Abstract
Studies of the effects of GH and the mechanisms of its actions frequently use rats or mice and various recombinant human GH preparations. Authors of many of these studies appear unaware of the fact that, in rodents, human GH signals through both GH and prolactin (PRL) receptors; thus, treatment with human GH is equivalent to a combined treatment with GH and PRL. GH receptors and PRL receptors are present in multiple cell types. Importantly, PRL exerts major effects on brain neuroendocrine action, female and male reproduction, metabolism, body composition, immune responses, and a host of other functions; thus, treatment of rodents with recombinant human GH could affect these important physiological parameters.
Lactogenic activity of human GH (hGH) in rodents was already known in the 1960s (1, 2) and contrasted with the purely somatotropic activity of the bovine, ovine, and porcine GH. Readers interested in history of endocrinology may be intrigued by the fact that, based on the difficulties of extracting biologically active prolactin (PRL) from human pituitaries collected postmortem and the luteotropic and lactogenic activity of hGH in rodents, it was believed that humans may be unique among mammals by not producing PRL and instead having a pituitary hormone that serves both somatotropic and lactotropic functions. In a review article on the discovery of human prolactin, Friesen (3) stated, “Although prolactin was discovered in the early 1930s in sheep, cows, birds etc, no human form had been purified because it was thought to be identical to human growth hormone. In fact, prior to 1970, most endocrinologists doubted human prolactin even existed.” This doubt was obviously discarded after successful identification and purification of human PRL in the early 1970s (4–6).
Many years later, several laboratories produced transgenic mice that expressed a variety of cloned GH genes. Remarkable increase in adult body size was a common characteristic of animals expressing human, bovine, ovine, or rat GH. However, transgenic mice expressing hGH exhibited various characteristics not seen in transgenics that expressed other species of GH (7–11). Prominent among them was stimulation of mammary glands leading to lactation. In a line of mice expressing high levels of hGH under the control of rat phosphoenolpyruvate carboxykinase promoter/enhancer, females experienced repeated pseudopregnancies consistent with the luteotropic activity of PRL receptor (PRLR) ligands in rodents, and virgin hGH transgenic females readily accepted foster pups and produced enough milk to raise normal-size litters to weaning (12). In a different line of transgenic mice with a lower level of hGH expression, activation of tuberoinfundibular dopaminergic neurons (a well documented target of PRL action in the brain) interfered with endogenous PRL release, leading to the infertility of females (13). Interestingly, some of the effects of expression of hGH in transgenic mice on hypothalamic neurotransmitter turnover, pituitary morphology, and hormone levels were opposite to the effects of expression of bovine GH on the same parameters (8, 15). Additionally, consistent with lactogenic activity of hGH in rodents, transgenic mice expressing hGH exhibit high frequency of mammary tumors (16, 17).
Evolutionally, GH and PRL are closely related as members of the same family and are similar in terms of size and structure. Both GH and PRL act primarily (and likely exclusively) by binding to dimeric cell surface receptors and activating the JAK2-STAT5 (Janus kinase-signal transducer and activator of transcription) signaling pathway to modulate the expression of numerous genes in target tissues. However, they exert many hormone-specific (and often also species specific) actions. For example, PRL provides the key luteotropic stimulus in rodents and carnivores (but not in ruminants or primates) and is importantly involved in the control of seasonal transitions between reproductive activity and quiescence (18, 19), whereas there is no evidence for GH sharing any of these actions. However, GH and PRL actions can also overlap. For example, hepatic IGF-1 expression, circulating IGF-1 levels and somatic growth are primarily regulated by GH, but PRL may also enhance IGF-1 expression, at least in fetal tissues (20), and stimulate somatic growth.
Although neuroendocrine control of the release of GH and PRL from the pituitary is very different involving distinct neuronal groups, neuropeptides, and neurotransmitters, in many species both PRL and GH are released in response to stress.
Cloning of GH and PRL genes from various species and elegant crystallographic studies elucidated details of three-dimensional structure of GH and PRL molecules and their interaction with the corresponding receptors (21, 22). These studies identified molecular regions responsible for the lactogenic (PRL-like) activity of hGH and for differences between human, as compared with ruminant, porcine, or rodent GHs (21, 22)
For example, nonprimate GHs are unable to bind to the human GH receptor (GHR), whereas primate GHs can bind to nonprimate GHRs and PRLRs with high affinity (21, 23–25). It has been suggested that nonprimate GHs are unable to bind to the human GHRs because of amino acid differences between the two hormones. In particular, Cunningham and Wells (22) have shown that nonprimate GHs possess substitutions of at least 10 of the 25 hGH amino acid-binding determinants (Ile4, Metl4, Ser62, Asn63, Arg64, Lys70, Tyr164, Asp171, Phe176, Ile179). These changes explain the lack of binding of nonprimate GH to human GHRs. An extension of this work by Souza et al (26) suggested that species-specific amino acid residues, primarily the substitution of Asp171 for His in all nonprimate GH relative to hGH, is responsible for this effect. Similarly to the GH induced intracellular signaling, effects of PRL vary with the species of hormone and cognate receptor origin. Utama et al (27) described major differences in the biological activities of PRLs derived from seven different mammalian species on human and rat PRLR. In the same study, mouse PRL was shown to be a competitive inhibitor of human PRL binding to human PRLR and to exhibit a partial antagonist activity in this system (27).
Major species differences in biological activity also extend to placental hormones from the same family, which exhibit various combinations of somatotropic and lactogenic activity. Bovine and porcine placental lactogen have been shown to bind to the somatogen receptor (28–31). Additionally, human placental GH, similarly to the human pituitary GH (and recombinant hGH), is lactogenic in rodents (32, 14).
We want to finish this brief article with an appeal to investigators, both authors and reviewers (and particularly those now entering this field), to carefully consider the biological differences between GHs derived from different species in designing their experiments and interpreting their results. Attention to the well-documented and powerful lactogenic effects of hGH in rodents and high affinity of this hormone for the rodent PRLR is of particular importance (Figure 1).
Figure 1.
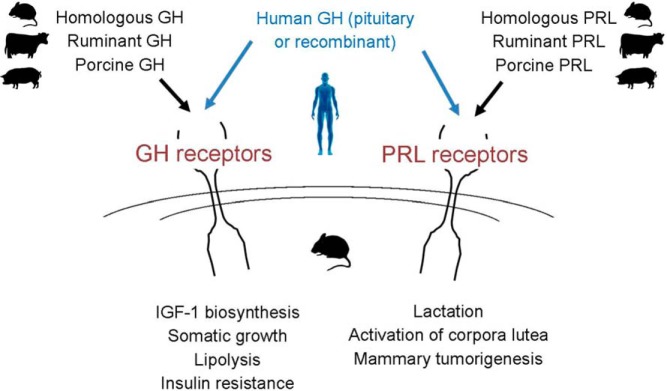
Actions of homologous (mouse, rat), ruminant (bovine, ovine), porcine or human GH in rodents.
Acknowledgments
This work was supported by the National Institute on Aging of the National Institutes of Health under Award R01AG019899 (to A.B.) and by the State of Ohio Eminent Scholars Program, including a gift by Milton and Lawrence Goll (to J.J.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- GHR
- GH receptor
- hGH
- human GH
- PRL
- prolactin
- PRLR
- prolactin receptor
References
- 1. Forsyth IA, Folley SJ, Chadwick A. Lactogenic and pigeon crop-stimulating activities of human pituitary growth hormone preparation. J Endocrinol. 1965;31:115–126. [DOI] [PubMed] [Google Scholar]
- 2. Hartree AS, Kovacic N, Thomas M. Growth-promoting and luteotrophic activities of human growth hormone. J Endocrinol. 1965;33:249–258. [DOI] [PubMed] [Google Scholar]
- 3. Friesen HG. The discovery of human prolactin: a very personal account. Clin Invest Med. 1995;18:66–72. [PubMed] [Google Scholar]
- 4. Gala RR. Prolactin production by the human anterior pituitary cultured in vitro. J Endocrinol. 1971;50:637–642. [DOI] [PubMed] [Google Scholar]
- 5. Lewis UJ, Singh RN, Seavey BK. Human prolactin: Isolation and some properties. Biochem Biophys Res Commun. 1971;44:1169–1176. [DOI] [PubMed] [Google Scholar]
- 6. Hwang P, Guyda H, Friesen H. Purification of human prolactin. Methods Enzymol. 1975;37:389–402. [DOI] [PubMed] [Google Scholar]
- 7. Brem G, Wanke R, Wolf E, et al. Multiple consequences of human growth hormone expression in transgenic mice. Mol Biol Med. 1989;6:531–547. [PubMed] [Google Scholar]
- 8. Steger RW, Bartke A, Parkening TA, et al. Effects of heterologous growth hormones on hypothalamic and pituitary function in transgenic mice. Neuroendocrinology. 1991;53:365–372. [DOI] [PubMed] [Google Scholar]
- 9. Bartke A, Naar EM, Johnson L, et al. Effects of expression of human or bovine growth hormone genes on sperm production and male reproductive performance in four lines of transgenic mice. J Reprod Fertil. 1992;95:109–118. [DOI] [PubMed] [Google Scholar]
- 10. Phelps CJ, Bartke A. Stimulatory effect of human, but not bovine, growth hormone expression on numbers of tuberinfundibular dopaminergic neurons in transgenic mice. Endocrinology. 1997;138:2849–2855. [DOI] [PubMed] [Google Scholar]
- 11. Kopchick J, Chen XZ, Li Y, et al. Differential in vivo activities of bovine growth hormone analogs. Transgen Res. 1998;7:61–71. [DOI] [PubMed] [Google Scholar]
- 12. Milton S, Cecim M, Li YS, Yun JS, Wagner TE, Bartke A. Transgenic female mice with high human growth hormone levels are fertile and capable of normal lactation without having been pregnant. Endocrinology. 1992;131:536–538. [DOI] [PubMed] [Google Scholar]
- 13. Bartke A, Steger RW, Hodges SL, et al. Infertility in transgenic female mice with human growth hormone expression: Evidence for luteal failure. J Exp Zool. 1988;248:121–124. [DOI] [PubMed] [Google Scholar]
- 14. Prins GS, Cecim M, Birch L, Wagner TE, Bartke A. Growth response and androgen receptor expression in seminal vesicles from aging transgenic mice expressing human or bovine growth hormone genes. Endocrinology. 1992;131:2016–2023. [DOI] [PubMed] [Google Scholar]
- 15. Bartke A, Cecim M, Tang K, Steger RW, Chandrashekar V, Turyn D. Neuroendocrine and reproductive consequences of overexpression of growth hormone in transgenic mice. Proc Soc Exp Biol Med. 1994;206:345–359. [DOI] [PubMed] [Google Scholar]
- 16. Tornell J, Carlsson B, Pohjanen P, Wennbo H, Rymo L, Isaksson O. High frequency of mammary adenocarcinomas in metallothionein promoter-human growth hormone transgenic mice created from two different strains of mice. J Steroid Biochem Mol Biol. 1992;43:237–242. [DOI] [PubMed] [Google Scholar]
- 17. Steger RW, Bartke A, Cecim M. Premature ageing in transgenic mice expressing different growth hormone genes. J Reprod Fertil Suppl. 1993;46:61–75. [PubMed] [Google Scholar]
- 18. Bartke A, Goldman BD, Bex FJ, et al. Effects of prolactin on testicular regression and recrudescence in the golden hamster. Endocrinology. 1980;106:167–172. [DOI] [PubMed] [Google Scholar]
- 19. Matt KS, Bartke A, Soares MJ, Talamantes F, Hebert A, Hogan MP. Does prolactin modify testosterone feedback in the hamster? Suppression of plasma prolactin inhibits photoperiod-induced decreases in testosterone feedback sensitivity. Endocrinology. 1984;115:2098–2103. [DOI] [PubMed] [Google Scholar]
- 20. Karabulut AK, Layfield R, Pratten MK. The mechanism of growth-promoting effects of prolactin in embryogenesis—links to growth factors. Cells Tissues Organs. 1999;164:2–13. [DOI] [PubMed] [Google Scholar]
- 21. Goffin V, Shiverick KT, Kelly PA, Martial JA. Sequence-function relationships within the expanding family of prolactin, growth hormone, placental lactogen, and related proteins in mammals. Endocr Rev. 1996;17:385–410. [DOI] [PubMed] [Google Scholar]
- 22. Cunningham BC, Wells JA. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989;244:1081–1085. [DOI] [PubMed] [Google Scholar]
- 23. Posner BI, Kelly PA, Shiu RPC, Paud R, Friesen HG. Studies of insulin, growth hormone and prolactin binding: tissue distribution, species variation and characterization. Endocrinology. 1974;95:521–531. [DOI] [PubMed] [Google Scholar]
- 24. Shiu RP, Elsholtz HP, Tanaka T, et al. Receptor-mediated mitogenic action of prolactin in a rat lymphoma cell line. Endocrinology. 1983;113:159–165. [DOI] [PubMed] [Google Scholar]
- 25. Cunningham BC, Grass S, Fuh G, Wells JA. Zinc mediation of the binding of human growth hormone to the human prolactin receptor. Science. 1990;250:1709–1712. [DOI] [PubMed] [Google Scholar]
- 26. Souza SC, Frick GP, Wang X, Kopchick JJ, Lobo RB, Goodman HM. A single arginine residue determines species specificity of the human growth hormone receptor. Proc Natl Acad Sci US A. 1995;92:959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Utama FE, LeBaron MJ, Neilson LM, et al. Human prolactin receptors are insensitive to mouse prolactin: Implications for xenotransplant modeling of human breast cancer in mice. J Endocrinol. 2006;188:589–601. [DOI] [PubMed] [Google Scholar]
- 28. Fiddes RJ, Brandon MR, Adams TE. Functional expression of an ovine growth hormone receptor in transfected Chinese hamster ovary cells. Mol Cell Endocrinol. 1992;86:37–47. [DOI] [PubMed] [Google Scholar]
- 29. Vashdi D, Elberg G, Sakal E, Gertler A. Biological activity of bovine placental lactogen in 3t3–f442a preadipocytes is mediated through a somatogenic receptor. FEBS Lett. 1992;305:101–104. [DOI] [PubMed] [Google Scholar]
- 30. Gertler A, Hauser SD, Sakal E, et al. Preparation, purification, and determination of the biological activities of 12 N terminus-truncated recombinant analogues of bovine placental lactogen. J Biol Chem. 1992;267:12655–12659. [PubMed] [Google Scholar]
- 31. Breier BH, Funk B, Surus A, et al. Characterization of ovine growth hormone (oGH) and ovine placental lactogen (oPL) binding to fetal and adult hepatic tissue in sheep: evidence that oGH and oPL interact with a common receptor. Endocrinology. 1994;135:919–928. [DOI] [PubMed] [Google Scholar]
- 32. Nickel BE, Kardami E, Cattini PA. The human placental growth hormone variant is mitogenic for rat lymphoma nb2 cells. Endocrinology. 1990;126:971–976. [DOI] [PubMed] [Google Scholar]


