Abstract
Insulin exerts pleiotropic effects on cell growth, survival, and metabolism, and its role in multiple tissues has been dissected using conditional knockout mice; however, its role in development has not been studied. Lineage tracing experiments have demonstrated that interscapular brown adipose tissue (BAT) arises from a Myf5-positive lineage shared with skeletal muscle and distinct from the majority of white adipose tissue (WAT) precursors. In this study, we sought to investigate the effects of impaired insulin signaling in the Myf5-expressing precursor cells by deleting the insulin receptor gene. Mice lacking insulin receptor in the Myf5 lineage (Myf5IRKO) have a decrease of interscapular BAT mass; however, muscle development appeared normal. Histologically, the residual BAT had decreased cell size but appeared mature and potentially functional. Expression of adipogenic inhibitors preadipocyte factor-1, Necdin, and wingless-type MMTV integration site member 10a in the residual BAT tissue was nonetheless increased compared with controls, and there was an enrichment of progenitor cells with impaired adipogenic differentiation capacity, suggesting a suppression of adipogenesis in BAT. Surprisingly, when cold challenged, Myf5IRKO mice did not show impaired thermogenesis. This resistance to cold could be attributed to an increased presence of uncoupling protein 1-positive brown adipocytes in sc WAT as well as increased expression of lipolytic activity in BAT. These data suggest a critical role of insulin signaling in the development of interscapular BAT from Myf5-positive progenitor cells, but it appears to be dispensable for muscle development. They also underscore the importance of compensatory browning of sc WAT in the absence of BAT for thermoregulation.
Insulin resistance is the hallmark of metabolic syndrome, which is a cluster of many of our most common medical conditions, including type 2 diabetes mellitus, dyslipidemias, nonalcoholic fatty liver, and cardiovascular disease (1). Insulin action in its target tissue is mediated by receptor binding at the cell surface. This initiates a complex cascade of intracellular signaling events that lead to the numerous cellular effects of insulin, including regulation of glucose uptake and fatty acid synthesis (2). Over the past decade, the function of insulin in its target tissues has been systemically dissected using the Cre-loxP system to disrupt insulin receptor (InsR) in a tissue-specific fashion (3). These studies not only underscore the essential role of insulin signaling in classic insulin sensitive tissues, such as skeletal muscle (4), fat (5–7), and liver (8), but also reveal novel functions of insulin in nonclassic insulin sensitive tissue, such as pancreatic β-cells (9) and brain (10).
In addition to its prominent role in glucose and fatty acid metabolism, insulin also regulates cell growth and differentiation. Mice with whole-body deletion of InsR are born at term with slight growth retardation, they develop glucose abnormality soon after birth, and die of ketoacidosis (11). IGF-I regulates embryonic and fetal growth and IGF-I receptor (IGF-IR) knock mice are growth retarded (12). Interestingly, mice lacking both InsR and IGF-IR display more severe growth impediment than mice carrying either single receptor deletion alone (13), suggesting that InsR also regulates embryonic growth. However, the function of InsR in embryonic tissue development has not yet been elucidated in a cell type-specific manner.
Recently, it has been demonstrated that the preformed brown adipose tissue (BAT) and skeletal muscle share a common developmental ancestry. Developmentally, BAT arises from a Myf5-positive lineage shared with skeletal muscle and separate from most WAT precursors (14–17). Myf5 is a member of the muscle-specific determination genes and plays an important role in skeletal muscle development (18). Although the role of InsR in adult skeletal muscle and mature brown adipocytes has been studied by using tissue-specific knockout mice (4–10), whether it could regulate the development of muscle and fat in the Myf5-expressing cells has not been explored.
BAT is specialized for energy expenditure and its mass/activity is inversely correlated to body mass index and percent body fat in humans making it an attractive target for antiobesity therapies (19–21). The thermogenic properties of BAT are due to the expression of uncoupling protein 1 (UCP1), which uncouples oxidative phosphorylation to generate heat. Cells that express UCP1 fall into 2 categories; those located in the interscapular region that constitutively express UCP1 and arise during embryogenesis, termed classical BAT, and those that can be induced in WAT in a process called browning that are termed beige, brite, or recruited BAT (14, 22). Transplantation studies in mice have demonstrated the ability of classical BAT to regulate glucose homeostasis and improve insulin sensitivity (23). BAT activity can also regulate fatty acid and glucose homeostasis in mice, highlighting an important role of this tissue in the regulation of nutrient metabolism (24, 25).
In vitro, the insulin signaling pathway affects brown adipocyte differentiation (26) through the cAMP response element binding protein (CREB) and Forkhead box protein O1 (FoxO1)-dependent pathways to decrease the expression level of the adipogenic inhibitor Necdin (27). Suppression of insulin signaling through genetic deletion of InsR substrate-1 (IRS-1) blocks brown preadipocyte differentiation and up-regulates preadipocyte factor-1 (Pref-1), Necdin, and Wnt10a (28). In vivo, specific deletion of InsR in mature adipocytes leads to reduced fat mass and protection from high-fat diet-induced obesity (29), whereas transgenic mice lacking InsR in UCP1-positive mature brown adipocytes develop glucose intolerance and an age-dependent loss of interscapular BAT; however, they do not develop insulin resistance (6). These models ablate insulin signaling in differentiated cells making it difficult to understand the role of insulin in early adipose development. In order to focus on the impact of insulin signaling in BAT progenitor cells, we generated a mouse model lacking the InsR in all cells descending from the Myf5 lineage (Myf5IRKO).
In this study, we show that Myf5IRKO mice display a severe paucity of interscapular BAT and an increase in the expression of several known targets of insulin signaling that act as negative regulators of adipogenesis. The reduction of interscapular BAT mass is mainly due to blockade of preadipocyte differentiation as primary brown preadipocytes isolated from KO animals exhibit a dramatic defect in in vitro differentiation. Myf5IRKO mice display compensatory browning of sc WAT (sWAT), which is sufficient to maintain normal thermogenesis. The effects of InsR disruption in the Myf5-positive on classical BAT do not seem to be associated with defective insulin signaling in muscle and Myf5IRKO muscle appears to develop normally, suggesting an indispensible role of InsR in BAT development that is compensated for in muscle development.
Materials and Methods
Animals
All animal procedures were approved by the Institutional Animal Use and Care Committee at Joslin Diabetes Center. Transgenic mice carrying floxed alleles for the InsR β-subunit were used to generate conditional gene deletions mouse models by intercrossing with Myf5-driven cre recombinase. Mice at room temperature were housed in a temperature controlled room kept between 20°C–22°C on a 12-hour light, 12-hour dark cycle. Mice subjected to cold challenge at 5 months of age were housed in a temperature controlled diurnal incubator (Caron Products & Services, Inc) at 4°C on a 12-hour light, 12-hour dark cycle. All mice were allowed ad libitum access to water and food unless otherwise noted. Body core temperature was determined by rectal probe measurements. Serum insulin was measured by ELISA (Crystal Chem, Inc).
Body composition analysis and energy expenditure
Mice were scanned using the IVIS Spectrum CT (PerkinElmer) using the standard 2-mouse resolution. To determine body composition, Image J software was used to generate histograms of voxel (3 dimensional pixels) intensity distributions of 8-bit image stacks. A 1-cm3 area in the interscapular region was selected, and the number of voxels in each intensity bin (from 1–255) was divided by the total number of voxels with an intensity value above background (intensity of 0) to determine the percentage of voxels of each intensity value (Supplemental Figure 3). In addition, DEXA was performed according to the manufacturer's instructions (GE Lunar PIXImus 2). DEXA and MicroCT were used to measure femur length and gave similar values. For the energy expenditure studies, 6 control and 6 Myf5IRKO male mice were monitored using the Comprehensive Laboratory Animal Monitoring System (Columbus Instruments). Oxygen consumption and carbon dioxide and heat production were measured by indirect calorimetry in 5-month-old animals. The respiratory exchange ratio (carbon dioxide production/oxygen consumption) was calculated from the gas exchange data.
Gene expression analysis
Total RNA was extracted from tissue or cells with TRIzol and purified using a spin column kit (Zymo Research). RNA (500 ng–1 μg) was reverse transcribed with a high-capacity cDNA reverse transcription kit (Applied Biosystems). Real-time PCR was performed starting with 10 ng of cDNA and forward and reverse oligonucleotide primers (300nM each) in a final volume of 10 μL with SYBR green PCR Master Mix (Roche). Fluorescence was determined and analyzed in an ABI Prism 7900 sequence detection system (Applied Biosystems). For adipose tissue, acidic ribosomal phosphoprotein P0 expression was used to normalize gene expression. For muscle tissue, TATA box-binding protein expression was used to normalize gene expression. Real-time PCR primer sequences are listed in Supplemental Table 1.
Cell culture and adipocyte differentiation
Adipose tissue was dissected and minced before digestion in 0.2% collagenase I (Worthington). After SVF cells were collected by centrifugation, red blood cells were lysed with NH4Cl and then the SVF was filtered through a 40-μm filter. SVF cells were maintained in DMEM (high glucose) containing 10% bovine serum at 37°C in a 5% CO2 incubator. Adipocyte differentiation was induced in 70% confluent cells by treating cells with an induction mixture containing insulin (10 μg/mL), indomethacin (50μM), 3-isobutyl-1-methylxanthine (0.5μM), dexamethasone (1μM), and triiodothyronine (1nM). After 48 hours of induction, cells were kept in medium containing insulin and triiodothyronine for the subsequent 5 days, changing the medium every 3 days. For BODIPY staining, the medium was supplemented with 5uM BODIPY FL C5 (Sigma) for the final 12 hours and then stained with 4,6-diamidino-2-phenylindole (DAPI) before imaging.
Histology
For H&E staining, sections were prepared, processed, and stained as described (30). For immunostaining, primary antibodies were incubated overnight at 4°C: perilipin, 1:500 (Sigma) and UCP1, 1:100 (Abcam). After primary incubation, sections were washed and either processed with a VECTASTAIN elite kit (Vector Labs) according the manufacturer's instructions or incubated with appropriate Alexa Fluor 488 antibodies (Life Technologies) at 1:200 dilution in the dark for 20 minutes. After secondary incubation, sections were washed with distilled water for DAPI staining (0.1 μg/mL in water for 3 min in the dark) and mounted. Slides were kept in the dark and analyzed on a fluorescence microscope (Olympus BX60F-3; Olympus Corp).
Western blot analysis
For signaling studies, mice were fasted for 6 hours before 5 units of human insulin (Eli Lilly) was injected retro orbitally into anesthetized animals. After 10 minutes, tissues were dissected and homogenized in RIPA buffer supplemented with protease inhibitor cocktail and phosphatase inhibitor cocktails 2 and 3 (Sigma) using a bullet blender and steel beads. For all other studies, mice were fed ad libitum until killing, and tissues were dissected and homogenized as described. Protein concentrations were determined with the bicinchoninic acid (BCA) assay assay (Pierce Biotechnology). Lysates were subjected to SDS-PAGE, transferred to polyvinylidene difluoride membranes (Amersham Biosciences), and blocked using blocking buffer (Roche). Antibodies to UCP1 (Abcam), TH (Millipore), tubulin (Sigma), InsR (Cell Signaling Technology), and phospho-InsR (Cell Signaling Technology) were stained using species-specific secondary antibodies labeled with either Alexa Fluor 488 or Alexa Fluor 594 (Life Technologies), and proteins on the membrane were visualized using the IVIS Spectrum CT with appropriate filter sets. Staining was quantified using Living Image software (PerkinElmer) to measure radiance in photons/s.
Muscle strength tests
Mice at 5 months of age were held by the tail and allowed to grip a wire cage attached to a dynamometer (Amatek), then pulled until they released the cage. This was repeated 3 times, after which each animal was allowed to rest for 15 minutes, and then the test was repeated. Average grip strength was calculated by taking the average of the 6 total trials.
Results
Loss of InsR in the Myf5 lineage impairs interscapular BAT formation
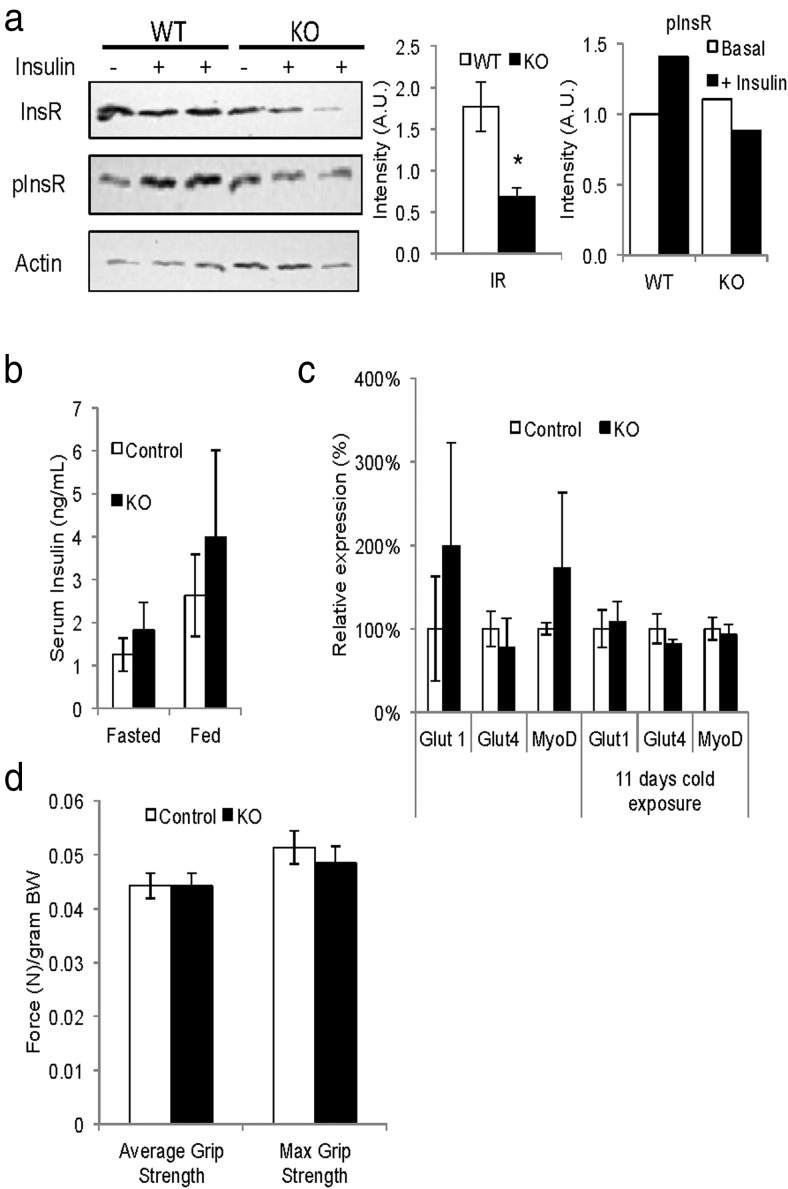
In order to determine the impact of insulin signaling on BAT and skeletal muscle development in vivo, we inactivated the InsR in Myf5-expressing precursor cells by crossing InsR floxed (4) mice to the myf5-cre line (31). At 3 and 5 months of age, myf5-cre;IRfl/fl conditional knockout (Myf5IRKO) mice showed no differences in total body weight (Figure 1A); however, as early as 3 weeks of age, Myf5IRKO mice were distinguishable from their littermates by a visible paucity of the interscapular BAT depot that was obvious when handling the mice. Gross morphology of skeletal muscle appeared to be normal, and there was no change in the sizes of different types of limb skeletal muscle (transversus abdominus, gastrocnemius, and soleus) (Figure 1B). Quantitative PCR confirmed that InsR mRNA expression was significantly decreased in skeletal muscle tissue compared with control animals but remained unchanged in sWAT, consistent with specific recombination of the floxed allele in the Myf5-positive lineage. InsR expression was unchanged in residual interscapular BAT tissue isolated from Myf5IRKO mice, raising the possibility that remaining tissue might arise from a Myf5-negative lineage, which constituted less than 5% of the progenitor population in interscapular BAT (17). InsR expression could also indicate inefficient recombination of the floxed allele. Insulin and IGF-I have overlapping function in the regulation of adipogenic gene expression; however, there was a trend towards decreased expression of IGF-IR and IRS-1 expression in classical BAT that did not reach significance and no change in sWAT or skeletal muscle tissue making compensation via enhanced IGF-I signaling unlikely (Figure 1C).
Figure 1.
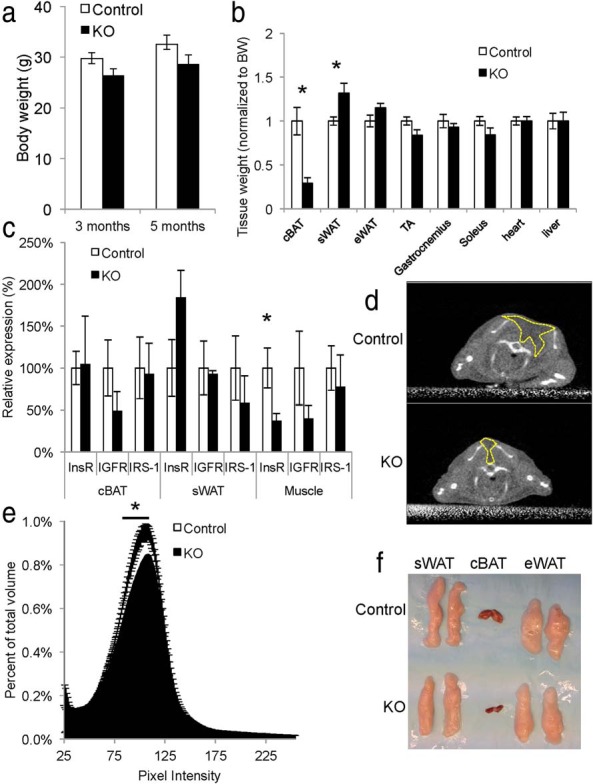
Loss of InsR in the Myf5 lineage impairs interscapular BAT formation. A, Body weight of control (n = 14) and Myf5IRKO mice (n = 13) at 3 and 5 months of age. B, Relative tissue weights from control (n = 6) and Myf5IRKO mice (n = 5). C, InsR, IGF-IR, and IRS-1 expression measured by real-time PCR in interscapular BAT, sWAT, and gastrocnemius muscle tissue from 4-month-old control (n = 6) and Myf5IRKO (KO, n = 5) mice. Control expression was set to 100%. D, Representative coronal MicroCT projections of control and Myf5IRKO mice at 5 months of age. Dashed lines surround the low-density interscapular fat pad. E, Interscapular tissue density distribution in a 1-cm3 region of interest in control (n = 7) and Myf5IRKO mice (n = 7) at 5 months of age. F, Representative images of interscapular BAT, sWAT, and eWAT from 5-month control and Myf5IRKO mice. All bar graphs are presented as mean ± SEM. Statistical significance was assessed by 2-tailed Student's t test; *, P < .05.
To determine adipose tissue volume in live animals, we developed a quantitative method using MicroCT scanning. Adipose tissue is characteristically lower in density (represented by pixel intensity) compared with lean tissue and bone and could be readily identified in the interscapular region (Figure 1C). In Myf5IRKO mice, we detected a decrease in the percentage of low-density tissue in the interscapular region, which can be attributed to a paucity of adipose tissue (Figure 1E). In older animals (12 mo of age), the lack of interscapular adipose tissue was still apparent by MicroCT imaging (Supplemental Figure 1A). Indeed, analysis of individual adipose tissue depots in Myf5IRKO mice demonstrated that the lack of interscapular tissue was mainly due to a paucity of classical BAT (Figure 1F). Myf5IRKO mice also had an increase in sWAT weight after normalization to body weight; however, epididymal WAT (eWAT), liver, heart, and muscle were unaffected (Figure 1B), and mice did not appear runted (Supplemental Figure 1, B and C). Thus, Myf5IRKO mice cannot regulate BAT tissue development, but the development of other tissues that are descendent from both Myf5-positive and negative lineages are unaffected.
InsR deletion in the Myf5 lineage does not impair muscle insulin signaling or function
Although disruption of InsR in Myf5-expressing progenitors did not affect skeletal muscle mass (Figure 1B), expression of InsR protein in mature intercostal muscle was significantly reduced (Figure 2A). In addition to a decrease in protein expression, we observed a slight decrease in phosphorylation of InsR in response to acute insulin treatment; however, mice had normal levels of insulin in both the fed and fasting state (Figure 2B). Expression of both of the major glucose transporters in gastrocnemius muscle, Glut1 and Glut4, remained unchanged, as was the expression of Myogenic differentiation 1 (MyoD) which coregulates myogenic precursor differentiation with Myf5 (Figure 2C) (18). To assess muscle function, we measured grip strength in control and Myf5IRKO animals using a dynamometer apparatus. Clearly, the KO mice did not display alteration in maximal and averaged grip strength (Figure 2D), suggesting muscle function and development is preserved.
Figure 2.
InsR deletion in the Myf5 lineage does not impair muscle function. A, Protein levels of InsR and phospho-InsR (pInsR) measured by Western blotting in intercostal muscle from representative Myf5IRKO and control mice that were treated acutely with insulin or vehicle and densitometry analysis of InsR and pInsR (n = 3 animals per group). C, Serum insulin levels from Myf5IRKO and control mice fasted for 12 hours or fed ad libitum. D, Expression of Glut1, Glut4, and MyoD measured by real-time PCR in gastrocnemius muscle from control and Myf5IRKO mice at room temperature and after cold challenge. Control expression was set to 100% (n = 5–8 animals per group). E, Grip strength measured by dynamometer in control and Myf5IRKO mice (n = 8 animals per group). All bar graphs are presented as mean ± SEM.
Specific ablation of InsR in Myf5-expressing cells inhibits differentiation of brown preadipocytes
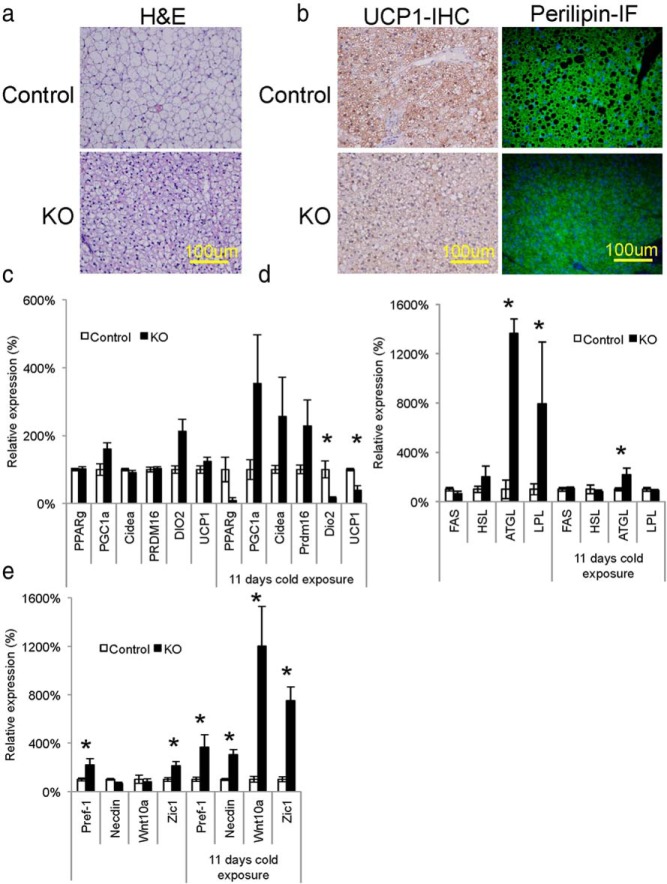
Histological analysis of the residual BAT tissue by hematoxylin and eosin (H&E) staining indicated a significant decrease in cell size due to reduction of lipid accumulation (Figures 3A). Interestingly, immunohistological analysis of the residual BAT in Myf5IRKO mice revealed detectable levels of UCP1 protein and the lipid droplet associated protein perilipin in the residual BAT of Myf5IRKO mice compared with wild-type mice (Figure 3B), suggesting the presence of mature brown adipocytes in this remaining tissue. Consistent with the presence of mature brown adipocytes, quantitative polymerase chain reaction analysis demonstrated no difference in the expression of general adipogenic and brown-fat selective markers, PPARγ, PPARγ coactivator 1α (PGC1α), cell death inducing DNA fragmentation factor alpha subunit-like effector a (Cidea), PR domain containing 16 (PRDM16), deiodinase 2 (Dio2), and UCP1 in interscapular BAT from Myf5IRKO mice compared with control. Cold exposure induces cell proliferation in classical BAT (32) and increases UCP1 expression (33); however, in Myf5IRKO mice that were cold-challenged for 11 days, expression levels of Dio2 and UCP1 were reduced, indicating that Myf5IRKO mice have a defect in the thermogenic program in classical BAT in response to cold challenge (Figure 3C).
Figure 3.
BAT morphology and gene expression is altered in Myf5IRKO mice. A, H&E-stained sections of interscapular BAT from control and Myf5IRKO mice at 4 months of age. B, Immunohistological staining for UCP1 (brown staining) and perilipin in interscapular BAT from control and Myf5IRKO mice at 4 months of age. C, Expression of PPARγ, PGC1α, Cidea, PRDM16, DIO2, and UCP1 measured by real-time PCR in interscapular BAT, sWAT, and gastrocnemius muscle tissue from control and Myf5IRKO mice that were either kept at room temperature or subjected to an 11-day cold challenge at 4°C. Control expression was set to 100% (n = 5–8 animals per group). D, Expression of FAS, HSL, ATGL, and LPL measured by real-time PCR in interscapular BAT, sWAT, and gastrocnemius muscle tissue from control and Myf5IRKO mice that were either kept at room temperature or subjected to an 11-day cold challenge at 4°C. Control expression was set to 100% (n = 5–8 animals per group). E, Expression of Pref-1, Necdin, Wnt10a, and Zic1 measured by real-time PCR in interscapular BAT, sWAT, and gastrocnemius muscle tissue from control and Myf5IRKO mice that were either kept at room temperature or subjected to an 11-day cold challenge at 4°C. Control expression was set to 100% (n = 5–8 animals per group). All bar graphs are presented as mean ± SEM. Statistical significance assessed by 2-tailed Student's t test; *, P < .05.
Adipocyte lipid content is controlled by a balance between fatty acid synthesis controlled by fatty acid synthase (FAS) and triglyceride breakdown, which is dependent on hormone-sensitive lipase (HSL), adipocyte triglyceride lipase (ATGL), and lipoprotein lipase (LPL). There was no difference in the expression of FAS or HSL; however, ATGL and LPL expression were significantly enhanced in classical BAT and the increase in ATGL expression was maintained after cold challenge, suggesting increased lipolysis in Myf5IRKO compared with controls which could contribute to the decrease in lipid droplet size that is apparent histologically (Figure 3A). We also detected increased expression of the preadipocyte markers Pref-1 and Zic1 in residual BAT tissue compared with wild-type controls. After 11 days of cold challenge at 4°C, Pref-1 and Zic1 expression, as well as Necdin and Wnt10a, 2 other insulin signaling targets that act as inhibitors blocking preadipocytes-adipocyte transition (28), were also increased (Figure 3E). These data suggest that the InsR KO brown preadipocytes fail to undergo adipocyte differentiation due to an impairment in removal of the suppressive signals that prevent the initiation of adipogenic program.
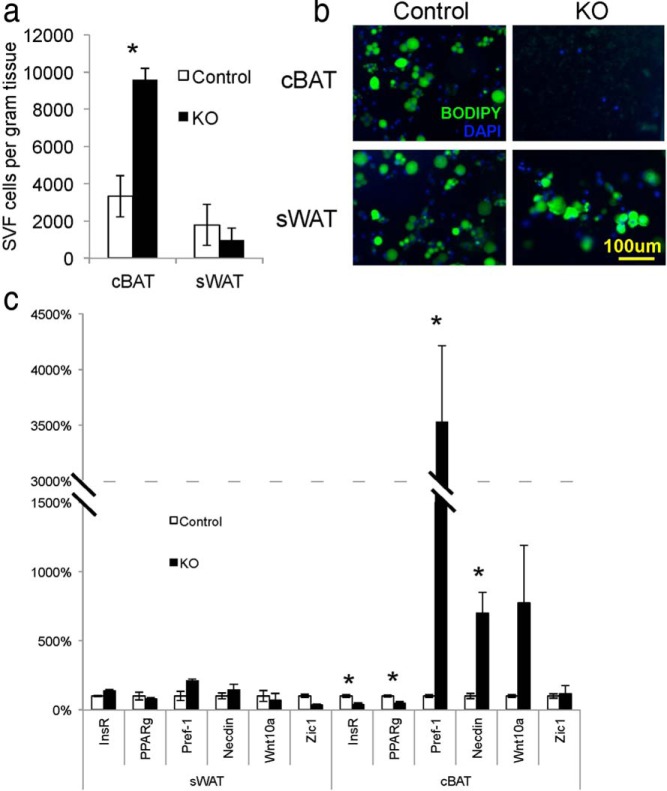
To test whether impaired insulin signaling in the Myf5-positive lineage decreases differentiation of these cells in vivo, we isolated stromal vascular fraction (SVF) cells from control and Myf5IRKO animals. Consistent with a block in the differentiation of progenitor cells in the Myf5-positive lineage, we observed a significant increase in the number of SVF cells per gram of tissue from interscapular BAT but no change in the ratio of SVF cells to tissue weight in sWAT (Figure 4A). In culture, SVF cells from Myf5IRKO mice were induced to differentiate and stained with BODIPY FL C5 to assess lipid accumulation. Although cells isolated from sWAT of Myf5IRKO and control mice effectively became lipid-laden cells, BAT SVF cells from Myf5IRKO mice failed to accumulate lipid (Figure 4B). Consistent with the blockage in differentiation, we observed a 35-fold increase of Pref-1 expression and an approximately 7-fold increase in the expression of Necdin, and a trend for increased Wnt10a in differentiated Myf5IRKO classical BAT cells but not sWAT cells. In accordance with the undifferentiated status of the cells, expression of PPARγ was significantly decreased in the Myf5IRKO culture (Figure 4C). Importantly, expression of InsR in these cells was also notably decreased (Figure 4C), suggesting that insulin signaling is required for brown preadipocyte differentiation in a cell-autonomous manner.
Figure 4.
Specific ablation of the InsR inhibits differentiation of brown adipose progenitor cells. A, Average number of SVF cells per gram of tissue isolated from interscapular BAT or sWAT of 14-month-old control and Myf5IRKO mice (n = 3 per group). B, BODIPY labeled lipid staining of control or Myf5IRKO preadipocytes from interscapular BAT or sWAT differentiated for 7 days. C, Expression of InsR, PPARγ, Pref-1, Necdin, Wnt10a, and Zic1 measured by real-time PCR in differentiated preadipocytes from control and Myf5IRKO interscapular BAT and sWAT. Control expression was set to 100% (n = 3 per group). All bar graphs are presented as mean ± SEM. Statistical significance was assessed by 2-tailed Student's t test; *, P < .05.
Loss of interscapular BAT in Myf5IRKO mice causes compensatory browning of WAT to maintain temperature homeostasis
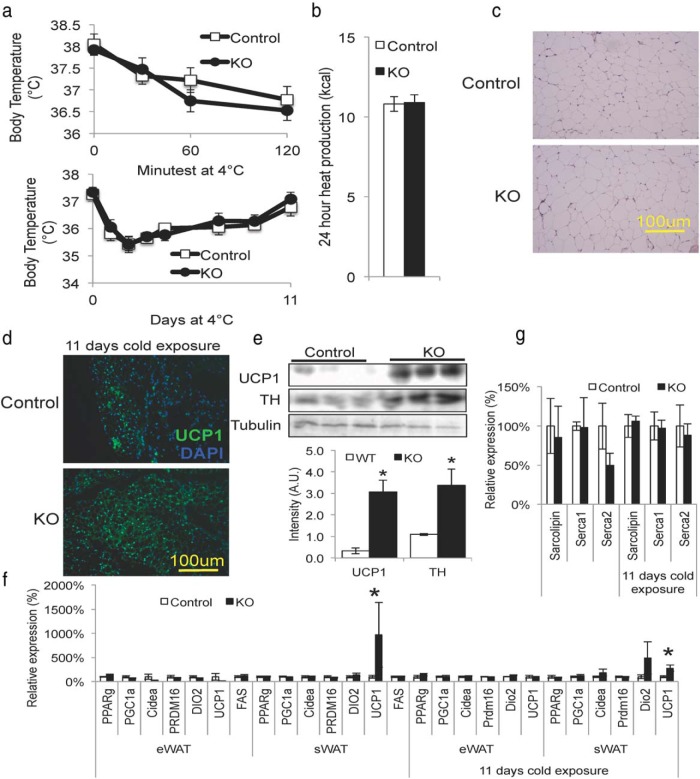
Because classical BAT plays an important role in thermoregulation, one would expect the extreme paucity of classical BAT tissue in Myf5IRKO mice would lead to cold sensitivity. However, Myf5IRKO mice were able to maintain body temperature similarly to control animals when cold challenged at 4°C for 2 hours up to 11 days (Figure 5A). When placed in metabolic cages at room temperature, which is slightly below thermoneutrality for mice, Myf5IRKO mice produced similar amounts of heat to control animals (Figure 5B and Supplemental Figure 2, B and C) and ate a similar amount of food (Supplemental Figure 2A). Although we did not observe a histological difference between sWAT from Myf5IRKO mice and controls maintained at room temperature (Figure 5C), the lack of impaired thermogenesis when cold challenged could be attributed to increased UCP1 expression in sWAT consistent with browning of WAT (Figure 5D). Immunoblotting of sWAT tissue from mice that were cold challenged demonstrated an approximately 6-fold increase in UCP1 protein expression and a 2-fold increase in the sympathetic marker tyrosine hydroxylase (TH) (Figure 5E). Gene expression analysis confirmed increased UCP1 expression in sWAT but not eWAT in Myf5IRKO mice at room temperature, which is slightly below thermoneutral for mice, and after cold challenge (Figure 5F).
Figure 5.
Loss of interscapular BAT in Myf5IRKO mice causes compensatory browning of WAT. A, Core body temperature of 5-month-old control or Myf5IRKO mice during an acute (2 h) or long-term cold challenge at 4°C (n = 8 animals per group). B, Total heat production of control or Myf5IRKO mice (n = 6 animals per group). C, H&E-stained sections of sWAT from control and Myf5IRKO mice at 4 months of age. D, Immunohistological staining for UCP1 in sWAT from control and Myf5IRKO mice after an 11-day cold challenge at 4°C. E, Protein levels of UCP1 and TH measured by Western blotting in sWAT from Myf5IRKO and control mice cold challenged at 4°C. Three representative animals are shown and densitometry analysis is shown below (n = 8 animals per group). F, Expression of PPARγ, PGC1α, Cidea, PRDM16, DIO2, and UCP1 measured by real-time PCR in eWAT and sWAT from control and Myf5IRKO mice at room temperature and after cold challenge. Control expression was set to 100% (n = 5–8 animals per group). G, Expression of Sarcolipin, Serca1, and Serca2 measured by real-time PCR in gastrocnemius muscle from control and Myf5IRKO mice at room temperature and after cold challenge. Control expression was set to 100% (n = 5–8 animals per group). All bar graphs are presented as mean ± SEM. Statistical significance was assessed by 2-tailed Student's t test; *, P < .05.
Sarcolipin, a recently identified regulator of the sarco/endoplasmic reticulum Ca(2+)-ATPase (Serca) pump, has been shown to mediate nonshivering thermogenesis in muscle tissue and cause futile cycling of the Serca pump (34). This mechanism does not appear to be compensating for the paucity of classical BAT in thermoregulation, because we detected no change in expression of Sarcolipin, Serca1, or Serca2 in Myf5IRKO mice at room temperature and after cold challenge (Figure 5G). Taken together, these results suggest compensatory browning of WAT mediated by increased sympathetic input is the major mechanism that preserves thermoregulation in Myf5IRKO mice.
Discussion
Insulin is known to regulate both cell growth and differentiation in many cell types, including muscle (35), mammary gland (36), adipose (37), and others. To understand the role of insulin signaling in the Myf5-positive cellular lineage, we created mice with a knockout of the InsR in Myf5-expressing cells. The resulting Myf5IRKO mice exhibit reduced interscapular BAT mass and increased expression of several negative regulators of adipogenesis in the remaining BAT tissue and decreased differentiation of brown preadipocytes when cultured in vitro. Nonetheless, these mice are not sensitive to cold exposure, at least in part due to normal expression of UCP1 in residual BAT tissue as well as compensatory browning of sWAT. Surprisingly, deletion of InsR in the Myf5 lineage does not seem to have an impact on muscle development and physiology or serum insulin levels.
MicroCT analysis of Myf5IRKO mice provides an exciting opportunity for highly sensitive analysis of depot-specific changes in tissue density and distribution that can be difficult to detect using other techniques, such as dual energy x-ray (DEXA) scanning. Initially, we did not detect any differences in whole-body composition using DEXA (Supplemental Figure 1C); however, using MicroCT, we were able to distinguish the interscapular region in 3 dimensions, allowing careful quantification of tissue composition in this specific region. MicroCT also allows longitudinal monitoring of interscapular adipose tissue, because animals do not need to be killed to determine tissue volume.
The phenotype of the Myf5IRKO differs drastically from the fat-specific knockout of the InsR. In fat-specific knockout of the InsR mice, cre expression is driven by the aP2 promoter, which is expressed mainly in mature brown and white adipocytes (38), leading to reduced WAT mass and no significant change in BAT mass (5, 39). In contrast, deletion of the InsR in brown adipose precursor cells, as reported in this study, demonstrates its indispensible role in BAT development. Mature brown adipocytes may be able to counteract the loss of InsR by signaling through IGF-I signaling, and indeed, combined deletion of both receptors in mature adipose tissue in FIGIRKO mice leads to a paucity of classical BAT (40). Importantly, IGF-I signaling does not seem to compensate InsR deletion in Myf5 progenitor cells. The increased expression of Pref-1, Necdin, and Wnt10a suggest that InsR-mediated signaling is required to remove the adipogenic block in brown preadipocytes and allow them to differentiate. The suppression of Pref-1 appears particularly critical to adipogenesis as bone morphogenetic protein (BMP) signaling can also suppress Pref-1 expression and rescue the differentiation defect of IRS-1 null brown preadipocytes (41). Mice lacking BMP receptor 1a in the Myf5 lineage (Myf5BMPr1a) show a similar paucity of classical BAT to Myf5IRKO mice (16).
Interestingly, the residual interscapular BAT tissue in Myf5IRKO mice seems to be capable of some level of thermogenesis, because we observed lipid droplets, lipid associated protein expression, and UCP1 expression. It is also possible that the residual BAT tissue has increased rates of lipolysis as indicated by increased ATGL and LPL expression, suggesting increased lipid fuel utilization to compensate for decreased tissue mass. From the data, InsR expression was not completely abolished in skeletal muscle or classical BAT, suggesting incomplete recombination that could have led to some mature cells escaping gene deletion. It is also possible that a Myf5-negative lineage of brown progenitors expanded to compensate for the loss of InsR in Myf5-positive cells (17). The development of intercostal muscle is dependent on Myf5 (42), and indeed, recombination could be observed in this tissue, resulting in a decrease of InsR protein contents in response to insulin treatment. Despite the reduced levels of InsR, development of skeletal muscle appears to be normal. These results suggest that although insulin signaling plays a crucial role in regulation of muscle glucose metabolism, it is nonessential for muscle development when deleted from the Myf5-expressing progenitors
We have previously reported compensatory browning of WAT in the context of defective classical BAT in Myf5BMPr1a (16). Compensatory browning in Myf5BMPr1a mice is mediated at least in part through increased sympathetic tone suggesting this mechanism may play a role in Myf5IRKO mice. Indeed, the Myf5IRKO mice also display enhanced browning of WAT for thermoregulation, which appears to be due to increased sympathetic input into sWAT. These data highlight a cross talk between the constitutive and recruitable brown fat cells for thermoregulation and maintaining energy homeostasis. Given that the residual interscapular BAT in Myf5IRKO mice appears to be functional, it is difficult to estimate the relative contribution of white adipose browning compared with constitutive BAT activity to thermoregulation in these animals; however, FIGIRKO mice are extremely cold sensitive due to the absence of both classical BAT and recruited BAT (40). Myf5IRKO mice have a similar paucity of classical BAT to FIGIRKO mice but insulin signaling of progenitor cells in sWAT remains unaffected, suggesting that recruited BAT that arise in Myf5IRKO animals are capable of compensating for most cold sensitivity when classical BAT is defective. The absence of compensatory uncoupling of calcium cycling in Myf5IRKO muscle further supports the important role of recruited BAT in thermoregulation in Myf5IRKO mice.
Recently, Sanchez-Gurmaches and Guertin (43) have reported a similar mouse model, which displays decreased BAT and an altered fat distribution. These data are in agreement with our observations; however, we did not observe the compensatory increase in WAT tissue weights that they observed. Their lineage tracing studies suggest a compensatory hyperplasia of Myf5-negative cells in BAT from Myf5IRKO mice; however, our results demonstrate a cell-autonomous defect in adipogenic differentiation of all BAT stromal precursor cells. These results leave in question the lineage of Myf5-negative cells in Myf5IRKO BAT.
The data generated in this study support previous reports that the InsR is dispensable for muscle development (4); however, the dramatic effect of InsR deletion in Myf5-positive brown preadipocytes suggests a possible divergence point in the skeletal muscle/brown adipocyte lineage, where InsR is required for brown preadipocytes differentiation but dispensable for muscle differentiation. Taken together, these results highlight the critical role of insulin signaling in regulating the differentiation and function of brown preadipocytes and support the current model whereby browning of WAT can compensate for deficiency of classical BAT in thermoregulation.
Acknowledgments
We thank M. Mulvey and A. Clermont of the Joslin Diabetes Center Physiology core for expert technical assistance. We also thank P. Soriano (Mount Sinai School of Medicine, New York, NY) and C.R. Kahn (Joslin Diabetes Center) for providing myf5-cre mice and floxed InsR mice, respectively.
This work was supported in part by the National Institute of Health (NIH) Grant R01DK077097 (to Y.-H.T.), the Joslin Diabetes Center's Diabetes Research Center Grant P30DK036836, a research grant from the American Diabetes Foundation (ADA 7–12-BS-191), and by funding from the Harvard Stem Cell Institute (to Y.-H.T.). M.D.L. was supported by NIH Fellowships T32DK007260 and F32DK102320. T.J.S. was supported by the Mary K. Iacocca Foundation and the German Research Foundation Grant DFG SCHU2445/1–1. A.J.P. was supported by the Harvard College Research Program.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATGL
- adipocyte triglyceride lipase
- BAT
- brown adipose tissue
- BMP
- bone morphogenetic protein
- BODIPY FL C5
- N-(4,4-Difluoro-5,7-Dimethyl-4-Bora-3a,4a-Diaza-s-Indacene-3-Pentanoyl)Sphingosine
- Cidea
- cell death-inducing DFFA-like effector a
- DEXA
- dual energy x-ray
- Dio2
- deiodinase 2
- eWAT
- epididymal WAT
- FAS
- fatty acid synthase
- FIGIRKO
- fat specific IGF1R and InsR KO mice
- H&E
- hematoxylin and eosin
- Glut
- glucose transporter
- HSL
- hormone-sensitive lipase
- IGF-IR
- IGF-I receptor
- InsR
- insulin receptor
- IRS-1
- InsR substrate-1
- KO
- knockout
- LPL
- lipoprotein lipase
- MicroCT
- micro computed tomography
- Myf5BMPr1a
- mice lacking BMP receptor 1a in the Myf5 lineage
- PGC1α
- PPARγ coactivator 1α
- PPAR
- peroxisome proliferator-activated receptor
- PRDM16
- PR domain containing 16
- Pref-1
- preadipocyte factor-1
- Serca
- sarco/endoplasmic reticulum Ca(2+)-ATPase
- SVF
- stromal vascular fraction
- sWAT
- sc WAT
- TH
- tyrosine hydroxylase
- UCP1
- uncoupling protein 1
- Wnt10a
- wingless-type MMTV integration site member 10a
- Zic1
- zinc finger protein of the cerebellum 1.
References
- 1. Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. [DOI] [PubMed] [Google Scholar]
- 2. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. [DOI] [PubMed] [Google Scholar]
- 3. Leroith D, Accili D. Mechanisms of disease: using genetically altered mice to study concepts of type 2 diabetes. Nat Clin Pract Endocrinol Metab. 2008;4:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brüning JC, Michael MD, Winnay JN, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. [DOI] [PubMed] [Google Scholar]
- 5. Blüher M, Michael MD, Peroni OD, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. [DOI] [PubMed] [Google Scholar]
- 6. Guerra C, Navarro P, Valverde AM, et al. Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance. J Clin Invest. 2001;108:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michael MD, Kulkarni RN, Postic C, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 9. Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. [DOI] [PubMed] [Google Scholar]
- 10. Brüning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. [DOI] [PubMed] [Google Scholar]
- 11. Accili D, Drago J, Lee EJ, et al. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12:106–109. [DOI] [PubMed] [Google Scholar]
- 12. Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 13. Rother KI, Accili D. Role of insulin receptors and IGF receptors in growth and development. Pediatr Nephrol. 2000;14:558–561. [DOI] [PubMed] [Google Scholar]
- 14. Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Timmons JA, Wennmalm K, Larsson O, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA. 2007;104:4401–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schulz TJ, Huang P, Huang TL, et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495:379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 2012;16:348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hopwood ND, Pluck A, Gurdon JB. Xenopus Myf-5 marks early muscle cells and can activate muscle genes ectopically in early embryos. Development. 1991;111:551–560. [DOI] [PubMed] [Google Scholar]
- 19. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. [DOI] [PubMed] [Google Scholar]
- 21. Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. [DOI] [PubMed] [Google Scholar]
- 22. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stanford KI, Middelbeek RJ, Townsend KL, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–205. [DOI] [PubMed] [Google Scholar]
- 25. Townsend KL, Tseng YH. Brown fat fuel utilization and thermogenesis. Trends Endocrinol Metab. 2014;25:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Entingh AJ, Taniguchi CM, Kahn CR. Bi-directional regulation of brown fat adipogenesis by the insulin receptor. J Biol Chem. 2003;278:33377–33383. [DOI] [PubMed] [Google Scholar]
- 27. Cypess AM, Zhang H, Schulz TJ, et al. Insulin/IGF-I regulation of necdin and brown adipocyte differentiation via CREB- and FoxO1-associated pathways. Endocrinology. 2011;152:3680–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tseng YH, Butte AJ, Kokkotou E, et al. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat Cell Biol. 2005;7:601–611. [DOI] [PubMed] [Google Scholar]
- 29. Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. [DOI] [PubMed] [Google Scholar]
- 30. Tseng YH, Kokkotou E, Schulz TJ, et al. New role of bone morphogeneticprotein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tallquist MD, Weismann KE, Hellström M, Soriano P. Early myotome specification regulates PDGFA expression and axial skeleton development. Development. 2000;127:5059–5070. [DOI] [PubMed] [Google Scholar]
- 32. Bukowiecki L, Collet AJ, Follea N, Guay G, Jahjah L. Brown adipose tissue hyperplasia: a fundamental mechanism of adaptation to cold and hyperphagia. Am J Physiol. 1982;242:E353–359. [DOI] [PubMed] [Google Scholar]
- 33. Carmona MC, Valmaseda A, Brun S, et al. Differential regulation of uncoupling protein-2 and uncoupling protein-3 gene expression in brown adipose tissue during development and cold exposure. Biochem Biophys Res Commun. 1998;243:224–228. [DOI] [PubMed] [Google Scholar]
- 34. Bal NC, Maurya SK, Sopariwala DH, et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18:1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mandel JL, Pearson ML. Insulin stimulates myogenesis in a rat myoblast line. Nature. 1974;251:618–620. [DOI] [PubMed] [Google Scholar]
- 36. Voytovich AE, Topper YJ. Hormone-dependent differentiation of immature mouse mammary gland in vitro. Science. 1967;158:1326–1327. [DOI] [PubMed] [Google Scholar]
- 37. Saad MJ, Folli F, Araki E, Hashimoto N, Csermely P, Kahn CR. Regulation of insulin receptor, insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-F442A adipocytes. Effects of differentiation, insulin, and dexamethasone. Mol Endocrinol. 1994;8:545–557. [DOI] [PubMed] [Google Scholar]
- 38. Barlow C, Schroeder M, Lekstrom-Himes J, et al. Targeted expression of Cre recombinase to adipose tissue of transgenic mice directs adipose-specific excision of loxP-flanked gene segments. Nucleic Acids Res. 1997;25:2543–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Katic M, Kennedy AR, Leykin I, et al. Mitochondrial gene expression and increased oxidative metabolism: role in increased lifespan of fat-specific insulin receptor knock-out mice. Aging Cell. 2007;6:827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boucher J, Mori MA, Lee KY, et al. Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nat Commun. 2012;3:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang H, Schulz TJ, Espinoza DO, et al. Cross talk between insulin and bone morphogenetic protein signaling systems in brown adipogenesis. Mol Cell Biol. 2010;30:4224–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kablar B, Krastel K, Ying C, Asakura A, Tapscott SJ, Rudnicki MA. MyoD and Myf-5 differentially regulate the development of limb versus trunk skeletal muscle. Development. 1997;124:4729–4738. [DOI] [PubMed] [Google Scholar]
- 43. Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. [DOI] [PMC free article] [PubMed] [Google Scholar]