Abstract
Our recent study indicates that a brief infusion (20 min) of estradiol (E2) benzoate (EB) into the stalk-median eminence (S-ME) stimulates GnRH release with a latency of approximately 10 minutes. In contrast to the effect induced by a brief infusion of EB, it has previously been shown that systemic EB administration suppresses release of GnRH, kisspeptin, and LH with a latency of several hours, which is known as the negative feedback action of E2. We speculated that the differential results by these 2 modes of EB administration are due to the length of E2 exposure. Therefore, in the present study, the effects of EB infusion for periods of 20 minutes, 4 hours, or 7 hours into the S-ME of ovariectomized female monkeys on the release of GnRH and kisspeptin were examined using a microdialysis method. To assess the effects of the EB infusion on LH release, serum samples were also collected. The results show that similar to the results with 20-minute infusion, both 4- and 7-hour infusions of EB consistently stimulated release of GnRH and kisspeptin from the S-ME accompanied by LH release in the general circulation. In contrast, sc injection of EB suppressed all 3 hormones (GnRH, kisspeptin, and LH) measured. It is concluded that regardless of the exposure period, direct E2 action on GnRH and kisspeptin neurons in the S-ME, where their neuroterminals are present, is stimulatory, and the E2-negative feedback effects do not occur at the S-ME level.
The concept that negative and positive feedback actions of ovarian steroids regulate female reproductive cyclicity is well established. A wealth of data indicates that circulating gonadal steroids reach the preoptic area-hypothalamus and anterior pituitary and stimulate or inhibit GnRH release as well as gonadotropin release. Specifically, an increase in estradiol (E2) inhibits GnRH/LH release with a latency of several hours and stimulates GnRH/LH release with a latency of approximately 24 hours (1, 2). Moreover, it has been well documented that the negative feedback actions of E2 occur at the medial basal hypothalamus (MBH), ie, in the arcuate nucleus (ARC) (3–7).
In contrast to these negative and positive feedback actions on GnRH/LH release, E2 also rapidly stimulates GnRH neurons in vitro (8–13). Surprisingly, the rapid E2 action on GnRH release is not limited to in vitro studies. A recent study in our laboratory shows that a brief (20 min) infusion of E2-benzoate (EB) in the stalk-median eminence (S-ME) induces GnRH release with a latency of approximately 10 minutes (14). In fact, this EB-induced GnRH release is accompanied by a release of neuro-E2 locally synthesized and rapidly secreted from the S-ME of ovariectomized (OVX) female monkeys (14).
However, it remains to be clarified what differentiates the underlying mechanism of rapid excitatory E2 action in vivo from the mechanisms governing the negative feedback action of E2. It is possible that the length of EB exposure of GnRH neurons is a determining factor, because the rapid EB action was examined with a 20-minute infusion of EB to the S-ME (14), whereas elevated levels of EB remain for several hours after systemic injection (2). Alternatively, the site of EB exposure within the hypothalamus may be a determinant, because direct EB infusion into the S-ME may be limited to the action in the GnRH neurons and their neuroterminals, whereas EB injected systemically diffuses extensively throughout the hypothalamus, including the ARC and S-ME (15), where interneurons mediate EB actions. Accordingly, to understand the underlying mechanisms of the rapid excitatory E2 action on GnRH release, we examined the effects of increasing the period of EB infusion into the S-ME on GnRH release using a microdialysis method in OVX female monkeys. Because the kisspeptin system is a target of the E2 feedback mechanism (16, 17), in the present study, we also measured changes in kisspeptin release. Finally, to assess the physiological consequence of the rapid EB action on the S-ME, we monitored changes in circulating LH levels.
Materials and Methods
Animals
A total of 16 OVX female rhesus monkeys (65.6 ± 6.1 mo of age) (Macaca mulatta) were used in the microdialysis experiments. All animals were OVX at least 1 month before experimentation. All animals were born and raised at the Wisconsin National Primate Research Center and housed in pairs (cages 172 × 86 × 86 cm) in a room with a lighting schedule of 12-hour light and 12-hour dark, at a controlled temperature (22°C). Animals were fed a standard diet of Teklad Primate Chow (Harlan) twice per day and water was available ad libitum. Enrichment, including fresh fruit, peanuts, and treats, was provided daily. The protocol was approved by the Animal Care and Use Committee, University of Wisconsin-Madison in accordance with the guidelines of the National Institute of Health and United States Department of Agriculture.
Experimental design
In experiment 1, we examined the effects of EB on kisspeptin release, because it is possible that EB also rapidly stimulates kisspeptin release, as cell bodies and neuroterminals of kisspeptin neurons are present in the primate S-ME (18, 19). After 60 minutes of control sampling, EB (100nM) was infused into the S-ME for 20 minutes, whereas dialysates were collected at 10-minute intervals for an additional 120 minutes. EB was prepared as previously described (14). Briefly, EB in oil (Schering) was sterilized in a boiling water bath for 30 minutes, dissolved in methanol, and then serially diluted to the final concentration with sterile artificial cerebrospinal fluid (aCSF). Vehicle control (oil) was likewise prepared with sterilization and dilution in aCSF solution.
In experiment 2, to examine the effects of a longer exposure to EB, we infused EB for a period of 4 or 7 hours. After 2 hours of control sampling, EB (100nM) was infused into the S-ME of adult OVX female monkeys, whereas dialysates were collected at 20-minute intervals for an additional 8 hours after the initiation of treatment. Vehicle control (7 hours) was similarly infused through the microdialysis probe, whereas dialysates were collected for 8 hours. This EB dose was chosen, as infusion for 20 minutes consistently stimulated GnRH release (14). In a few cases the effects of 7-hour EB infusion at a dose of 1nM and 1μM were also examined. Dialysates (40 μL) from experiment 2 were divided into 2 (20 μL) samples for measurement of GnRH and kisspeptin peptide levels. Note that previous studies indicate that the concentration of chemicals infused through the semipermeable membrane of the microdialysis probe is approximately 10% (14, 20). As such, actual concentrations that the tissues were exposed to are approximately one-tenth of the infused EB, ie, 0.1nM, 10nM, and 100nM rather than 1nM, 100nM, and 1μM, respectively.
In experiment 3, to compare the effect of EB infusion with the effects of systemic injection of EB on release of GnRH and kisspeptin, after 2 hours of control sampling EB at 50 μg/kg was injected sc, whereas dialysates were collected at 20-minute intervals for 8 hours. This treatment was similar to that used previously (2). Dialysates (40 μL) from experiment 3 were divided into 2 (20 μL) samples for measurement of GnRH and kisspeptin peptide levels.
In experiment 4, to determine whether the changes in circulating LH levels reflect changes in GnRH and kisspeptin release induced by EB treatments (direct EB/vehicle infusion for 7 hours or sc EB injection), LH levels in serum before, 6 hours, and approximately 8 hours after the initiation of EB treatments were measured. That is, “blood sampling at 6 hours after the initiation of EB” means 1 hour before completion of a 7-hour EB/vehicle infusion, and “blood sampling at 8 hours after the initiation of EB” means 1 hour after the completion of a 7-hour EB/vehicle infusion. Control bloods before EB treatments were collected immediately before guide cannula placement surgery, which was approximately 4–5 hours before the initiation of EB/vehicle infusion or EB injection; whereas, the approximately 8 hours after blood samples were collected at the end of microdialysis experiments, which was approximately 8 hours after the initiation of EB treatments. All before and approximately 8 hours after blood samples were obtained by saphenous vein puncture in a tabletop restraint device, whereas 6 hours after blood samples were obtained through a catheter while the animals were in chairs. In this experiment, we did not conduct oil control experiments, because it has been repeatedly shown that oil injection results in no effects (2, 21–23).
Cranial pedestal implantation and guide cannula insertion
All animals were well adapted to primate chairs, experimental conditions, and researchers before experimentation. At least 1 month before experimentation, all animals were implanted with cranial pedestals under isoflurane anesthesia as previously described (14, 20, 24). On the day of the microdialysis experiment, blood samples for LH measurement were taken, and then animals were anesthetized with ketamine and dexmedetomidine, a microdrive unit was secured onto the pedestal, and a guide cannula with an inner stylet was inserted into the brain 5 mm above the S-ME region. Ventriculographs were used for positioning the site of dialysate sample collection within the S-ME as previously described (20, 24).
Microdialysis
The microdialysis procedures were similar to those previously described (14, 20). Briefly, immediately after guide cannula placement, the monkey was placed in a primate chair and a microdialysis probe with a polyarylethersulfone membrane (20-kDA molecular mass cutoff, 5.0 mm in length, 0.5 mm in diameter) was inserted into the guide cannula so that the tip of the probe was located in the S-ME. aCSF (Harvard Apparatus) containing bacitracin (4 U/mL) was infused through the influx tubing at 2 μL/min using a 2.5-mL Hamilton syringe (Hamilton) and a CMA/102 microdialysis pump. Dialysates were collected at 10- or 20-minute intervals with a fraction collector (model FC203B; Gilson) for less than 12 hours. RIA buffer (0.3% BSA, 0.01M PO4, 0.15M NaCl, and 0.1% NaN3; pH 7.8) was added to dialysates for GnRH and kisspeptin assays. All samples were then immediately frozen on dry ice and stored at −80°C until assayed.
Radioimmunoassays
RIA for GnRH measurement was conducted using the R42 antiserum as previously described (24). Assay sensitivity was 0.02 pg/tube. Intra- and interassay coefficients of variation were 8.1% and 11.3%, respectively. RIA for kisspeptin measurement was conducted using the GQ2 antiserum as previously described (25, 26). Assay sensitivity was 0.05 pg/tube. Intra- and interassay coefficients of variation were 10.1% and 14.3%, respectively. LH RIA was conducted using the recombinant cynomolgus LH kit from the Hormone and Peptide Program. The reference standard used was AFP-6936A, provided by National Hormone and Peptide Program as previously described (2). Assay sensitivity was 0.01 ng/tube. The intra- and interassay coefficients of variation were 3.2% and 5.0% respectively.
Statistical analyses
In order to conserve limited resources, all 16 OVX monkeys were randomly assigned to experiments 1–4. For each animal, at least a 3-week period was given between each microdialysis experiment. In experiment 1, mean kisspeptin release was calculated in 20-minute intervals for both EB and vehicle control infusions (8 experiments in 4 monkeys, 2 per animal). The control period used for examining EB effects on mean GnRH and kisspeptin levels in experiment 1 was the 1-hour period before EB infusion. In experiments 2 and 3, mean GnRH and kisspeptin release were calculated in 1-hour intervals. In experiment 2, the 4-hour EB infusion data were calculated from 7 experiments in 7 monkeys, the 7-hour EB infusion data were from 8 experiments in 4 monkeys (2 per animal), and vehicle infusion data were from 8 experiments in 8 monkeys. In experiment 3, the effects of sc EB injection were determined by calculation of 6 experiments in 6 monkeys. The control period for experiments 2 and 3 was a 2-hour period before each treatment. For LH measurements in experiment 4, data were calculated from 3 experiments in 3 monkeys for each treatment group.
Pulses of GnRH and kisspeptin were identified by the PULSAR program (27) using the same parameters as previously described (14, 25). To examine the effects of EB treatments on GnRH and kisspeptin pulsatility, the next parameters of the 2-hour period before and an 8-hour period after the initiation of EB/vehicle infusions were compared: 1) mean release (mean levels) was derived from the mean of all GnRH and kisspeptin values; 2) baseline was calculated from the mean of the troughs; 3) pulse amplitude was calculated from the difference between peak and trough; 4) pulse duration was the length of time of each pulse; 5) interpulse interval was the time between 2 peaks; and 6) latency to the response was the time to the peak of the first pulse after initiation of each treatment. Two-way ANOVA with repeated measures followed by Bonferroni's post hoc test was applied for statistical comparisons. The concordance rate of GnRH and kisspeptin pulses was assessed as described previously (25, 28). There was a concordance if the kisspeptin peak occurred at the same time or 1 time point preceding a GnRH peak. As a control, a set of kisspeptin data from an experiment was cross-compared with a set of mismatched GnRH data from another monkey. Subsequently, the frequency of concordance from the monkey in which kisspeptin and GnRH were measured in the same experiment was compared with the frequency of random coincidence from a mismatched dataset. Statistical significance was evaluated with one-way ANOVA with repeated measures followed by Bonferroni's post hoc test. For all analysis, differences were considered significant at P ≤ .05.
Results
Experiment 1. A brief infusion of EB in OVX female monkeys stimulates kisspeptin release
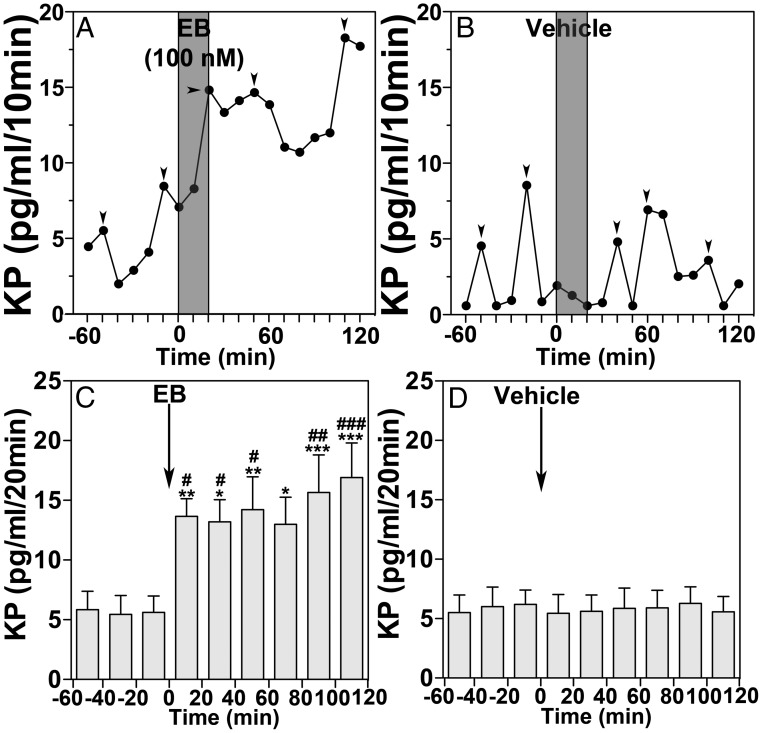
Previously, we have shown that 20-minute EB infusion into the S-ME of OVX female monkeys stimulated GnRH release (14). It is possible that a similar 20-minute EB infusion also stimulates kisspeptin release. Indeed, the results indicated that 20-minute EB infusion increased kisspeptin release with a latency of 20 minutes, and this increase lasted for the entire 120-minute sampling period (Figure 1A). Vehicle control had no effect (Figure 1B). The group data (Figure 1, C and D) indicated that the EB-induced increase in kisspeptin release was significantly larger than that during the 60-minute control period before (P = .0048, n = 8) as well as that of the vehicle control (P < .0001, n = 8).
Figure 1.
EB infused into the S-ME of OVX female monkeys rapidly stimulates kisspeptin (KP) release. A 20-minute infusion of EB into the S-ME stimulated KP release within 20 minutes (A) and remained elevated throughout the treatment period, whereas vehicle control had no effect on KP release (B). Mean data show that EB stimulated mean KP release within 20 minutes and remained elevated for the entire 120-minute recording period (C). Vehicle control had no effect on mean KP release (D). Bonferroni's post hoc analysis indicates that KP release is significantly higher than the 60-minute control period before EB infusion (*, P < .05; **, P < .01; ***, P < .001 vs before). EB-induced KP release was also significantly elevated from vehicle control at the corresponding time period (#, P < .05; ##, P < .01; ###, P < .001 vs vehicle). Arrowheads indicate peaks identified by PULSAR analysis (27).
Experiment 2. Prolonged infusions of EB stimulate GnRH and kisspeptin release
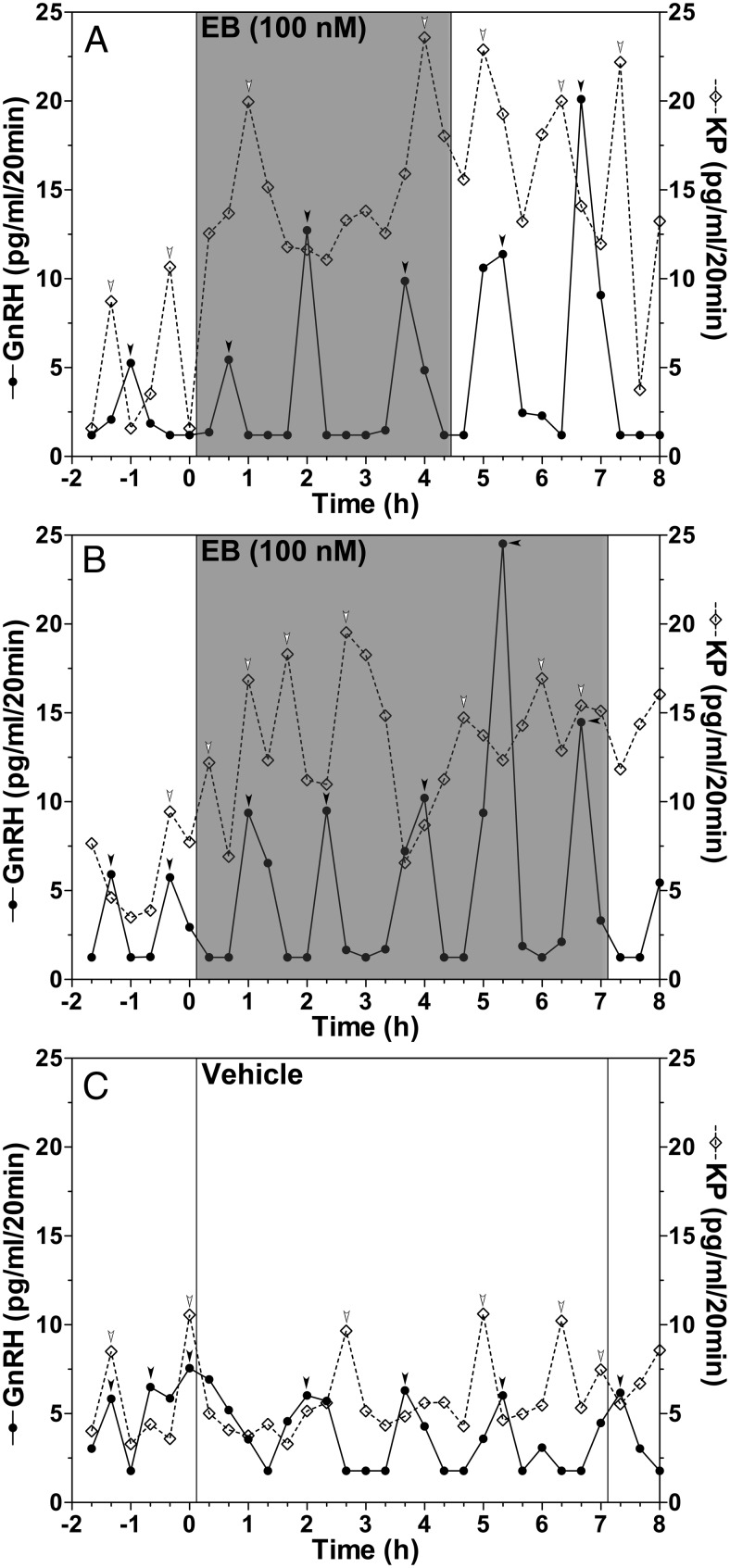
We hypothesized that the length of time that the S-ME is exposed to EB is an important factor in determining what effect EB has on GnRH release. We chose to use the infusion time periods of 4 and 7 hours, because these were significantly longer than approximately 2-hour latency of the EB-induced suppression of GnRH and LH release by systemic injection (2). The results indicate that infusion of EB for 4 hours (Figure 2A) or 7 hours (Figure 2B) into the S-ME stimulated both GnRH and kisspeptin release.
Figure 2.
EB infusions into the S-ME of OVX female monkeys for 4 and 7 hours stimulate GnRH (closed circles with solid line) and kisspeptin (KP) (open diamonds with dashed line) release. Representative cases of a 4-hour EB infusion (A), a 7-hour EB infusion (B), and a 7-hour vehicle infusion (C) experiment are shown. Arrowheads indicate peaks as determined by PULSAR analysis (27).
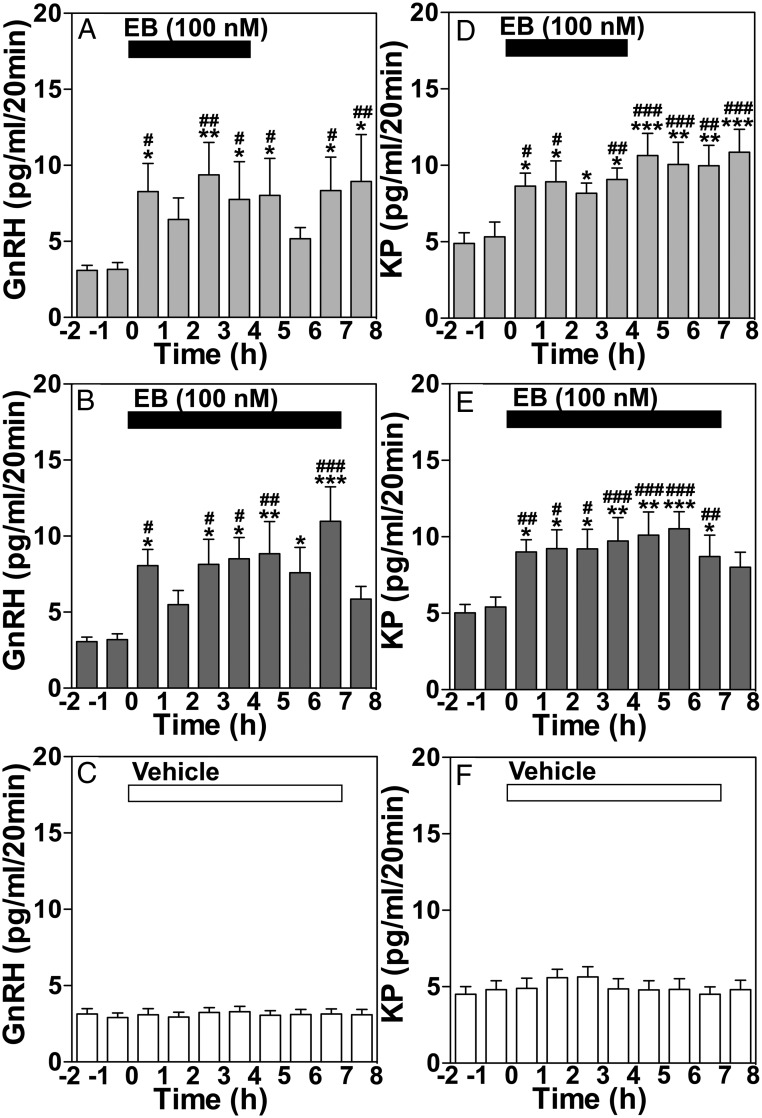
The stimulation of GnRH release by a prolonged EB infusion was characterized by increased mean release and pulse amplitude of GnRH throughout the treatment period (Figure 3, A and B, and Table 1), whereas stimulation of kisspeptin release was characterized by an elevated mean and baseline, but not amplitude, resulting in a sustained increase in kisspeptin release (Figure 3, D and E, and Table 1). Moreover, although EB infusion for 4 and 7 hours had no effect on interpulse interval and pulse duration of GnRH and kisspeptin release, EB infusion for both 4 and 7 hours resulted in a significantly shorter latency to first peak for both GnRH and kisspeptin release as compared with vehicle control (Table 1). Importantly, the effects of a 4-hour EB treatment on GnRH and kisspeptin release lasted well past the end of the EB infusion (Figure 3, A and D). Infusion of vehicle control for 7 hours did not induce any significant effect on GnRH and kisspeptin release (Figures 2C and 3, C and F).
Figure 3.
Group data (mean ± SEM) showing the effect of a 4-hour EB infusion on GnRH (A) and kisspeptin (KP) (D) release, or a 7-hour EB infusion on GnRH (B) and KP (E) release, and vehicle infusion on GnRH (C) and KP (F) release. Mean GnRH release in either the 4-hour (P = .0181, n = 7) or 7-hour (P < .0001, n = 8) treatment groups was significantly higher than the 2 hours respective control period before treatment as well as vehicle control (P < .0001 for both, n = 8). Similarly, the EB-induced increase in KP levels in both the 4- and 7-hour EB infusion treatment groups were significantly higher than the 2-hour control period before (P < .0001 for both) and vehicle control (P < .0001 for both). Bonferroni's post hoc analysis indicates that in 4- and 7-hour EB infusion experiments mean GnRH and KP release are significantly higher than the 2-hour period before EB infusion as indicated (*, P < .05; **, P < .01; ***, P < .001 vs before) and also significantly elevated compared with vehicle control at the corresponding time period as indicated (#, P < .05; ##, P < .01; ###, P < .001 vs vehicle).
Table 1.
Effects of EB Infusion (4 or 7 h) or Vehicle Infusion (7 h) Into the S-ME on the Parameters of GnRH and Kisspeptin Release in OVX Female Rhesus Monkeys
| Measured Neuropeptides Treatments | GnRH |
Kisspeptin |
||||
|---|---|---|---|---|---|---|
| EB 4 h | EB 7 h | Vehicle | EB 4 h | EB 7 h | Vehicle | |
| Mean levels (pg/mL) | ||||||
| Before | 3.1 ± 0.4* | 3.1 ± 0.3 | 3.0 ± 0.3 | 5.0 ± 0.8 | 5.2 ± 0.6 | 4.5 ± 0.5 |
| After | 7.8 ± 2.0a,d | 7.9 ± 1.5b,e | 3.1 ± 0.3 | 9.5 ± 1.2c,e | 9.3 ± 1.2c,e | 4.6 ± 0.6 |
| Baseline levels (pg/mL) | ||||||
| Before | 1.3 ± 0.1 | 1.5 ± 0.2 | 1.4 ± 0.1 | 2.6 ± 0.2f | 2.8 ± 0.2f | 2.7 ± 0.5f |
| After | 1.2 ± 0.1 | 1.4 ± 0.2 | 1.3 ± 0.1 | 5.5 ± 1.0b,d,g | 5.3 ± 0.4b,d,g | 2.5 ± 0.7 |
| Pulse amplitude (pg/mL) | ||||||
| Before | 4.0 ± 0.9 | 3.7 ± 0.7 | 3.3 ± 0.6 | 5.6 ± 1.3 | 5.3 ± 0.5 | 4.7 ± 0.5 |
| After | 10.1 ± 1.9c,e | 7.6 ± 1.5a,d | 3.2 ± 0.5 | 7.0 ± 1.4 | 6.4 ± 0.8 | 4.8 ± 0.8 |
| Pulse duration (pg/mL) | ||||||
| Before | 51.4 ± 8.3 | 52.6 ± 4.5 | 49.5 ± 5.7 | 40.0 ± 5.2 | 35.5 ± 4.5 | 36.1 ± 4.6 |
| After | 51.6 ± 8.2 | 50.0 ± 6.0 | 42.7 ± 7.3 | 46.8 ± 1.9 | 39.2 ± 5.0 | 37.5 ± 4.0 |
| Interpulse interval (min) | ||||||
| Before | 77.1 ± 6.4 | 78.1 ± 8.6 | 82.9 ± 12.2 | 66 ± 8.3 | 58.7 ± 12.2 | 69.4 ± 7.2 |
| After | 88.0 ± 6.1 | 74.1 ± 4.1 | 85.0 ± 13.3 | 72.6 ± 4.1 | 59.3 ± 3.5 | 69.6 ± 4.8 |
| Latency to first peak (min) | 23.3 ± 3.1b | 26.7 ± 3.3b | 62.9 ± 16.0 | 28.0 ± 4.9a | 32.0 ± 4.9a | 64.4 ± 9.9 |
An asterisk indicates mean ± SEM; the comparisons were made between before (2-h pretreatment period) and after (8-h respective treatment periods).
P < .05 vs respective GnRH or KP vehicle treatment.
P < .01 vs respective GnRH or KP vehicle treatment.
P < .001 vs respective GnRH or KP vehicle treatment.
P < .05 vs before.
P < .01 vs before.
P < .05 vs GnRH.
P < .001 vs GnRH.
We conducted concordance analysis between kisspeptin and GnRH pulses, although the sampling frequency was half of the previous study (25). As such, the estimate is less accurate. The results indicated that the concordance between the 2 peptides was lost during EB infusion for 7 hours. That is, under vehicle infusion, kisspeptin pulses occurred synchronously with GnRH pulses 65.0 ± 5.6% of the time, which was significantly (P < .01) higher than random concordance (42.3 ± 4.1%). Although the concordance under EB infusion for a 4-hour period (56.9 ± 4.5%) remained significant (P < .05) over random concordance, the concordance between kisspeptin and GnRH pulses under EB infusion for 7 hours (54.3 ± 3.6%) was no longer different from random concordance (P > .05).
Experiment 3. Systemic EB administration suppresses GnRH and kisspeptin release
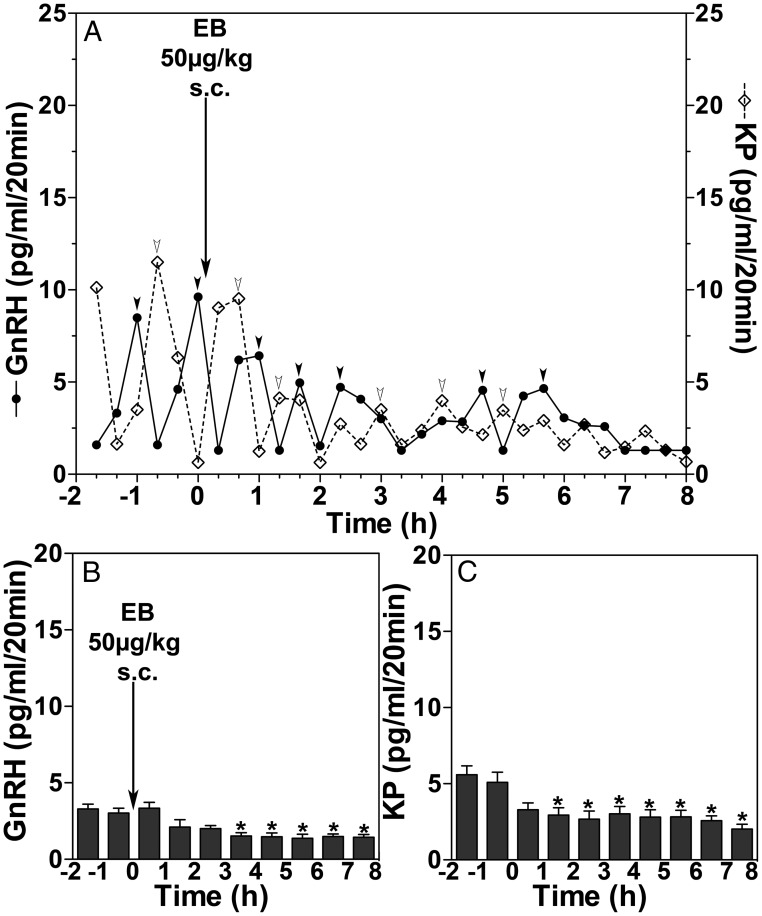
Because prolonged infusion of EB stimulated release of GnRH and kisspeptin, suppressive effects of EB by systemic administration may be attributable to the site of EB exposure in the brain, we next examined the systemic administration of EB on GnRH and kisspeptin release. As seen in Figure 4A, EB at 50 μg/kg sc suppressed both GnRH and kisspeptin release. The group data indicate that EB administered systemically (n = 6) (Figure 4B) significantly reduced GnRH release with a latency of 3 hours, when compared with the control period before (P = .0404) (Figure 4B). EB administered systemically also suppressed kisspeptin release with a latency of 1 hour (Figure 4C), when compared with the 2-hour control period before (P = .0079) (Figure 4C).
Figure 4.
EB injection (sc) suppressed GnRH and kisspeptin (KP) release. A representative case (A) of the effect of sc EB injection on GnRH release (closed circles with solid line) and KP release (open diamonds with dashed line) and group data (mean ± SEM) of GnRH (B) and KP (C) release are shown. Arrowheads indicate peaks identified by PULSAR algorithm (27). Group data indicate that sc EB injection significantly suppressed GnRH and KP release when compared with the 2-hour period before injection (*, P < .05).
Experiment 4. Effects of EB on LH release
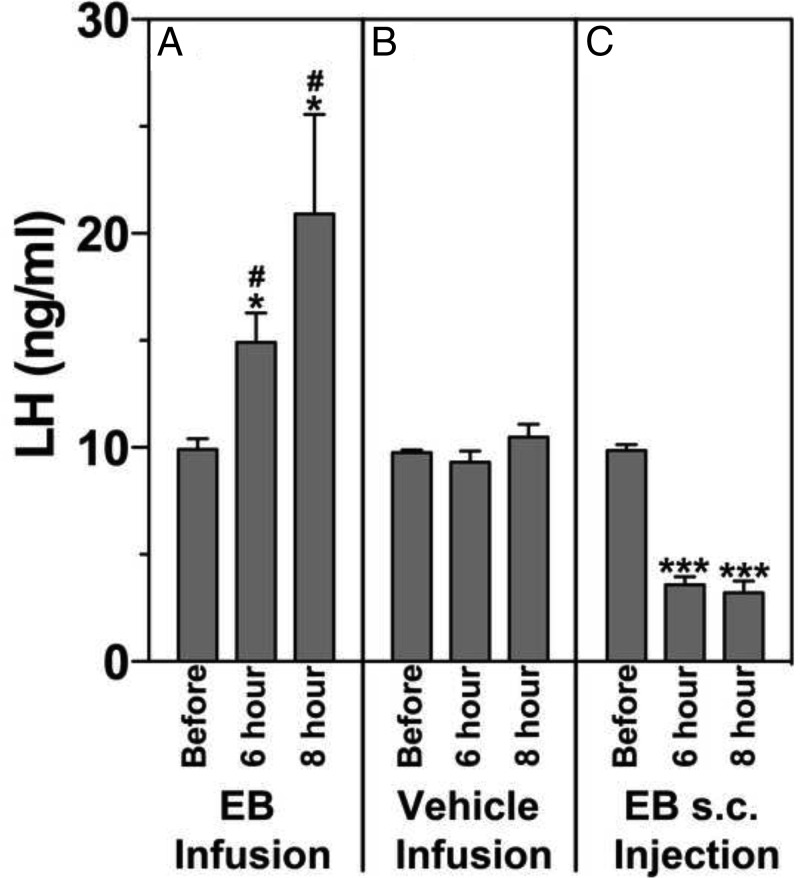
To determine whether the changes in circulating LH levels reflect changes in GnRH and kisspeptin release induced by direct EB infusion or sc EB injection, LH levels in serum were assessed. Results indicated that LH levels after EB infusion into the S-ME were significantly higher than that before the infusion at both 6 and 8 hours after initiation of a 7-hour EB infusion (P = .007, n = 3) (Figure 5A) and when compared with vehicle control experiments (P = .039, n = 3) (Figure 5B). In contrast, consistent with our previous observation (2), EB administered sc suppressed LH release at 6 and 8 hours after EB injection when compared with before treatment (P = .0001, n = 3) (Figure 5C).
Figure 5.
Changes in LH release measured from serum samples taken before, 6 hours, and approximately 8 hours after the initiation of a 7-hour EB infusion (A) or a 7-hour vehicle infusion (B) and sc EB injection (C) in OVX female monkeys. LH levels at 6 hours or approximately 8 hours after EB infusion into the S-ME were significantly elevated as compared with those before EB infusion (A) (*, P < .05) and corresponding time after vehicle infusion (B) (#, P < .05). LH levels at 6 hours and approximately 8 hours after sc EB injection were also significantly lower than those before EB injection (C) (***, P < .001).
Analysis of sites of EB infusion
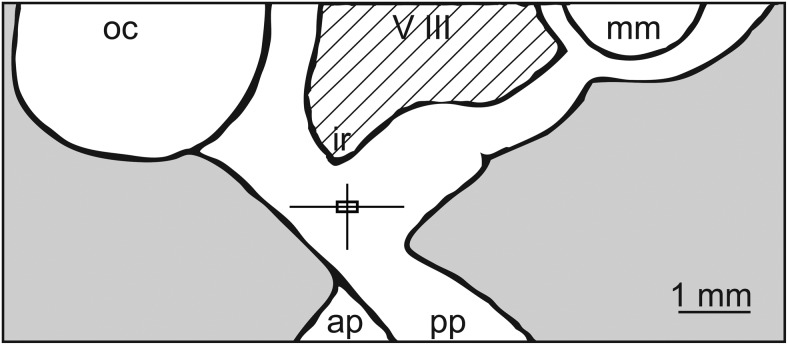
The location of the microdialysis probe tips in all experiments were examined using x-ray ventriculography. Results indicated that the probe tips were located in the S-ME (Figure 6). Therefore, microdialysis infusion is restricted to the S-ME.
Figure 6.
Midsagittal diagram of the hypothalamus showing locations of the microdialysis probe tip plotted from a total of 45 experiments. The vertical and horizontal lines of the box plot indicate the dorsal-ventral and anterior-posterior ranges of sampling locations. The intersections of these lines indicate the mean cannula location, and the box delineates the SEM. The lateral position measured from midline was 0.3 ± 0.1 mm. ap, anterior pituitary; ir, infundibular recess; mm, mammillary body; oc, optic chiasm; pp, posterior pituitary; V III, third ventricle.
Discussion
In the present study, we first found that a brief direct infusion of EB into the S-ME stimulates kisspeptin release. This observation suggests that rapid action of E2 is not limited to GnRH neurons/neuroterminals but also occurs at kisspeptin neurons/neuroterminals. Second, we observed that prolonged infusion of EB to the S-ME did not suppress release of GnRH and kisspeptin. It rather stimulated release of both peptides. Finally, we confirmed that systemic injection of EB suppressed GnRH release and further found that it also suppressed kisspeptin release.
It is well documented that systemic administration of EB suppresses release of GnRH and LH, known as the negative feedback effect of E2. In fact, in past (2, 26) and present studies, we have shown that sc injection of EB suppresses release of GnRH, kisspeptin, and LH with a latency of approximately 2–3 hours. However, the results of previous (14) and present studies indicate that a brief EB infusion to the S-ME stimulates release of GnRH and kisspeptin within 20 minutes. We hypothesized that the differences in inhibitory vs stimulatory action by the 2 modes of EB administration were due to 1) the exposure period of the S-ME to EB, because systemic EB administration elevates circulating E2 levels for several hours (2); 2) the EB concentration at the S-ME; or 3) the sites of EB exposure within the hypothalamus. First, the results of this study suggest that the exposure period of the S-ME to EB does not account for the difference in the 2 modes of EB administration. Similar to the results of a brief EB infusion, direct EB infusion for a prolonged period (4 or 7 h) stimulates both GnRH and kisspeptin release and does not mimic systemically administered EB.
Second, the concentration of EB at the S-ME may differentiate stimulatory from inhibitory E2 action. This was the case in an in vitro experiment with GT-1 cells, in which picomolar levels of E2 suppressed, whereas nanomolar levels of E2 stimulated, cAMP production (29). In contrast, in vivo studies suggest that dose may not alone be an important determining factor. Ferin et al (3) show that similar to 800 ng E2 iv injection (0.6nM), 800 pg E2 injection in 3 μL (∼600nM) into several sites of the MBH (not in the S-ME) suppressed LH release in OVX female monkeys. In the present study, 100nM EB consistently stimulated, whereas sc EB injection suppressed, GnRH and kisspeptin release. A calculated E2 concentration by sc EB injection (50 μg/kg) in the S-ME is approximately 1nM–1.5nM, as circulating E2 levels reach 300- to 400-pg/mL concentrations (2). Furthermore, our results from a few cases, examining the effect of lower or higher doses of EB, indicated that 1nM and 1μM EB infusions for 7 hours induced a stimulatory effect (data not shown). Therefore, stimulatory effects of EB infusion into the S-ME are not due to the difference in concentrations at the S-ME.
Third, the data presented in this study indicate that the site of EB exposure is critical for the regulation of GnRH and kisspeptin release in OVX female monkeys. The tips of the microdialysis probes in this study are all within the S-ME, as determined by x-ray ventriculography (Figure 6), and consequently, microdialysis infusion is restricted to the S-ME (an infused chemical diffuses ∼1 mm3) (21). In contrast, systemically administered EB reaches a large portion of the brain, as well as the pituitary gland, as shown by a study with radioautography (15). Classical studies with lesions of the ARC and E2 implantation in the MBH (5, 30), as well as more recent findings of estrogen receptor (ER)α-mediated inhibitory E2 action in ARC neurons (7, 31), indicate that exposure of neurons in the ARC to EB is essential for the negative feedback action of E2. Although there is a report showing that neither sc EB injection nor EB infusion into the S-ME induces any significant effects on GnRH release (32), there are considerable differences in methodology and analysis. Most notably, infusion of substantial amounts of cholesterol before EB infusion would likely change local steroid synthesis and consequently could alter EB action in the S-ME. Cholesterol is a precursor molecule for the steroidogenic pathway. Nevertheless, the results of the present study demonstrate that direct stimulatory action of E2 in the S-ME is completely different from the classical negative feedback action of E2.
A growing body of literature suggests that there is region-specific differential regulation of the GnRH neuron. Using fast scan cyclic voltammetry, Moenter and colleagues (33, 34) demonstrate that mouse GnRH neurons in the POA and the S-ME are differentially regulated by kisspeptin, neurokinin B, and gonadotropin inhibitory hormone inputs. Additionally, in the brain, GnRH neurons in mice exhibit a broad range of firing patterns (35), and activity is rapidly modulated by E2 in context-specific manners (12, 36). These observations suggest that there is region-specific differential regulation of GnRH neuronal activity. The findings of the present work demonstrate that, in the monkey as well, E2-induced changes in GnRH release are location dependent.
Direct infusion of EB in the S-ME stimulates release of GnRH and kisspeptin with a latency of less than 20 minutes, whereas systemic administration of EB suppressed release of both neuropeptides with a latency approximately 2 hours. The presence of dense fibers and some cell bodies of GnRH and kisspeptin neurons in the S-ME of primates (18, 19), and reports showing that activation of kisspeptin neuronal input readily stimulates GnRH release from GnRH neuroterminals in the mouse S-ME (33, 37), suggests that GnRH and kisspeptin neuroterminals in the S-ME can be stimulated by direct infusion of EB. Because systemically administered E2 would reach the S-ME and the ARC responsible for negative feedback within a similar time frame (38), despite the S-ME being free from the blood-brain barrier, we speculate that the mechanism of EB action infused in the S-ME is fundamentally different from EB action induced by systemic injection.
One can argue that the difference in EB action by the 2 modes of administration is due to the difference in EB metabolism in the S-ME and peripheral circulation. There is very little information as to the differential metabolism of EB vs E2 in peripheral tissues/circulation. Eaton et al (39) reported that using thin-layer chromatography 3H-EB in serum was metabolized to 3H-E2 in 1 hour in vitro. In a previous study (14), however, we were unable to confirm this conversion. In fact, conversion of EB to E2 in the S-ME measured by liquid chromatography and tandem mass spectrometry is negligible for several hours (14). Although the differential metabolism between EB and E2 in the brain and peripheral tissues and the detailed cellular mechanism of EB action remains to be determined, our observation that E2 and EB at the same dose result in indistinguishable effects on GnRH release (Kenealy, B., and E. Terasawa, unpublished data) suggests that EB can directly modify the activity of neurons in the S-ME, because it binds ERα (40).
We propose that diverse ER signaling is responsible for the difference in the latency of EB action. It has been well documented that the negative and positive feedback actions of E2 requires inputs from interneurons expressing ERα to GnRH neurons (31, see Ref. 41). In contrast, receptor mechanisms responsible for rapid E2 action within the S-ME in vivo are unknown. Evidence from in vitro studies indicate that E2 stimulates GnRH neurons through membrane receptor-initiated estrogen action and that rapid action of E2 does not need to enter the cell and is initiated by nonclassical membrane ERs (see Ref. 42). In fact, we and others have shown that the involvement of non-ERα membrane receptors, such as GPR30, STX-sensitive receptors, and ERβ, is important for rapid signaling in GnRH neurons (12, 13, 43–45). Moreover, experiments using rat S-ME fragments show that activation of membrane bound ERs by E2 or E2 conjugated to BSA induces nitric oxide production from endothelial cells of the hypothalamus, leading to GnRH release (46). Although the mechanisms mediating rapid EB action in vivo in the S-ME are likely more complex than in vitro models, because the MBH, including S-ME, is composed of many types of neurons and glia, we speculate that membrane receptor-initiated estrogen action plays a significant role in the rapid E2 action.
Previously, we observed that kisspeptin pulses occurred with GnRH pulses at the concordance rate of 75%. Because EB-induced release pattern of kisspeptin differs from EB-induced release pattern of GnRH, we examined the concordance between kisspeptin and GnRH pulses. Under vehicle infusion (spontaneous condition), the concordance rate was 65%. This rate is not as high as the rate obtained in the previous work, because the sample frequency of this study was half of the previous work (25). Nevertheless, EB infusion of the S-ME resulted in a tendency to lose concordance between the 2 peptide pulses. In fact, under 7-hour EB infusion, the concordance rate was reduced to 54%, which was no longer different from random concordance. It is not surprising to see the loss of concordance during EB infusion, as discussed above, diverse E2 action likely occurs independently in the 2 types of neurons. A similar nonlinear relationship between GnRH and kisspeptin release is seen in OVX pubertal monkeys (47).
The findings of this and a previous study (14), showing that direct EB infusion into the S-ME of OVX monkeys rapidly stimulates GnRH and kisspeptin release, are quite unique. Past research in primates have determined the time course of the negative and positive feedback effects of E2 and the site of negative feedback within the hypothalamus (1, 3). Additionally, several studies from Knobil and coworkers indicate that positive feedback of E2 in monkeys primarily occurs at the pituitary level (48, 49). In all of these studies, however, the role of the S-ME region, part of which is outside the blood-brain barrier, was overlooked. Nevertheless, the results of the present study indicate that GnRH release stimulated by direct EB infusion to the S-ME is accompanied by an increase in LH, whereas systemic injection of EB suppressed release of both GnRH and LH. Therefore, it appears that the final output of GnRH release induced by E2 is a balance between inhibitory/excitatory inputs from interneurons in the hypothalamus, especially within the ARC, and stimulatory input at the S-ME. Additional studies are necessary to prove these views.
In the present study, we have shown that direct infusion of EB, a synthetic E2, in the region where GnRH and kisspeptin neuroterminals reside, stimulates the neurosecretion of the peptide hormones, regardless of the dose and the exposure period of E2. Presently, the question remains whether priming of the hypothalamus, including the S-ME with a low dose of E2, will alter the effects of EB infusion into the S-ME of OVX monkeys. Additionally, we do not know which ER subtypes are involved for each mode of EB administration. Nevertheless, the findings of this study provide new insights on the underlying mechanism of E2 action in the primate brain.
Acknowledgments
We thank Dr Joseph Kurian and Dr Hemanta Shrestha for their technical help. We also thank the veterinary and animal care staff at the Wisconsin National Primate Research Center for their dedicated work on animal wellness.
This work was supported National Institutes of Health Grants R01HD015433 and R21HD077447 (for E.T.) and by OD011106/RR00061 (to the Wisconsin National Primate Research Center).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- arcuate nucleus
- E2
- estradiol
- EB
- E2-benzoate
- ER
- estrogen receptor
- MBH
- medial basal hypothalamus
- OVX
- ovariectomized
- S-ME
- stalk-median eminence.
References
- 1. Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology. 1985;117:711–721. [DOI] [PubMed] [Google Scholar]
- 2. Mizuno M, Terasawa E. Search for neural substrates mediating inhibitory effects of oestrogen on pulsatile luteinizing hormone-releasing hormone release in vivo in ovariectomized female rhesus monkeys (Macaca mulatta). J Neuroendocrinol. 2005;17:238–245. [DOI] [PubMed] [Google Scholar]
- 3. Ferin M, Carmel PW, Zimmerman EA, Warren M, Perez R, Vande Wiele RL. Location of intrahypothalamic estrogen-responsive sites influencing LH secretion in the female rhesus monkey. Endocrinology. 1974;95:1059–1068. [DOI] [PubMed] [Google Scholar]
- 4. Krey LC, Butler WR, Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the monkey. I. Gonadotropin secretion. Endocrinology. 1975;96:1073–1087. [DOI] [PubMed] [Google Scholar]
- 5. Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta). Endocrinology. 1978;102:52–62. [DOI] [PubMed] [Google Scholar]
- 6. Cogen Ph, Antunes JL, Louis KM, Dyrenfurth I, Ferin M. The effects of anterior hypothalamic disconnection on gonadotropin secretion in the female rhesus monkey. Endocrinology. 1980;107:677–683. [DOI] [PubMed] [Google Scholar]
- 7. Yeo SH, Herbison AE. Estrogen-negative feedback and estrous cyclicity are critically dependent upon estrogen receptor-α in the arcuate nucleus of adult female mice. Endocrinology. 2014;155:2986–2995. [DOI] [PubMed] [Google Scholar]
- 8. Abrahám IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor β mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Temple JL, Laing E, Sunder A, Wray S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci. 2004;24:6326–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology. 2005;146:4312–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology. 2008;149:1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci. 2009;29:5616–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol. 2008;23:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kenealy BP, Kapoor A, Guerriero KA, et al. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci. 2013;33:19051–19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pfaff DW, Gerlach JL, McEwen BS, Ferin M, Carmel P, Zimmerman EA. Autoradiographic localization of hormone-concentrating cells in the brain of the female rhesus monkey. J Comp Neurol. 1976;170:279–293. [DOI] [PubMed] [Google Scholar]
- 16. Alçin E, Sahu A, Ramaswamy S, et al. Ovarian regulation of kisspeptin neurons in the arcuate nucleus of the rhesus monkey (Macaca mulatta). J Neuroendocrinol. 2013;25:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeo SH, Clarkson J, Herbison AE. Kisspeptin-GPR54 signaling at the GnRH neuron is necessary for negative feedback regulation of luteinizing hormone secretion in female mice. Neuroendocrinology. 2014;100:191–197. [DOI] [PubMed] [Google Scholar]
- 18. Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hrabovszky E, Ciofi P, Vida B, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31:1984–1998. [DOI] [PubMed] [Google Scholar]
- 20. Frost SI, Keen KL, Levine JE, Terasawa E. Microdialysis methods for in vivo neuropeptide measurement in the stalk-median eminence in the rhesus monkey. J Neurosci Methods. 2008;168:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Terasawa E, Krook C, Eman S, et al. Pulsatile luteinizing hormone (LH) release during the progesterone-induced LH surge in the female rhesus monkey. Endocrinology. 1987;120:2265–2271. [DOI] [PubMed] [Google Scholar]
- 22. Chongthammakun S, Terasawa E. Negative feedback effects of estrogen on luteinizing hormone-releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology. 1993;132:735–743. [DOI] [PubMed] [Google Scholar]
- 23. Mizuno M, Gearing M, Terasawa E. The role of neuropeptide Y in the progesterone-induced luteinizing hormone-releasing hormone surge in vivo in ovariectomized female rhesus monkeys. Endocrinology. 2000;141:1772–1779. [DOI] [PubMed] [Google Scholar]
- 24. Gearing M, Terasawa E. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull. 1988;21:117–121. [DOI] [PubMed] [Google Scholar]
- 25. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149:4151–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guerriero KA, Keen KL, Terasawa E. Developmental increase in kisspeptin-54 release in vivo is independent of the pubertal increase in estradiol in female rhesus monkeys (Macaca mulatta). Endocrinology. 2012;153:1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982;243:E310–E318. [DOI] [PubMed] [Google Scholar]
- 28. Woller MJ, McDonald JK, Reboussin DM, Terasawa E. Neuropeptide Y is a neuromodulator of pulsatile luteinizing hormone-releasing hormone release in the gonadectomized rhesus monkey. Endocrinology. 1992;130:2333–2342. [DOI] [PubMed] [Google Scholar]
- 29. Navarro CE, Abdul Saeed S, Murdock C, et al. Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotropin-releasing hormone neurons. Mol Endocrinol. 2003;17:1792–1804. [DOI] [PubMed] [Google Scholar]
- 30. Ramirez VD, Abrams RM, McCann SM. Effects of estradiol implants in the hypothalamo-hypophysial region of the rat on the secretion of luteinizing hormone. Endocrinology. 1964;75:243–248. [DOI] [PubMed] [Google Scholar]
- 31. Cheong RY, Porteous R, Chambon P, Abrahám I, Herbison AE. Effects of neuron-specific estrogen receptor (ER) α and ERβ deletion on the acute estrogen negative feedback mechanism in adult female mice. Endocrinology. 2014;155:1418–1427. [DOI] [PubMed] [Google Scholar]
- 32. Pau KY, Gliessman PM, Hess DL, Ronnekleiv OK, Levine JE, Spies HG. Acute administration of estrogen suppresses LH secretion without altering GnRH release in ovariectomized rhesus macaques. Brain Res. 1990;517:229–235. [DOI] [PubMed] [Google Scholar]
- 33. Gaskins GT, Glanowska KM, Moenter SM. Activation of neurokinin 3 receptors stimulates GnRH release in a location-dependent but kisspeptin-independent manner in adult mice. Endocrinology. 2013;154:3984–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Glanowska KM, Moenter SM. Differential regulation of GnRH secretion in the preoptic area (POA) and the median eminence (ME) in male mice. Endocrinology. 2015;156:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Constantin S, Iremonger KJ, Herbison AE. In vivo recordings of GnRH neuron firing reveal heterogeneity and dependence upon GABAA receptor signaling. J Neurosci. 2013;33:9394–9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Romanó N, Herbison AE. Activity-dependent modulation of gonadotrophin-releasing hormone neurone activity by acute oestradiol. J Neuroendocrinol. 2012;224:1296–1303. [DOI] [PubMed] [Google Scholar]
- 37. d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khayum MA, de Vries EF, Glaudemans AW, Dierckx RA, Doorduin J. In vivo imaging of brain estrogen receptors in rats: a 16α-18F-fluoro-17β-estradiol PET study. J Nucl Med. 2014;55:481–487. [DOI] [PubMed] [Google Scholar]
- 39. Eaton GG, Goy RW, Resko JA. Brain uptake and metabolism of estradiol benzoate and estrous behavior in ovariectomized guinea pigs. Horm Behav. 1975;6:81–97. [DOI] [PubMed] [Google Scholar]
- 40. Matthews J, Celius T, Halgren R, Zacharewski T. Differential estrogen receptor binding of estrogenic substances: a species comparison. J Steroid Biochem Mol Biol. 2000;74:223–234. [DOI] [PubMed] [Google Scholar]
- 41. Herbison AE. Physiology of the adult gonadotropin-releasing hormone neuronal network. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill's Physiology of Reproduction. Boston, MA: Elsevier; 2014:399–443. [Google Scholar]
- 42. Kelly MJ, Rønnekleiv OK. Membrane-initiated actions of estradiol that regulate reproduction, energy balance and body temperature. Front Neuroendocrinol. 2012;33:376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang C, Kelly MJ, Rønnekleiv OK. 17 β-estradiol rapidly increases ATP-sensitive potassium channel activity in gonadotropin-releasing hormone neurons via a protein kinase signaling pathway. Endocrinology. 2010;151:4477–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kenealy BP, Keen KL, Rønnekleiv OK, Terasawa E. STX, a novel nonsteroidal estrogenic compound, induces rapid action in primate GnRH neuronal calcium dynamics and peptide release. Endocrinology. 2011;152:3182–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Terasawa E, Kenealy BP. Neuroestrogen, rapid action of estradiol, and GnRH neurons. Front Neuroendocrinol. 2012;33:364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prevot V, Croix D, Rialas CM, et al. Estradiol coupling to endothelial nitric oxide stimulates gonadotropin-releasing hormone release from rat median eminence via a membrane receptor. Endocrinology. 1999;140:652–659. [DOI] [PubMed] [Google Scholar]
- 47. Guerriero KA, Keen KL, Millar RP, Terasawa E. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female rhesus monkeys (Macaca mulatta): implication for the mechanism of puberty. Endocrinology. 2012;153:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakai Y, Plant TM, Hess DL, Keogh EJ, Knobil E. On the sites of the negative and positive feedback action of estradiol in the control of gonadotropin secretion in the rhesus monkey. Endocrinology. 1978;102:1008–1014. [DOI] [PubMed] [Google Scholar]
- 49. Ordög T, Goldsmith JR, Chen MD, Connaughton MA, Hotchkiss J, Knobil E. On the mechanism of the positive feedback action of estradiol on luteinizing hormone secretion in the rhesus monkey. J Clin Endocrinol Metab. 1998;83:4047–4053. [DOI] [PubMed] [Google Scholar]