Abstract
AIM: To discuss the helical computed tomography (CT) characteristics of gastric cancer and evaluate the diagnostic value of contrast-enhanced helical hydro-CT (HHCT) in staging gastric cancer.
METHODS: A total of 50 patients with gastric cancer were included in this study. The CT findings in them were retros-pectively analyzed and correlated with pathologic findings at surgery. All patients were preoperatively imaged by plain and contrast-enhanced helical CT after orally ingesting 1 000-1 500 mL water. Peristalsis was minimized by intra-venous administration of spasmolytics.
RESULTS: The foci of gastric cancer became more prominent in all the 50 patients and showed strong enhancement in contrast-enhanced HHCT. The tumor was located at the gastric cardia in 14 cases, at the gastric fundus in 3 cases, at the gastric body in 8 cases, at the gastric antrum in 4 cases, at the gastric fundus and the body in 8 cases, at the gastric body and antrum in 11 cases, and at three segments of the stomach in 2 cases. The CT features of gastric cancer were focal or diffuse mural thickening, soft tissue mass, cancerous ulcer, stenosis of stomach, infiltration to adjacent tissues, lymph node and distant metastases. Strong contrast enhancement of the gastric wall was closely related to gastric cancer. The accuracy rate of contrast-enhanced HHCT in staging gastric cancer was 86% (43/50). The detection rate of lymph node metastases by CT was 60% (12/20).
CONCLUSION: Contrast-enhanced HHCT is a reliable method to diagnose and stage gastric cancer.
Keywords: Stomach, Neoplasm, Tomography, X-ray, Staging
INTRODUCTION
Gastric cancer is one of the most common malignancies in China. Its accurate staging contributes to its pre-operative management[1-4]. In the evaluation of gastric diseases, gastroscopy is used for the detection of gastric abnormality. Computed tomography (CT) is a valuable tool in addition to gastroscopy in the evaluation of gastric diseases[5-7]. With the clinical application of helical CT and the improvement of CT equipments, helical CT has been used for the detection of gastric abnormality and in staging of gastric cancer[8-11]. Since January 1999, 50 patients with gastric cancer have been scanned. CT results are correlated with surgical and pathologic findings. This work aimed to analyze the characteristic findings of gastric cancer, stage the tumor and evaluate the clinical value of CT imaging of the stomach.
MATERIALS AND METHODS
Patients
A total of 50 cases of gastric cancer were examined by helical hydro-CT (HHCT) and their clinical data were collected from January 1999 to April 2004. There were 36 male and 14 female patients with an age range of 39-81 years (mean 58.3 years).
CT examination
All patients included in the study were fasting for at least 5 h before CT imaging with a commercially available scanner (Hispeed CT/i unit GE Medical Systems, Milwaukee, WI, USA). Thirty minutes before scanning, the patients ingested 500-1 000 mL of water, and were then examined in the supine position during full inspiration. Immediately before helical scanning, the patients received 20-40 mg of anisodamine (raceanisodamine hydrochloride, Hangzhou, China) intramuscularly to minimize peristalsis. An additional 500 mL of water was then offered to improve distension of the proximal part of the stomach. First, a plain scan of the upper abdomen was obtained using 10-mm sections. If the tumor was located at the antral or pyloric antrum, patients were then examined in the prone position, followed by injection of 80-100 mL of contrast material (Omnipaque, Schering, Germany) at a rate of 2.5-3.0 mL/s (Madrid power injector, SA) into the antecubital vein. Helical scanning of the stomach was performed at 30, 60-70, 150-180 s after contrast material was injected. It started above the diaphragm to cover the gastric cardia by obtaining contrast images with 5- or 7-mm collimation, a pitch of 1.5:1.0 or 1.1:1.0 (120 kV, 210 mA), and a matrix size of 512×512, then 3-mm axial scans were reconstructed for image evaluation.
Image interpretation
Without prior knowledge of endoscopic, surgical, or histological findings, one reviewer interpreted all CT studies. First, the gastric wall was evaluated for the presence of tumor. Carcinoma of the stomach was suspected if thickening and marked contrast enhancement of the gastric wall were visible on CT. The depth of infiltration and perigastric invasion of gastric tumors were evaluated according to the TNM system[11].
CT staging of gastric tumor was performed as follows: T1, invasion of the lamina propria or submucosa, when thickening was limited to the inner layer; T2, invasion of the muscularis propria, when all three layers of the gastric wall were grossly thickened and replaced by a homogeneous or inhomogeneous soft tissue mass with no serosal irregularity; T3, tumor invasion of the serosa, when high-density irregularities of the outer layer or micronodularity or strands in the fat planes contiguous to the lesion were evident; T4, invasion of adjacent organs and structures, when cleavage fat planes between neoplastic gastric wall and contiguous organs were replaced by mass or when the invasion was clearly demonstrated.
Soft tissue nodules were classified as lymph nodes, which were divided into loco regional peri-gastric lymph nodes (N1/N2 = 3 cm away from the gastric tumor) and distant nodes which were classified as metastatic disease (M1) according to the TNM system if it has tumor infiltration. All visible lymph nodes were judged to be infiltrated by carcinoma independent of size or contrast enhancement. In abdominal M staging, any hepatic or splenic lesion other than a cyst was regarded as potentially malignant. Nodular thickening of the peritoneum or ascites without signs of liver cirrhosis was assumed to be peritoneal carcinomatosis.
CT-histopathologic correlation
All resected specimens were carefully examined both macroscopically and microscopically. A lesion-by-lesion analysis was performed, and gastric carcinoma was staged according to the American Joint Committee on Cancer (AJCC) classification[11]. The histological studies were correlated with the CT data, and comparative evaluation of location, size, depth of tumor infiltration, and extent of the primary tumor was performed. The presence of lymph node metastasis was histologically determined, and the histological N stage of the tumor was correlated with CT findings. In all patients who underwent surgery, the abdomen was carefully explored to exclude metastatic disease.
Statistical analysis
According to the AJCC classification, TNM staging criteria were applied to all cases. The accuracy of CT imaging to predict the T, N, and M stage of gastric cancer was determined. The overall detection rate of gastric cancer was determined. The maximum diameter of each cancer was calculated and compared with the maximum diameter of the tumor determined by pathology. The largest tumor diameter was used for correlation with the pathologic size of the tumor.
RESULTS
All CT studies were successfully completed. A lesion-by-lesion analysis revealed that 43 of 50 (86%) gastric cancer were correctly staged by HHCT. Gastrectomy was performed in 46 patients, 4 patients with T1 tumor underwent partial gastrectomy. In all cases, histology revealed malignant ulcer corresponding to early gastric cancer.
Location and histological classification of the tumor
The maximum diameter of the resected gastric tumors ranged 0.7-15 cm (mean 5.3 cm). Fourteen cancers were located at the gastric cardia, three of them showed infiltration of the distal esophagus, three of them invaded the gastric body, and two of them infiltrated both the distal esophagus and the gastric body. The remaining cancers were located at the gastric fundus (n = 3), the gastric body (n = 8), the gastric antrum (n = 4), the gastric fundus and the body (n = 8), and the gastric body and antrum (n = 11). In two cases, the gastric cancer involved three segments of the stomach (linitis plastica).
The histological classifications were papillary adeno-carcinoma (5 cases), mucous carcinoma (2 cases), low-grade adenocarcinoma (35 cases), undifferentiated carcinoma (2 cases), squamous carcinoma (3 cases), and adenosquamous carcinoma (3 cases).
Contrast-enhanced HHCT findings of gastric cancer
In 13 of 50 cases (26%), single layer normal gastric wall was demonstrated. In the remaining 37 cases (74%), two- or three-layer structure was shown at arterial phase of contrast-enhanced HHCT scans. Abnormal local gastric wall thickening and area of strong contrast medium enhancement were found in one of four early gastric cancers confirmed by pathology. Two layers of gastric wall were found in 3 patients with early gastric cancer and in 5 of 46 patients with advanced gastric cancer. In the three advanced gastric cancers, low attenuation was found with no enhancement below the strong enhancement inner layer, their pathological diagnosis was mucous carcinoma. Single or triple layer gastric wall with homogeneous or heterogeneous strong enhancement was shown in the remaining advanced gastric cancer, their mean increase of CT values was 26.47±6.67 HU. Gastric wall thickening was found in 37 cases (Figures 1A and B), intramural soft mass in 23 cases (Figure 1C), lymph node metastasis in 12 cases, tumor spread to perigastric area and infiltration to adjacent organs in 9 cases, and distant metastasis in 7 cases. In our study, the detection rate of lymph node metastasis by CT was 60% (12/20).
Figure 1.
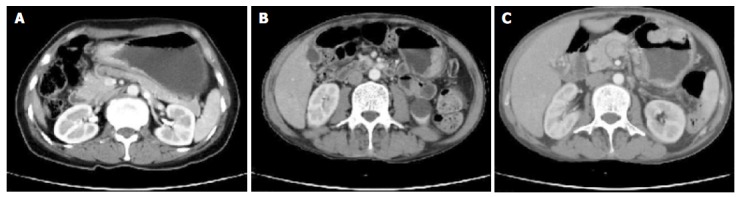
Enhancement patterns of gastric cancer on contrast-enhanced HHCT. A: Focal wall thickening in gastric antrum with marked enhancement of mucosal layer and narrowing of gastric lumina; B: focal wall thickening with marked enhancement of mucosal layer in greater curvature of the gastric body; C: homogeneous enhancement of soft tissue mass in anterior wall of the gastric body.
Gastric cancer staging by CT and pathology
The TNM staging of gastric cancer by contrast-enhanced HHCT was compared to the pathological results in 50 cases (Table 1). In 43 of 50 cases, HHCT staging of gastric cancer was in accordance with pathological staging. The accurate rate of T staging by HHCT was 86.0% (43/50). Findings at HHCT were concordant with histological findings in 2 of 4 T1 tumors (50%), in 17 of 20 T2 tumors (85%), in 15 of 17 T3 tumors (83.3%), and in 7 of 9 T4 tumors (77.8%). Overstaging occurred in 2 T1 tumors and in 3 T2 tumors on CT images. In addition, 2 T4 tumors were understaged.
Table 1.
T staging of gastric carcinoma (n = 50)
| Staging at CT | Staging at pathology | |||
| T1 | T2 | T3 | T4 | |
| T1 | 2 | |||
| T2 | 2 | 17 | 2 | |
| T3 | 3 | 15 | 2 | |
| T4 | 7 | |||
DISCUSSION
Normal architecture of gastric wall and enhancement pattern of gastric cancer on contrast-enhanced HHCT
Normal gastric wall is histologically classified as four slices: mucosa, submucosa, muscular layer, and serosa. The structure of gastric wall is shown as a single slice in conventional CT. The excellent spatial resolution of helical CT makes it possible to identify the triple-layer structure of the gastric wall after contrast material is intravenously injected. The inner layer with high attenuation corresponds to the mucosa and its muscular layer, the middle layer with low attenuation corresponds to the submucosal layer, and the outer layer with slightly high attenuation corresponds to the muscular layer and serosa[12-15]. The results of our study are concordant with the previous reports[16,17]. After gastric lumen was sufficiently filled, the mean thickness of normal gastric wall was 2-3 mm on CT image, while it was 4-5 mm at cardia and antrum[18]. Otherwise it was regarded as abnormal. The thickness of gastric lumen may be equal to 1.0 cm or above, if not being sufficiently filled. Therefore, it is a prerequisite to fill gastric lumen before CT examination.
Significance of contrast-enhanced HHCT in staging gastric cancer
CT is most frequently used in staging gastric cancer. Helical CT in combination with the water-filling method has led to marked improvements in the detection and characterization of gastric cancer[19]. The main CT findings of gastric cancer are local or diffuse thickenings of gastric wall with variable enhancement and intraluminal soft tissue mass. In this study, gastric wall thickening was found in 37 cases, intraluminal soft tissue mass in 13 cases, local and distant lymph metastasis in 12 cases. Tumor can also infiltrate into perigastric tissue, encroach on adjacent organs and metastasize to distant places. Contrast-enhanced HHCT can reflect blood supply to gastric cancer.
Reports on staging gastric cancer by conventional CT prior to surgery are available[20]. Recently more and more studies have been focused on staging gastric cancer by helical CT[21]. Our study demonstrated that TNM staging by CT was closely related with pathology. The accuracy of HHCT (86.0%) in our study was higher than that of conventional CT (72%)[13]. TNM staging by HCT depends upon the improvement of CT equipments and stomach filling with water. Contrast-enhanced HHCT can further improve the accuracy of gastric cancer staging before operation.
Our results showed 60.0% of all lymph nodes were detected by HHCT. The sensitivity of detecting lymph node metastasis was not high. The detection of regional lymph nodes can be improved by hypotension of the stomach, because the delineation of regional lymph nodes and gastric wall can be improved[22]. Enlarged lymph nodes may be missed if they are located close to the gastric cancer. For the assessment of lymph node metastasis, the size of lymph nodes is the most frequently used criterion. But there is no agreement concerning the upper limits of normal lymph node size. The diameter of normal lymph nodes in the upper abdomen may vary from 6 to 11 mm depending on their location[23-25]. Lymph nodes in the gastro-hepatic ligament region have to be considered as abnormal if they exceed 8 mm in diameter. A diameter of lymph nodes in 8-15 mm is used in studies as the upper limit for normal lymph nodes[26-28]. However, when these criteria are used, false-negative and false-positive results may occur because micrometastases do not result in enlargement or inflammatory changes in lymph nodes[4,13,29,30]. Contrast enhancement of lymph nodes can be useful in differentiating lymph nodes with and without metastasis[11]. Further study is needed to improve methods for the detection of lymph node metastasis such as dynamic CT[4,31].
In conclusion, the primary pathologic condition of gastric cancer and its metastasis to adjacent or distant structures can be demonstrated by contrast-enhanced HHCT. HHCT can provide more information for surgeons to make an appropriate therapy for gastric cancer patients.
Footnotes
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Dehn TC, Reznek RH, Nockler IB, White FE. The pre-operative assessment of advanced gastric cancer by computed tomography. Br J Surg. 1984;71:413–417. doi: 10.1002/bjs.1800710603. [DOI] [PubMed] [Google Scholar]
- 2.Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Winawer SJ, Urmacher C, Brennan MF. Preoperative staging of gastric cancer: comparison of endoscopic US and dynamic CT. Radiology. 1991;181:426–432. doi: 10.1148/radiology.181.2.1924784. [DOI] [PubMed] [Google Scholar]
- 3.D'Elia F, Zingarelli A, Palli D, Grani M. Hydro-dynamic CT preoperative staging of gastric cancer: correlation with pathological findings. A prospective study of 107 cases. Eur Radiol. 2000;10:1877–1885. doi: 10.1007/s003300000537. [DOI] [PubMed] [Google Scholar]
- 4.Hundt W, Braunschweig R, Reiser M. Assessment of gastric cancer: value of breathhold technique and two-phase spiral CT. Eur Radiol. 1999;9:68–72. doi: 10.1007/s003300050630. [DOI] [PubMed] [Google Scholar]
- 5.Shirakawa T, Fukuda K, Tada S. New method for evaluation of perigastric invasion of gastric cancer by right lateral position CT. Eur Radiol. 1996;6:358–361. doi: 10.1007/BF00180611. [DOI] [PubMed] [Google Scholar]
- 6.Lee DH, Ko YT. Advanced gastric carcinoma: the role of three-dimensional and axial imaging by spiral CT. Abdom Imaging. 1999;24:111–116. doi: 10.1007/s002619900456. [DOI] [PubMed] [Google Scholar]
- 7.Lee DH. Two-dimensional and three-dimensional imaging of gastric tumors using spiral CT. Abdom Imaging. 2000;25:1–6. doi: 10.1007/s002619910001. [DOI] [PubMed] [Google Scholar]
- 8.Tsuda K, Hori S, Murakami T, Nakamura H, Tomoda K, Nakanishi K, Shiozaki H. Intramural invasion of gastric cancer: evaluation by CT with water-filling method. J Comput Assist Tomogr. 1995;19:941–947. doi: 10.1097/00004728-199511000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Düx M, Richter GM, Hansmann J, Kuntz C, Kauffmann GW. Helical hydro-CT for diagnosis and staging of gastric carcinoma. J Comput Assist Tomogr. 1999;23:913–922. doi: 10.1097/00004728-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Sohn KM, Lee JM, Lee SY, Ahn BY, Park SM, Kim KM. Comparing MR imaging and CT in the staging of gastric carcinoma. AJR Am J Roentgenol. 2000;174:1551–1557. doi: 10.2214/ajr.174.6.1741551. [DOI] [PubMed] [Google Scholar]
- 11.Ba-Ssalamah A, Prokop M, Uffmann M, Pokieser P, Teleky B, Lechner G. Dedicated multidetector CT of the stomach: spectrum of diseases. Radiographics. 2003;23:625–644. doi: 10.1148/rg.233025127. [DOI] [PubMed] [Google Scholar]
- 12.Kuntz C, Herfarth C. Imaging diagnosis for staging of gastric cancer. Semin Surg Oncol. 1999;17:96–102. doi: 10.1002/(sici)1098-2388(199909)17:2<96::aid-ssu3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Rossi M, Broglia L, Maccioni F, Bezzi M, Laghi A, Graziano P, Mingazzini PL, Rossi P. Hydro-CT in patients with gastric cancer: preoperative radiologic staging. Eur Radiol. 1997;7:659–664. doi: 10.1007/BF02742921. [DOI] [PubMed] [Google Scholar]
- 14.Kadowaki K, Murakami T, Yoshioka H, Kim T, Takahashi S, Tomoda K, Narumi Y, Nakamura H. Helical CT imaging of gastric cancer: normal wall appearance and the potential for staging. Radiat Med. 2000;18:47–54. [PubMed] [Google Scholar]
- 15.Bhandari S, Shim CS, Kim JH, Jung IS, Cho JY, Lee JS, Lee MS, Kim BS. Usefulness of three-dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest Endosc. 2004;59:619–626. doi: 10.1016/s0016-5107(04)00169-5. [DOI] [PubMed] [Google Scholar]
- 16.Cho JS, Kim JK, Rho SM, Lee HY, Jeong HY, Lee CS. Preoperative assessment of gastric carcinoma: value of two-phase dynamic CT with mechanical iv. injection of contrast material. AJR Am J Roentgenol. 1994;163:69–75. doi: 10.2214/ajr.163.1.8010251. [DOI] [PubMed] [Google Scholar]
- 17.Fukuya T, Honda H, Kaneko K, Kuroiwa T, Yoshimitsu K, Irie H, Maehara Y, Masuda K. Efficacy of helical CT in T-staging of gastric cancer. J Comput Assist Tomogr. 1997;21:73–81. doi: 10.1097/00004728-199701000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Scatarige JC, DiSantis DJ. CT of the stomach and duodenum. Radiol Clin North Am. 1989;27:687–706. [PubMed] [Google Scholar]
- 19.Takao M, Fukuda T, Iwanaga S, Hayashi K, Kusano H, Okudaira S. Gastric cancer: evaluation of triphasic spiral CT and radiologic-pathologic correlation. J Comput Assist Tomogr. 1998;22:288–294. doi: 10.1097/00004728-199803000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Rossi M, Broglia L, Graziano P, Maccioni F, Bezzi M, Masciangelo R, Rossi P. Local invasion of gastric cancer: CT findings and pathologic correlation using 5-mm incremental scanning, hypotonia, and water filling. AJR Am J Roentgenol. 1999;172:383–388. doi: 10.2214/ajr.172.2.9930788. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Jeong YK, Kim DH, Go BK, Woo YJ, Ham SY, Yang SO. Two-phase helical CT for detection of early gastric carcinoma: importance of the mucosal phase for analysis of the abnormal mucosal layer. J Comput Assist Tomogr. 2000;24:777–782. doi: 10.1097/00004728-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Davies J, Chalmers AG, Sue-Ling HM, May J, Miller GV, Martin IG, Johnston D. Spiral computed tomography and operative staging of gastric carcinoma: a comparison with histopathological staging. Gut. 1997;41:314–319. doi: 10.1136/gut.41.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180:319–322. doi: 10.1148/radiology.180.2.2068292. [DOI] [PubMed] [Google Scholar]
- 24.De Gaetano AM, Vecchioli A, Minordi LM, Parrella A, Gaudino S, Masselli G, Savino G. Role of diagnostic imaging in abdominal lymphadenopathy. Rays. 2000;25:463–484. [PubMed] [Google Scholar]
- 25.Lee DH, Ko YT, Park SJ, Lim JW. Comparison of hydro-US and spiral CT in the staging of gastric cancer. Clin Imaging. 2001;25:181–186. doi: 10.1016/s0899-7071(01)00280-7. [DOI] [PubMed] [Google Scholar]
- 26.Fukuya T, Honda H, Hayashi T, Kaneko K, Tateshi Y, Ro T, Maehara Y, Tanaka M, Tsuneyoshi M, Masuda K. Lymph-node metastases: efficacy for detection with helical CT in patients with gastric cancer. Radiology. 1995;197:705–711. doi: 10.1148/radiology.197.3.7480743. [DOI] [PubMed] [Google Scholar]
- 27.Tunaci M. Carcinoma of stomach and duodenum: radiologic diagnosis and staging. Eur J Radiol. 2002;42:181–192. doi: 10.1016/s0720-048x(02)00035-9. [DOI] [PubMed] [Google Scholar]
- 28.Gossios K, Tsianos E, Prassopoulos P, Papakonstantinou O, Tsimoyiannis E, Gourtsoyiannis N. Usefulness of the non-distension of the stomach in the evaluation of perigastric invasion in advanced gastric cancer by CT. Eur J Radiol. 1998;29:61–70. doi: 10.1016/s0720-048x(98)00024-2. [DOI] [PubMed] [Google Scholar]
- 29.Balfe DM, Mauro MA, Koehler RE, Lee JK, Weyman PJ, Picus D, Peterson RR. Gastrohepatic ligament: normal and pathologic CT anatomy. Radiology. 1984;150:485–490. doi: 10.1148/radiology.150.2.6691106. [DOI] [PubMed] [Google Scholar]
- 30.Einstein DM, Singer AA, Chilcote WA, Desai RK. Abdominal lymphadenopathy: spectrum of CT findings. Radiographics. 1991;11:457–472. doi: 10.1148/radiographics.11.3.1852937. [DOI] [PubMed] [Google Scholar]
- 31.Magnusson A, Andersson T, Larsson B, Hagberg H, Sundström CH. Contrast enhancement of pathologic lymph nodes demonstrated by computed tomography. Acta Radiol. 1989;30:307–310. [PubMed] [Google Scholar]


