stable. GFP- cells from cocultures showed a stable expression of AFP (B), CK-19 (C) or albumin (E), and a weak signal for CK-18 expression (D). The GFP+ cells showed a stable expression of the liver specific genes AFP (B), CK-18 (D), and albumin (E), and a weak expression of CK-19 (C) over the whole observation period.
Liver specific gene-expression (AFP, CK-19, CK-18, albumin)
GAPDH expression in cultured rMSC, hepatocytes, GFP+ and GFP-FACS-sorted cells was similar at all time points (not shown). Although we used the same amounts of cDNA for all analyses, we found slightly different signals. Therefore, only semiquantitative assessments were possible. Cultures of rMSC alone showed no expression for AFP, CK-19, CK-18, or albumin (Figure 6B-E, lane rMSC). Cultured hepatocytes were found to express AFP and CK-19 weakly, and CK-18 and albumin stable over the whole culture period (Figure 6 B-E, lane rHep). In mixed cultures, the GFP-cells (lane GFP-cocultures) showed a strong expression of AFP (Figure 6B), albumin (Figure 6E) and CK-19 (Figure 6C), and a weak expression of CK-18 (Figure 6D). The GFP+ cells (lane GFP+ cocultures) showed a stable expression of AFP (Figure 6B), CK-18 (Figure 6D), and albumin (Figure 6E), and weak expression of CK-19 (Figure 6C), respectively. Negative controls without template were negative at all times and probes (not shown).
DISCUSSION
In this study, cloned GFP+ rMSC from passage ≥9 were used to analyze hepatic differentiation potential of MSC. For liver specific differentiation, cells were cultured on a fibronectin matrix in serum-free medium containing the hepatocytic growth factors- HGF, EGF, FGF-4, and the stem cell growth factor- SCF. The impact of liver cells on hepatic differentiation was assessed in cocultures of GFP+ rMSC with freshly isolated rat hepatocytes (GFP-negative) and was compared to pure cultures of rMSC or hepatocytes, respectively. For gene-expression analysis, cells from cocultures were separated in GFP+ or GFP-cells by FACS-sorting before RT-PCR analysis (Figure 1).
AFP in the liver is a marker of immature (e.g. fetal) liver cells or oval cells in adult liver[36]. CK19 has been shown to be expressed on hepatic oval cells as well as on adult biliary epithelial cells. Albumin is a typical marker of mature hepatocytes, whereas CK18 is expressed by several liver cells types, including biliary epithelial cells and hepatic oval cells[37]. Our data indicated, that rMSC possess a differentiation potential towards hepatocytic cells in vitro; expression of the liver specific genes CK-18, albumin, CK-19, and AFP was demonstrated in GFP+ cells of the cocultures for the observation period of 3 wk. Thy1 is a marker for CD34-positive stem cells, activated endothelial cells and fetal liver cells[38,39]. In the adult liver, Thy1 is expressed on oval cells but not on mature hepatocytes[37,40]. It has also been found on mesenchymal stem cells generated from rat bone marrow[41,42]. Expression of Thy1 in cultured MSC suggests that the rMSC should have the potential to differentiate toward hepatic cells by exhibition of a liver stem cell-like gene expression profile, as it is found in adult liver stem cells (oval cells). Oval cells are tissue residing liver stem cells, which possess a bipotential ability to generate biliary and hepatocytic cells, which can clonally repopulate the regenerating liver under certain conditions (reviewed in Ref.[37]). Also, for other multipotential stem cell types from bone marrow, a hepatocytic differentiation potential was shown: sorted hematopoietic stem cells[14,23], side population cells in the liver derived from bone marrow[43], or multipotential adult progenitor cells[30] were found to express liver specific genes, when cultured in the presence of certain growth factors. Previous studies in our laboratory have shown the tri-lineage different-ation potential of the cloned rMSC used, e.g. into adipogenic, chondrogenic, or osteogenic cells[41]. In the present study, an additional differentiation potential towards hepatic progenitor cells was shown for the first time for clonally derived MSC. Thus, MSC seem to possess a multilineage differentiation potential, including a potential to differentiate into hepatocyte-like cells at least in vitro.
In our study, we found that hepatocyte-specific gene expression was induced by the coculture of isolated hepatocytes. Cultured rMSC alone did not express any of the liver specific genes studied in the presence of a FN-coating and hepatic growth factors, suggesting that the added growth factors are not sufficient to induce hepatic differentiation. In contrast, a hepatocyte-like gene expression profile was observed in rMSC-derived cells in cocultures with hepatocytes. Additionally, we found binucleated GFP+ cells beginning with wk 1 (in the adult rat liver ≈10% are binucleated cells). Very recently, a first report described the differentiation of rMSC into hepatocytic cells induced by growth factors. However, the authors used non-clonal cells of early passages. According to our experience, MSC isolated from bone marrow of rodents in early passages still contain hematopoietic cells. Thus, in these experiments a differentiation of contaminating cells into hepatic lineages cannot be conclusively excluded. In our experiments, a differentiation of MSC into hepatic cells without coculture was not successful. This could be due to either an impaired differentiation capability of the chosen clonal MSC or the high purity of MSC without contamination of HSC.
It has been shown that MSC express HGF as well as the respective receptor, c-met[44]. Thus, MSC should be accessible for differentiation induction via this pathway. Furthermore, cocultured liver cells (GFP-) also showed a stable liver specific gene expression and viability over the whole observation period, whereas the hepatocyte controls rapidly lost cell viability and differentiation within the first week of culture. This is consistent with findings of other groups that investigated the differentiation of stem cells into hepatic lineages using coculture models. A positive influence for the induction of hepatocyte-specific genes in stem cells was found to be dependent on the presence of hepatocytes, even in cultures with separation of the cells by a semi-permeable membrane[23,24]. A coculture of hepatocytes with stromal bone marrow cells allowing cell-cell contacts was first established by Mizuguchi et al[45]. They found that in cocultures the proliferation of hepatocytes and small hepatocytes was significantly enhanced by marrow stromal cells compared to controls. Furthermore, a prolonged hepatocyte-specific gene expression was demonstrated by analysis of the markers, albumin[45] and tryptophan-dioxygenase, and activated hepatocyte-specific transcription factors were revealed by Northern blot analysis. By immunolabeling, the presence of Jagged1 protein was found in the cocultured marrow stromal cells[24] and it was suggested to mediate differentiation events via the Notch signal pathway. However, no analysis of differentiation of the cocultured MSC was performed. Okumoto et al[24], highlighted the role of the Jagged1 and Notch expression as one pathway of bone marrow cell differentiation towards hepatocytic cells. Thus, a liver-specific differentiation of stroma cells might be possible but remained unknown from these studies. The cells used in our study were similarly generated from bone marrow, at least they were characterized by plastic adherence and clonal expansion. It is important to note, that the coculture of MSC and hepatocytes seemed also to improve the viability of the cultured hepatocytes.
Our in vitro data indicate that mesenchymal stem cells from rat bone marrow possess a differentiation capacity towards hepatocytic cells in vitro. Furthermore, we showed a strong influence of cocultures with isolated liver cells permitting cell-to-cell contacts for the induction of liver specific gene expression of cultured stem cells. Bone marrow-derived liver stem cells, and herein the MSC are promising candidates for new cell-based approaches for the treatment of liver diseases[46]: (1) these cells can easily be harvested from adult bone marrow and expanded tremendiously in vitro; (2) transduction of MSC may result in the expansion of “cured” daughter cells; (3) the use of adult stem cells is favorable over other stem cells, such as embryonic stem cells or fetal stem cells, regarding ethical issues; (4) MSC have been proven to suppress T-cell activation in unrelated donor-recipient situations and seem to be an alternative in some areas of tissue regeneration[47]. The differentiation of such cells toward liver cells by cocultures may permit the generation of artificial liver tissue for tissue engineering of the liver or liver cell transplantation[48]. In our experiments, we have not investigated the influence of fusion on differentiation of MSC into hepatic cells. Still, the debate about fusion or true 搕ransdifferentiation continues. For liver cells, fusion seems to be a common process. However, for our experimental setting the mechanism remains to be explored in future. But even in the case of fusion, the generated cells would be equipped with two new informations: expansion and hepatic differentiation. Thus, the potential of MSC to differentiate towards potential liver cells should be of high interest for new cell-based therapies. Therefore, coculture of rMSC with hepatocytes is a model worth studying for further characterization of the mechanisms underlying a differentiation of stem cell into the hepatocytic lineage.
ACKNOWLEDGMENTS
We thank Arne Düsedau, Heinrich Pette Institute of the University of Hamburg, for FACS-sorting and Mrs. B. Roth, Department of Pediatric Surgery, for technical assistance.
Footnotes
Supported by the “Rudolf Bartling Foundation” and “Foerderge-meinschaft Kinder-Krebs-Zentrum Hamburg e.V.”
Language Editor Elsevier HK
References
- 1.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 2.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- 3.Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- 4.Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- 5.Körbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, Champlin RE, Estrov Z. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346:738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 6.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 8.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Ge S, McNamara G, Hao QL, Crooks GM, Nolta JA. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003;101:4201–4208. doi: 10.1182/blood-2002-05-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa F, Drake CJ, Yang S, Fleming P, Minamiguchi H, Visconti RP, Crosby CV, Argraves WS, Harada M, Key LL, et al. Transplanted human cord blood cells give rise to hepatocytes in engrafted mice. Ann N Y Acad Sci. 2003;996:174–185. doi: 10.1111/j.1749-6632.2003.tb03245.x. [DOI] [PubMed] [Google Scholar]
- 11.Newsome PN, Johannessen I, Boyle S, Dalakas E, McAulay KA, Samuel K, Rae F, Forrester L, Turner ML, Hayes PC, et al. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology. 2003;124:1891–1900. doi: 10.1016/s0016-5085(03)00401-3. [DOI] [PubMed] [Google Scholar]
- 12.Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 13.Oh SH, Miyazaki M, Kouchi H, Inoue Y, Sakaguchi M, Tsuji T, Shima N, Higashio K, Namba M. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun. 2000;279:500–504. doi: 10.1006/bbrc.2000.3985. [DOI] [PubMed] [Google Scholar]
- 14.Fiegel HC, Lioznov MV, Cortes-Dericks L, Lange C, Kluth D, Fehse B, Zander AR. Liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells. 2003;21:98–104. doi: 10.1634/stemcells.21-1-98. [DOI] [PubMed] [Google Scholar]
- 15.Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu-Saito K, Hara Y, Teramoto K, Arii S, Sato C, Takase K, et al. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003;21:217–227. doi: 10.1634/stemcells.21-2-217. [DOI] [PubMed] [Google Scholar]
- 16.Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos GK. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reid LM. Stem cell biology, hormone/matrix synergies and liver differentiation. Curr Opin Cell Biol. 1990;2:121–130. doi: 10.1016/s0955-0674(05)80042-0. [DOI] [PubMed] [Google Scholar]
- 18.Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. J Cell Physiol. 1992;151:497–505. doi: 10.1002/jcp.1041510308. [DOI] [PubMed] [Google Scholar]
- 19.Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB J. 1996;10:1471–1484. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- 20.Guguen-Guillouzo C, Clément B, Baffet G, Beaumont C, Morel-Chany E, Glaise D, Guillouzo A. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp Cell Res. 1983;143:47–54. doi: 10.1016/0014-4827(83)90107-6. [DOI] [PubMed] [Google Scholar]
- 21.Shimaoka S, Nakamura T, Ichihara A. Stimulation of growth of primary cultured adult rat hepatocytes without growth factors by coculture with nonparenchymal liver cells. Exp Cell Res. 1987;172:228–242. doi: 10.1016/0014-4827(87)90109-1. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki M, Akiyama I, Sakaguchi M, Nakashima E, Okada M, Kataoka K, Huh NH. Improved conditions to induce hepatocytes from rat bone marrow cells in culture. Biochem Biophys Res Commun. 2002;298:24–30. doi: 10.1016/s0006-291x(02)02340-9. [DOI] [PubMed] [Google Scholar]
- 23.Avital I, Inderbitzin D, Aoki T, Tyan DB, Cohen AH, Ferraresso C, Rozga J, Arnaout WS, Demetriou AA. Isolation, characterization, and transplantation of bone marrow-derived hepatocyte stem cells. Biochem Biophys Res Commun. 2001;288:156–164. doi: 10.1006/bbrc.2001.5712. [DOI] [PubMed] [Google Scholar]
- 24.Okumoto K, Saito T, Hattori E, Ito JI, Adachi T, Takeda T, Sugahara K, Watanabe H, Saito K, Togashi H, et al. Differentiation of bone marrow cells into cells that express liver-specific genes in vitro: implication of the Notch signals in differentiation. Biochem Biophys Res Commun. 2003;304:691–695. doi: 10.1016/s0006-291x(03)00637-5. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 26.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 27.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 28.Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci USA. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Schwieger M, Lange C, Kraunus J, Sun H, van den Akker E, Modlich U, Serinsöz E, Will E, von Laer D, et al. Predictable and efficient retroviral gene transfer into murine bone marrow repopulating cells using a defined vector dose. Exp Hematol. 2003;31:1206–1214. doi: 10.1016/j.exphem.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Kühlcke K, Fehse B, Schilz A, Loges S, Lindemann C, Ayuk F, Lehmann F, Stute N, Fauser AA, Zander AR, et al. Highly efficient retroviral gene transfer based on centrifugation-mediated vector preloading of tissue culture vessels. Mol Ther. 2002;5:473–478. doi: 10.1006/mthe.2002.0566. [DOI] [PubMed] [Google Scholar]
- 34.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 35.Kaufmann PM, Kneser U, Fiegel HC, Pollok JM, Kluth D, Izbicki JR, Herbst H, Rogiers X. Is there an optimal concentration of cotransplanted islets of Langerhans for stimulation of hepatocytes in three dimensional matrices? Transplantation. 1999;68:272–279. doi: 10.1097/00007890-199907270-00020. [DOI] [PubMed] [Google Scholar]
- 36.Brill S, Holst P, Sigal S, Zvibel I, Fiorino A, Ochs A, Somasundaran U, Reid LM. Hepatic progenitor populations in embryonic, neonatal, and adult liver. Proc Soc Exp Biol Med. 1993;204:261–269. doi: 10.3181/00379727-204-43662. [DOI] [PubMed] [Google Scholar]
- 37.Thorgeirsson SS. Hepatic stem cells in liver regeneration. FASEB J. 1996;10:1249–1256. [PubMed] [Google Scholar]
- 38.Fiegel HC, Park JJ, Lioznov MV, Martin A, Jaeschke-Melli S, Kaufmann PM, Fehse B, Zander AR, Kluth D. Characterization of cell types during rat liver development. Hepatology. 2003;37:148–154. doi: 10.1053/jhep.2003.50007. [DOI] [PubMed] [Google Scholar]
- 39.Fiegel HC, Kluth J, Lioznov MV, Holzhüter S, Fehse B, Zander AR, Kluth D. Hepatic lineages isolated from developing rat liver show different ways of maturation. Biochem Biophys Res Commun. 2003;305:46–53. doi: 10.1016/s0006-291x(03)00662-4. [DOI] [PubMed] [Google Scholar]
- 40.Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology. 1998;27:433–445. doi: 10.1002/hep.510270218. [DOI] [PubMed] [Google Scholar]
- 41.Lange C, Jaquet K, Krause K, Kuck KH, Zander AR. Myogenic differentiation of rat mesenchymal stem cells [abstract] Exp Hematol. 2003;31(Suppl 1):181. [Google Scholar]
- 42.Javazon EH, Colter DC, Schwarz EJ, Prockop DJ. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19:219–225. doi: 10.1634/stemcells.19-3-219. [DOI] [PubMed] [Google Scholar]
- 43.Wulf GG, Luo KL, Jackson KA, Brenner MK, Goodell MA. Cells of the hepatic side population contribute to liver regeneration and can be replenished with bone marrow stem cells. Haematologica. 2003;88:368–378. [PubMed] [Google Scholar]
- 44.Neuss S, Becher E, Wöltje M, Tietze L, Jahnen-Dechent W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22:405–414. doi: 10.1634/stemcells.22-3-405. [DOI] [PubMed] [Google Scholar]
- 45.Mizuguchi T, Hui T, Palm K, Sugiyama N, Mitaka T, Demetriou AA, Rozga J. Enhanced proliferation and differentiation of rat hepatocytes cultured with bone marrow stromal cells. J Cell Physiol. 2001;189:106–119. doi: 10.1002/jcp.1136. [DOI] [PubMed] [Google Scholar]
- 46.Mitaka T. Hepatic stem cells: from bone marrow cells to hepatocytes. Biochem Biophys Res Commun. 2001;281:1–5. doi: 10.1006/bbrc.2001.4270. [DOI] [PubMed] [Google Scholar]
- 47.Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, Koç ON. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 48.Mitaka T. Reconstruction of hepatic organoid by hepatic stem cells. J Hepatobiliary Pancreat Surg. 2002;9:697–703. doi: 10.1007/s005340200096. [DOI] [PubMed] [Google Scholar]


