Abstract
Previously, we demonstrated that skin cells metabolize melatonin to 6-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine and 5-methoxytryptamine. In this study, we determined that N1-acetyl-5-methoxykynuramine (AMK) is endogenously produced in the human epidermis from melatonin through the kynuric pathway. The epidermal content of AMK (average from 13 subjects) is 0.99 ± 0.21 ng/mg protein, being significantly higher in African Americans (1.50 ± 0.36 ng/mg protein) than in Caucasians (0.56 ± 0.09 ng/mg protein). It is especially high in young African Americans. The levels do not differ significantly between males and females. In vitro testing using HaCaT keratinocytes has shown that exogenously added melatonin is metabolized to AMK in a dose dependent manner with a Vmax = 388 pg/million cells and Km = 185 μM. AMK production is higher in melanized than in amelanotic melanoma cells. Testing of DNA incorporation shows that AMK has antiproliferative effects in HaCaT and SKMEL-188 cells (nonpigmented and pigmented). AMK also inhibits growth of normal melanocytes but has no significant effect on melanogenesis or cell morphology. These findings indicate that antiproliferative effects of AMK are not related to melanin pigmentation. In summary, we show for the first time that AMK is produced endogenously in the human epidermis, that its production is affected by melanin skin pigmentation, and that AMK exhibits antiproliferative effects in cultured keratinocytes and melanoma cells.
Melatonin is synthesized not only in the pineal gland, brain, and retina (1) but also in several peripheral sites (1–3), including skin (4). Both centrally and peripherally produced melatonin regulates or modifies body functions in a context-dependent fashion through activation of specific melatonin receptors, namely types 1 and 2 (5–7) or through receptor-independent mechanisms (1). The final phenotypic effects of melatonin depends on the local concentration of melatonin that is secondary to its local production/accumulation and rate of metabolic transformation to molecules that show different biological activity or are inactive (1). There are 2 main routes of melatonin metabolism, including indolic and kynuric pathways (1, 8). The kynuric pathway involves both enzymatic and nonenzymatic transformation of melatonin with major final products represented by N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and/or N1-acetyl-5-methoxykynuramine (AMK) (8).
The epidermal layer of the skin, although being situated at the interphase between external and internal environments, plays a crucial role in preservation of body homeostasis (9, 10). Its functions are in part coordinated by a local neuroendocrine system (11), which also encompasses cutaneous serotoninergic/melatoninergic system (4, 12). Thus, mammalian skin cells produce serotonin and metabolize it to melatonin (13–17). Melatonin is also metabolized by skin cells through indolic and kynuric pathways (13, 17–20), whereas AFMK can also be produced nonenzymatically after exposure of the skin to UVB radiation (18).
The role of the local melatoninergic system composed of melatonin as well as its metabolites in the regulation of human epidermal functions has become gradually appreciated (21–23). These phenotypic effects of melatonin also depend on its metabolism and the chemical nature of the final product (19–21, 24). Here, we tested whether a final product of the kynuric pathway, AMK, is produced in the human skin and performed an initial evaluation of its biological activity using keratinocytes and melanocytes. We also investigated endogenous accumulation of AMK in relation to race, sex and age of the donor.
Materials and Methods
Chemicals
Charcoal-treated fetal bovine serum (ctFBS) was purchased from Atlanta Biologicals, whereas DMEM, Ham's F-10 media, trypsin/EDTA, and antibiotic/antimycotic mixture were from Mediatech, Inc. Melatonin was from Sigma-Aldrich, whereas AMK was purchased from Toronto Research Chemicals, Inc. Acetonitrile, methanol, water (Fisher Scientific), and formic and acetic acid (Sigma-Aldrich) were of HPLC grade. Fresh stock of 100mM melatonin in ethanol and of 10mM AMK in ethanol or dimethylsulfoxide were prepared before experiments and stored at −80°C until studies were performed. Trichloroacetic acid and 3,4-dihydroxy-L-phenylalanine were purchased from Sigma-Aldrich, and [3H]-thymidine was purchased from Moravek Biochemicals, Inc.
Human skin and processing of the epidermis
Human skin was obtained from the University Methodist Hospital (Memphis, TN), whereas human foreskin was from the Regional One Health (Memphis, TN), through protocols approved by the University of Tennessee Health Science Center (UTHSC) Institutional Review Board. The skin donors' (n = 13) age range were from 30 to 90 years and from black and white races and from both males and females. The epidermal samples were collected as described before and stored at −80°C (20).
The epidermal fractions were homogenized with Poly Tron (PT 2100) (Kinematica) after adding 3.2 vol (vol/wt) of PBS and further addition to acetonitrile to 75% (20). After centrifuge at 4000 rpm, the supernatant was filtered using a syringe filter (PES, 0.45 μm, 30 mm; Celltreat) followed by dry using Speed vac plus (Savant Instruments, Inc) and stored at −80°C.
Cell culture conditions
HaCaT keratinocytes were grown in DMEM, whereas human SKMEL-188 and AbC-1 melanoma cells in Ham's F-10 media supplemented with 10% ctFBS and 1% antibiotic/antimycotic mixture at 37°C with 5% CO2 in the atmosphere (25–27). Induction of melanogenesis in melanoma cells was achieved either by addition of 400μM L-tyrosine to the media (25) or replacing Ham's F-10 with media high in L-tyrosine, eg, 75%DMEM/25% Ham's F-10 (26) as indicated. Cultures of normal human neonatal melanocytes from foreskin of African American (AA) patients were established as described previously (28). Normal melanocytes were cultured in MBM-4 with MGM-4 supplements (Lonza) (28).
Production of AMK in skin cells
To test the production of AMK in human epidermal HaCaT keratinocytes and melanoma SKMEL-188 cells, the cells were detached with trypsin, washed with PBS, and then incubated (3 × 106 cells) with melatonin in 1 mL of HEPES-buffered media (120mM NaCl, 5mM KCl, 1mM EDTA, 1mM MgSO4, 15mM sodium acetate, 100mM HEPES, 10mM glucose, 1% BSA, 2mM isocitrate, and 0.2mM NADP; pH 7.4) (19). After 18 hours of incubation at 37°C with shaking at 60 rpm, the reaction mixtures were extracted with 75% acetonitrile and processed further as described above and previously (19).
Detection of AMK
To detect AMK, the dried samples were redissolved in methanol and analyzed by liquid chromatography-mass spectrometry (LC-MS) consisting of a Waters Acquity UPLC system (Waters Corp) and a Xevo G2 quadrupole-time-of-flight (qTOF) mass spectrometer equipped with an ESI source (Waters MS Technologies). A Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 1.8 μm) (Agilent Technology) was used for sample analysis and the flow rate was 0.3 mL/min with linear gradient of acetonitrile containing 0.1% formic acid: 5%–10% for 1 minute, 10%–15% for 2.5 minutes, 15%–20% for 3.5 minutes, 20%–25% for 0.5 minutes, 25%–70% for 1 minute, 70%–85% for 1 minute, and 85%–100% for 0.5 minutes (19, 20). In selected cases, fractions with retention time (RT) of AMK were prepurified before LC-MS analysis by RP-HPLC using a dual pump chromatography system (Waters 2695 Alliance) equipped with a Waters Atlantis dC18 column (100 × 4.6 mm, 5-μm particle size) at a flow rate of 0.75 mL/min in 25% acetonitrile and 0.1% acetic acid isocratically (19, 20).
Cell proliferation assays
[3H]-thymidine incorporation into DNA was measured as described previously (19, 27). Briefly, HaCaT keratinocytes were grown in DMEM plus 5% of ctFBS, whereas SKMEL-188 melanoma cells were cultured either in Ham's F-10 media (amelanotic phenotype) or Ham's F-10 plus 400μM L-tyrosine (melanotic phenotype), supplemented with 5% ctFBS. After reaching 75% confluence cells were detached as described previously (27). Equal amount of cells were inoculated into 24-well plates, and next day when cells reached 30% confluence, the media were changed to corresponding media with vehicle or graded concentrations of AMK (10−11M to 10−5M), and after 72 hours, [3H]-thymidine was added at the concentration of 0.25 μCi/mL for additional 4 hours. Measurement of incorporation of into DNA followed protocols described previously and radioactivity was counted with Packard Matrix 9600 direct β-counter (Packard) (27, 28).
Normal human epidermal melanocytes were inoculated into 25-cm2 flasks at a concentration 50 000 cells/flask and cultured in MBM-4 with MGM-4 plus 10−5M AMK or ethanol (0.1%) as a control. After 6 days, the cells were harvested, counted using a hemacytometer chamber (Fisher Scientific) and pelleted to study melanin pigmentation (25, 26).
In addition, equal numbers of SKMEL-188 or AbC-1 melanoma cells were inoculated in 25 cm2 flasks for 1 day before the media were changed to DMEM/Ham's F-10 (75%/25%) to induce melanin pigmentation; thereafter they were further cultured for 3 or 2 days, respectively, in the presence of 10−5M AMK or vehicle control (dimethylsulfoxide 0.1%). The cells were counted, pelleted by centrifugation and cell pellets were used to study melanogenesis (see below).
Evaluation of melanogenesis
To define relative melanin content, cell pellets were photographed (25, 26). To measure 3,4-dihydroxyphenylalanine (Dopa) oxidase activity of tyrosinase, the cell pellets were lysed in ice cold 0.1M sodium phosphate buffer (pH 6.8; 0.5% Triton X-100 and 0.1mM phenylmethylsulfonyl fluoride, cell debris was removed by centrifuged at 16 000g for 10 minutes, and supernatant was used for enzymatic assay as described previously (20, 25, 26). Briefly, the increase of absorbance in the presence of 1mM 3,4-dihydroxy-L-phenylalanine was measured at 475 nm, and the activity was presented as micromoles of dopachrome formed during 1 hour of incubation per milligram of protein.
Results and Discussion
Production of AMK in keratinocytes and melanoma cells in vitro
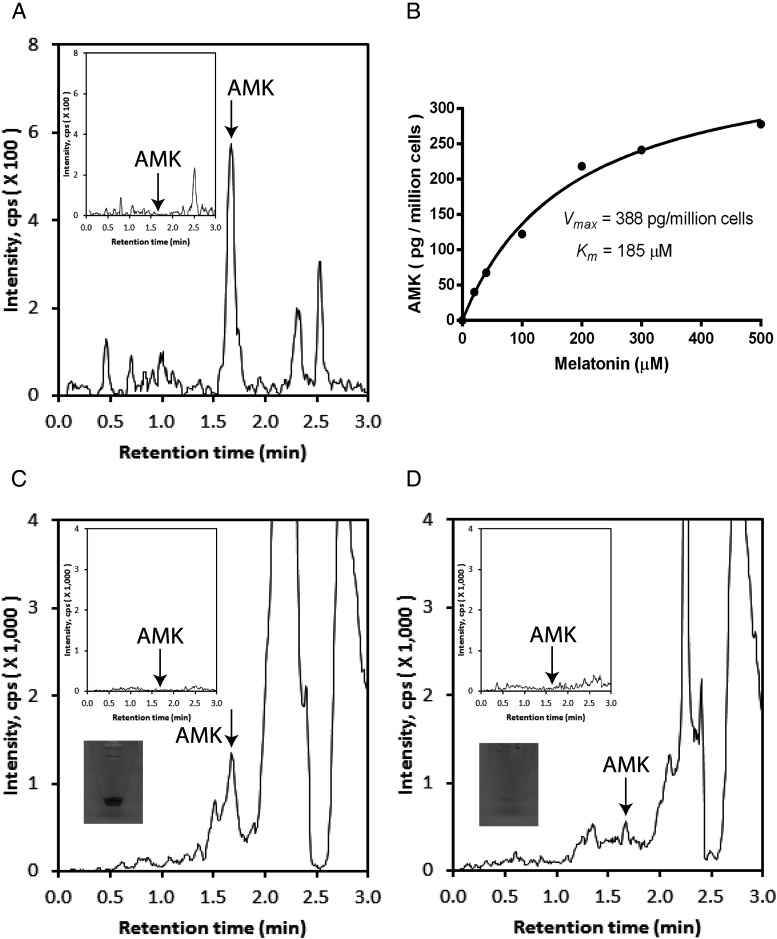
Tests performed with HaCaT keratinocytes and human melanoma cells showed that exogenously added melatonin is metabolized to AMK (Figure 1, A and B). This transformation occurred in a dose dependent manners with Vmax = 0.39 ng/106 cells and Km = 185μM (Figure 1B). Regarding melanoma cells, a higher production of AMK was seen in pigmented in comparison with nonpigmented cells (Figure 1, C and D).
Figure 1.
LC-MS qTOF monitoring of melatonin transformation to AMK by human epidermal keratinocytes and melanoma cells. A, Although production of AMK is undetectable in HaCaT keratinocytes incubated without substrate (inset), AMK is easily detectable (m/z = 259.1 [M+Na]+) in cells cultured in the presence of melatonin. B, Dose-dependent production of AMK by HaCaT keratinocytes. C and D, Production of AMK (m/z = 237.1 [M+H]+) in SKMEL-188 cells is enhanced in pigmented (C) in comparison with nonpigmented cells (D). The insets show controls (cells incubated without substrate) and color of cell pellets corresponding to the melanin content. Arrows show RT of AMK standard. Melatonin was added at concentration 500μM in C and D.
We found for the first time, that human skin cells (keratinocytes and melanoma cells) metabolize melatonin to AMK. Higher production of AMK in pigmented cells may be secondary to an oxidative environment generated by an active melanogenesis (29, 30). In fact, metabolism of melatonin through the kynuric pathway can be accelerated or mediated by reactive oxygen species (ROS) (8) and active melanogenesis generates ROS (30).
AMK is endogenously produced in the human epidermis
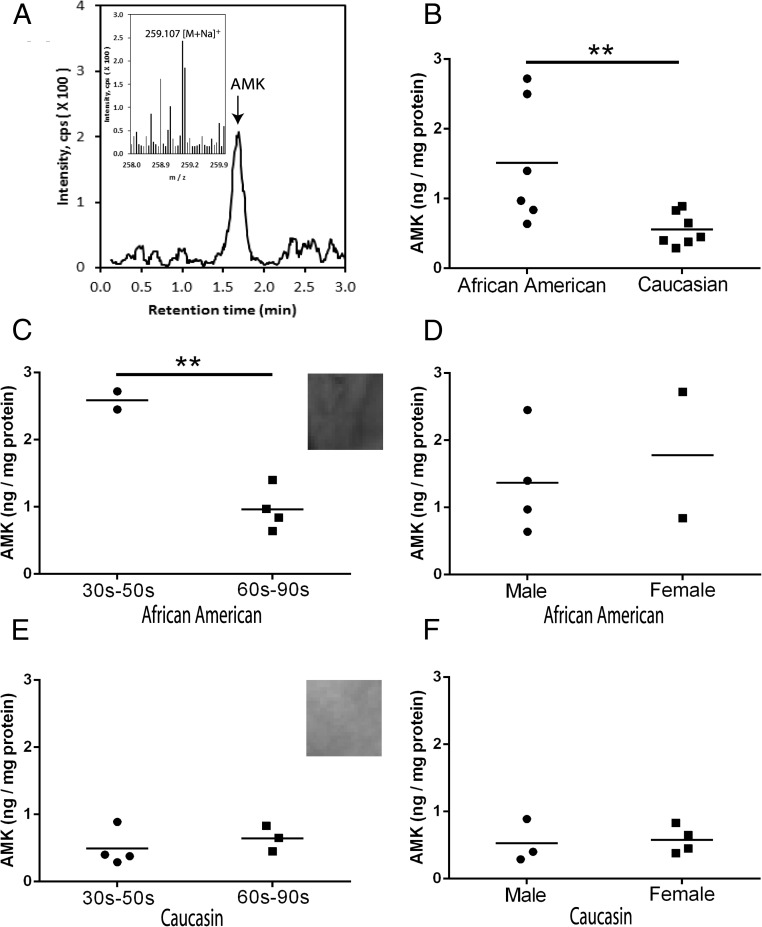
Acetonitrile extracted samples from 13 patients demonstrated the presence of AMK ions at m/z = 259.1 [M+Na]+ and m/z =237.1 [M+H]+ with a RT corresponding to AMK standard in the human epidermis (Figure 2, see qTOF and mass spectra). Further AMK quantification was performed using AMK ion at m/z = 259.1 [M+Na]+ with help of extracted-ion chromatogram. As shown in Figure 2, AMK was detected in all of the tested samples of the epidermis with average amount of 0.99 ± 0.21 ng/mg ranging from 0.29 to 2.72 ng/mg protein. The pigmented epidermis of AAs produced more AMK (1.5 ± 0.36 ng/mg protein) than the lesser pigmented epidermis of Caucasian (C) individuals (0.56 ± 0.09 ng/mg protein) (P < .01). The production of AMK was higher (P < .01) in young individuals (30–50 y old) vs old age (60–90 y old) in AA but not in C (lack of correlation with age). There were no differences in the AMK content in relation to the gender or between different age groups when analyzing all samples without racial substratification. AMK was below detectability in human serum samples from 2 middle age individuals (data not shown), suggesting that its peripheral production is solely for local use in the skin.
Figure 2.
Detection of AMK in the human epidermis. A, Detection of an AMK ion at m/z = 259.1 [M+Na]+ with RT of the AMK standard. B–F, Levels of endogenous production of AMK in relation to race, age, and gender. B, All subjects compared. C and D, AAs. E and F, Cs. Quantification was performed using ion at m/z = 237.1 [M+H]+, and data were calculated from the standard curve obtained with chemically synthesized AMK. **, P < .01 at Student's t test.
This is the first, although preliminary, demonstration that AMK is endogenously produced in the human epidermis in vivo and that its relative content depends on the skin melanin pigmentation dictated by the racial background of the donors. The higher levels of AMK in AA is consistent with studies on isolated cells in which induction of melanin pigmentation also enhanced production of AMK (see Figure 1, C and D). The latter indicates that the presence of melanin pigment or active melanogenesis affect epidermal accumulation of AMK. A possible explanation may lie in ROS generation by melanogenesis (30), which plays an important role in melatonin metabolism through kynuric pathway (8).
Effects of AMK on cell proliferation and melanogenesis
AMK inhibited DNA synthesis in a dose-dependent manner in keratinocytes and amelanotic SKMEL-188 melanoma cells, respectively (Supplemental Figure 1, A and B). We also tested whether induction of melanogenesis by L-tyrosine or already established melanotic phenotype can affect the antiproliferative action of AMK; this study showed that when melanoma cells were exposed concomitantly to AMK and 400μM L-tyrosine the antiproliferative action of AMK was retained (Supplemental Figure 1C). However, this effect disappeared when already pigmented SKMEL-188 cells were treated with AMK (Supplemental Figure 1D). Because it is well documented that melanin pigmentation can dramatically change the behavior of melanoma cells (29, 31), the attenuation of the AMK effect in melanotic cells may represent an additional example how melanization can change responsiveness to external factors.
Supplemental Figure 1, E and F, shows that culturing of normal epidermal melanocytes and human melanoma cells in the presence of AMK leads to a reduction in cell number in comparison with cells treated with vehicle only. This confirms an antiproliferative effect of AMK in skin cells (see Supplemental Figure 1, A–C).
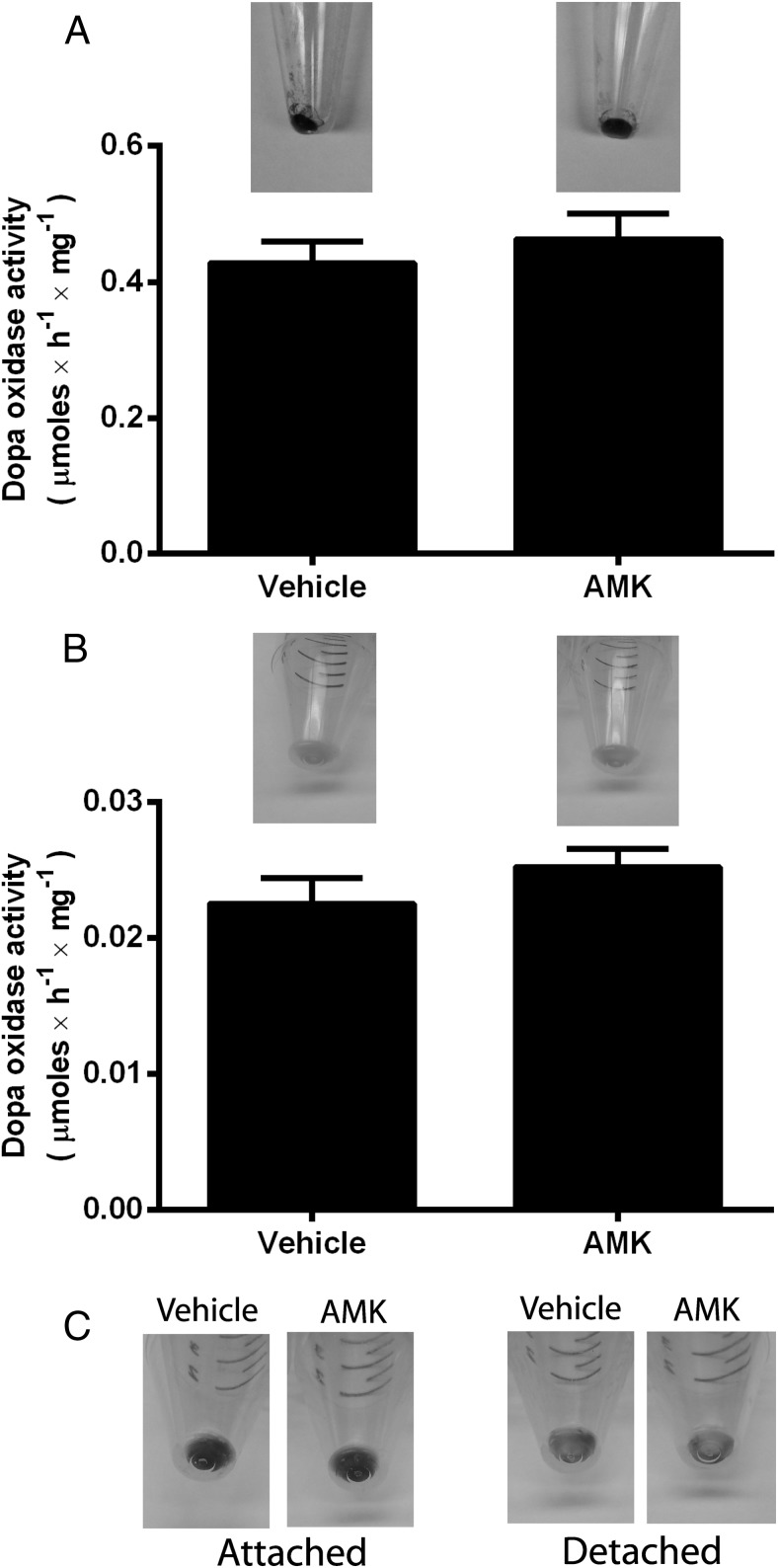
Because melatonin has shown antimelanogenic activity in rodent melanocytes (32, 33) and, to a lesser degree, in human melanocytes (inhibition of tyrosinase activity) (20), we tested the effect of AMK on melanin pigmentation using normal human epidermal melanocytes, melanoma SKMEL-188 cells, and hamster AbC-1 melanoma cells (Figure 3). AMK at a 10−5M concentration neither influenced tyrosinase activity nor melanin pigmentation in either normal human melanocytes (Figure 3A) or in human melanoma cells cultured in media enriched with melanin precursors (Figure 3B). Similarly, AMK had no effect on melanin production in hamster AbC-1 melanoma cells (Figure 3C). In addition, there was a lack of noticeable effect of AMK on the morphology of melanocytes or melanoma cells in comparison with cells treated with vehicle only (data not shown). Thus, one can safely conclude that AMK, a product of kynuric pathway of melatonin metabolism, has no direct effect on differentiated functions of normal or malignant melanocytes.
Figure 3.
Effect of AMK on melanin pigmentation and tyrosinase activity. A, Normal human melanocytes. B, SKMEL-188 human melanoma. C, AbC-1 hamster melanoma. Normal human melanocytes were grown in MBM-4 with MGM-4 media. SKMEL-188 and AbC-1 cells were grown in DMEM (75%)/Ham's F-10 (25%) media plus 10% ctFBS. Data are presented as means ± SD (n = 3).
Concluding remarks
Previously we have documented the production of melatonin in human skin (14, 34), which can further be metabolized through indolic and kynuric pathways (18–20), as could be predicted from the studies on rodent skin (13). In this study, we show for the first time that cultured skin cells not only metabolize melatonin to AMK, but AMK also accumulates in vivo in the epidermis. Therefore, we propose that the kynuric pathway of melatonin metabolism in the skin follows the sequence: melatonin → AFMK → AMK.
Interestingly, epidermal AMK content is affected by the skin melanin pigmentation with the higher levels of AMK seen in AA in comparison with C, which is consistent with higher levels of consecutive precursor molecules, melatonin and AFMK in AA vs C (20). Because melatonin, AFMK and AMK serve as powerful antioxidants (35, 36) and protectors of epidermal integrity against solar radiation (21, 24, 37, 38), we suggest that the local melatoninergic system, which includes kynuric metabolism of melatonin, serves a complementary action to the melanin pigmentation line of defense against solar radiation in individuals with high levels of the skin melanin content.
Finally, AMK shows antiproliferative properties towards skin cells that are similar to those of melatonin and AFMK; however, it had no effect on melanogenesis which contrasts with the actions of melatonin and AFMK. In conclusion, we show for the first time that human skin can produce biologically active AMK, which could act as an endogenous bioregulator and skin protector.
Acknowledgments
This article is dedicated to Dr Aaron B. Lerner, a discoverer of melatonin.
This work was supported by National Institutes of Health Grants 1R01AR056666-01A2, 2RO1 AR052190-06A2 and R21 AR066505-01A1 (to A.S.) and 1S10OD010678-01 (to W.L.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- African American
- AFMK
- N1-acetyl-N2-formyl-5-methoxykynuramine
- AMK
- N1-acetyl-5-methoxykynuramine
- C
- Caucasian
- ctFBS
- charcoal-treated fetal bovine serum
- LC-MS
- liquid chromatography-mass spectrometry
- ROS
- reactive oxygen species
- RT
- retention time.
References
- 1. Reiter RJ, Tan DX, Galano A. Melatonin: exceeding expectations. Physiology (Bethesda). 2014;29:325–333. [DOI] [PubMed] [Google Scholar]
- 2. Acuña-Castroviejo D, Escames G, Venegas C, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71:2997–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lanoix D, Lacasse AA, Reiter RJ, Vaillancourt C. Melatonin: the watchdog of villous trophoblast homeostasis against hypoxia/reoxygenation-induced oxidative stress and apoptosis. Mol Cell Endocrinol. 2013;381:35–45. [DOI] [PubMed] [Google Scholar]
- 4. Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol Metab. 2008;19:17–24. [DOI] [PubMed] [Google Scholar]
- 5. Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351:152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. [DOI] [PubMed] [Google Scholar]
- 7. Li DY, Smith DG, Hardeland R, et al. Melatonin receptor genes in vertebrates. Int J Mol Sci. 2013;14:11208–11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hardeland R, Tan DX, Reiter RJ. Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pineal Res. 2009;47:109–126. [DOI] [PubMed] [Google Scholar]
- 9. Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii,, 1–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elias PM, Crumrine D, Paller A, Rodriguez-Martin M, Williams ML. Pathogenesis of the cutaneous phenotype in inherited disorders of cholesterol metabolism: therapeutic implications for topical treatment of these disorders. Dermatoendocrinol. 2011;3:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocrine Rev. 2000;21:457–487. [DOI] [PubMed] [Google Scholar]
- 12. Semak I, Naumova M, Korik E, Terekhovich V, Wortsman J, Slominski A. A novel metabolic pathway of melatonin: oxidation by cytochrome C. Biochemistry. 2005;44:9300–9307. [DOI] [PubMed] [Google Scholar]
- 13. Slominski A, Baker J, Rosano TG, et al. Metabolism of serotonin to N-acetylserotonin, melatonin, and 5-methoxytryptamine in hamster skin culture. J Biol Chem. 1996;271:12281–12286. [DOI] [PubMed] [Google Scholar]
- 14. Slominski A, Pisarchik A, Semak I, et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002;16:896–898. [DOI] [PubMed] [Google Scholar]
- 15. Kobayashi H, Kromminga A, Dunlop TW, et al. A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 2005;19:1710–1712. [DOI] [PubMed] [Google Scholar]
- 16. Slominski A, Pisarchik A, Semak I, Sweatman T, Szczesniewski A, Wortsman J. Serotoninergic system in hamster skin. J Invest Dermatol. 2002;119:934–942. [DOI] [PubMed] [Google Scholar]
- 17. Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, Wortsman J. Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002;511:102–106. [DOI] [PubMed] [Google Scholar]
- 18. Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006;20:1564–1566. [DOI] [PubMed] [Google Scholar]
- 19. Kim TK, Kleszczynski K, Janjetovic Z, et al. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013;27:2742–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim TK, Lin Z, Tidwell WJ, Li W, Slominski AT. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol Cell Endocrinol. 2015;404:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Slominski AT, Kleszczyński K, Semak I, et al. Local melatoninergic system as the protector of skin integrity. Int J Mol Sci. 2014;15:17705–17732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fischer TW, Slominski A, Tobin DJ, Paus R. Melatonin and the hair follicle. J Pineal Res. 2008;44:1–15. [DOI] [PubMed] [Google Scholar]
- 23. Kleszczynski K, Fischer TW. Melatonin and human skin aging. Dermatoendocrinol. 2012;4:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janjetovic Z, Nahmias ZP, Hanna S, et al. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J Pineal Res. 2014;57:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Słominski A, Moellmann G, Kuklinska E, Bomirski A, Pawelek J. Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, L-tyrosine and L-dopa. J Cell Sci. 1988;89:287–296. [DOI] [PubMed] [Google Scholar]
- 26. Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2009;124:1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Slominski AT, Kim TK, Janjetovic Z, et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2011;300:C526–C541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slominski AT, Janjetovic Z, Kim TK, et al. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012;32:3733–3742. [PMC free article] [PubMed] [Google Scholar]
- 29. Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. [DOI] [PubMed] [Google Scholar]
- 31. Slominski A, Kim TK, Brożyna AA, et al. The role of melanogenesis in regulation of melanoma behavior: melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch Biochem Biophys. 2014;563:79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Slominski A, Chassalevris N, Mazurkiewicz J, Maurer M, Paus R. Murine skin as a target for melatonin bioregulation. Exp Dermatol. 1994;3:45–50. [DOI] [PubMed] [Google Scholar]
- 33. Slominski A, Pruski D. Melatonin inhibits proliferation and melanogenesis in rodent melanoma cells. Exp Cell Res. 1993;206:189–194. [DOI] [PubMed] [Google Scholar]
- 34. Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19:176–194. [DOI] [PubMed] [Google Scholar]
- 35. Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J Pineal Res. 2013;54:245–257. [DOI] [PubMed] [Google Scholar]
- 36. Reiter RJ, Tan DX, Galano A. Melatonin reduces lipid peroxidation and membrane viscosity. Front Physiol. 2014;5:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kleszczyński K, Tukaj S, Kruse N, Zillikens D, Fischer TW. Melatonin prevents ultraviolet radiation-induced alterations in plasma membrane potential and intracellular pH in human keratinocytes. J Pineal Res. 2013;54:89–99. [DOI] [PubMed] [Google Scholar]
- 38. Fischer TW, Kleszczyński K, Hardkop LH, Kruse N, Zillikens D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J Pineal Res. 2013;54:303–312. [DOI] [PubMed] [Google Scholar]