Abstract
AIM: To investigate the expression of cyclooxygenase-2 (COX-2) in gastric cancer and its relation with the liver metastasis and prognosis.
METHODS: Expression of COX-2 mRNA and protein was examined in gastric cancer and its paired substantial normal tissue by semi-quantitative reverse transcription-polymerase chain reaction and immunohistochemistry. The relation between COX-2 expression and prognosis was investigated in 195 cases.
RESULTS: The expression of COX-2 mRNA in gastric cancer tissue was significantly higher than that in normal tissue in 47 cases (w = 792, P<0.01). The COX-2 mRNA in pT3-4 tissue expressed higher than that in pT1-2 tissue (w = 204, P<0.05). The positive expression rate of COX-2 protein was 57.9% (113/195). The COX-2 expression was significantly related to histological type, lymphnode metastasis, venous invasion and liver metastasis (P<0.05). No relation was found between COX-2 expression and invasion depth, peritoneal metastasis and International Union against Cancer TNM stage. The multiple regression analysis showed that the COX-2 expression and venous invasion were obviously associated with liver metastasis (P<0.05). However, there was no significant correlation between COX-2 immunoreactivity and prognosis.
CONCLUSION: COX-2 may play an important role in the development of gastric cancer, and the over-expression of COX-2 protein may be a high risk factor for liver metastasis.
Keywords: Gastric cancer, Cyclooxygenase-2, Neoplasm metastasis, Long-term prognosis
INTRODUCTION
Gastric cancer is one of the most common malignancies worldwide, and yet little is known about its molecular process of development and progression. Epidemiological studies showed that non-steroidal anti-inflammatory drugs (NSAID) can reduce the incidence of cancer of digestive tract[1-4]. Although the mechanism of NSAID remains unclear, it may inhibit COX[5]. COX, a key enzyme in conversion of arachidonic acid to prostaglandin, has two isoforms, namely COX-1 and cyclooxygenase-2 (COX-2). COX-1 is constitutively expressed in various tissues and involved in various physiological processes, whereas COX-2 is induced by pathological stimuli, such as inflammation, growth factors and cytokines produced by tumor cells[6,7]. Abnormal expression of COX-2 has been supposed to be related to the pathogenesis of human cancers[6,8,9].
We attempted to determine the expression of COX-2 in gastric cancer, and the relation between COX-2 and the pathological features of gastric cancer. In addition, the role of COX-2 in liver metastasis of gastric cancer and its prognostic value were explored.
MATERIALS AND METHODS
Patients and tissue samples for RT-PCR
Tissue specimens were taken from 47 gastric cancer patients who underwent resection (15 women and 32 men, aged 29-82 years). Paired samples of cancer tissue and normal gastric mucosa were obtained from each patient during surgery. Resected specimens were snap frozen in liquid nitrogen and stored at -80 °C. All patients were treated at Department of General Surgery, First Affiliated Hospital, Medical College of Zhejiang University, in 2001-2002. According to the criteria of International Union against Cancer (UICC), the depth of invasion was divided into pT1, pT2, pT3, and pT4.
Patients and tissue samples for immunohistochemistry
One hundred and ninety-five patients undergoing resection for primary gastric cancer were examined at Department of Gastrointestinal Surgery, First Affiliated Hospital, Medical School, Zhejiang University in 1993-1997 (57 women and 138 men, aged 27-85 years). Resected specimens were fixed in 40 g/L formaldehyde and embedded in paraffin. The cancers were reviewed according to the rules of clinical and pathological studies on gastric cancer for histological type, depth of invasion, lymphatic invasion, and venous invasion. Liver metastasis was found in 25 cases (12.8%). Peritoneal metastasis was found in 35 cases (17.9%).
Reverse transcription-PCR analysis for COX-2
Total RNA was isolated using TRIzol reagent (Life Technologies, Inc., Gaithersburg, MD, USA) according to standard acid-guanidium-phenol-chloroform method. About 2 µg of RNA from each sample was reverse transcribed at 42 °C for 60 min in a 20 µL reaction volume using RevertAid M-MuLV reverse transcriptase (MBI Fermentas). cDNA was incubated at 95 °C for 5 min to inactivate the reverse transcriptase, and served as template DNA for 32 cycles of amplification using the GeneAmp PCR System 2400 (Perkin-Elmer Applied Biosystems, CA, USA). PCR was performed in a standard 25 µL reaction mixture containing 1.5 µL of 25 mmol/L magnesium chloride, 1 µL 10 mmol/L dNTP, 25 µmol/L of each sense and antisense primer and 1.5 U of Taq DNA polymerase (Promega). Amplification was performed (at 94 °C for 1 min, at 57 °C for 1 min and at 72 °C for 1.5 min) after heat-start for 5 min. Finally, an additional extension step was carried out for 10 min. As a negative control, the DNA template was omitted in the reaction. The primer sequences and PCR product sizes were as follows. COX-2: forward primer, 5’-TGA AAC CCA CTC CAA ACA CAG-3’; reverse primer, 5’-TCA TCA GGC ACA GGG AGG AAG-3’, 232 bp; β-actin: forward primer, 5’-TCG TGA TGG ACT CCG GTG AC-3’; reverse primer, 5’-TCG TGG ATG CCA CAG GAC TC-3’, 370 bp. The amplified products were evaluated in 2% agarose gel and visualized by ethidium bromide staining under UV light. The stained bands were analyzed with a digital gel documentation system and associated densitometry software (EDAS 290 and 1D software; Kodak Digital Science, Rochester, NY, USA). To estimate COX-2 expression levels, the expression ratio was designated as a band density ratio of COX-2 to β-actin.
Immunohistochemistry
The EnVision’ method was used for immunohistochemical staining. Five micrometer-thick sections were deparaffinized with xylene and progressively dehydrated in decreasing concentrations of alcohol. Endogenous peroxidase activity was blocked by hydrogen peroxidase (3%) in Tris-buffered saline (TBS) for 30 min. Sections were boiled (pressure-cooking) in citrate buffer, pH 6.0, for 3 min for antigen retrieval. Non-specific binding was blocked with 5% goat serum in TBS for 15 min, and the tissues were incubated with monoclonal antibody to COX-2 (1:200; Cayman Chemical, Ann Arbor, MI, USA; catalog no. 160112) in TBS containing 2% rabbit serum and 1% bovine serum albumin for 1 h. The sections were washed with TBS, and incubated with EnVision (goat anti-mouse/HRP) for 60 min. The color was developed in diaminobenzidine solution (Sigma Chemical Co., St. Louis, MO, USA) and counterstained with Mayer’s hematoxylin. Tissues were incubated with TBS containing 2% rabbit serum and 1% bovine serum albumin without the primary antibody as control. Furthermore, each run included a positive control side, which was shown previously to be strongly positive for COX-2. The scientists who performed the immunohistochemical analysis were blinded to pathological features. Tissues stained more than 10% were classified as COX-2 protein-positive[11].
Statistical analysis
Statistical analysis was performed by use of Wilcoxon signed-ranks, χ2 test and Kaplan-Meier test. The risk factors for liver metastasis were evaluated by a multivariate logistic regression analysis, variables with a P-value less than 0.1 in univariate analysis were included. P<0.05 was considered statistically significant.
RESULTS
RT-PCR analysis
The expression of COX-2 mRNA in gastric cancer was apparently elevated in comparison to the normal gastric mucosa (1.080.65 vs 0.330.21, P<0.01). In addition, the COX-2 mRNA in pT3, pT4 tissues was significantly higher than that in pT1, pT2 (0.750.43 vs 1.140.67, P<0.05) (Figure 1).
Figure 1.
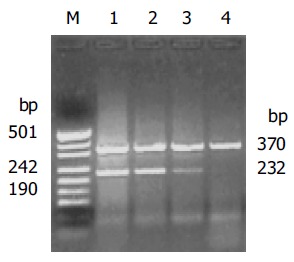
Expression of COX-2 mRNA in gastric cancer. M: pUC19 DNA/MspI marker; lanes 1 and 2: gastric cancer tissues; lanes 3 and 4: matched normal gastric mucosal tissue.
Relation between COX-2 expression and clinicopathological features
As shown in Figure 2, the COX-2 protein was expressed intensely, mainly in cytoplasm and nuclear membrane of cancer cells. In the 195 specimens, 113 (58%) tumor tissue specimens showed positive immunoreactivity for the mAb COX-2. The COX-2 protein was expressed more frequently and intensely in differentiated adenocarcinoma than in diffuse-type carcinoma. The samples with lymph node metastasis, venous invasion and liver metastasis were significantly stained by COX-2, compared to those without lymph node metastasis. No statistical correlation was revealed between COX-2 immunoreactivity and depth of invasion, peritoneal metastasis and UICC TNM stage (Table 1).
Figure 2.
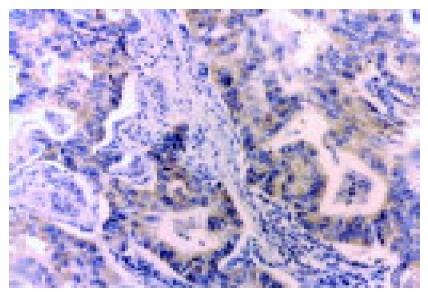
Immunostaining of COX-2 in gastric cancer.
Table 1.
Correlation between clinicopathologic features of gastric cancer and COX-2 expression
| Variable | n | COX-2 positive cases (%) | P (χ²) |
| Histologic type | |||
| Differentiated | 84 | 59 (70.2) | 0.002 (9.15) |
| Diffuse | 111 | 54 (48.6) | |
| Depth of invasion | |||
| T2 | 84 | 47 (60.0) | 0.483 (1.46) |
| T3 | 88 | 50 (56.8) | |
| T4 | 23 | 16 (69.6) | |
| UICC stage | |||
| I | 14 | 6 (42.8) | |
| II | 23 | 11 (47.8) | 0.410 (2.88) |
| III | 61 | 38 (62.3) | |
| IV | 97 | 58 (59.8) | |
| Venous invasion | |||
| Negative | 80 | 39 (48.8) | 0.030 (4.71) |
| Positive | 115 | 74 (64.3) | |
| Lymph node metastasis | |||
| Negative | 20 | 7 (35.0) | 0.028 (4.82) |
| Positive | 175 | 106 (60.6) | |
| Liver metastasis | |||
| Negative | 170 | 93 (54.7) | 0.017 (5.72) |
| Positive | 25 | 20 (80.0) | |
| Peritoneal metastasis | |||
| Negative | 160 | 95 (59.4) | 0.388 (0.74) |
| Positive | 35 | 18 (51.4) |
Risk factors for liver metastasis of gastric cancer
Based on the results of the univariate analysis, histological type (P = 0.066), depth of invasion (P = 0.601), venous invasion (P = 0.011), lymph node metastasis (P = 0.810), COX-2 immunoreactivity (P = 0.008), and venous invasion were included as covariables in the multivariate regression analysis. The result revealed that COX-2 immunoreactivity and venous invasion were the significant risk factors for liver metastasis (Table 2).
Table 2.
Multivariate logistic regression analysis of independent risk factors for liver metastasis in 195 gastric cancer patients
| Independent risk factor | Relative risk | 95%CI | P |
| Venous invasion | |||
| Negative | 1 | ||
| Positive | 2.698 | 1.093–6.660 | 0.031 |
| COX-2 immunoreactivity | |||
| Negative | 1 | ||
| Positive | 2.857 | 1.159–7.041 | 0.023 |
Expression of COX-2 and prognosis
According to the expression of COX-2, the Kaplan-Meier survival curves showed no significant correlation between COX-2 immunoreactivity and prognosis of gastric cancer (Figure 3).
Figure 3.
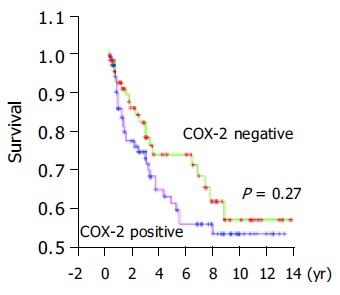
Overall survival curves of patients with gastric cancer.
DISCUSSION
In the current study, we demonstrated that both COX-2 mRNA and protein were expressed in gastric cancer tissue. The COX-2 mRNA level was associated with the depth of tumor invasion. Ristimaki et al[8], initially reported, that COX-2 mRNA level is elevated in gastric cancer. Uefuji et al[11], found that the level of COX-2 protein is higher in gastric cancer than in paired normal gastric mucosa by Western blot analysis. Furthermore, Ohno et al[12], revealed, that COX-2 mRNA level increases gradually with the depth of gastric cancer invasion. But the exact mechanism is still unknown. The COX-2 specific antibody identified that COX-2 protein is located in cytoplasm and nuclear membrane of gastric cancer cells, but not in stroma surrounding cancerous tissues, the COX-2 protein is also observed in smooth muscle cells, fibroblasts, and inflammatory mononuclear cells[12-14]. Additionally, epithelial cells showing intestinal metaplasia and adenoma are also strongly immunoreactive to anti-COX-2 Ab. The above data demonstrate that COX-2 is upregulated in gastric cancer, and high COX-2 expression may be an early event in the carcinogenesis of stomach.
In our study, the COX-2 protein was expressed higher in differentiated adenocarcinoma than in diffuse-type carcinoma. Yamagata et al[15], reported, that COX-2 expression increases in early intestinal-type gastric cancer, and is significantly higher than that in diffuse-type carcinoma. However, no correlation is found between the histologic type of gastric cancer and COX-2 protein in other reports[12].
Recent studies have shown that COX-2 expression is correlated with lymph node metastasis and lymphatic invasion in gastric carcinoma. Murata et al[17], showed that COX-2 overexpression is associated significantly with tumor invasion of gastric wall lymphatic vessels and lymph node metastasis. The same results are also disclosed in other reports[13,17]. Taken together, these results suggest that COX-2 may influence lymphatic involvement and promote tumor invasion.
Our study showed that COX-2 expression was associated with venous invasion and liver metastasis of gastric cancer. The multivariate analysis revealed that the COX-2 immunoreactivity was an independent risk factor for liver metastasis of gastric carcinoma. COX-2 can enhance the adhesive ability of endothelial cells in colonic carcinoma cells, and fortify liver metastatic potential via secretion of sialyl Lewis antigens[18,19]. Nagatsuka et al[16], also discovered that the selective COX-2 inhibitor (JTE 522) has inhibitory effects on liver metastasis of colon cancer. On the contrary, some reports showed that COX-2 does not play a major role in the process of distant metastasis of gastric cancer[11,12,17]. The reason why there is such a discrepancy is not clear, but the differences in methods (antibody, results evaluation) might be an important reason.
It was reported that COX-2 protein overexpression is significantly associated with the UICC TNM stage and the prognosis of gastric cancer patients[16,17]. Ristimaki et al[20], have connected COX-2 overexpression to an unfavorable distant disease-free survival in breast cancer patients (n = 1 576). However, no significant correlation was found between COX-2 immunoreactivity and peritoneal metastasis, UICC TNM stage and prognosis in our study. Joo et al[21], also have not found any significant correlation between them. A large group and long term follow-up are necessary to assess the prognostic value of COX-2 in gastric cancer.
Our results suggest that COX-2 is overexpressed in gastric cancer, and COX-2 may play an important role in liver and lymphatic metastasis of gastric cancer. Inhibiting the activity of COX-2 may provide therapeutic benefit to gastric cancer.
Footnotes
Supported by the Natural Science Foundation of Zhejiang Province, No. 302048
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Farrow DC, Vaughan TL, Hansten PD, Stanford JL, Risch HA, Gammon MD, Chow WH, Dubrow R, Ahsan H, Mayne ST, et al. Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:97–102. [PubMed] [Google Scholar]
- 2.García-Rodríguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12:88–93. doi: 10.1097/00001648-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Morgan G. Beneficial effects of NSAIDs in the gastrointestinal tract. Eur J Gastroenterol Hepatol. 1999;11:393–400. doi: 10.1097/00042737-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Funkhouser EM, Sharp GB. Aspirin and reduced risk of esophageal carcinoma. Cancer. 1995;76:1116–1119. doi: 10.1002/1097-0142(19951001)76:7<1116::aid-cncr2820760703>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (Part II) J Natl Cancer Inst. 1998;90:1609–1620. doi: 10.1093/jnci/90.21.1609. [DOI] [PubMed] [Google Scholar]
- 6.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 7.Williams CS, DuBois RN. Prostaglandin endoperoxide synthase: why two isoforms? Am J Physiol. 1996;270:G393–G400. doi: 10.1152/ajpgi.1996.270.3.G393. [DOI] [PubMed] [Google Scholar]
- 8.Ristimäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276–1280. [PubMed] [Google Scholar]
- 9.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- 10.Saukkonen K, Nieminen O, van Rees B, Vilkki S, Härkönen M, Juhola M, Mecklin JP, Sipponen P, Ristimäki A. Expression of cyclooxygenase-2 in dysplasia of the stomach and in intestinal-type gastric adenocarcinoma. Clin Cancer Res. 2001;7:1923–1931. [PubMed] [Google Scholar]
- 11.Uefuji K, Ichikura T, Mochizuki H, Shinomiya N. Expression of cyclooxygenase-2 protein in gastric adenocarcinoma. J Surg Oncol. 1998;69:168–172. doi: 10.1002/(sici)1096-9098(199811)69:3<168::aid-jso9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, Ichikawa W, Nihei Z, Sugihara K. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876–1881. [PubMed] [Google Scholar]
- 13.Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, Kim HS, Nam DK, Lee KB, Kim HC. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000;6:519–525. [PubMed] [Google Scholar]
- 14.van Rees BP, Saukkonen K, Ristimäki A, Polkowski W, Tytgat GN, Drillenburg P, Offerhaus GJ. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol. 2002;196:171–179. doi: 10.1002/path.1033. [DOI] [PubMed] [Google Scholar]
- 15.Yamagata R, Shimoyama T, Fukuda S, Yoshimura T, Tanaka M, Munakata A. Cyclooxygenase-2 expression is increased in early intestinal-type gastric cancer and gastric mucosa with intestinal metaplasia. Eur J Gastroenterol Hepatol. 2002;14:359–363. doi: 10.1097/00042737-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Nagatsuka I, Yamada N, Shimizu S, Ohira M, Nishino H, Seki S, Hirakawa K. Inhibitory effect of a selective cyclooxygenase-2 inhibitor on liver metastasis of colon cancer. Int J Cancer. 2002;100:515–519. doi: 10.1002/ijc.10508. [DOI] [PubMed] [Google Scholar]
- 17.Murata H, Kawano S, Tsuji S, Tsuji M, Sawaoka H, Kimura Y, Shiozaki H, Hori M. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999;94:451–455. doi: 10.1111/j.1572-0241.1999.876_e.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto H, Itoh F, Fukushima H, Hinoda Y, Imai K. Overexpression of cyclooxygenase-2 protein is less frequent in gastric cancers with microsatellite instability. Int J Cancer. 1999;84:400–403. doi: 10.1002/(sici)1097-0215(19990820)84:4<400::aid-ijc12>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 19.Kakiuchi Y, Tsuji S, Tsujii M, Murata H, Kawai N, Yasumaru M, Kimura A, Komori M, Irie T, Miyoshi E, et al. Cyclooxygenase-2 activity altered the cell-surface carbohydrate antigens on colon cancer cells and enhanced liver metastasis. Cancer Res. 2002;62:1567–1572. [PubMed] [Google Scholar]
- 20.Ristimäki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 21.Joo YE, Oh WT, Rew JS, Park CS, Choi SK, Kim SJ. Cyclooxygenase-2 expression is associated with well-differentiated and intestinal-type pathways in gastric carcinogenesis. Digestion. 2002;66:222–229. doi: 10.1159/000068366. [DOI] [PubMed] [Google Scholar]


