Abstract
Testosterone treatment induces erythrocytosis that could potentially affect blood viscosity and cardiovascular risk. We thus investigated the effects of testosterone administration on blood viscosity and erythrocyte deformability using mouse models. Blood viscosity, erythrocyte deformability, and hematocrits were measured in normal male and female mice, as well as in females and castrated males after short-term (2 wk) and long-term (5–7 mo) testosterone intervention (50 mg/kg, weekly). Castrated males for long-term intervention were studied in parallel with the normal males to assess the effect of long-term testosterone deprivation. An additional short-term intervention study was conducted in females with a lower testosterone dose (5 mg/kg). Our results indicate no rheological difference among normal males, females, and castrated males at steady-state. Short-term high-dose testosterone increased hematocrit and whole-blood viscosity in both females and castrated males. This effect diminished after long-term treatment, in association with increased erythrocyte deformability in the testosterone-treated mice, suggesting the presence of adaptive mechanism. Considering that cardiovascular events in human trials are seen early after intervention, rheological changes as potential mediator of vascular events warrant further investigation.
The use of testosterone as a prescription drug has increased dramatically in recent years. Several studies raised concerns about the cardiovascular risk of testosterone therapy (1–3). Epidemiologic studies suggested that both very low and very high testosterone levels are linked to cardiovascular and cerebral risk (4, 5), but the mechanism remains unclear. Elevated blood viscosity is associated with cardiovascular and cerebral risk, especially in the context of therapeutic administration of erythropoiesis-stimulating agents (6–9). Likewise, testosterone administration consistently increases hematocrits in men (10, 11). Because erythrocyte mass is a major contributor to blood viscosity, testosterone administration might thus increase blood viscosity. However, relevant literature is scant and inconclusive (12–14). On the other hand, increased erythrocyte deformability has been reported in erythropoietin-transgenic mice, which allows the mice to offset their whole-blood viscosity to a much lower level than that predicted from their exceptionally high hematocrits (15). It is unclear whether men or animals acquire similar adaptations to testosterone-induced erythrocytosis. Here, we report the effects of testosterone on blood viscosity and erythrocyte deformability in mice after short-term and long-term interventions.
Materials and Methods
Animals
For long-term intervention experiments, precastrated male, sham male, and female C57BL/6 mice were purchased from The Jackson Laboratory at 6 weeks of age. For short-term experiments, precastrated male and female mice were purchased as retired breeders (7–8 mo). At the end point, all mice were about 8–9 months. Mice were randomized to vehicle or testosterone groups with matching levels of hematocrits at baseline. Testosterone propionate was injected sc weekly at 50 mg/kg in 100-μL medium-chain triglyceride oil (Life Enhancement) for either 2 weeks (females and castrated males) or 5 months (females) and 7 months (castrated males). In response to the reviewers' suggestion, additional female mice were tested with low-dose testosterone propionate (5 mg/kg). All control mice were weekly injected with 100-μL vehicle oil. The number of animals for each experiment is listed in the corresponding figure legend. All mice were housed in the Center for Animal Resources at Harvard Medical School with controlled temperature at 21°C and a 12-hour light, 12-hour dark cycle with free access to water and standard chow. The use of animals was approved by the Institutional Animal Care and Use Committee of Harvard Medical School.
Blood analysis
Erythrocyte packing difference (EPD) was measured as a marker for erythrocyte sedimentation rate (16). Briefly, a microhematocrit tube was filled with tail-vein blood and spun at 100g for 30 minutes at room temperature for the apparent hematocrit reading. Then the same tube was spun in CritSpin (Iris Sample Processing, Inc) to obtain the standard hematocrit reading. EPD was calculated as the difference between these 2 readings.
Complete blood counts were obtained from the Hematology Core at Boston Children's Hospital (Boston, MA). Blood viscosity was measured as described (15). Blood was obtained via cardiac puncture into an EDTA blood collection tube and analyzed within 5–10 minutes using a rotation viscosimeter (DVIII_ Rheometer; Brookfield Engineering Laboratories). Viscosity was recorded at 37°C under different shear rates. Plasma viscosity was analyzed similarly except that samples were prestored at −80°C. Erythrocyte deformability was measured as the elongation rate (15). Whole blood (0.3 mL) was mixed with 3 mL of PBS containing 14.4% Dextran (number D6030; USbiological) with a viscosity of 10 mPas, close to human peripheral artery blood viscosity at low shear rate (17). For selected experiments, a high viscosity Dextran solution was also tested (24 mPas). The osmolarity of both solutions was adjusted to 310–320 mOsm/kg. The erythrocyte suspension was used to measure erythrocyte elongation rate using a Laser diffractometer (Rheodyn-SSD; Myrenne).
Serum testosterone was measured using liquid chromatography-tandem mass spectrometry, an assay certified by the Hormone Assay Standardization Program of the Centers for Disease Control as described previously (18). Because of the small volume available from some animals, serum samples were pooled for some analyses. Serum testosterone were undetectable (<0.01 ng/mL) in vehicle-treated castrated males and increased with testosterone treatment (9.3 ± 3.4 ng/mL, mean ± SD, n = 5, pooled plasma from 13 animals). Testosterone levels were also elevated in testosterone-treated females (7.1 ± 3.0 and 0.88 ± 0.37 ng/mL for high- and low-dose testosterone, respectively, n = 7; for the low-dose group, n = 3, pooled from 8 animals for the high-dose group), as compared with that in vehicle-treated females (0.04 ± 0.13 ng/mL, n = 15). The mean testosterone level in normal males was 0.57 ± 0.66 ng/mL. Thus, high-dose testosterone administration in females and castrated males resulted in supraphysiologic testosterone levels, whereas low-dose treatment in females resulted in a level close to that in normal adult males.
Because pharmacokinetics of testosterone are characterized by peaks of testosterone levels shortly after injection followed by gradual decline over the ensuing dosing interval, we obtained additional samples to assess the time-dependent changes in serum testosterone after injection. As displayed in Supplemental Figure 1, serum testosterone declines rapidly after reaching a peak. Effects of treatment on kidney and spleen weights are shown in Supplemental Table 1; kidney mass, but not spleen, responded to testosterone treatment, as expected (19).
Statistics
Statistical analyses were performed using SAS 9.3 software (SAS Institute) and Prism software (GraphPad Software, Inc). Results are shown as mean ± SD. For hematological parameters, comparison between the vehicle- vs testosterone-treated groups (and nontreated females vs nontreated males) were done using t test for independent samples and paired comparisons. For blood viscosity and erythrocyte elongation rate, ANOVA with repeated measures was performed. To assess the interaction between main effect and shear force (or shear rate), Mauchly's sphericity test was performed and Greenhouse-Geisser adjusted P values were calculated. If the interaction was found to be nonsignificant, overall main effect was tested. For each shear force (rate), t test for main effects was also performed. Statistical significance was evaluated at 0.05 level of α.
Results
Comparison between normal male and female mice
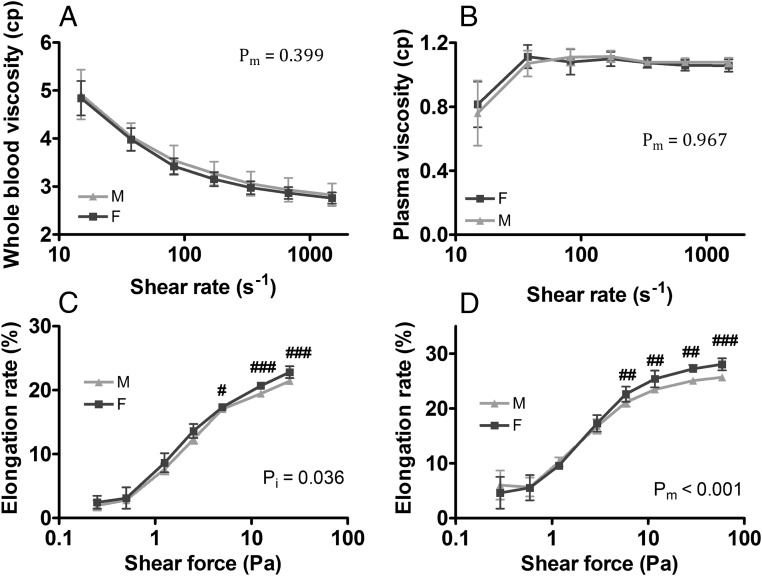
The red blood cell indices were generally similar between male and female mice with a trend towards higher hematocrit level in the males (Supplemental Table 2). Females had higher reticulocyte count but lower mean corpuscular volume (MCV) than males. EPD was higher in the males than the females (6.5 ± 1.1% vs 4.0 ± 0.8%, P < .01), replicating the findings in humans (16). No sex difference was detected in whole-blood viscosity and plasma viscosity (Figure 1, A and B). Female mice showed slightly higher erythrocyte deformability at high shear forces (Figure 1, C and D), as would be predicted by their higher reticulocyte counts (Supplemental Table 2).
Figure 1.
Whole-blood viscosity, plasma viscosity, and erythrocyte deformability in adult mice. Adult male and female mice were studied without intervention: whole-blood viscosity (A), plasma viscosity (B), erythrocyte deformability (elongation rate) at near physiological viscosity (10 mPas) (C), and erythrocyte elongation rate at supraphysiological viscosity (24 mPas) (D). M, male; F, females. Results are shown as mean ± SD, n = 8–12 mice in each group; pm, main effect; pi, interactions; #, P < .05; ##, P < .01; ###, P < .001; t test.
Effect of short-term testosterone treatment in female and castrated male mice
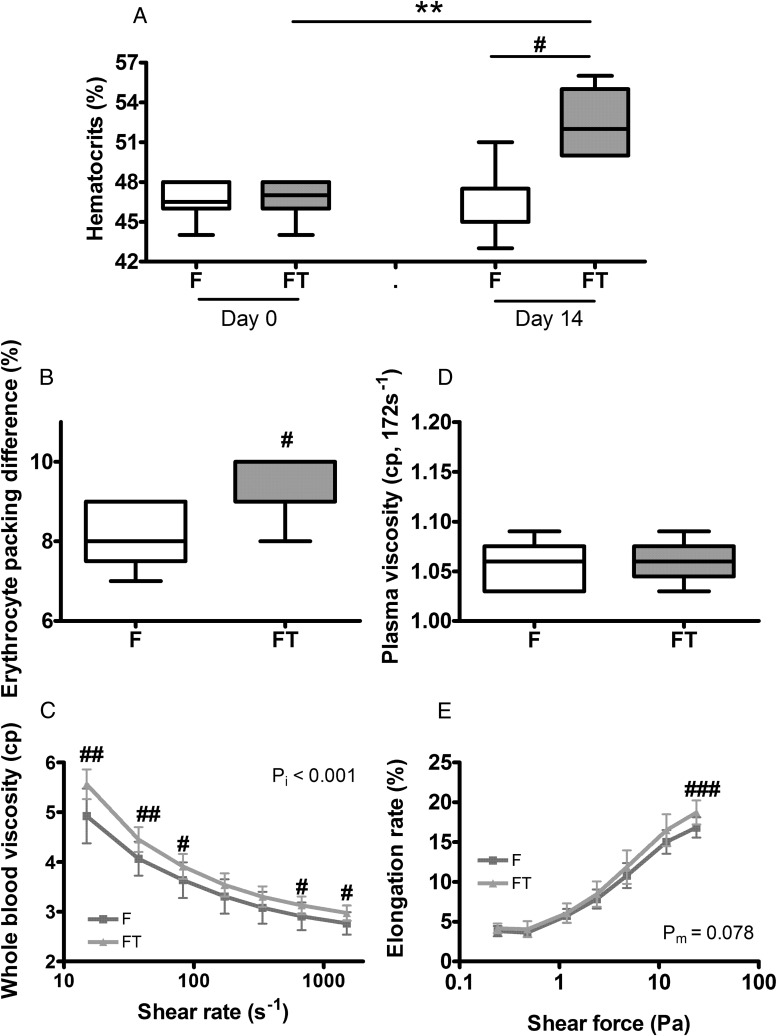
Testosterone treatment at high dose (50 mg/kg) for 2 weeks increased hematocrits (Figure 2A) and EPD (Figure 2B) in female mice. Expected increase in other RBC indices were also observed (data not shown), as we had recently reported (20). This treatment was associated with higher whole-blood viscosity (Figure 2C). A maximal increase of approximately 13% was observed at 15 s−1, a shear rate that falls in the diastolic range (7). The difference became smaller but remained significant at higher shear rates in the systolic range also (Figure 2C). Plasma viscosity (Figure 2D) was not different between the 2 groups. An increase in erythrocytes deformability was detected in testosterone-treated females but only at the highest shear force tested (Figure 2E). Similarly, short-term testosterone treatment at the same high dose increased hematocrits in castrated male mice (Supplemental Figure 2A), EPD (Supplemental Figure 2B), and other RBC indices (Supplemental Table 2), in association with an increase in whole-blood viscosity (Supplemental Figure 2C) but with no effect on plasma viscosity (Supplemental Figure 2D) and erythrocyte deformability (Supplemental Figure 2E).
Figure 2.
Effect of short-term testosterone treatment in female mice. Adult female mice were injected weekly with vehicle or testosterone propionate (50 mg/kg) for 2 weeks and measured for: hematocrits (A), EPD (B), whole-blood viscosity (C), plasma viscosity, measured at shear rate of 172s−1 (D), and erythrocyte elongation rate (E). F, females on vehicle; FT, females treated with testosterone. Results are shown as mean ± SD, n = 8–12; pm, main effect; pi, interactions; **, P < .01, paired t test; #, P < .05; ##, P < .01; ###, P < .001; unpaired t test.
In contrast, when female mice were treated with a low-dose testosterone (5 mg/kg) for 2 weeks, there was no detectable change in blood viscosity (Supplemental Figure 3A) and erythrocyte deformability (Supplemental Figure 3B). Short-term treatment with low dosage did not affect hematocrit and other RBC indices (Supplemental Table 2), as previously reported (21), but this dose still increased kidney weight moderately (Supplemental Table 1).
Effect of long-term testosterone treatment on female and castrated male mice
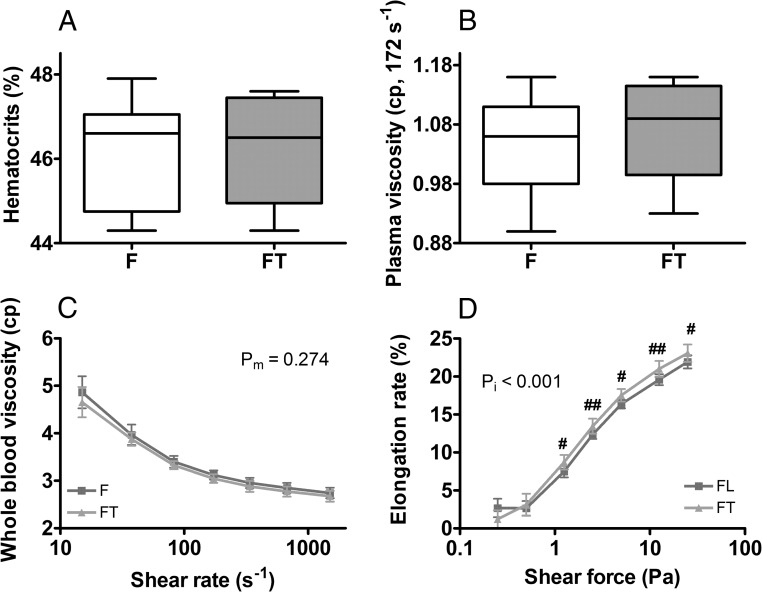
During prolonged intervention (5–7 mo) with high-dose testosterone, serum testosterone levels remained elevated at supraphysiologic levels. In female mice, hematocrits returned to baseline and did not differ between vehicle- and testosterone-treated groups (Figure 3A). EPD was not different between the 2 groups (data not shown). Other RBC indices were similar except that testosterone-treated group had higher red cell distribution width (RDW) and MCV (Supplemental Table 2). Plasma viscosity and whole-blood viscosity were not different (Figure 3, B and C). However, erythrocyte deformability was significantly increased in the testosterone-treated group (Figure 3D). In castrated male mice, hematocrits remained about 2% higher in the testosterone-treated group (Supplemental Figure 4A), which also had higher values for MCV, RDW, and reticulocyte hemoglobin content (Supplemental Table 2). Importantly, prolonged administration of high-dose testosterone did not negatively affect plasma and whole-blood viscosity in castrated males (Supplemental Figure 4, B and C) but increased the erythrocyte deformability (Supplemental Figure 4D).
Figure 3.
Effect of long-term testosterone treatment in female mice. Female mice were treated weekly with testosterone propionate (50 mg/kg, sc) for 5 months and measured for: hematocrits (A), plasma viscosity (B), whole-blood viscosity (C), and erythrocyte deformability (D). F, females on vehicle; FT, females treated with testosterone. Results are shown are mean ± SD, n = 12–14 per group; pm, main effect; pi, interactions; #, P < .05; ##, P < .01; t test.
We also compared the intact males and long-term castrated males and found no significant effect of prolonged testosterone deprivation on whole-blood viscosity and erythrocyte deformability (Supplemental Figure 5).
Discussion
The increased clinical use of testosterone in hypogonadal men, as well as its consistent effect of erythrocytosis with its controversial cardiovascular risks, argues for a need to better understand the rheological effect of testosterone. To our knowledge, this is the first study to comprehensively assess the effects of testosterone on whole-blood and plasma viscosity, erythrocyte deformability, and to compare sex differences after short-term as well as longer-term treatments. Our results indicate that long-term intervention did not adversely affect whole-blood viscosity and plasma viscosity in adult mice supplemented to supraphysiologic levels of testosterone. In contrast, short-term administration of high-dose testosterone transiently raised whole-blood viscosity in association with increased hematocrits in female and castrated male mice. At shear rate of 15 s−1, whole-blood viscosity was increased by 13% and 15% for females and castrated males, respectively. Although moderate, such an effect may increase risk for adverse clinical outcomes in subjects with comorbidities (22). Importantly, our data suggest the presence of adaptive mechanisms that restored whole-blood viscosity to normal during prolonged testosterone administration.
The effect of short-term administration of high-dose testosterone on whole-blood viscosity was associated with the expected increase in hematocrits at this time point. Although hematocrits is a determinant of whole-blood viscosity, its contribution is considered important at high shear rates, whereas erythrocyte aggregation and plasma factors are thought to be important determinants at low shear rates (7, 9). However, no testosterone effect on plasma viscosity was found during this work. Although we did not directly measure erythrocyte aggregation, we show that EPD was increased after short-term high-dose testosterone treatment in both females and castrated males, suggesting that erythrocytes in these mice were more buoyant and thus less likely to form shear-resistant aggregates, in agreement with the predicted increase of young erythrocytes (20, 23). Hence, a mechanism to explain the increase of blood viscosity after short-term high-dose testosterone intervention remains unclear, if due to factors other than the obvious increase in hematocrits.
In agreement with the well-known sex differences that men have higher hematocrits and lower RDW than women (24), we recorded a slightly higher hematocrit in male mice than in female mice, although the difference did not reach statistical significance. Adult men generally have higher blood viscosity and greater erythrocyte fragility than women of reproductive age. We do not know whether this is due to regular blood renewal in women or the testosterone effect in men (25). However, we did not detect a difference in blood viscosity or erythrocyte deformability between adult male and female mice, which, unlike women, do not experience menstrual blood loss. The mechanism of sex differences in hematocrit and blood viscosity in humans is not entirely clear. It is possible that the estrogens may have independent effects on erythropoiesis. Estradiol has been shown to directly affect lymphopoiesis and osteoclasts, which are of hematopoietic origin (26), but it has not been shown to directly affect erythropoiesis or erythrocyte survival. Studies of men with aromatase deficiency suggest that testosterone's conversion to estradiol is not required for mediating its effects on erythropoiesis and that estradiol does not significantly hemoglobin and hematocrit (27). It is therefore likely that the narrowing of the sex difference in hemoglobin and hematocrit after menopause is at least in part due to the cessation of menstrual blood loss in women.
The apparent lack of difference shown in Figure 1 suggests, but does not prove, that testosterone per se has no negative effect on blood viscosity and erythrocyte deformability. This argument is also supported by the observation that long-term deprivation of testosterone in male mice did not elicit detectable improvement in blood viscosity or erythrocyte deformability compared with normal males. Furthermore, long-term treatment with supraphysiologic testosterone did not elicit detectable adverse perturbations to blood viscosity in both females and castrated males. Interestingly, erythrocyte deformability was increased after long-term high-dose testosterone treatment. Whether the increase in erythrocyte deformability was a direct effect of testosterone on erythrocytes remains to be investigated. It was recently reported that testosterone replacement in castrated prostate cancer patients improves erythrocyte membrane lipid profile (28), an effect that would predict improved erythrocyte deformability (9). Because erythrocyte lifespan is typically 45–50 days in adult mice (29), most of the circulating erythrocytes tested in the short-term experiments were formed before testosterone intervention. After months of treatment, all circulating erythrocytes were formed under supraphysiological levels of testosterone. This may play a role to allow detection of the small changes in erythrocyte deformability.
Our study has notable strengths and limitations. We performed a comprehensive assessment of the rheological effect in female and castrated male mice after both short-term and long-term testosterone interventions. The testosterone dosage clearly raised the circulating testosterone concentrations into the supraphysiologic range. These studies were conducted in healthy adult mice. Hence, the relevance to aged and diseased individuals remains to be investigated. In addition, we used intact female mice for this work. Although this has the advantage for assessing the physiologically relevant sex difference, treatment with exogenous testosterone could potentially disturb ovarian cycling and estrogen production. How this may contribute to the observations also remains to be studied. In summary, although short-term administration of high-dose testosterone increased whole-blood viscosity in association with increased hematocrits, the effects did not sustain long-term interventions. Additional preclinical studies using older animals with comorbidities, as well as clinical trials, are required to further define the rheological effect of testosterone supplementation and their consequences in men, especially those with coronary artery disease.
Acknowledgments
We thank Dr David E. Clapham of Boston Children's Hospital for generously offering us access to the Vapor Pressure Osmometer (Wescor, Inc) and the accessory supplies, and Dr Paul G. DeCaen for helping us to operate this instrument.
This work was supported by the National Institutes of Health Grant R01AG037193 (to S.Bh.). Additional support was provided by the Boston Claude D. Pepper Older Americans Independence Center for Function promoting Anabolic Therapies.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- EPD
- erythrocyte packing difference
- MCV
- mean corpuscular volume
- RDW
- red cell distribution width.
References
- 1. Vigen R, O'Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. [DOI] [PubMed] [Google Scholar]
- 2. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeap BB, Alfonso H, Chubb SA, et al. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014;99:E9–E18. [DOI] [PubMed] [Google Scholar]
- 5. Soisson V, Brailly-Tabard S, Helmer C, et al. A J-shaped association between plasma testosterone and risk of ischemic arterial event in elderly men: the French 3C cohort study. Maturitas. 2013;75:282–288. [DOI] [PubMed] [Google Scholar]
- 6. Smith BD, La Celle PL. Blood viscosity and thrombosis: clinical considerations. Prog Hemost Thromb. 1982;6:179–201. [PubMed] [Google Scholar]
- 7. Cowan AQ, Cho DJ, Rosenson RS. Importance of blood rheology in the pathophysiology of atherothrombosis. Cardiovasc Drugs Ther. 2012;26:339–348. [DOI] [PubMed] [Google Scholar]
- 8. Jeong SK, Cho YI, Duey M, Rosenson RS. Cardiovascular risks of anemia correction with erythrocyte stimulating agents: should blood viscosity be monitored for risk assessment? Cardiovasc Drugs Ther. 2010;24:151–160. [DOI] [PubMed] [Google Scholar]
- 9. Simmonds MJ, Meiselman HJ, Baskurt OK. Blood rheology and aging. J Geriatr Cardiol. 2013;10:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bachman E, Feng R, Travison T, et al. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab. 2010;95:4743–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci. 2014;69:725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Basaria S, Nguyen T, Rosenson RS, Dobs AS. Effect of methyl testosterone administration on plasma viscosity in postmenopausal women. Clin Endocrinol (Oxf). 2002;57:209–214. [DOI] [PubMed] [Google Scholar]
- 13. Anderson FH, Francis RM, Faulkner K. Androgen supplementation in eugonadal men with osteoporosis-effects of 6 months of treatment on bone mineral density and cardiovascular risk factors. Bone. 1996;18:171–177. [DOI] [PubMed] [Google Scholar]
- 14. Zhao C, Moon du G, Park JK. Effect of testosterone undecanoate on hematological profiles, blood lipid and viscosity and plasma testosterone level in castrated rabbits. Can Urol Assoc J. 2013;7:E221–E225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vogel J, Kiessling I, Heinicke K, et al. Transgenic mice overexpressing erythropoietin adapt to excessive erythrocytosis by regulating blood viscosity. Blood. 2003;102:2278–2284. [DOI] [PubMed] [Google Scholar]
- 16. Judkiewicz L, Ciszewski R, Bartosz G. The effect of donor age on the packing susceptibility of erythrocytes. Mech Ageing Dev. 1988;46:83–87. [DOI] [PubMed] [Google Scholar]
- 17. Forconi S, Guerrini M, Ravelli P, et al. Arterial and venous blood viscosity in ischemic lower limbs in patients affected by peripheral obliterative arterial disease. J Cardiovasc Surg (Torino). 1979;20:379–384. [PubMed] [Google Scholar]
- 18. Bhasin S, Zhang A, Coviello A, et al. The impact of assay quality and reference ranges on clinical decision making in the diagnosis of androgen disorders. Steroids. 2008;73:1311–1317. [DOI] [PubMed] [Google Scholar]
- 19. Broulik PD. The effect of castration and androgen treatment on glomerular volume in mice. Exp Clin Endocrinol. 1983;82:115–117. [DOI] [PubMed] [Google Scholar]
- 20. Guo W, Bachman E, Li M, et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell. 2013;12:280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Delev DP, Davcheva DP, Kostadinov ID, Kostadinova II. Effect of testosterone propionate on erythropoiesis after experimental orchiectomy. Folia Med (Plovdiv). 2013;55:51–57. [DOI] [PubMed] [Google Scholar]
- 22. Li RY, Cao ZG, Li Y, Wang RT. Increased whole blood viscosity is associated with silent cerebral infarction. Clin Hemorheol Microcirc. Published online ahead of print August 29, 2013. DOI: 10.3233/CH-131760. [DOI] [PubMed] [Google Scholar]
- 23. Naets JP, Wittek M. The mechanism of action of androgens on erythropoiesis. Ann NY Acad Sci. 1968;149:366–376. [DOI] [PubMed] [Google Scholar]
- 24. Kameneva MV, Garrett KO, Watach MJ, Borovetz HS. Red blood cell aging and risk of cardiovascular diseases. Clin Hemorheol Microcirc. 1998;18:67–74. [PubMed] [Google Scholar]
- 25. Gorodeski GI. Impact of the menopause on the epidemiology and risk factors of coronary artery heart disease in women. Exp Gerontol. 1994;29:357–375. [DOI] [PubMed] [Google Scholar]
- 26. Henning P, Ohlsson C, Engdahl C, et al. The effect of estrogen on bone requires ERα in nonhematopoietic cells but is enhanced by ERα in hematopoietic cells. Am J Physiol Endocrinol Metab. 2014;307:E589–E595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rochira V, Zirilli L, Madeo B, Maffei L, Carani C. Testosterone action on erythropoiesis does not require its aromatization to estrogen: insights from the testosterone and estrogen treatment of two aromatase-deficient men. J Steroid Biochem Mol Biol. 2009;113:189–194. [DOI] [PubMed] [Google Scholar]
- 28. Angelova P, Momchilova A, Petkova D, Staneva G, Pankov R, Kamenov Z. Testosterone replacement therapy improves erythrocyte membrane lipid composition in hypogonadal men. Aging Male. 2012;15:173–179. [DOI] [PubMed] [Google Scholar]
- 29. Wang S, Dale GL, Song P, Viollet B, Zou MH. AMPKα1 deletion shortens erythrocyte life span in mice: role of oxidative stress. J Biol Chem. 2010;285:19976–19985. [DOI] [PMC free article] [PubMed] [Google Scholar]