Abstract
AIM: To observe the relationship between ethanol-induced oxidative damage in human primary cultured hepatocytes and cytochrome P450 2E1 (CYP2E1) activity, in order to address if inhibition of CYP2E1 could attenuate ethanol-induced cellular damage.
METHODS: The dose-dependent (25-100 mmol/L) and time-dependent (0-24 h) exposures of primary human cultured hepatocytes to ethanol were carried out. CYP2E1 activity and protein expression were detected by spectrophotometer and Western blot analysis respectively. Hepatotoxicity was investigated by determination of lactate dehydrogenase (LDH) and aspartate transaminase (AST) level in hepatocyte culture supernatants, as well as the intracellular formation of malondialdehyde (MDA).
RESULTS: A dose-and time-dependent response between ethanol exposure and CYP2E1 activity in human hepatocytes was demonstrated. Moreover, there was a time-dependent increase of CYP2E1 protein after 100 mmol/L ethanol exposure. Meanwhile, ethanol exposure of hepatocytes caused a time-dependent increase of cellular MDA level, LDH, and AST activities in supernatants. Furthermore, the inhibitor of CYP2E1, diallyl sulfide (DAS) could partly attenuate the increases of MDA, LDH, and AST in human hepatocytes.
CONCLUSION: A positive relationship between ethanol-induced oxidative damage in human primary cultured hepatocytes and CYP2E1 activity was exhibited, and the inhibition of CYP2E1 could partly attenuate ethanol-induced oxidative damage.
Keywords: Ethanol, CYP2E1, Oxidative damage, Human primary hepatocytes
INTRODUCTION
The enzymes that are believed to be primarily responsible for the oxidation of ethanol are alcohol dehydrogenase, catalase, and cytochrome P450 2E1 (CYP2E1), all of which appear to be relatively widely distributed in mammalian cell types[1,2]. Ethanol has long been known to have cytotoxic effects on a wide variety of animal cell types, even at relatively low doses[3,4]. In several cell types and organs such as liver[5], it is now believed that the toxicity of ethanol stems principally from free radicals produced during oxidation[6] that can then damage cellular components such as DNA, proteins, and membrane lipids.
CYP2E1 is of special interest because of its ability to metabolize and activate numerous hepatotoxic substrates in the liver such as ethanol, carbon tetrachloride, acetaminophen, and N-nitroso dimethylamine, to more toxic products. CYP2E1 exhibits enhanced reduced form of nicotinamide adenine dinucleotide phosphate oxidase activity and is very reactive in catalysis of lipid peroxidation and production of reactive oxygen intermediates (ROI) such as H2O2 in higher amounts relative to other P450 isoforms[7]. Induction of cytochrome CYP2E1 by ethanol appears to be one of the central pathways by which ethanol generates a state of oxidative stress. In addition, oxidation of ethanol by CYP2E1 produces acetaldehyde, a highly reactive compound that may contribute to the toxic effect of ethanol[8]. Hence, there is considerable interest in the role of reactive oxygen species (ROS) during ethanol metabolism by which ethanol is hepatotoxic.
CYP2E1 is shown to be more effective in catalyzing lipid peroxidation compared to several other forms of cytochrome P450 enzymes. Increases in formation of ROS by microsomes isolated from ethanol-treated rats were prevented by anti-CYP2E1 IgG, thus linking them to induction of CYP2E1[9]. In the intragastric ethanol feeding, significant alcoholic injury occurred[10]. In addition, large increases in lipid peroxidation have been observed, and the ethanol-induced liver pathology has been shown to correlate with CYP2E1 levels and elevated lipid peroxidation, and to be blocked by inhibitors of CYP2E1. Moreover, CYP2E1 inhibitors also reduced ethanol-induced ROS formation, suggesting that CYP2E1 contributes to ROS formation. Inhibitors of CYP2E1 were also shown to prevent ethanol cytotoxicity in transduced HepG2 cells[11].
However, question arises whether the responses to expression of different forms of cytochromes P450 are similar in rats and humans. The concentrations of ethanol required to increase CYP2E1 in cultured human hepatocytes are dramatically lower than those required to induce these forms of P450 in cultured rat hepatocytes. The activity of alcohol dehydrogenase, a major enzyme involved in ethanol metabolism, is lower in human liver than in rat liver. Thus, in the human hepatocyte cultures, more ethanol may be available intracellularly to induce the P450s due to lower metabolism. Alternatively, the mechanism of induction of these forms of P450s may be different in human than in rat hepatocytes[12]. In this study, we investigated the effects of ethanol administration on human primary culture hepatocytes in order to determine if there is a correlation between CYP2E1 activity and ethanol-induced oxidative damage in human hepatocytes, and further address if diallyl sulfide (DAS), an inhibitor of CYP2E1[13], could partly attenuate ethanol-induced oxidative damage.
MATERIALS AND METHODS
Materials
Williams medium E (with Glutamax-1), HEPES, and penicillin/streptomycin were obtained from Life Technologies (Karlsruhe, Germany), insulin and hydrocortisone were supplied from Sigma (Deisenhofen, Germany), while calf serum was purchased from PAA Ltd (Linz, Austria), anti-mouse CYP2E1 antibody was purchased from Amersham (Germany). Western blot development kits were purchased from Amersham (ECL, Buckinghamshire, UK). All other reagents were obtained from Sigma unless indicated otherwise.
Isolation and culture of human hepatocytes
Human liver tissue weighing 5-10 g was obtained from liver resections of cholecystectomy of patients who had no known liver pathology, nor had they received medication during 4 wk prior to surgery. None of the patients was a habitual consumer of alcohol or other drugs. The collection of tissue was done according to institutional guideline, and the patient written consent. Immediately after resection, a wedge section of the normal tissue was transferred under sterile conditions to the laboratory in culture media. Human hepatocytes were isolated by a two-step collagenase perfusion technique followed by a Percoll centrifugation step as previously described[14]. Hepatocyte purity assessed under light microscope was over 95% and viability consistently exceeded 93% by trypan blue exclusion. The freshly harvested human hepatocytes were then seeded onto rat-tail collagen-coated Petri dishes or 12-well culture trays. The medium consisted of Williams E medium supplemented with 1 µmol/L insulin, 15 mmol/L HEPES, 1.4 µmol hydrocortisone, 10% calf serum, penicillin (100 U/mL), and streptomycin (100 µg/mL). Cells were incubated in a humidified incubator in 950 mL/L air and 50 mL/L CO2 at 37 °C until cell attachment. On the following day, cells were exposed to various concentrations of ethanol (25-100 mmol/L) for 9 h. Time-dependent studies were undertaken in human hepatocytes using 100 mmol/L ethanol between 0.5 and 24 h. At the corresponding time intervals, cells and supernatants were collected in agreement with the applied technique.
CYP 2E1 enzyme activity
Human hepatocytes were seeded onto 12-well dishes at a concentration of 0.5106 cells. The cells were cultured overnight, fresh medium alone or medium containing various concentrations of ethanol or DAS (25-100 µmol/L) was added, and the cells were incubated for various lengths of time. Cells were harvested with a cell scraper and washed with PBS twice. The activity of CYP2E1 was determined by the rate of hydroxylation of p-nitrophenol[15], at 546 nm. Plates were washed with saline to remove traces of phenol red and incubated with 0.5 mmol/L p-nitrophenol up to 60 min. The extinction coefficient for p-nitrophenol is 10.28 mmol/(L·cm). Results were expressed as formed p-nitrophenol pmols/min/mg protein.
Cellular damage
LDH and AST measurement Both enzymes, LDH and AST, were measured using a commercially available test kit. Results were expressed as units per liter.
Lipid peroxidation measurement MDA, formed from the breakdown of polyunsaturated fatty acids, was used as a convenient index for determining the extent of lipid peroxidation reactions. It was assayed in human hepatocytes using the thiobarbituric acid reaction as described by Wrighton et al[12]. The absorbance of the resulting organic layer was measured spectrophotometrically at 532 nm and calculated in relative to an external standard (1,1,3, 3-tetraethoxypropane) of MDA. Results were expressed as nmol/mg of protein.
Western blot analysis Cells were washed twice with PBS and homogenized in a buffer containing protease inhibitors. Protein concentrations were determined by the method of Lowry, using bovine serum albumin as the standard. Proteins were separated on a 10% SDS polyacrylamide gel, and then transferred onto polyvinylidene difluoride membranes[16,17]. Nonspecific binding sites were blocked by overnight incubation of membranes in nonfat milk (5/100 g) solution solved in PBS/Tween-20 at 4 °C. After washing with PBS/Tween-20, the membranes were incubated with a CYP2E1 antibody, followed by an incubation with a horseradish-peroxidase-conjugated antibody at room temperature for 1 h. Then, membranes were washed again with PBS/Tween-20 for 1 h, and the immune complexes were developed using a chemilumin-escence detection system. Equal loading of total protein was verified using a commercially available antibody against β-actin[18].
Statistical analysis
Values were expressed as mean±SD of three values per experiment and experiments were repeated at least twice. Differences were analyzed by using the ANOVA test. Statistical significance was established at a P value <0.05.
RESULTS
Dose- and time-dependent CYP2E1 activity by ethanol administration
As depicted in Figure 1A, we observed a dose-dependent increase in CYP2E1 activity, which reached its maximum at 100 mmol/L ethanol after 9 h exposure as compared to untreated control cultures (CT: 50.096.68 pmol/mg protein per min vs 100 mmol/L ethanol: 130.712.61 pmol/mg protein per min; P<0.01). We found that the exposure to 100 mmol/L ethanol led to a continuous increase in CYP2E1 activity in human hepatocytes (Figure 1B). CYP2E1 activity increased rapidly and reached its maximum between 6 and 12 h after ethanol exposure. Then, by 24 h we saw a decline of CYP2E1 activity. CYP2E1 activity between 3 and 24 h was significantly higher compared to untreated control cultures.
Figure 1.
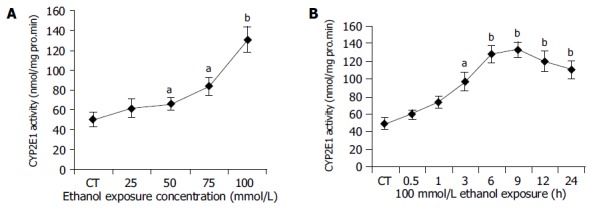
Dose- and time-dependent CYP2E1 activity by ethanal administration. A: Ethanol dose-dependent induction of CYP450 2E1. Hepatocytes were incubated with various concentrations of ethanol (0-100 mmol/L) for 9 h. CYP2E1 enzyme activity was measured as described in Materials and methods. CT: control; aP<0.05, bP<0.01 vs untreated control cultures. Each point represents the mean±SE of triplicates of five independent experiments; B: Ethanol time-dependent induction of CYP2E1 enzyme activity in human hepatocytes. Hepatocytes were incubated with medium in the presence or absence of 100 mmol/L ethanol for various lengths of time (0-24 h). CYP2E1 enzyme activity was measured. CT: control; aP<0.05, bP<0.01 vs untreated control cultures. Each point represents the mean±SE of triplicates of five independent experiments.
Time-dependent CYP2E1 protein expression after ethanol exposure
Figure 2 shows a time-dependent expression of CYP2E1 protein in human hepatocytes. We demonstrated that the exposure to 100 mmol/L ethanol of human hepatocytes induced continuous increase of CYP2E1 protein expression during the investigation period.
Figure 2.
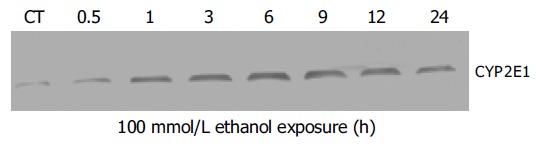
Western blot analysis of time-dependent induction of CYP2E1 protein expression in human hepatocytes. Human hepatocytes were incubated with medium alone or medium containing 100 mmol/L ethanol for 0-24 h. Each was loaded with 100 mg of the sample. Experiments were carried out as described under Materials and methods (CT: control).
Cytotoxicity of ethanol in human hepatocytes
The formation of various radicals was closely linked to oxidative injury. A typical characteristic of radical formation was lipid peroxidation, where malondialdehyde (MDA) was a by-product that could be easily measured in cells and tissue. Figure 3 shows that MDA levels increased with time, reaching first time significant level already after 3 h (P<0.05 vs controls) of ethanol exposure. The maximum MDA formation in human hepatocytes after ethanol exposure was seen at 9 h and thereafter a slow decline of the lipid peroxidation could be seen. Moreover, there appeared to be a positive relation between CYP2E1 activity and MDA level (r = 0.9724, P<0.01).
Figure 3.
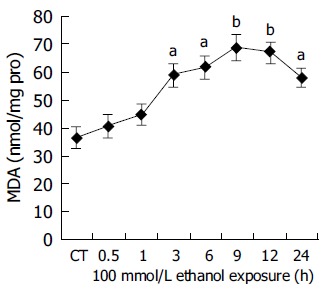
Ethanol-dependent formation of MDA in human hepatocytes. Hepatocytes were incubated with 100 mmol/L ethanol for various lengths of time (0-24 h). MDA formation was measured as described in Materials and methods. CT: control; aP<0.05, bP<0.01 vs untreated control cultures. Each point represents the mean±SE of triplicates of five independent experiments.
Further, the degree of cellular injury caused by ethanol can be estimated by the leakage of enzymes from the hepatocytes. In order to evaluate the hepatocellular damage caused by ethanol, supernatants taken from hepatocyte cultures were screened for the presence of LDH and AST. In our experiments, ethanol caused a clear time-dependent release of LDH and AST. (LDH: CT = 15.61.66 U/L, 100 mmol/L ethanol (24 h): 84.57.86 U/L, P<0.01 vs CT; Figure 4. AST: CT = 94.229.52 U/L, 100 mmol/L ethanol (24 h): 181.9111.55 U/L, P<0.01 vs CT Figure 5.) The above results indicated that ethanol was toxic to human hepatocytes, and there was positive relationship between CYP2E1 activity induced by ethanol and ethanol-induced cellular damage.
Figure 4.
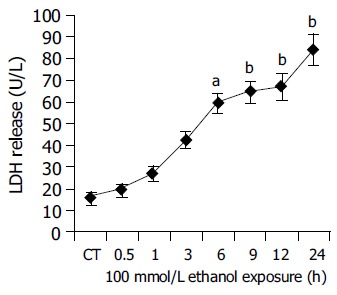
Time course experiments for LDH release after ethanol (100 mmol/L) exposure between 0.5 and 24 h. CT: control; aP<0.05, bP<0.01 vs untreated control cultures. Each point represents the mean±SE of triplicates of five independent experiments.
Figure 5.
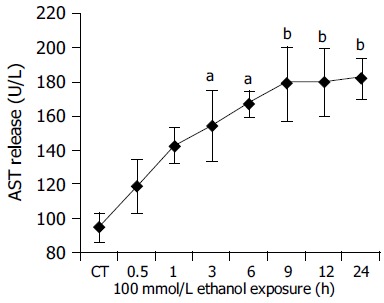
Time course experiments for AST release after ethanol 100 mmol/L exposure between 0.5 and 24 h. CT: control; aP<0.05, bP<0.01 vs untreated control cultures. Each point represents the mean±SD of triplicates of five independent experiments.
Protection from ethanol-induced oxidative damage by CYP2E1 inhibitors
In the following set of experiments we addressed the question whether the inhibition of CYP2E1 could protect human hepatocytes from ethanol-induced cellular damage. First, we found that the co-induction of ethanol with 25 or 50 µmol/L DAS, produced no cellular protection against the ethanol-induced cytotoxicity. In contrast, when cells were co-inducted with 100 µmol/L DAS and ethanol for 9 h, we saw a profound reduction of MDA formation caused by ethanol (Table 1). However, MDA formation was still higher than that of untreated control cultures. This reversing effect of DAS was also seen with regard to the release of LDH and AST (Table 1). There was no effect on MDA, LDH, and AST, when 25-100 µmol/L DAS was incubated with ethanol, respectively (Table 1). Thus, the inhibition of CYP2E1 could partly attenuate ethanol-induced cytotoxicity in human primary hepatocytes.
Table 1.
Ethanol exposure of hepatocytes causes cellular damage in human hepatocytes
| Group | MDA (nmol/mg protein) | LDH (U/L) | AST (U/L) |
| CT | 36.37±3.64 | 15.6±1.66 | 94.22±9.52 |
| 100 mmol/L ethanol | 69.05±4.41 | 84.5±7.86 | 181.91±11.55 |
| 100 mmol/L ethanol plus | 65.31±9.32 | 80.4±8.86 | 174.51±17.12 |
| 25 µmol/L DAS | |||
| 100 mmol/L ethanol plus | 59.54±7.02 | 75.5±7.06 | 160.42±17.86 |
| 50 µmol/L DAS | |||
| 100 mmol/L ethanol plus | 56.13±6.32a | 70.8±6.24a | 156.42±15.55a |
| 100 µmol/L DAS | |||
| 25 µmol/L DAS | 37.43±3.45 | 16.7±1.85 | 93.056±9.64 |
| 50 µmol/L DAS | 36.43±4.15 | 15.8±1.33 | 92.16±9.04 |
| 100 µmol/L DAS | 38.43±4.05 | 17.6±1.95 | 96.32±10.08 |
P<0.05 vs 100 mmol/L ethanol (n = 5).
DISCUSSION
Ethanol consumption and its effects on CYP2E1 in animals have long been studied in the past. However, reports about the influence of CYP2E1 inhibition on human primary hepatocytes and its possible positive effects on ethanol-induced toxicity are very limited. The concentrations of ethanol required to increase CYP2E1 in cultured human hepatocytes are dramatically lower than those required to induce the same forms of P450 in cultured rat hepatocytes. The activity of alcohol dehydrogenase, a major enzyme involved in ethanol metabolism, is lower in human liver than in rat liver. Thus, in the human hepatocyte cultures, more ethanol may be available intracellularly to induce the P450s due to lower metabolism. Alternatively, the mechanism of induction of these forms of P450s may be different or more sensitive in human than in rat hepatocytes[12]. It was reported that 25-100 mmol/L ethanol could lead to sustained cellular damage such as teratogenesis in guinea pigs, ethanol-induced unconsciousness or even death in humans[19]. In the present study, 25-100 mmol/L ethanol was used to investigate the relationship between ethanol exposure and CYP2E1 gene expression. Meanwhile, cellular LDH, AST, and MDA levels were chosen to assess hepatocyte damage caused by ethanol exposure.
CYP2E1 is induced by a broad variety of chemicals, such as ethanol. The production of ROS via CYP2E1 induction may contribute to the development of alcoholic liver disease or at least increase cytotoxic effects of alcohol[20]. Ethanol is an essential CYP2E1 inducer in human hepatocytes, which was also confirmed in a recent study by Ponsoda et al[21] showing that human hepatocytes incubated with 100 mmol/L ethanol showed a two- to threefold increase in CYP2E1 enzyme activity compared with untreated control cultures. By use of recombinant retroviral expression, in addition to ethanol (20-100 mmol/L) exposure, the culture medium of the HepG2 cell line exhibited both a large increase of CYP2E1 content and enzyme activity[22]. Our data confirmed these results, clearly showing a dose-dependent and time-dependent relationship between CYP2E1 enzyme activity in human hepatocytes and ethanol exposure. Western blot analysis further proved ethanol-induced CYP2E1 expression in a time-dependent manner.
Lipid peroxidation (and associated membrane damage) is a key feature in alcoholic liver injury. It resulted in increased excretion of MDA in the urine of rats following short- and long-term administration of relatively low doses of ethanol[23]. Lipid peroxidation results directly from the increased oxygen radical production inducted by CYP2E1. In the present work, we have demonstrated that time-dependent increased MDA levels were directly linked to the presence of ethanol and there was a clear relationship between MDA levels and CYP2E1 changes in hepatocytes after ethanol administration.
The increase in LDH activity is seen in many pathologic conditions. Release of the intracytoplasmic enzyme LDH into cell culture medium is frequently used as a measure of cellular injury. AST is present only in hepatocytes, located in mitochondria and cytoplasm. Like LDH, its increase is an early sign of cellular injury. In the present study, ethanol clearly caused a time-dependent release of LDH and AST, and there was a positive relationship between the induction of CYP2E1 activity and the release of LDH and AST. Ethanol exposure could cause marked cellular damage in human primary cultured hepatocytes.
It was demonstrated that ethanol cytotoxicity was directly related to CYP2E1 enzyme activity. Cytotoxicity of ethanol in rat hepatocytes was prevented either by alcohol/aldehyde dehydrogenase inhibitors or by CYP2E1 inhibitors. Cederbaum observed that ethanol-induced ROI formation was also reduced in the presence of CYP2E1 inhibitors in HepG2 cells, suggesting that CYP2E1 directly contributes to ROI formation[24]. Leclercq et al[25] using the knockout mice observed that other CYPs, notably CYP4A10 and CYP4A14, were upregulated in the CYP2E1 knockout but not the wild-type mice; these CYPs were, like CYP2E1, active generators of reactive oxygen and catalysts of lipid peroxidation, and in the absence of CYP2E1 served as alternative initiators of oxidative stress. Furthermore, French and collaborators found that the ethanol-induced oxidative inactivation of the proteasome and increase in oxidized proteins were completely prevented in these CYP2E1 knockout mice[26]. Our experiments showed that DAS, the inhibitor of CYP2E1, could partly protect human hepatocytes from ethanol-induced cellular injury, or reduce cellular MDA, LDH and AST levels. However, in contrast to these observations, studies by Thurman and colleagues have suggested that CYP2E1 may not play a role in alcoholic liver injury based upon studies in CYP2E1 knockout mice[1,27,28]. In some cells, ethanol-induced damage may not be the consequence of oxidative reactions, but may result directly from the direct interaction of ethanol with cell membranes or from its non-oxidative incorporation into fatty acid ethyl esters[29,30]. Clearly, further studies are necessary to resolve the above discrepancies.
In summary, this study revealed that an innocuous concentration of DAS significantly decreased the ethanol-induced oxidative damage in human primary cultured hepatocytes. This protection appeared to be, at least in part, due to the attenuation of oxidative stress. These findings warrant future in vivo studies of DAS for the prevention and/or treatment of alcoholic liver disease. The inhibition of CYP2E1 in cells or organs could lead to new strategies for better prevention and treatment of ethanol-induced oxidative damage in human liver.
ACKNOWLEDGMENT
We thank Professor Andreas K Nussler, Department of General-, Visceral-, and Transplantation Surgery, Humboldt University, Charit, Campus Virchow, Berlin, Germany, for his countless help in the performance of this study.
Footnotes
Supported by the National Science Foundation of China, No. 30271130
Science Editor Zhu LH and Guo SY Language Editor Elsevier HK
References
- 1.Oneta CM, Lieber CS, Li J, Rüttimann S, Schmid B, Lattmann J, Rosman AS, Seitz HK. Dynamics of cytochrome P4502E1 activity in man: induction by ethanol and disappearance during withdrawal phase. J Hepatol. 2002;36:47–52. doi: 10.1016/s0168-8278(01)00223-9. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal DP. Genetic polymorphisms of alcohol metabolizing enzymes. Pathol Biol (Paris) 2001;49:703–709. doi: 10.1016/s0369-8114(01)00242-5. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SM, Cunningham CC. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:11–16. doi: 10.1016/s0891-5849(01)00769-9. [DOI] [PubMed] [Google Scholar]
- 4.Person RE, Chen H, Fantel AG, Juchau MR. Enzymic catalysis of the accumulation of acetaldehyde from ethanol in human prenatal cephalic tissues: evaluation of the relative contributions of CYP2E1, alcohol dehydrogenase, and catalase/peroxidases. Alcohol Clin Exp Res. 2000;24:1433–1442. [PubMed] [Google Scholar]
- 5.Diehl AM. Liver disease in alcohol abusers: clinical perspective. Alcohol. 2002;27:7–11. doi: 10.1016/s0741-8329(02)00204-5. [DOI] [PubMed] [Google Scholar]
- 6.Albano E. Free radical mechanisms in immune reactions associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:110–114. doi: 10.1016/s0891-5849(01)00773-0. [DOI] [PubMed] [Google Scholar]
- 7.Cervino V, Benaim G, Carafoli E, Guerini D. The effect of ethanol on the plasma membrane calcium pump is isoform-specific. J Biol Chem. 1998;273:29811–29815. doi: 10.1074/jbc.273.45.29811. [DOI] [PubMed] [Google Scholar]
- 8.Neuman MG, Shear NH, Jacobson-Brown PM, Katz GG, Neilson HK, Malkiewicz IM, Cameron RG, Abbott F. CYP2E1-mediated modulation of valproic acid-induced hepatocytotoxicity. Clin Biochem. 2001;34:211–218. doi: 10.1016/s0009-9120(01)00217-x. [DOI] [PubMed] [Google Scholar]
- 9.Kukielka E, Cederbaum AI. DNA strand cleavage as a sensitive assay for the production of hydroxyl radicals by microsomes: role of cytochrome P4502E1 in the increased activity after ethanol treatment. Biochem J. 1994;302(Pt3):773–779. doi: 10.1042/bj3020773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanji AA, Zhao S, Sadrzadeh SM, Dannenberg AJ, Tahan SR, Waxman DJ. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol Clin Exp Res. 1994;18:1280–1285. doi: 10.1111/j.1530-0277.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 11.Cederbaum AI, Wu D, Mari M, Bai J. CYP2E1-dependent toxicity and oxidative stress in HepG2 cells. Free Radic Biol Med. 2001;31:1539–1543. doi: 10.1016/s0891-5849(01)00743-2. [DOI] [PubMed] [Google Scholar]
- 12.Kostrubsky VE, Strom SC, Wood SG, Wrighton SA, Sinclair PR, Sinclair JF. Ethanol and isopentanol increase CYP3A and CYP2E in primary cultures of human hepatocytes. Arch Biochem Biophys. 1995;322:516–520. doi: 10.1006/abbi.1995.1495. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Shankar K, Bucci TJ, Warbritton A, Mehendale HM. Diallyl sulfide inhibition of CYP2E1 does not rescue diabetic rats from thioacetamide-induced mortality. Toxicol Appl Pharmacol. 2001;173:27–37. doi: 10.1006/taap.2001.9165. [DOI] [PubMed] [Google Scholar]
- 14.Nussler AK, Liu ZZ, Di Silvio M, Sweetland MA, Geller DA, Lancaster JR, Billiar TR, Freeswick PD, Lowenstein CL, Simmons RL. Hepatocyte inducible nitric oxide synthesis is influenced in vitro by cell density. Am J Physiol. 1994;267:C394–C401. doi: 10.1152/ajpcell.1994.267.2.C394. [DOI] [PubMed] [Google Scholar]
- 15.Sapone A, Affatato A, Canistro D, Broccoli M, Trespidi S, Pozzetti L, Biagi GL, Cantelli-Forti G, Paolini M. Induction and suppression of cytochrome P450 isoenzymes and generation of oxygen radicals by procymidone in liver, kidney and lung of CD1 mice. Mutat Res. 2003;527:67–80. doi: 10.1016/s0027-5107(03)00055-1. [DOI] [PubMed] [Google Scholar]
- 16.Raghavendra V, Kulkarni SK. Possible antioxidant mechanism in melatonin reversal of aging and chronic ethanol-induced amnesia in plus-maze and passive avoidance memory tasks. Free Radic Biol Med. 2001;30:595–602. doi: 10.1016/s0891-5849(00)00447-0. [DOI] [PubMed] [Google Scholar]
- 17.Hannon-Fletcher MP, O'Kane MJ, Moles KW, Barnett YA, Barnett CR. Lymphocyte cytochrome P450-CYP2E1 expression in human IDDM subjects. Food Chem Toxicol. 2001;39:125–132. doi: 10.1016/s0278-6915(00)00119-8. [DOI] [PubMed] [Google Scholar]
- 18.Jung M, Drapier JC, Weidenbach H, Renia L, Oliveira L, Wang A, Beger HG, Nussler AK. Effects of hepatocellular iron imbalance on nitric oxide and reactive oxygen intermediates production in a model of sepsis. J Hepatol. 2000;33:387–394. doi: 10.1016/s0168-8278(00)80274-3. [DOI] [PubMed] [Google Scholar]
- 19.Cook MN, Marks GS, Vreman HJ, Nakatsu K, Stevenson DK, Brien JF. Heme oxygenase activity and acute and chronic ethanol exposure in the hippocampus, frontal cerebral cortex, and cerebellum of the near-term fetal guinea pig. Alcohol. 1997;14:117–124. doi: 10.1016/s0741-8329(96)00114-0. [DOI] [PubMed] [Google Scholar]
- 20.Cederbaum AI. Iron and CYP2E1-dependent oxidative stress and toxicity. Alcohol. 2003;30:115–120. doi: 10.1016/s0741-8329(03)00104-6. [DOI] [PubMed] [Google Scholar]
- 21.Ponsoda X, Bort R, Jover R, Gómez-Lechón MJ, Castell JV. Increased toxicity of cocaine on human hepatocytes induced by ethanol: role of GSH. Biochem Pharmacol. 1999;58:1579–1585. doi: 10.1016/s0006-2952(99)00249-x. [DOI] [PubMed] [Google Scholar]
- 22.Carroccio A, Wu D, Cederbaum AI. Ethanol increases content and activity of human cytochrome P4502E1 in a transduced HepG2 cell line. Biochem Biophys Res Commun. 1994;203:727–733. doi: 10.1006/bbrc.1994.2242. [DOI] [PubMed] [Google Scholar]
- 23.Goasduff T, Cederbaum AI. CYP2E1 degradation by in vitro reconstituted systems: role of the molecular chaperone hsp90. Arch Biochem Biophys. 2000;379:321–330. doi: 10.1006/abbi.2000.1870. [DOI] [PubMed] [Google Scholar]
- 24.Cederbaum AI. Ethanol-related cytotoxicity catalyzed by CYP2E1-dependent generation of reactive oxygen intermediates in transduced HepG2 cells. Biofactors. 1998;8:93–96. doi: 10.1002/biof.5520080116. [DOI] [PubMed] [Google Scholar]
- 25.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bardag-Gorce F, Yuan QX, Li J, French BA, Fang C, Ingelman-Sundberg M, French SW. The effect of ethanol-induced cytochrome p4502E1 on the inhibition of proteasome activity by alcohol. Biochem Biophys Res Commun. 2000;279:23–29. doi: 10.1006/bbrc.2000.3889. [DOI] [PubMed] [Google Scholar]
- 27.Isayama F, Froh M, Bradford BU, McKim SE, Kadiiska MB, Connor HD, Mason RP, Koop DR, Wheeler MD, Arteel GE. The CYP inhibitor 1-aminobenzotriazole does not prevent oxidative stress associated with alcohol-induced liver injury in rats and mice. Free Radic Biol Med. 2003;35:1568–1581. doi: 10.1016/j.freeradbiomed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Kono H, Bradford BU, Yin M, Sulik KK, Koop DR, Peters JM, Gonzalez FJ, McDonald T, Dikalova A, Kadiiska MB, et al. CYP2E1 is not involved in early alcohol-induced liver injury. Am J Physiol. 1999;277:G1259–G1267. doi: 10.1152/ajpgi.1999.277.6.G1259. [DOI] [PubMed] [Google Scholar]
- 29.You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1–G6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Hu W, Baldassare JJ, Bora PS, Chen S, Poulos JE, O'Neill R, Britton RS, Bacon BR. The ethanol metabolite, linolenic acid ethyl ester, stimulates mitogen-activated protein kinase and cyclin signaling in hepatic stellate cells. Life Sci. 2003;73:1083–1096. doi: 10.1016/s0024-3205(03)00383-7. [DOI] [PubMed] [Google Scholar]


