Abstract
AIM: To investigate the visceral response to acute retrograde gastric electrical stimulation (RGES) in healthy humans and to derive optimal parameters for treatment of patients with obesity.
METHODS: RGES with a series of effective parameters were performed via a bipolar mucosal electrode implanted along the great curvature 5 cm above pylorus of stomach in 12 healthy human subjects. Symptoms associated with dyspepsia and other discomfort were observed and graded during RGES at different settings, including long pulse and pulse train. Gastric myoelectrical activity at baseline and during different settings of stimulation was recorded by a multi-channel electrogastrography.
RESULTS: The gastric slow wave was entrained in all the subjects at the pacing parameter of 9 cpm in frequency, 500 ms in pulse width, and 5 mA in amplitude. The frequently appeared symptoms during stimulation were satiety, bloating, discomfort, pain, sting, and nausea. The total symptom score for each subject significantly increased as the amplitude or pulse width was adjusted to a higher scale in both long pulse and pulse train. There was a wide diversity of visceral responses to RGES among individuals.
CONCLUSION: Acute RGES can result in a series of symptoms associated with dyspepsia, which is beneficial to the treatment of obesity. Optimal parameter should be determined according to the individual sensitivity to electrical stimulation.
Keywords: Visceral response, Retrograde gastric electrical stimulation, Symptom, Obesity
INTRODUCTION
Motility is one of the most critical physiological functions of the stomach. Coordinated gastric contractions are necessary for the emptying of ingested food, and impairment in gastric motility may result in delayed gastric emptying. Gastric motility (contractile activity) is regulated by the myoelectrical activity of the stomach, called slow waves[1]. The gastric slow wave originates in the proximal stomach, propagates distally toward the pylorus, and dominates the maximum frequency, propagation velocity and propagation direction of gastric contractions. Abnormalities in gastric slow waves including uncoupling and gastric dysrhythmia lead to gastric motor disorders and are frequently observed in patients with functional disorders of the stomach, such as gastroparesis, functional dyspepsia, anorexia, etc. Gastric myoelectrical abnormalities are believed to be the fundamental factors for gastric hypomotility[2-5]. Gastric prokinetics are used to treat patients with gastric hypomotility and cisapride has been proved to be effective on both gastric hypomotility and myoelectrical abnormality[6]. But there are still a lot of patients who are refractory or could not tolerate these drugs.
Gastric electrical stimulation is used to affect gastric motility with the rationale as a forward gastric pacing to trigger the gastric myoelectrical propagation from proximal towards pylorus of stomach. It has been accepted as a therapy for chronic gastroparesis associated with drug refractory nausea and vomiting secondary to diabetic or idiopathic etiology[7,8]. Researchers adopt its mechanism with converse effects and have developed implantable gastric stimulation (IGS) to treat obesity that is attributed to rapid gastric emptying or hypermotility[9-11].
IGS for weight loss is an exciting new concept for the treatment of obesity[12]. It is unique in that it relies on neither gastric restriction nor intestinal malabsorption but instead induces early satiety. IGS is also a relatively invasive procedure that does not alter the gastrointestinal anatomy. The system comprises an electrical pulse generator similar to a cardiac pacemaker and a serosal lead implanted in the muscular layer of the distal gastric wall. Both animal[13] and human studies[14-18] revealed that it results in decreased food intake and a substantial weight loss.
Up to now, whether there is responsive diversity among individuals to gastric electrical stimulation is unknown. An optimal parameter, which should be of high efficiency, low energy expenditure and acceptability, has not been determined. This study aimed to investigate the visceral response to RGES with mucosal electrode, and to derive optimal parameter for therapy of obesity.
MATERIALS AND METHODS
Subjects
Twelve healthy volunteers were recruited in this study, including six males and six females, with their age being 29.4±8.6 years, body weight being 62.63±8.29 kg (48-80 kg) and body mass index being 23.18±2.62 kg/m2. None of the subjects had gastrointestinal diseases or symptoms or a history of gastrointestinal surgery. All women were studied during their follicular phase of the menses to minimize possible hormonal influences[19]. No medications were used by any of the participants except for oral contraceptives 2 wk prior to the study. Organic diseases of stomach, such as erosive gastritis, gastric ulcer, esophagitis, etc., were ruled out by endoscopy. Subjects with swallowing disorders such as dysphagia, achalasia, and hypersensitivity to nasal intubation were excluded. Females in pregnancy, or nursing period were also excluded. Written consent form was signed by each subject before the study, and the protocol was approved by the Ethical Committee of the University Hospital.
Implantation of mucosal electrodes
The subjects were fasted for 8 h or more before implantation of the electrodes. A temporary transvenous cardiac pacing lead system (Model 6416, Medtronic, Netherlands) composed of an active fixation, a lead with bipolar electrodes and a soft-tipped, lubricated guide catheter was used (Figure 1). This lead was intubated into the stomach through a nasal cavity before endoscopy. At a good exposure of the antrum under endoscopy, the distal electrode was screwed into the mucosa along the greater curvature 5 cm above the pylorus and fixed with a titanium clamp. The proximal electrode was affixed to the surface of the gastric mucosa with one or two titanium clamps (Figure 2). After the placement, the guide catheter was pulled out of the stomach. An X-ray picture was taken once daily to ensure that the electrodes were located at the original positions in the stomach.
Figure 1.

Model 6416 temporary transvenous pacing lead system.
Figure 2.

Placement of mucosal electrode. Arrow 1 indicates the distal electrode; arrow 2 indicates the proximal electrode.
A multi-channel electrogastrograph (POLYGRAM NETTM, Medtronic Functional Diagnostics A/S, Denmark) was used to record gastric myoelectrical activity. Two channel gastric myoelectrical recordings were obtained by connecting each of the mucosal electrodes to a common reference electrode placed on the leg of the subject. These myoelectrical recordings were used to further confirm the attachment of electrodes to the gastric mucosa. A recording of rhythmic 3 cpm gastric slow waves would be indicative of a good attachment of the electrodes. The entrainment of gastric myoelectrical activity by RGES was also observed to assess its electrophysiological effectiveness.
Experimental protocol
After a baseline recording of gastric slow waves for 30 min, symptomatic responses to gastric electrical stimulation with various parameters were assessed in the fasting state. Visceral sensation (symptomatic response) to gastric electrical stimulation was assessed by three parameters: initial sensation, maximum tolerance, and the symptom score. The initial sensation was defined as the stimulation with which the subject first reported one or more of any symptoms. The maximum tolerance was defined as the stimulation with which the subject reported the maximally tolerable symptoms.
Symptoms, including satiety, bloating, discomfort (the character and location of a symptom could not be described clearly and precisely), upper abdominal pain, sting, belching, nausea, vomiting, were recorded and graded during RGES at different settings. Each symptom was graded from 0 to 3 (0: no symptom; 1: mild symptoms, requiring intention to feel the symptoms; 2: moderate symptoms, being aware of the symptoms, but not interfering with daily activities; 3: severe symptoms, interfering with daily activities). The total symptom score in each subject was derived and its correlation with the stimulation energy was determined.
Retrograde gastric electrical stimulation
RGES was performed via the bipolar electrodes attached to the mucosa/submucosa of the distal antrum using a universal pulse generator (Acupulser, model A310, World Precision Instrument, Inc., Sarasota, FL, USA). Two models of electric stimulation, long pulse and pulse train, were applied to the pacing electrodes in the constant current mode. The long pulse was composed of periodic rectangular pulses with a frequency of 9 cpm, pulse width of 500 ms, and adjustable pulse amplitude (current) consisting three settings of 5, 10, and 15 mA. The pulsed train was on for a period of 2 s and off for 3 s. The pulse frequency was 20 Hz, an adjustable pulse width consisting three settings of 5, 10, and 20 ms. The amplitude was adjusted from 10 to 15 mA. If maximal intolerable symptom did not appear, the amplitude was adjusted to 20 mA. Each session of stimulation lasted for about 20 min and there was a 10 min or more resting period between consecutive stimulation sessions. For all tests, the subject was blinded about the stimulation and stimulation parameters.
Stimulation energy was calculated for each stimulation session. The stimulation energy was defined as t×I2R, where t is stimulation time, I is stimulation current and R is the impedance between two stimulation electrodes. In this study, R was ignored since it was constant for various stimulation sessions, I was the pulse amplitude and t was the total on-time of pulses within a minute. For long pulses, t = pulse frequency (pulses/min)×pulse width. For pulse trains, t = number of trains/min×number of pulses/train×pulse width. The unit of stimulation energy used in this study was s (mA)2 or simply smA2.
Statistical analysis
Results of specific symptom scores and total symptom score in each subject to different parameters were expressed as mean±SE. Statistical analysis was performed by one-way ANOVA. Pearson’s linear correlation and regression method was used to assess the correlation between responsive severity and stimulation energy. P<0.05 was considered statistically significant.
RESULTS
All the 12 subjects completed the study with good compliance. Regular gastric slow waves were recorded from stimulation electrodes attached to the gastric mucosa at baseline. Typical tracings are presented in Figure 3. The dominant frequency of the gastric slow waves was 2.71±0.16 cpm. The gastric slow waves in all the subjects were completely entrained by the signal of RGES at the frequency 9 cpm, pulse width 500 ms and amplitude 5 mA (112.5 smA2, Figure 4).
Figure 3.
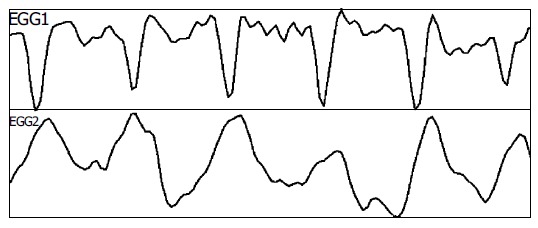
Intrinsic myoelectrical activities from mucosa/submucosa electrodes in stomach.
Figure 4.
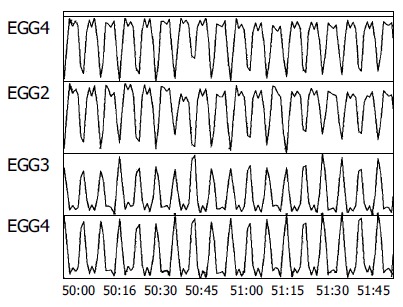
Entrainment of gastric myoelectrical activity by RGES.
Frequently appeared symptoms during stimulation were satiety, bloating, discomfort, upper abdominal pain, sting, and nausea. The sum of these six symptoms was used as an overall total symptom score. The severity of specific symptoms to different parameters is shown in Table 1.
Table 1.
Symptom scores at different parameters (mean±SE)
| Long pulse | Long pulse | Long pulse | Pulse train | Pulse train | Pulse train | Pulse train | |
| 112.5 smA2 | 450 smA2 | 1 012.5 smA2 | 240 smA2 | 480 smA2 | 960 smA2 | 2 160 smA2 | |
| Satiety | 0.08±0.08 | 0 | 0.08±0.08 | 0.08±0.08 | 0.17±0.17 | 0.25±0.18 | 0.42±0.23 |
| Bloating | 0.08±0.18 | 0.21±0.17 | 0.33±0.19 | 0.25±0.18 | 0.50±0.19 | 0.58±0.26 | 1.00±0.31 |
| Discomfort | 0.38±0.18 | 0.5±0.27 | 0.67±0.28 | 0.75±0.12 | 0.50±0.19 | 0.50±0.23 | 0.42±0.26 |
| Pain | 0.33±0.18 | 0.8±0.26 | 1.54±0.27 | 0.16±0.21 | 1.33±0.19 | 1.92±0.24 | 2.50±0.27 |
| Sting | 0.17±0.17 | 0.17±0.17 | 0.25±0.18 | 0.08±0.17 | 0.25±0.21 | 0.25±0.18 | 0.33±0.22 |
| Nausea | 0.17±0.17 | 0.21±0.21 | 0.25±0.25 | 0.08±0.08 | 0.21±0.17 | 0.29±0.22 | 0.75±0.35 |
The output energy of electrical stimulation for inducing initial sensation showed a wide distribution among individuals. The initial sensation appeared in three subjects at 112.5 smA2 (long pulse: 9 cpm, 500 ms, 5 mA), six subjects at 240 smA2 (pulse train: 2 s-on, 3 s-off, 20 Hz, 5 ms, 10 mA), one subject at 450 smA2 (long pulse: 9 cpm, 500 ms, 10 mA), and two subjects at 480 smA2 (pulse train: 2 s-on, 3 s-off, 20 Hz, 10 ms, 10 mA). The maximal energy for inducing initial sensation was more than four times that of the minimum. The initial sensation occurred as different symptoms. Bloating appeared in three subjects, satiety in two, discomfort and pain in five, sting and nausea in one, respectively.
The output energy for inducing maximal tolerable sensation also showed a wide diversity among individuals. The maximal tolerable sensation was recorded at 480 smA2 in two subjects, at 2 160 smA2 (pulse train: 2 s-on, 3 s-off, 20 Hz, 20 ms, 15 mA) in nine subjects and at 3 840 smA2 (pulse train: 2 s-on, 3 s-off, 20 Hz, 20 ms, 20 mA) in one subject. The maximal energy for maximal tolerable sensation was eight times that of the minimum. The major symptoms resulted from the maximally tolerable stimulation included upper abdominal pain in nine subjects, nausea in two subjects, and both pain and nausea in one subject. Some mild to moderate symptoms were simultaneously noted with the maximally tolerable stimulation, including satiety, bloating, discomfort, belching, and nausea.
The most common symptomatic response to stronger RGES was upper abdominal pain. It appeared synchronously with stimulus signals, such as 9 cpm at long pulse, and became persistent in three subjects when the maximal stimulation was applied. It disappeared immediately as the stimulation terminated in seven subjects and gradually ceased within 4 min in five subjects. Other symptoms, such as bloating, discomfort, nausea, etc., also disappeared as soon as the stimulation ended.
During stimulation with long pulse, the severity of the symptoms including bloating, discomfort, and pain significantly increased when the pulse amplitude was adjusted to a higher scale (P<0.01, Figure 5A). The severity of symptoms including satiety, bloating, pain, and nausea also significantly increased when the pulse width or amplitude was set to higher scales during pulse train (P<0.05, Figure 5B). The mean total symptom score for the seven settings of gastric electrical stimulation was linearly correlated with stimulation energy (r = 0.96, P<0.001, Figure 6). That is, the total symptom score for each subject significantly increased as the output energy was adjusted to a higher scale in both long pulse and pulse train.
Figure 5.

Symptom scores at different parameters of long pulse (A) and pulse train (B).
Figure 6.

Correlation of total symptom score with output energy of stimulation.
DISCUSSION
Obesity, defined by an excess of body fat, is a highly prevalent disorder in the Western world. In the USA, it has been estimated that one of three adults is obese[20]. Obesity predisposes to or aggravates many clinical conditions, such as hypertension, hyperlipidemia, diabetes, gout, atherosclerotic heart disease, etc.[21]. Obesity is of a multifactorial pathogenesis. The basic mechanism is believed that obesity results from food intake greater than energy needs. The quantity of food intake is directly determined by sensory and motor function of stomach, i.e., gastric accommodation (capacity) and emptying. Previous studies using an intragastric balloon to assess proximal stomach capacity showed that it is larger in both moderate and severe obese individuals[22,23]. In the studies of gastric capacity in obese people, distal gastric volume is also found larger in obese individuals in the fasting state from imaging with single photon emission computed tomography[24], suggesting that the increased gastric volume causes changes in the sensation of satiety with a consequent increase of food intake in these subjects.
In addition to stomach capacity, the retaining time of food in stomach also influences mechano- and chemosensitive satiety signals and it is a reasonable hypothesis that an enhanced rate of gastric emptying predisposes to overeating. Although the contribution of changes in gastrointestinal motility to the pathogenesis of obesity is unclear, several noteworthy changes in gastrointestinal motility have been observed in obesity[25]. For example, some studies suggest more rapid gastric emptying in obesity[9-11], although normal[26-29] or even slower[30-32] emptying has also been reported in other studies. But in a better designed study of 77 subjects including 46 obese and 31 age-, sex-, and racematched nonobese individuals, obese subjects are found to have a more rapid emptying rate than nonobese subjects[9].
Based on the pathogenesis of obesity, great efforts from different fields have been tried to conquer obesity. Medicines and neuroendocrine agents or analogs are found to be able to inhibit appetite or to delay gastric emptying[33,34]. Surgical approaches can weaken the gastric capacity, as implantation of balloon in the stomach[35-37]. By doing this, symptoms associated with dyspepsia, such as satiety, bloating, anorexia, discomfort or pain of upper abdomen, are duplicated in fasting state or postprandial. The occurrence of these symptoms results in reduction of food intake, eventually loss of body weight, which is the goal for the treatment of obesity.
As the stomach contractility is controlled by gastric myoelectrical activity, electrophysiological approaches can alter stomach motility. A recent preliminary study indicates the potential of gastric electrical stimulation for weight loss[13]. The first human study using a gastric stimulator for the treatment of morbid obesity was performed in 1995, which results in decreased food intake and a substantial weight loss[14]. IGS for human obesity has been investigated in different institutes in the world since then[15-18].
Recently, we have proposed retrograde gastric electrical stimulation (RGES). In this method, electrical stimulation is performed at a tachygastrial frequency and via a pair of mucosal electrodes placed in the distal antrum, mimicking an ectopic pacemaker generating tachygastria. Experiments in dogs have revealed a successful inhibition of antral contractions in the fed state and a delayed gastric emptying of liquid[38,39]. This study was designed to observe the acute symptomatic responses induced by RGES via bipolar mucosal electrodes. During seven settings of RGES, the frequently appeared symptoms were satiety, bloating, discomfort, pain, sting, and nausea, which are all associated with dyspepsia. These symptoms were typically seen during initial sensation by less energy. Even during maximum stimulation, the frequently appeared symptom was pain of upper abdomen, which is the key symptom of dyspepsia. Furthermore, some mild to moderate symptoms simultaneously occurred such as bloating, discomfort, nausea, satiety, and belching. These symptoms are all associated with dyspepsia. When these symptoms are present with activation of gastric stimulation, the subjects will change their eating behavior and ingest less food, eventually leading to their weight loss. All these are expected for the treatment of obese patients.
In this study, we found that the severity of some symptoms, such as bloating, discomfort and pain, significantly increased when the output energy of stimulation was adjusted to a higher scale. The total symptom score for each subject also simultaneously increased with the increase of energy, suggesting that there is a linear correlation between symptom score and stimulation energy, which is consistent with that of dosage-efficacy seen in pharmacology. In this sense, RGES meets the essential qualification for a potential therapy of obesity.
IGS can induce weight loss in patients with morbid obesity, but its mechanism remains largely unknown. Recent animal studies suggested that this increased satiety is attributed to gastric distention induced by gastric stimulation[38]. Gastric distention contributes to the feeling of fullness or satiety. The mechanism is unclear, but distending the stomach stimulates gastric stretch receptors, thus triggering vagal discharges that activate hypothalamic neurons[40] and induce the feeling of satiety[41]. The stimulation-induced gastric relaxation or distention is mediated by the intrinsic nitrergic pathway, as the inhibitor of nitric oxide synthase blocks the effect whereas vagatomy does not[42].
Satiety as a signal of reduction of food intake is also regulated by endocrine system especially the peptides in gastrointestinal tract[43]. Peptides like cholecystokinin (CCK), glucagon-like peptide (GLP)-1, leptin and ghrelin have been shown to evoke satiety, thereby reducing food intake[43,44]. Cigaina and Hirschberg[16] have investigated the mechanism behind the changed eating behavior in patients treated with IGS. Gastric electrical stimulation can lead to significant weight loss and decrease in plasma levels of CCK, somatostatin, GLP-1, and leptin. Weight loss correlates significantly with decreased leptin levels. In this study, RGES induced a series of symptoms associated with dyspepsia, suggesting that the stimulation results in gastric distention via neuroendocrine pathways. As gastric electrical stimulation is a novel and promising therapy for morbid obesity, more studies are necessary to elucidate the correlations between satiety, weight loss, and digestive neuro-hormone changes.
The results of our study reveal a wide diversity of visceral sensitivity to RGES among individuals and we should determine the optimal parameters according to the individual responsive severity. However, we should not select the pulse amplitude that is more than 15 mA, at which it would induce intolerable symptoms to most human subjects. The subjects who get initial sensation with low stimulation energy may be expected to get desirable therapeutic effect from RGES, but those insensitive to RGES may not. As for those insensitive subjects, we should include them in this therapy with deliberation. They may take other effective option such as bariatric surgery.
Footnotes
Science Editor Wang XL and Li WZ Language Editor Elsevier HK
References
- 1.Chen JD, McCallum RW. Electrogastrographic parameters and their clinical significance. In: Chen JDZ, McCallum RW, Eds , editors. The Electrogastrography: principles and clinical applications. New York: Raven Press; 1994. pp. 45–73. [Google Scholar]
- 2.Chen JD, McCallum RW. Clinical applications of electrogastrography. Am J Gastroenterol. 1993;88:1324–1336. [PubMed] [Google Scholar]
- 3.Chen JD, Schirmer BD, McCallum RW. Serosal and cutaneous recordings of gastric myoelectrical activity in patients with gastroparesis. Am J Physiol. 1994;266:G90–G98. doi: 10.1152/ajpgi.1994.266.1.G90. [DOI] [PubMed] [Google Scholar]
- 4.You CH, Lee KY, Chey WY, Menguy R. Electrogastrographic study of patients with unexplained nausea, bloating, and vomiting. Gastroenterology. 1980;79:311–314. [PubMed] [Google Scholar]
- 5.Chen JD, Pan J, McCallum RW. Clinical significance of gastric myoelectrical dysrhythmias. Dig Dis. 1995;13:275–290. doi: 10.1159/000171508. [DOI] [PubMed] [Google Scholar]
- 6.Chen JD, Ke MY, Lin XM, Wang Z, Zhang M. Cisapride provides symptomatic relief in functional dyspepsia associated with gastric myoelectrical abnormality. Aliment Pharmacol Ther. 2000;14:1041–1047. doi: 10.1046/j.1365-2036.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen JD, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401–409. doi: 10.1053/gast.2003.50048. [DOI] [PubMed] [Google Scholar]
- 8.McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 9.Wright RA, Krinsky S, Fleeman C, Trujillo J, Teague E. Gastric emptying and obesity. Gastroenterology. 1983;84:747–751. [PubMed] [Google Scholar]
- 10.Zahorska-Markiewicz B, Jonderko K, Lelek A, Skrzypek D. Gastric emptying in obesity. Hum Nutr Clin Nutr. 1986;40:309–313. [PubMed] [Google Scholar]
- 11.Sasaki H, Nagulesparan M, Dubois A, Straus E, Samloff IM, Lawrence WH, Johnson GC, Sievers ML, Unger RH. Hypergastrinemia in obese noninsulin-dependent diabetes: a possible reflection of high prevalence of vagal dysfunction. J Clin Endocrinol Metab. 1983;56:744–750. doi: 10.1210/jcem-56-4-744. [DOI] [PubMed] [Google Scholar]
- 12.Shikora SA. Implantable gastric stimulation for the treatment of severe obesity. Obes Surg. 2004;14:545–548. doi: 10.1381/096089204323013596. [DOI] [PubMed] [Google Scholar]
- 13.Xing J, Brody F, Brodsky J, Rosen M, Larive B, Ponsky J, Soffer E. Gastric electrical-stimulation effects on canine gastric emptying, food intake, and body weight. Obes Res. 2003;11:41–47. doi: 10.1038/oby.2003.8. [DOI] [PubMed] [Google Scholar]
- 14.Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg. 2002;12 Suppl 1:12S–16S. doi: 10.1007/BF03342141. [DOI] [PubMed] [Google Scholar]
- 15.D'Argent J. Gastric electrical stimulation as therapy of morbid obesity: preliminary results from the French study. Obes Surg. 2002;12 Suppl 1:21S–25S. doi: 10.1381/096089202762552638. [DOI] [PubMed] [Google Scholar]
- 16.Cigaina V, Hirschberg AL. Gastric pacing for morbid obesity: plasma levels of gastrointestinal peptides and leptin. Obes Res. 2003;11:1456–1462. doi: 10.1038/oby.2003.195. [DOI] [PubMed] [Google Scholar]
- 17.Favretti F, De Luca M, Segato G, Busetto L, Ceoloni A, Magon A, Enzi G. Treatment of morbid obesity with the Transcend Implantable Gastric Stimulator (IGS): a prospective survey. Obes Surg. 2004;14:666–670. doi: 10.1381/096089204323093462. [DOI] [PubMed] [Google Scholar]
- 18.Miller K, Höller E, Hell E. Intragastric stimulation (IGS) for treatment of morbid obesity. Zentralbl Chir. 2002;127:1049–1054. doi: 10.1055/s-2002-36378. [DOI] [PubMed] [Google Scholar]
- 19.Parkman HP, Harris AD, Miller MA, Fisher RS. Influence of age, gender, and menstrual cycle on the normal electrogastrogram. Am J Gastroenterol. 1996;91:127–133. [PubMed] [Google Scholar]
- 20.Wickelgren I. Obesity: how big a problem? Science. 1998;280:1364–1367. doi: 10.1126/science.280.5368.1364. [DOI] [PubMed] [Google Scholar]
- 21.Seidell JC. Societal and personal costs of obesity. Exp Clin Endocrinol Diabetes. 1998;106 Suppl 2:7–9. doi: 10.1055/s-0029-1212029. [DOI] [PubMed] [Google Scholar]
- 22.Granström L, Backman L. Stomach distension in extremely obese and in normal subjects. Acta Chir Scand. 1985;151:367–370. [PubMed] [Google Scholar]
- 23.Geliebter A. Gastric distension and gastric capacity in relation to food intake in humans. Physiol Behav. 1988;44:665–668. doi: 10.1016/0031-9384(88)90333-2. [DOI] [PubMed] [Google Scholar]
- 24.Kim DY, Camilleri M, Murray JA, Stephens DA, Levine JA, Burton DD. Is there a role for gastric accommodation and satiety in asymptomatic obese people? Obes Res. 2001;9:655–661. doi: 10.1038/oby.2001.89. [DOI] [PubMed] [Google Scholar]
- 25.Wisén O, Hellström PM. Gastrointestinal motility in obesity. J Intern Med. 1995;237:411–418. doi: 10.1111/j.1365-2796.1995.tb01195.x. [DOI] [PubMed] [Google Scholar]
- 26.Barkin JS, Reiner DK, Goldberg RI, Phillips RS, Janowitz WR. The effects of morbid obesity and the Garren-Edwards gastric bubble on solid phase gastric emptying. Am J Gastroenterol. 1988;83:1364–1367. [PubMed] [Google Scholar]
- 27.French SJ, Murray B, Rumsey RD, Sepple CP, Read NW. Preliminary studies on the gastrointestinal responses to fatty meals in obese people. Int J Obes Relat Metab Disord. 1993;17:295–300. [PubMed] [Google Scholar]
- 28.Verdich C, Madsen JL, Toubro S, Buemann B, Holst JJ, Astrup A. Effect of obesity and major weight reduction on gastric emptying. Int J Obes Relat Metab Disord. 2000;24:899–905. doi: 10.1038/sj.ijo.0801250. [DOI] [PubMed] [Google Scholar]
- 29.Horowitz M, Collins PJ, Harding PE, Shearman DJ. Abnormalities of gastric emptying in obese patients. Gastroenterology. 1983;85:983–985. [PubMed] [Google Scholar]
- 30.Horowitz M, Collins PJ, Shearman DJ. Effect of increasing the caloric/osmotic content of the liquid component of a mixed solid and liquid meal on gastric emptying in obese subjects. Hum Nutr Clin Nutr. 1986;40:51–56. [PubMed] [Google Scholar]
- 31.Maddox A, Horowitz M, Wishart J, Collins P. Gastric and oesophageal emptying in obesity. Scand J Gastroenterol. 1989;24:593–598. doi: 10.3109/00365528909093095. [DOI] [PubMed] [Google Scholar]
- 32.Geliebter A, Melton PM, Gage D, McCray RS, Hashim SA. Gastric balloon to treat obesity: a double-blind study in nondieting subjects. Am J Clin Nutr. 1990;51:584–588. doi: 10.1093/ajcn/51.4.584. [DOI] [PubMed] [Google Scholar]
- 33.Foxx-Orenstein A, Camilleri M, Stephens D, Burton D. Effect of a somatostatin analogue on gastric motor and sensory functions in healthy humans. Gut. 2003;52:1555–1561. doi: 10.1136/gut.52.11.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nozu T, Martinez V, Rivier J, Taché Y. Peripheral urocortin delays gastric emptying: role of CRF receptor 2. Am J Physiol. 1999;276:G867–G874. doi: 10.1152/ajpgi.1999.276.4.G867. [DOI] [PubMed] [Google Scholar]
- 35.Sagar PM. Surgical treatment of morbid obesity. Br J Surg. 1995;82:732–739. doi: 10.1002/bjs.1800820606. [DOI] [PubMed] [Google Scholar]
- 36.NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956–961. [PubMed] [Google Scholar]
- 37.Geliebter A, Melton PM, McCray RS, Gage D, Heymsfield SB, Abiri M, Hashim SA. Clinical trial of silicone-rubber gastric balloon to treat obesity. Int J Obes. 1991;15:259–266. [PubMed] [Google Scholar]
- 38.Xing JH, Brody F, Brodsky J, Larive B, Ponsky J, Soffer E. Gastric electrical stimulation at proximal stomach induces gastric relaxation in dogs. Neurogastroenterol Motil. 2003;15:15–23. doi: 10.1046/j.1365-2982.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 39.Ouyang H, Yin J, Chen JD. Therapeutic potential of gastric electrical stimulation for obesity and its possible mechanisms: a preliminary canine study. Dig Dis Sci. 2003;48:698–705. doi: 10.1023/a:1022824406648. [DOI] [PubMed] [Google Scholar]
- 40.Anand BK, Pillai RV. Activity of single neurones in the hypothalamic feeding centres: effect of gastric distension. J Physiol. 1967;192:63–77. doi: 10.1113/jphysiol.1967.sp008288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deutsch JA, Young WG, Kalogeris TJ. The stomach signals satiety. Science. 1978;201:165–167. doi: 10.1126/science.663647. [DOI] [PubMed] [Google Scholar]
- 42.McMinn JE, Baskin DG, Schwartz MW. Neuroendocrine mechanisms regulating food intake and body weight. Obes Rev. 2000;1:37–46. doi: 10.1046/j.1467-789x.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Barachina MD, Martínez V, Wei JY, Taché Y. Synergistic interaction between CCK and leptin to regulate food intake. Regul Pept. 2000;92:79–85. doi: 10.1016/s0167-0115(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 44.Gutzwiller JP, Göke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D, Beglinger C. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81–86. doi: 10.1136/gut.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]


