Abstract
AIM: To demonstrate the prevalence of sonographic fatty liver, overweight and ischemic heart disease (IHD) among the male workers in Taiwan, and to investigate the possible association of these three factors.
METHODS: From July to September 2003, a total of 2 088 male aircraft-maintenance workers aged from 22 to 65 years (mean 40.5) underwent an annual health examination, including anthropometrical evaluation, blood pressure measurement, personal medical history assessment, biochemical blood analysis, abdominal ultrasonographic examination and digital electrocardiography (ECG). The Student’s t-test, χ2 test and multivariate logistic regression analysis were utilized to evaluate the relationship between IHD and salient risk factors.
RESULTS: The all-over prevalence of overweight was 41.4%, and that of fatty liver was 29.5% (mild, moderate and severe fatty liver being 14.5%, 11.3%, and 3.7%, respectively); while the prevalence of ischemic changes on ECG was 17.1% in this study. The abnormal rates for conventional IHD risk factors including hypertension, dyslipidemia, hyperglycemia and overweight increased in accordance with the severity of fatty liver. Overweight and severity of fatty liver were independently associated with increased risks for developing IHD. Overweight subjects had a 1.32-fold (95%CI: 1.01-1.73) increased IHD risk. Participants with mild, moderate, and severe fatty liver had a 1.88-fold (95%CI: 1.37-2.6), 2.37-fold (95%CI: 1.66-3.37) and 2.76-fold (95%CI: 1.62-4.72) increased risk for developing IHD. The prevalence of ischemic ECG for the fatty liver-affected subjects with or without overweight was 30.1% and 19.1%, while that of overweight subjects free from fatty liver was 14.4%. Compared to the subjects without fatty liver nor overweight, IHD risk for the three subgroups above was as follows: OR: 2.95 (95%CI: 2.31-4.09), OR: 1.60 (95%CI: 1.07-2.39) and OR: 1.11 (95%CI: 0.78-1.56), respectively.
CONCLUSION: The presence of fatty liver and its severity should be carefully considered as independent risk factors for IHD. Results of the study suggest the synergistic effect between fatty liver and overweight for developing IHD. Abdominal sonographic examination may provide valuable information for IHD risk assessment in addition to limited report about liver status, especially for overweight males.
Keywords: Fatty liver, Ischemic heart disease, Overweight, Male, Middle-aged
INTRODUCTION
Fatty change in the liver is closely associated with overweight status and metabolic impairments such as hyperglycemia, and dyslipidemia[1-3] which are also regarded as factors for atherosclerosis[1,4,5] and ischemic heart disease (IHD)[6-9]. However, the association between fatty liver and IHD is waiting for epidemiological investigation.
Since resting electrocardiogram (ECG) and abdominal sonographic examination are two routine, non-invasive health examinations used in medical check-ups in Taiwan[10-15], we had the opportunity to examine the association between fatty liver and ischemic ECG changes, the hallmark of IHD and strong predictor for cardiac events[16-18].
The purpose of this study was to evaluate the relationships between fatty liver and IHD utilizing epidemic data. Data analyses were controlled for conventional risk factors, especially overweight.
MATERIALS AND METHODS
Subjects
Records from a total of 2 088 male aircraft maintenance workers who underwent a periodic health examination from July to September 2003.
Methods
The health examinations included anthropometrical evaluation, measurement of weight and height, systolic and diastolic blood pressure. Definition of overweight was BMI ≥25 kg/m2, based on WHO criteria[19]. A questionnaire about personal medical history, including alcohol (usage more than once a week: yes vs no) and tobacco (current usage: yes vs no) consumption was filled by the examinees. Biochemical blood tests were conducted by Hitachi autoanalyzer model 7150 (Hitachi Corp, Tokyo, Japan), including fasting plasma glucose, levels of triglyceride, and total, low, and high-density lipoprotein (LDL, HDL) cholesterol. The definition of hypertension was systolic blood pressure ≥18.7 kPa or diastolic blood pressure ≥12 kPa. The cut points of hyperglycemia, hypocholesterolemia, hypercholesterolemia, and hypertriglyceridemia were fasting sugar ≥6.1 mmol/L, HDL <1.0 mmol/L, total cholesterol≥5.2 mmol/L and triglyceride ≥17.0 mmol/L.
Abdominal ultrasonographic examinations were performed using convex-type real-time electronic scanners (Toshiba SSA-340 with 3.75 MHz convex-type transducer) by three gastrointestinal specialists who were blind to the medical history and blood test results of the examinees. The definition of ultrasonic fatty liver was based on a comparative assessment of image brightness relative to the kidneys, in line with previously reported diagnostic criteria[10,20-23]. Severity of fatty liver was classified according to the following modified scoring system[10,13,15,22,23]: brightness compared to kidneys (0-3), blurring of gall bladder wall (0-3), blurring of hepatic veins (0-3), blurring of portal vein (0-3), far gain attenuation (0-3). Severity was defined as mild (total scores of 2-6), moderate (7-10), and severe (11-15) fatty liver.
A digital electrocardiograph recorder (Kenz Cardico 1207; Suzuken Co., Ltd 8, Higashi Kataha-machi, Higashi-ku Nagoya 461-8701, Japan.) was used for IHD assessment. IHD was defined based on evidence of resting ECG ischemic abnormalities, as expressed in computerized Minnesota code (1.1. ×-1.3. ×, 4.1. ×-4.4. ×, 5.1. ×-5.3. ×)[12,16-18].
The Student’s t and χ2 tests were used for analyzing continuous variables and categorical variables, respectively. Multivariate logistic regression was utilized to evaluate the relationship between IHD and salient risk factors. SAS software was used for statistical analysis (Version 8.0; SAS Institute, Cary, NC, USA).
RESULTS
After 63 cases whose data were incomplete (e.g., biochemical blood test, questionnaire) were excluded, a total of 2 025 subjects were enrolled in the final analysis. The excluded subjects had a similar distribution of anthropometric measurement and biochemical data as subjects in final analysis.
As shown in Table 1, the age for this sample population ranged from 22 to 63 years (mean, 40.5), the mean value of BMI was 24.6 kg/m2. The over-all prevalence of overweight was 41.4%, while that of fatty liver was 29.5%. The prevalence of ischemic changes in the resting ECG was 17.1%. The means and standard deviations for serum blood sugar and atherogenic lipid profile were 5.7±1.1 mmol/L of fasting sugar, 5.1±0.9 mmol/L of total cholesterol, 1.3±0.3 mmol/L of HDL cholesterol, 3.3±0.8 mmol/L of LDL cholesterol, and 17.1±13.9 mmol/L of triglyceride.
Table 1.
Baseline characteristics of middle-aged male workers in Taiwan from a periodic health examination (mean±SD, n = 2 025)
| Risk factor | Value | Range |
| Age (yr) | 40.5±9.9 | 22.0-63.0 |
| Height (cm) | 169.6±6.3 | 150.1-191.1 |
| Body weight (kg) | 70.9±10.6 | 42.8-121.9 |
| BMI (body mass index) (kg/m2) | 24.6±3.3 | 15.6-40.6 |
| Systolic blood pressure (kPa) | 17.1±2.2 | 11.7-27.5 |
| Diastolic blood pressure (kPa) | 10.6±1.6 | 6.7-17.3 |
| Fasting sugar (mmol/L) | 5.7±1.1 | 3.0-24.5 |
| Cholesterol total (mmol/L) | 5.1±0.9 | 2.6-9.3 |
| Cholesterol HDL (mmol/L) | 1.3±0.3 | 0.2-4.0 |
| Cholesterol LDL (mmol/L) | 3.3±0.8 | 1.2-7.1 |
| Triglyceride (mmol/L) | 17.1±13.9 | 3.1-171.1 |
| ECG with ischemic changes (n, %) | 347 | -17.1 |
| Fatty liver (n, %) | 597 | -29.5 |
| Overweight (n, %) | 839 | -41.4 |
| Smoking (n, %) | 702 | -34.7 |
| Alcohol use (n, %) | 444 | -21.9 |
Risk-factor distribution among subgroups stratified according to the severity of fatty liver, is presented in Table 2. The prevalence of mild, moderate and severe fatty liver was 14.5%, 11.3%, and 3.7%, respectively. The abnormal rates for conventional IHD risk factor including hypertension, dyslipidemia, hyperglycemia and overweight increased in accordance with the severity of fatty liver (Figure 1).
Table 2.
Assessment of risk factors stratified according to severity of fatty liver (FL)
|
n = 2 205 |
||||
| Risk factors | Non FL 1 428 (70.5%) | Mild FL 294 (14.5%) | Moderate FL 228 (11.3%) | Severe FL 75 (3.7%) |
| Age (yr) | 39.8±9.9 | 41.8±9.9a | 42.7±9.4c | 42.6±9.5e |
| BMI (body mass index) (kg/m2) | 23.9±3.1 | 25.0±2.6a | 26.9±2.7c | 29.0±3.1e |
| Systolic blood pressure (kPa) | 16.9±2.2 | 17.2±2.1a | 17.5±2.2c | 18.3±2.6e |
| Diastolic blood pressure (kPa) | 10.5±1.6 | 10.6±1.4a | 10.9±1.5c | 11.4±1.7e |
| Fasting sugar (mmol/L) | 5.6±1.0 | 5.8±1.2a | 6.0±1.5c | 6.1±1.6e |
| Cholesterol total (mmol/L) | 5.0±0.9 | 5.2±0.9a | 5.3±0.9c | 5.3±0.9e |
| Cholesterol HDL (mmol/L) | 1.3±0.3 | 1.2±0.2a | 1.1±0.2c | 1.1±0.2e |
| Cholesterol LDL (mmol/L) | 3.3±0.8 | 3.5±0.8a | 3.4±0.8c | 3.4±0.9e |
| Triglyceride (mmol/L) | 15.0±11.1 | 18.2±13.1a | 26.3±20.9c | 27.3±22.3e |
| ECG with ischemic changes (n, %) | 191 (13.4) | 67 (22.8)a | 64 (28.1)c | 25 (33.3)e |
| Overweight (n, %) | 457 (32.0) | 143 (48.6)a | 170 (74.6)c | 69 (92.0)e |
| Smoking (n, %) | 491 (34.4) | 106 (36.1) | 84 (36.8) | 21 (28.0) |
| Alcohol usea (n, %) | 304 (21.3) | 73 (24.8) | 54 (23.7) | 13 (17.3) |
P<0.05 vs non FL group;
P<0.05 vs non FL group;
P<0.05 vs non FL group.
Figure 1.
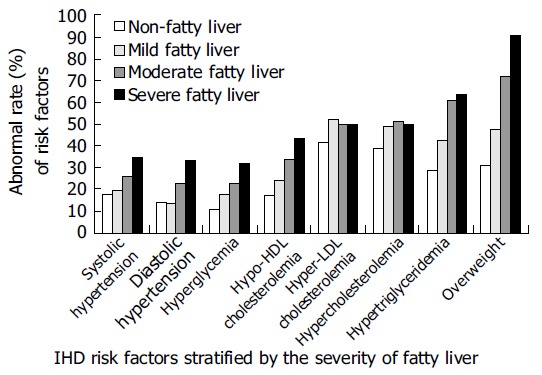
Abnormal rates of IHD risk factors for middle-aged male workers in Taiwan.
Multivariable analysis and odds ratios for IHD are summarized in Table 3. Results showed that overweight, increased systolic blood pressure and fatty liver severity were independently associated with IHD risk. Overweight subjects had a 1.32-fold (95%CI: 1.01-1.73) increased IHD risk. Participants with mild, moderate, and severe fatty liver experienced a 1.88-fold (95%CI: 1.37-2.60), 2.37-fold (95%CI: 1.66-3.37), and 2.76-fold (95%CI: 1.62-4.72) increased risk for developing IHD.
Table 3.
Multivariate logistic regression analysis for the risk factors for IHD
| Risk factors | Odds ratio | 95% CI |
| Age (yr) | 0.99 | 0.97-1.00 |
| Systolic blood pressure | 1.01a | 1.003-1.03 |
| Diastolic blood pressure | 0.99 | 0.97-1.01 |
| Fasting sugar | 1 | 1.00-1.006 |
| Cholesterol total | 0.99 | 0.98-1.007 |
| Cholesterol HDL | 1.01 | 1.00-1.03 |
| Cholesterol LDL | 1.01 | 0.99-1.02 |
| Triglyceride | 1 | 1.00-1.003 |
| Overweight | 1.32a | 1.01-1.73 |
| Fatty liverb | ||
| Mild fatty liver | 1.88a | 1.37-2.60 |
| Moderate fatty liver | 2.37a | 1.66-3.37 |
| Severe fatty liver | 2.76d | 1.62-4.72 |
| Smoking | 0.85 | 0.65-1.10 |
| Alcohol consumption | 1.054 | 0.79-1.41 |
P<0.05 vs Non FL group;
P<0.001 vs Non FL group;
P <0.0001 vs Non FL group.
The prevalence of ischemic ECG and odds ratios (OR) for IHD of the middle-aged male workers in Taiwan, stratified according to overweight and fatty liver status, are presented in Table 4. The prevalence of ischemic ECG and the risk for IHD of the fatty liver-affected subjects with or without overweight and the overweight subjects free from fatty liver was 30.1%, OR: 2.95 (95% CI: 2.31-4.09), 19.1%, OR:1.60 (95% CI: 1.07-2.39) and 14.4%, OR: 1.11 (95% CI: 0.78-1.56), respectively, compared to the subjects without fatty liver nor overweight. Result of test for interaction between fatty liver and overweight was significant (P<0.05).
Table 4.
Odds ratio for IHD stratified according to fatty liver (FL) and overweight status
|
Non-overweight (n = 1 186) |
Overweight (n = 839) |
|||
| Non-FL (n = 970) | FL (n = 216) | Non-FL (n = 458) | FL (n = 381) | |
| Ischemic ECG (%) | 125 (12.9) | 41 (19.1) | 66 (14.4) | 115 (30.1) |
| 1OR (95%CI) | 1.0a (-) | 1.60c (1.07-2.39) | 1.11 (0.78-1.56) | 2.95b (2.31-4.09) |
P<0.05 vs overweight and fatty, adjusted for age, blood pressure, blood sugar and lipid profile;
P<0.001 vs non-overweight and non-FL group;
P<0.05 vs non-overweight and non-FL group;
OR: adjusted odds ratio; CI: confidence interval.
DISCUSSION
Stress test or even coronary catheterization examination naturally has better specificitiy in IHD diagnosis, and biopsy of liver is the gold standard for hepatosteatosis. However, in the viewpoints of safety, ethic and screening purpose, resting ECG and abdominal sonographic examination have acceptable reliability and are practical tools of epidem-iological survey[10,16-18,21].
Our study indicates that adult male workers with fatty liver are more likely to develop IHD compared to subjects without fatty liver. This finding is compatible to previous studies demonstrating that fatty liver, as a developer of oxidative stress plays a cardinal role in cardiac dysfunction[24,25]. Of particular significance is the fact that non-overweight subjects with fatty liver experience a significantly increased IHD risk (OR: 1.6). As Park et al[26], concluded, for non-obese men with fatty liver, systemic inflammatory response increases, and systemic inflammatory response is the integral part of the atherosclerotic process[27,28]. IHD prevention for non-overweight subjects having fatty liver should be emphasized in clinical practice.
Findings of this study show a synergistic interaction between fatty liver and overweight, this combination makes middle-aged males have significantly highest IHD risk (OR: 2.95) in the four entities (Table 4). Similar findings have been shown in studies about insulin resistance[7,10,26,29-31], these studies manifested that both overweight and fatty liver are closely correlated with insulin resistance, which aggravates the atherogenic metabolic process[32,33], accelerating the development of atherosclerosis[34] and IHD[35]. For overweight middle-aged male workers with fatty liver, a comprehensive management for IHD risk reduction is needed.
Serum sugar and lipids had insignificant effects on developing IHD in this study, these findings are similar to our previous study based on Eastern population[36]. Genetic differences[37] and differences in diet components[38] may have affected these findings.
Smoking and drinking did not show significant effects on developing IHD in this study, the partial explanation might be that binary questionnaire could provide only limited information about the dose-effect, which is important in the development of IHD, and then leads to the unsteady results[36,39].
The findings of this study demonstrate that fatty liver is an independent risk factor for IHD. Abdominal sonographic examination may not only provide limited report about the liver status, but also can provide valuable information for IHD risk assessment, especially for those who are over-weight.
ACKNOWLEDGMENTS
The authors would like to acknowledge the personnel of the Department of Family Medicine and Internal Medicine, Shin Kong Wu Ho-Su Memorial Hospital for their full support and generous assistance.
Footnotes
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Akahoshi M, Amasaki Y, Soda M, Tominaga T, Ichimaru S, Nakashima E, Seto S, Yano K. Correlation between fatty liver and coronary risk factors: a population study of elderly men and women in Nagasaki, Japan. Hypertens Res. 2001;24:337–343. doi: 10.1291/hypres.24.337. [DOI] [PubMed] [Google Scholar]
- 2.Angelico F, Del Ben M, Conti R, Francioso S, Feole K, Maccioni D, Antonini TM, Alessandri C. Non-alcoholic fatty liver syndrome: a hepatic consequence of common metabolic diseases. J Gastroenterol Hepatol. 2003;18:588–594. doi: 10.1046/j.1440-1746.2003.02958.x. [DOI] [PubMed] [Google Scholar]
- 3.Knobler H, Schattner A, Zhornicki T, Malnick SD, Keter D, Sokolovskaya N, Lurie Y, Bass DD. Fatty liver--an additional and treatable feature of the insulin resistance syndrome. QJM. 1999;92:73–79. doi: 10.1093/qjmed/92.2.73. [DOI] [PubMed] [Google Scholar]
- 4.Chilton RJ. Recent discoveries in assessment of coronary heart disease: impact of vascular mechanisms on development of atherosclerosis. J Am Osteopath Assoc. 2001;101:S1–S5. [PubMed] [Google Scholar]
- 5.Ganz P, Creager MA, Fang JC, McConnell MV, Lee RT, Libby P, Selwyn AP. Pathogenic mechanisms of atherosclerosis: effect of lipid lowering on the biology of atherosclerosis. Am J Med. 1996;101:4A10S–4A16S. [PubMed] [Google Scholar]
- 6.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Bonadonna RC, Muggeo M. Carotid atherosclerosis and coronary heart disease in the metabolic syndrome: prospective data from the Bruneck study. Diabetes Care. 2003;26:1251–1257. doi: 10.2337/diacare.26.4.1251. [DOI] [PubMed] [Google Scholar]
- 7.Indulski JA, Lutz W. Ischaemic heart disease as an effect of obesity-related metabolic disturbances. Cent Eur J Public Health. 1999;7:122–129. [PubMed] [Google Scholar]
- 8.Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. High triglycerides/low high-density lipoprotein cholesterol, ischemic electrocardiogram changes, and risk of ischemic heart disease. Am Heart J. 2003;145:103–108. doi: 10.1067/mhj.2003.45. [DOI] [PubMed] [Google Scholar]
- 9.Lamarche B, Després JP, Moorjani S, Cantin B, Dagenais GR, Lupien PJ. Triglycerides and HDL-cholesterol as risk factors for ischemic heart disease. Results from the Québec cardiovascular study. Atherosclerosis. 1996;119:235–245. doi: 10.1016/0021-9150(95)05653-x. [DOI] [PubMed] [Google Scholar]
- 10.Hsiao TJ, Chen JC, Wang JD. Insulin resistance and ferritin as major determinants of nonalcoholic fatty liver disease in apparently healthy obese patients. Int J Obes Relat Metab Disord. 2004;28:167–172. doi: 10.1038/sj.ijo.0802519. [DOI] [PubMed] [Google Scholar]
- 11.Lai SW, Tan CK, Ng KC. Epidemiology of fatty liver in a hospital-based study in Taiwan. South Med J. 2002;95:1288–1292. [PubMed] [Google Scholar]
- 12.Chen CH, Chuang JH, Kuo HS, Chang MS, Wang SP, Chou P. Prevalence of coronary heart disease in Kin-Chen, Kinmen. Int J Cardiol. 1996;55:87–95. doi: 10.1016/0167-5273(96)02622-8. [DOI] [PubMed] [Google Scholar]
- 13.Leung KW, Liu JD, Chen PH, Wang CS, Wang CK, Huang MJ, Siauw CP, Yuan CY, Chen TY. Clinical significance and diagnosis of fatty liver in Taiwan. Taiwan YiXueHui ZaZhi. 1986;85:149–160. [PubMed] [Google Scholar]
- 14.Lu SN, Wang LY, Chang WY, Chen CJ, Su WP, Chen SC, Chuang WL, Hsieh MY. Abdominal sonographic screening in a single community. Gaoxiong YiXue KeXue ZaZhi. 1990;6:643–646. [PubMed] [Google Scholar]
- 15.Yang PM, Huang GT, Lin JT, Sheu JC, Lai MY, Su IJ, Hsu HC, Chen DS, Wang TH, Sung JL. Ultrasonography in the diagnosis of benign diffuse parenchymal liver diseases: a prospective study. Taiwan Yi Xue Hui Za Zhi. 1988;87:966–977. [PubMed] [Google Scholar]
- 16.Hampton JR. The importance of minor abnormalities in the resting electrocardiogram. Eur Heart J. 1984;5 Suppl A:61–63. doi: 10.1093/eurheartj/5.suppl_a.61. [DOI] [PubMed] [Google Scholar]
- 17.Okin PM, Devereux RB, Kors JA, van Herpen G, Crow RS, Fabsitz RR, Howard BV. Computerized ST depression analysis improves prediction of all-cause and cardiovascular mortality: the strong heart study. Ann Noninvasive Electrocardiol. 2001;6:107–116. doi: 10.1111/j.1542-474X.2001.tb00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kannel WB, Anderson K, McGee DL, Degatano LS, Stampfer MJ. Nonspecific electrocardiographic abnormality as a predictor of coronary heart disease: the Framingham Study. Am Heart J. 1987;113:370–376. doi: 10.1016/0002-8703(87)90280-8. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB Anonymous. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1-253. [PubMed] [Google Scholar]
- 20.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 21.Steinmaurer HJ, Jirak P, Walchshofer J, Clodi PH. Accuracy of sonography in the diagnosis of diffuse liver parenchymal diseases--comparison of sonography and liver histology. Ultraschall Med. 1984;5:98–103. doi: 10.1055/s-2007-1012076. [DOI] [PubMed] [Google Scholar]
- 22.Tam KM, Wu JS. Ultrasonographic diagnosis of fatty liver. Taiwan Yi Xue Hui Za Zhi. 1986;85:45–53. [PubMed] [Google Scholar]
- 23.Yajima Y, Ohta K, Narui T, Abe R, Suzuki H, Ohtsuki M. Ultrasonographical diagnosis of fatty liver: significance of the liver-kidney contrast. Tohoku J Exp Med. 1983;139:43–50. doi: 10.1620/tjem.139.43. [DOI] [PubMed] [Google Scholar]
- 24.Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quiñones L, Varela N, Contreras J, Lazarte R, Csendes A, et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond) 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 25.Koenig W. Heart disease and the inflammatory response. BMJ. 2000;321:187–188. doi: 10.1136/bmj.321.7255.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SH, Kim BI, Yun JW, Kim JW, Park DI, Cho YK, Sung IK, Park CY, Sohn CI, Jeon WK, et al. Insulin resistance and C-reactive protein as independent risk factors for non-alcoholic fatty liver disease in non-obese Asian men. J Gastroenterol Hepatol. 2004;19:694–698. doi: 10.1111/j.1440-1746.2004.03362.x. [DOI] [PubMed] [Google Scholar]
- 27.Bandyopadhyay D, Chattopadhyay A, Ghosh G, Datta AG. Oxidative stress-induced ischemic heart disease: protection by antioxidants. Curr Med Chem. 2004;11:369–387. doi: 10.2174/0929867043456016. [DOI] [PubMed] [Google Scholar]
- 28.Rothenbacher D, Hoffmeister A, Brenner H, Koenig W. Physical activity, coronary heart disease, and inflammatory response. Arch Intern Med. 2003;163:1200–1205. doi: 10.1001/archinte.163.10.1200. [DOI] [PubMed] [Google Scholar]
- 29.Goto T, Onuma T, Takebe K, Kral JG. The influence of fatty liver on insulin clearance and insulin resistance in non-diabetic Japanese subjects. Int J Obes Relat Metab Disord. 1995;19:841–845. [PubMed] [Google Scholar]
- 30.Strang BD, Bertics SJ, Grummer RR, Armentano LE. Relationship of triglyceride accumulation to insulin clearance and hormonal responsiveness in bovine hepatocytes. J Dairy Sci. 1998;81:740–747. doi: 10.3168/jds.S0022-0302(98)75630-9. [DOI] [PubMed] [Google Scholar]
- 31.Grundy SM. Metabolic complications of obesity. Endocrine. 2000;13:155–165. doi: 10.1385/ENDO:13:2:155. [DOI] [PubMed] [Google Scholar]
- 32.Cohn G, Valdes G, Capuzzi DM. Pathophysiology and treatment of the dyslipidemia of insulin resistance. Curr Cardiol Rep. 2001;3:416–423. doi: 10.1007/s11886-001-0059-0. [DOI] [PubMed] [Google Scholar]
- 33.Garg A. Insulin resistance in the pathogenesis of dyslipidemia. Diabetes Care. 1996;19:387–389. doi: 10.2337/diacare.19.4.387. [DOI] [PubMed] [Google Scholar]
- 34.Dandona P. Insulin resistance and endothelial dysfunction in atherosclerosis: implications and interventions. Diabetes Technol Ther. 2002;4:809–815. doi: 10.1089/152091502321118829. [DOI] [PubMed] [Google Scholar]
- 35.Lamarche B, Tchernof A, Mauriège P, Cantin B, Dagenais GR, Lupien PJ, Després JP. Fasting insulin and apolipoprotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart disease. JAMA. 1998;279:1955–1961. doi: 10.1001/jama.279.24.1955. [DOI] [PubMed] [Google Scholar]
- 36.Lin YC, Chu FY, Fu CC, Chen JD. Prevalence and risk factors for angina in elderly Taiwanese. J Gerontol A Biol Sci Med Sci. 2004;59:161–165. doi: 10.1093/gerona/59.2.m161. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell BD, Hazuda HP, Haffner SM, Patterson JK, Stern MP. High prevalence of angina pectoris in Mexican-American men. A population with reduced risk of myocardial infarction. Ann Epidemiol. 1991;1:415–426. doi: 10.1016/1047-2797(91)90011-z. [DOI] [PubMed] [Google Scholar]
- 38.Burchfiel CM, Abbott RD, Sharp DS, Curb JD, Rodriguez BL, Yano K. Distribution and correlates of lipids and lipoproteins in elderly Japanese-American men. The Honolulu Heart Program. Arterioscler Thromb Vasc Biol. 1996;16:1356–1364. doi: 10.1161/01.atv.16.11.1356. [DOI] [PubMed] [Google Scholar]
- 39.Gaziano JM, Buring JE, Breslow JL, Goldhaber SZ, Rosner B, Van Denburgh M, Willett W, Hennekens CH. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med. 1993;329:1829–1834. doi: 10.1056/NEJM199312163292501. [DOI] [PubMed] [Google Scholar]


