Abstract
AIM: To produce an antibody against rat eosinophil cationic protein (ECP) and to examine the effects of the antibody in rats with dextran sulfate sodium (DSS)-induced colitis.
METHODS: An antibody was raised against rat ECP. Rats were treated with 3% DSS in drinking water for 7 d and received the antibody or normal serum. The colons were examined histologically and correlated with clinical symptoms. Immunohistochemistry and Western blot analysis were estimated as a grade of inflammation.
RESULTS: The ECP antibody stained the activated eosinophils around the injured crypts in the colonic mucosa. Antibody treatment reduced the severity of colonic ulceration and acute clinical symptoms (diarrhea and/or blood-stained stool). Body weight gain was significantly greater and the colon length was significantly longer in anti-ECP-treated rats than in normal serum-treated rats. Expression of ECP in activated eosinophils was associated with the presence of erosions and inflammation. The number of Ki-67-positive cells in the regenerated surface epithelium increased in anti-ECP-treated rats compared with normal serum-treated rats. Western blot analysis revealed reduced expression of macrophage migration inhibitory factor (MIF) in anti-ECP-treated rats.
CONCLUSION: Our results indicate that treatment with ECP antibody, improved DSS-induced colitis in rats, possibly by increasing the regenerative activity of the colonic epithelium and downregulation of the immune response, and suggest that anti-ECP may promote intestinal wound healing in patients with ulcerative colitis (UC).
Keywords: Ulcerative colitis, Eosinophil cationic protein, Dextran sulfate sodium
INTRODUCTION
Eosinophil accumulation in the gastrointestinal tract is a common feature of numerous gastrointestinal disorders, including classic IgE-mediated food allergy, eosinophilic gastroenteritis, allergic colitis, eosinophilic esophagitis, inflammatory bowel disease (IBD)[1-3] and gastroesophageal reflux disease. In IBD, eosinophils usually represent only a small percentage of the infiltrating leukocytes[3,4] but their levels has been proposed as a negative prognostic indicator[4,5]. Several studies have found an association between allergic colitis and later development of IBD, but this association is controversial[6].
Eosinophils and one of their granule proteins, eosinophil cationic protein (ECP)[7] and eosinophil protein X (EPX), are generally recognized as being involved in the host defense against invading parasites. They are markedly cationic proteins with cytotoxic capacities that can potentially cause tissue destruction and could act as modulators of immune response[8]. The eosinophil may also be involved in the pathogenesis of IBD because we have already reported activation of eosinophils in patients with active ulcerative colitis (UC), using the techniques of indirect immunoenzymatic method and electron microscopic examination of eosinophils, measurement of serum ECP[9,10], as well as increased percentages of hypodense eosinophils in the peripheral blood. Moreover, bowel biopsies from patients with IBD have demonstrated infiltration of eosinophils in the lamina propria and marked extracellular deposits of ECP[11,12]. There is also an excess release of the eosinophil proteins ECP and EPX in the luminal fluid and fecal material of patients with UC or Crohn's disease[13]. However, the pathophysiological role played by activated eosinophils in the inflammatory process in UC has not yet been elucidated.
The aim of this study was to produce an antibody against rat ECP and to examine the antibody in dextran sulfate sodium (DSS)-induced colitis model. Rats treated with DSS develop severe colorectal damage mimicking IBD in humans[14,15]. We herein present evidence for the effectiveness of ECP antibody against DSS-induced colitis in rats, possibly by increasing the regenerative activity of the colonic epithelium and downregulation of the immune response.
MATERIALS AND METHODS
Antibody raised against rat ECP
TRAQWFAIQHISLNPPR[16] was synthesized based on human ECP amino acid sequence[17], which has homology to rat ECP[18]. Anti-ECP sera were generated by immunizing New Zealand white rabbits. In brief, rabbits were inoculated intradermally with 100 µg of ECP diluted in complete Freund’s adjuvant at weeks 2, 4, 6, 8, 10, 12, and 14. The serum was prepared according to the protocol provided by the manufacturer.
Induction of experimental colitis
Male Wistar rats/IZM (8 wk old) were obtained from Charles River Japan, Inc. Rats were kept in a specific pathogen-free environment at the Animal Center in accordance with the rules and regulations of the Institutional Animal Care and Use Committee of Nagasaki University. Food as well as drinking water with or without DSS (MW 5 000; Wako Pure Chemical Industries, Osaka, Japan) were provided ad libitum. The rats were treated with 3% DSS in drinking water for 7 d[19], and then received either antibodies or normal serum intraperitoneally. The rats were weighed daily and visually inspected for gross rectal bleeding and diarrhea. The colons were examined histologically and correlated with clinical symptoms. Hematoxylin and eosin (HE)-stained sections were prepared from the distal colon. The severity of ulceration was quantified by the Ulcer Index (ulcer length/circumference length, %) in the distal colon. Furthermore, the severity of colitis was evaluated by assessment of colon length and histological examination. Similarly, we evaluated the effect of anti-ECP antibodies with regard to clinical signs and pathologic features.
Administration of antibodies specific for ECP
The polyclonal ECP antibody (0.25 mL/rat at d 0 and 1, 0.5 mL/rat at d 2, 3, 4, 5, 6, and 7 after DSS treatment) or non-immune rabbit serum was injected intraperitoneally.
Immunohistochemical studies
Anti-ECP antibody as eosinophils, anti-ED1 antibody (Serotic, Oxford, UK) as activated macrophages, anti-Ki-67 antibody (MIB-5, Dako Cytomation Denmark A/S, Denmark) as proliferative cells were used. The sections were deparaffinized and rehydrated. Then, they were microwaved for 10 min in 0.01 mol/L citrate buffer, pH 6.0, to unmask the antigenicity, and trypsin digested for 15 min, for Ki-67 and for ED1 as pre-treatment, respectively. The slides were placed in methanol with 0.3% H2O2 for 10 min to block endogenous peroxidase activity. They were then incubated with ECP, Ki-67, and ED1 for 60 min and further processed for immunohistochemistry using the Histofine Simple Stain kit (Nichirei, Tokyo, Japan). Peroxidase activity was developed in diaminobenzidine as a chromogen. In the procedures described above, tissue sections that were not incubated with ECP, ED1, or Ki-67 served as negative controls.
Western blot analysis
Western blots were prepared for expression of ED1 and macrophage migration inhibitory factor (MIF). For this purpose, the colonic tissue was collected from the lesion area, and then suspended in 5 volumes of ice-cold 50 mmol/L Tris-HCl (pH 7.2) containing 150 mmol/L NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.05% SDS, broken into pieces on ice, and subjected to three freeze-thaw cycles. After centrifugation at 15 000 g for 10 min at 4 °C, 2-mercaptoethanol and bromophenol blue were added to the supernatant at final concentrations of 2% and 0.001%, respectively. The tissue extracts (30 μg protein) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the separated proteins transferred onto Hybond ECL nitrocellulose membrane. After blocking nonspecific binding sites with 5% skim milk, the membrane was incubated with 1 000 diluted antibody, against ED1 and MIF (N-20, Santa Cruz Biotechnology, Inc.) at room temperature for 1 h. The bound antibodies were detected using an enhanced chemiluminescence detection kit (ECL Plus, Amersham Life Science, Buckinghamshire, UK) and the amount of each protein was quantified by densitometric analysis[20].
Statistical analysis
All data were expressed as mean±SE. Differences between groups were examined for statistical significance using the Student’s t-test or Cochran and Cox test. A P value less than 0.05 denoted the presence of a statistically significant difference.
RESULTS
Clinicopathological findings
The body weight gain was significantly greater in anti-ECP-treated rats at d 4 and 7 after DSS treatment compared with normal serum-treated rats (Figure 1). ECP antibody clearly reduced acute clinical symptoms (diarrhea and/or blood-stained stool, Table 1). Moreover, ECP antibody improved various mucosal parameters; it reduced colonic ulceration severity (Ulcer Index) from 61.1±25.5% (DSS) to 43.1±29.4% (DSS+anti-ECP). The colon length was 13.4±0.4 cm in anti-ECP-treated rats and 12.3±0.6 cm in normal serum-treated rats at 7 d after DSS treatment (Table 2). DSS-induced shortening of the colon length was significantly abrogated in anti-ECP-treated rats (P<0.05).
Figure 1.
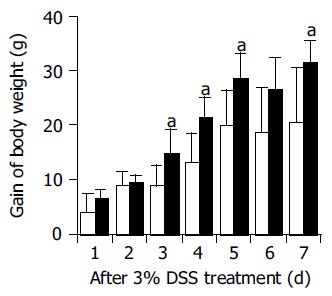
Changes in body weight in normal serum- (open columns), anti-ECP- (closed columns) treated rats during DSS treatment. The body weight gain after DSS treatment was significantly greater in anti-ECP-treated rats compared with normal serum-treated rats. Rats treated with the antibodies or normal serum intraperitoneally were also treated with 3% DSS in drinking water for 7 d (n = 7, aP<0.05 vs DSS+normal serum).
Table 1.
Clinical findings
| Time (d) |
Gross bleeding |
Diarrhea |
||
| NS | Anti-ECP | NS | Anti-ECP | |
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 14.3 | 14.3 | 14.3 |
| 3 | 14.3 | 0 | 14.3 | 14.3 |
| 4 | 0 | 0 | 0 | 0 |
| 5 | 14.3 | 0 | 28.6 | 14.3 |
| 6 | 42.9 | 14.3 | 28.6 | 0 |
| 7 | 42.9 | 28.6 | 0 | 0 |
Data are percentage of animals (n = 7).
Table 2.
Effects of anti-ECP antibody on ulcer index and colon length (mean±SE, n = 7)
| Group | Ulcer index (%)1 | Colon length (cm) |
| DSS+normal serum | 61.1±25.5 | 12.3±0.6 |
| DSS+anti-ECP | 43.1±29.4a | 13.4±0.4b |
Ulcer length/circumference length´100.
P<0.05 vs DSS+normal serum.
P<0.01 vs DSS+normal serum.
ECP expression in normal colonic mucosa and in colitis
HE- and immunohistochemically-stained sections showed eosinophils and ECP-positive eosinophils in close proximity to damaged crypts in the lamina propria and partially in the extracellular interstitium of the colon of rats with DSS-induced colitis (Figures 2A and B). The ECP antibody stained activated eosinophils. In the normal colon, eosinophil infiltration was hardly observed and ECP-positive activated eosinophils were never seen in the colonic tissue (data not shown).
Figure 2.
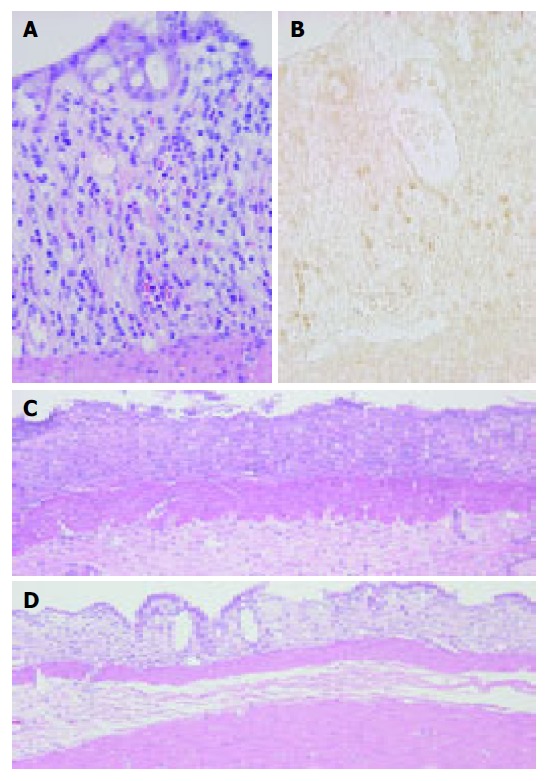
Colonic mucosa at 3 d post-DSS treatment showing eosinophils in the lamina propria. HE staining (A) and immunostaining for ECP (B) as described under Materials and methods. ECP-positive cells (brown) appear in close proximity to damaged crypts in the lamina propria and partially in the extracellular interstitium of DSS-induced colitis. Original magnification, ×400. Colonic mucosa at 7-d treatment of DSS. (C) Colonic ulceration in normal serum-treated rats. (D) Reduced severity of colonic mucosal ulceration in anti-ECP-treated rats. HE stain. Original magnification, ×100.
Histological findings
ECP antibody treatment clearly suppressed DSS-induced colitis as confirmed in HE-stained sections (Figures 2C and D). Moreover, immunohistochemical studies for Ki-67 and ED1 revealed that the ECP antibody treatment increased the regenerative activity of the colonic epithelium and downregulation of immune response (Figure 3). Expression of ECP in activated eosinophils was associated with the presence of erosions and inflammation. The number of Ki-67-positive cells in the regenerative surface epithelium/circumference significantly increased in anti-ECP-treated rats (21.3±10.4) compared with normal serum-treated rats (14.0±6.5) at d 7 after DSS treatment (P<0.05, Table 3 and Figure 3).
Figure 3.
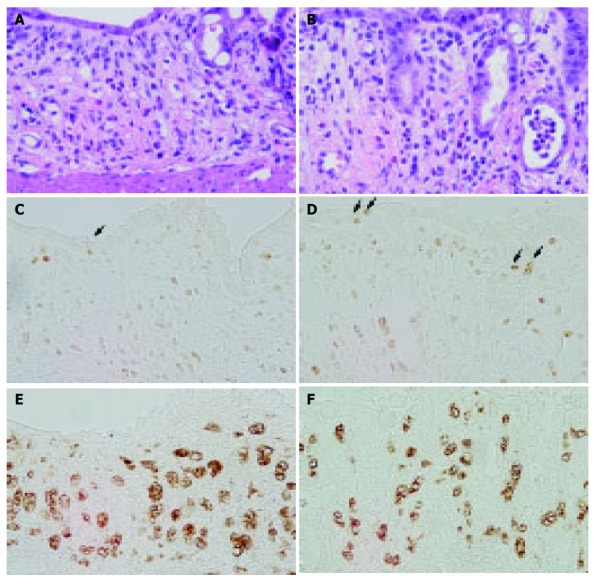
Colonic mucosa in normal serum-treated rats (A, C, and E) and anti-ECP1-treated rats (B, D, and F) at 7-d treatment of DSS. HE staining (A and B), immunostaining for Ki-67 (C and D) and ED1 (E and F) as described under Materials and methods. Treatment with ECP antibody increased the number of Ki-67-positive cells in the regenerated surface epithelium and reduced the size of activated macrophages present in the lamina propria. Original magnification, ×400.
Table 3.
Effects of anti-ECP antibody on the Ki-67-positive cells in the regenerative surface of colonic epithelium at d 7 after DSS treatment (mean±SE, n = 7)
Number of Ki-67-positive cells/circumference.
P<0.05 vs DSS+normal serum.
The number of ED1-positive macrophages in the lamina propria of anti-ECP-treated rats at d 7 after DSS treatment was less than that in normal serum-treated rats. Moreover, the size of ED1-positive macrophages in the lamina propria of anti-ECP-treated rats at d 7 after DSS treatment was smaller than that of normal serum-treated rats.
Western blot analysis
ECP antibody treatment significantly downregulated macrophage activation during 7-d treatment of DSS (Figure 4). The expressions of both ED1 and MIF in the damaged colonic tissue at d 7 after DSS treatment decreased significantly in anti-ECP-treated rats. The relative ED1 expression was significantly lower in anti-ECP-treated rats (1.25±0.79) than in normal serum-treated rats (3.62±1.83, P<0.05, n = 7). Furthermore, the relative expression of MIF was significantly lower in anti-ECP-treated rats (0.74±0.08) than in normal serum-treated rats (0.94±0.08, P<0.05, n = 7).
Figure 4.
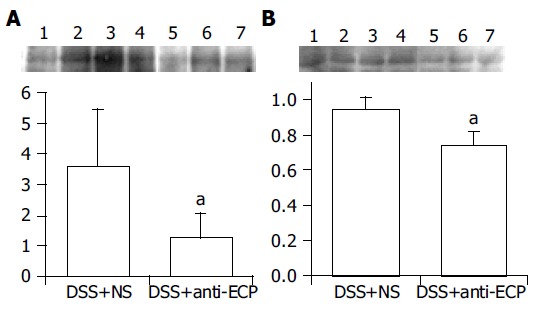
Detection of ED1 (A) and MIF (B) by Western blot analysis in colonic tissues after 7 d of DDS treatment in normal serum-treated rats (lanes 1-4) and anti-ECP-treated rats (lanes 5-7). The colonic tissue was collected from the lesion area and examined by Western blot analysis as described under Materials and methods. The relative expression levels of ED1 and MIF in the damaged colonic tissue were significantly lower in anti-ECP-treated rats than in normal serum-treated rats. The relative protein expression was quantified by densitometric analysis (n = 7, aP<0.05 vs DSS+normal serum).
DISCUSSION
A variety of clinical and experimental models has revealed that eosinophils promote pro-inflammatory changes mediated by their ability to release various cytotoxic substances and a variety of lipid mediators and cytokines. In type-2 helper T (Th2) cells-associated gastrointestinal inflammatory conditions, increased levels of eosinophils occur in the lamina propria in an eotaxin 1-dependent manner[21]. After mucosal allergen challenge, eosinophils under the regulation of IL-5 accumulate in the esophagus, an organ normally devoid of eosinophils. However, the main role of IL-5 in DSS-induced colonic inflammation is to attract a population of eosinophils that do not appear to contribute significantly to the initiation or development of tissue damage in this model of colitis[22].
In 1974, the existence of an eosinophil-granule protein, ECP, with a highly cytotoxic action, was reported by Olsson and Venge[7]. ECP is antibacterial[23], helminthotoxic[24], elicits the Gordon phenomenon when injected intrathecally into rabbits, and is cytotoxic to tracheal epithelium[25,26]. Although the mechanism of its cytotoxicity is not completely understood, it is suggested to be due to the pore-forming activity of ECP, which destabilizes lipid membranes[27], but is unrelated to RNase activity[28]. We demonstrated here that a polyclonal antibody to eosinophil granule protein ECP exhibits beneficial effects on various mucosal parameters of DSS-induced colitis in rats. ECP expressed in activated eosinophils of the colonic mucosa in DSS-induced colitis were associated with the presence of injured crypts and inflammation. The body weight gain was significantly greater in anti-ECP-treated rats, compared with control rats. The ECP antibody reduced acute clinical symptoms (diarrhea and/or blood-stained stool), severity of colonic ulceration and shortening of the colon length. Immunohistochemical studies for Ki-67 and ED1 revealed that ECP antibody treatment increased the regenerative activity of the colonic epithelium and downregulation of the immune response.
Mucosal repair involves both the rapid migration of cryptal enterocytes into the injured area of the mucosa, and replacement of the mucosa by cell replication[29]. Various peptide growth factors regulate epithelial cell function within the mucosal epithelium of the gastrointestinal tract. Recently, Sinha et al[30], reported preliminary data suggesting that epidermal growth factor enemas are effective in the treatment of active left-sided UC. Furthermore, Dignass et al[31], demonstrated that hepatocyte growth factor (HGF) modulates intestinal epithelial cell proliferation and migration, thus enhancing epithelial cell restitution, the initial step of gastrointestinal wound healing, in an in vitro model. Moreover, it was demonstrated that administration of recombinant human HGF lessened colitis-associated weight loss in rats as well as improved the clinical signs of colitis in vivo[32]. In our rat model, mucosal erosion was observed on d 5 and accelerated on d 7, whereas the colon length was reduced on d 7. Importantly, the ECP antibody enhanced epithelial regeneration, leading to a reduction in size of colonic mucosal erosions, although it was administered concomitantly with DSS. In this regard, enhanced mucosal repair allows for a more rapid recovery of epithelial barrier function leading to reduced exposure to various luminal agents that contribute to persistent colitis. Accordingly, treatment with ECP antibody should reduce the inflammatory response to these luminal stimuli.
MIF was originally identified as a lymphokine derived from activated T cells that inhibits the random migration of macrophages in vitro and is involved in delayed-type hypersensitivity[33]. Moreover, it is postulated that tumor necrosis factor (TNF)-α and interferon (IFN)-γ upregulate MIF production in macrophages and, conversely, MIF induces TNF-α production, forming a proinflammatory loop within the cytokine network[34]. Gut monocyte macrophages are responsible for production of MIF, which in turn stimulates Th-1-type cytokines and features characteristic of colitis. Swollen macrophages induced by DSS have been seen in the mucosa with gland dropout and inflammatory cell infiltration even under intact colonic epithelium[35]. It seems that macrophage dysfunction, alterations of luminal bacteria, and DSS toxic effects on the colonic epithelium acted together to result in inflammatory ulcerative changes of the colonic mucosa. Therefore, in our study, the ECP antibody reduced macrophage activation such as the size of ED1-positive macrophages and the expressions of ED1 and MIF proteins in damaged colonic mucosa induced by DSS, resulting in downregulation of the immune response.
ECP is one of the major components of eosinophilic granules with a molecular mass ranging from 16 to 21.4 ku. It exhibits various biological effects both in vitro and in vivo[8,36]. It is classified as a member of the ribonuclease (RNase) A supergene family based on the homology of both nucleotide and amino acid sequences. The homology of amino acid sequence between rat ECP[18] and human ECP[37] is 54%. When the amino acid sequence of rat ECP was compared with that of human ECP, eight structural cysteines and three amino acids, H15, K38, and H128, that are required for RNase activity[37] were found to be highly conserved. The length of the signal peptide of rat ECP is equivalent to that of human ECP. Maeda et al[38], demonstrated that ECP is growth-inhibitory and this ability of ECP to bind to heparin or other carboxy hydrates on the cell surface is dependent on tryptophan residues, W10 and W35. Interestingly, W10 is located at the P2 subsite of the catalytic domain of RNase and controls the weak RNase activity of the protein[39]. ECP alters the coagulation cascades[40], augments fibrinolysis[41], and regulates the classical pathway of complement[42]. Our results showed that the ECP antibody clearly reduced acute clinical symptoms of blood-stained stool. Our evaluation of the ECP molecule including W10 and H15 in human ECP enabled us to test the beneficial effects of anti-ECP treatment on inflammatory disease, in which uncontrolled cell growth could contribute to a delay in wound healing. Further studies are needed to investigate this issue.
In summary, we demonstrated that treatment with an antibody to ECP improved DSS-induced colitis in rats possibly by increasing the regenerative activity of the colonic epithelium and downregulation of the immune response. This supports the concept that humanized anti-ECP treatment could also be an effective therapy for IBDs.
Footnotes
Supported by a grant-in-aid from the Ministry of Science, Education, Sports and Culture of Japan, No. 14570193
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Dvorak AM. Ultrastructural evidence for release of major basic protein-containing crystalline cores of eosinophil granules in vivo: cytotoxic potential in Crohn's disease. J Immunol. 1980;125:460–462. [PubMed] [Google Scholar]
- 2.Sarin SK, Malhotra V, Sen Gupta S, Karol A, Gaur SK, Anand BS. Significance of eosinophil and mast cell counts in rectal mucosa in ulcerative colitis. A prospective controlled study. Dig Dis Sci. 1987;32:363–367. doi: 10.1007/BF01296289. [DOI] [PubMed] [Google Scholar]
- 3.Walsh RE, Gaginella TS. The eosinophil in inflammatory bowel disease. Scand J Gastroenterol. 1991;26:1217–1224. doi: 10.3109/00365529108998617. [DOI] [PubMed] [Google Scholar]
- 4.Desreumaux P, Nutten S, Colombel JF. Activated eosinophils in inflammatory bowel disease: do they matter? Am J Gastroenterol. 1999;94:3396–3398. doi: 10.1111/j.1572-0241.1999.01657.x. [DOI] [PubMed] [Google Scholar]
- 5.Nishitani H, Okabayashi M, Satomi M, Shimoyama T, Dohi Y. Infiltration of peroxidase-producing eosinophils into the lamina propria of patients with ulcerative colitis. J Gastroenterol. 1998;33:189–195. doi: 10.1007/s005350050068. [DOI] [PubMed] [Google Scholar]
- 6.Geajardo JR, Rothenberg ME. Eosinophilic esophagitis, gastroenteritis, gastroenterocolitis, and colitis. In: Food allergy: adverse reactions to foods and additives., editor. 3rd ed, edited by Metcalfe DD, Sampson HA, Simon RA, Malden (MA): Blackwell Publishing; 2003. pp. 217–226. [Google Scholar]
- 7.Olsson I, Venge P. Cationic proteins of human granulocytes. II. Separation of the cationic proteins of the granules of leukemic myeloid cells. Blood. 1974;44:235–246. [PubMed] [Google Scholar]
- 8.Giembycz MA, Lindsay MA. Pharmacology of the eosinophil. Pharmacol Rev. 1999;51:213–340. [PubMed] [Google Scholar]
- 9.Yamasaki K, Makiyama K, Iwanaga S, Mizuta Y, Kubo K. Immunohistochemical study of eosinophil cationic protein on colonic mucosa of patients with ulcerative colitis. Dig Organ Immunol. 1989;22:74–77. [Google Scholar]
- 10.Yamasaki K, Makiyama K. Eosinophil cationic protein (ECP) in ulcerative colitis. Acta Med Nagasaki. 1994;39:67–71. [Google Scholar]
- 11.Makiyama K, Kanzaki S, Yamasaki K, Zea-Iriarte W, Tsuji Y. Activation of eosinophils in the pathophysiology of ulcerative colitis. J Gastroenterol. 1995;30 Suppl 8:64–69. [PubMed] [Google Scholar]
- 12.Raab Y, Fredens K, Gerdin B, Hällgren R. Eosinophil activation in ulcerative colitis: studies on mucosal release and localization of eosinophil granule constituents. Dig Dis Sci. 1998;43:1061–1070. doi: 10.1023/a:1018843104511. [DOI] [PubMed] [Google Scholar]
- 13.Saitoh O, Kojima K, Sugi K, Matsuse R, Uchida K, Tabata K, Nakagawa K, Kayazawa M, Hirata I, Katsu K. Fecal eosinophil granule-derived proteins reflect disease activity in inflammatory bowel disease. Am J Gastroenterol. 1999;94:3513–3520. doi: 10.1111/j.1572-0241.1999.01640.x. [DOI] [PubMed] [Google Scholar]
- 14.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 15.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 16.Makiyama K, Tsuzimura N. Synthesis and evaluation of an ECP polypeptide as a polyclonal antibody for staining of migrating inflammatory cells in the colonic mucosa of patients with ulcer ative colitis (in Japanese). Annual Report of The Research Committee of Inflammatory Bowel Disease The Japan Ministry of Health Labour and Welfare. 2004. [Google Scholar]
- 17.Barker RL, Loegering DA, Ten RM, Hamann KJ, Pease LR, Gleich GJ. Eosinophil cationic protein cDNA. Comparison with other toxic cationic proteins and ribonucleases. J Immunol. 1989;143:952–955. [PubMed] [Google Scholar]
- 18.Nittoh T, Hirakata M, Mue S, Ohuchi K. Identification of cDNA encoding rat eosinophil cationic protein/eosinophil-associated ribonuclease. Biochim Biophys Acta. 1997;1351:42–46. doi: 10.1016/s0167-4781(97)00024-9. [DOI] [PubMed] [Google Scholar]
- 19.Shichijo K, Gottfried M, Sekine I, Pappas TN. Dextran sulfate sodium-induced colitis in immunodeficient rats. Dig Dis Sci. 2000;45:2320–2326. doi: 10.1023/a:1005678606273. [DOI] [PubMed] [Google Scholar]
- 20.Shichijo K, Ihara M, Matsuu M, Ito M, Okumura Y, Sekine I. Overexpression of heat shock protein 70 in stomach of stress-induced gastric ulcer-resistant rats. Dig Dis Sci. 2003;48:340–348. doi: 10.1023/a:1021939829515. [DOI] [PubMed] [Google Scholar]
- 21.Hogan SP, Mishra A, Brandt EB, Royalty MP, Pope SM, Zimmermann N, Foster PS, Rothenberg ME. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2:353–360. doi: 10.1038/86365. [DOI] [PubMed] [Google Scholar]
- 22.Stevceva L, Pavli P, Husband A, Matthaei KI, Young IG, Doe WF. Eosinophilia is attenuated in experimental colitis induced in IL-5 deficient mice. Genes Immun. 2000;1:213–218. doi: 10.1038/sj.gene.6363654. [DOI] [PubMed] [Google Scholar]
- 23.Lehrer RI, Szklarek D, Barton A, Ganz T, Hamann KJ, Gleich GJ. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol. 1989;142:4428–4434. [PubMed] [Google Scholar]
- 24.Ackerman SJ, Gleich GJ, Loegering DA, Richardson BA, Butterworth AE. Comparative toxicity of purified human eosinophil granule cationic proteins for schistosomula of Schistosoma mansoni. Am J Trop Med Hyg. 1985;34:735–745. doi: 10.4269/ajtmh.1985.34.735. [DOI] [PubMed] [Google Scholar]
- 25.Fredens K, Dybdahl H, Dahl R, Baandrup U. Extracellular deposit of the cationic proteins ECP and EPX in tissue infiltrations of eosinophils related to tissue damage. APMIS. 1988;96:711–719. doi: 10.1111/j.1699-0463.1988.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 26.Motojima S, Frigas E, Loegering DA, Gleich GJ. Toxicity of eosinophil cationic proteins for guinea pig tracheal epithelium in vitro. Am Rev Respir Dis. 1989;139:801–805. doi: 10.1164/ajrccm/139.3.801. [DOI] [PubMed] [Google Scholar]
- 27.Young JD, Peterson CG, Venge P, Cohn ZA. Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature. 1986;321:613–616. doi: 10.1038/321613a0. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg HF. Recombinant human eosinophil cationic protein. Ribonuclease activity is not essential for cytotoxicity. J Biol Chem. 1995;270:7876–7881. doi: 10.1074/jbc.270.14.7876. [DOI] [PubMed] [Google Scholar]
- 29.Silen W, Ito S. Mechanisms for rapid re-epithelialization of the gastric mucosal surface. Annu Rev Physiol. 1985;47:217–229. doi: 10.1146/annurev.ph.47.030185.001245. [DOI] [PubMed] [Google Scholar]
- 30.Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med. 2003;349:350–357. doi: 10.1056/NEJMoa013136. [DOI] [PubMed] [Google Scholar]
- 31.Dignass AU, Lynch-Devaney K, Podolsky DK. Hepatocyte growth factor/scatter factor modulates intestinal epithelial cell proliferation and migration. Biochem Biophys Res Commun. 1994;202:701–709. doi: 10.1006/bbrc.1994.1987. [DOI] [PubMed] [Google Scholar]
- 32.Tahara Y, Ido A, Yamamoto S, Miyata Y, Uto H, Hori T, Hayashi K, Tsubouchi H. Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J Pharmacol Exp Ther. 2003;307:146–151. doi: 10.1124/jpet.103.054106. [DOI] [PubMed] [Google Scholar]
- 33.Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 34.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oshitani N, Sawa Y, Hara J, Adachi K, Nakamura S, Matsumoto T, Arakawa T, Kuroki T. Functional and phenotypical activation of leucocytes in inflamed human colonic mucosa. J Gastroenterol Hepatol. 1997;12:809–814. doi: 10.1111/j.1440-1746.1997.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg HF. The eosinophil ribonucleases. Cell Mol Life Sci. 1998;54:795–803. doi: 10.1007/s000180050208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg HF, Ackerman SJ, Tenen DG. Human eosinophil cationic protein. Molecular cloning of a cytotoxin and helminthotoxin with ribonuclease activity. J Exp Med. 1989;170:163–176. doi: 10.1084/jem.170.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeda T, Kitazoe M, Tada H, de Llorens R, Salomon DS, Ueda M, Yamada H, Seno M. Growth inhibition of mammalian cells by eosinophil cationic protein. Eur J Biochem. 2002;269:307–316. doi: 10.1046/j.0014-2956.2001.02653.x. [DOI] [PubMed] [Google Scholar]
- 39.Boix E, Leonidas DD, Nikolovski Z, Nogués MV, Cuchillo CM, Acharya KR. Crystal structure of eosinophil cationic protein at 2.4 A resolution. Biochemistry. 1999;38:16794–16801. doi: 10.1021/bi9919145. [DOI] [PubMed] [Google Scholar]
- 40.Venge P, Dahl R, Hällgren R. Enhancement of factor XII dependent reactions by eosinophil cationic protein. Thromb Res. 1979;14:641–649. doi: 10.1016/0049-3848(79)90119-1. [DOI] [PubMed] [Google Scholar]
- 41.Dahl R, Venge P. Enhancement of urokinase-induced plasminogen activation by the cationic protein of human eosinophil granulocytes. Thromb Res. 1979;14:599–608. doi: 10.1016/0049-3848(79)90115-4. [DOI] [PubMed] [Google Scholar]
- 42.Weiler JM, Edens RE, Bell CS, Gleich GJ. Eosinophil granule cationic proteins regulate the classical pathway of complement. Immunology. 1995;84:213–219. [PMC free article] [PubMed] [Google Scholar]


