Abstract
Background
Chronic kidney disease is a risk factor for death in the year following myocardial infarction or coronary angioplasty. Whether the risk is similar after coronary stenting, whether impaired renal function is associated with an increased risk of cardiovascular death or myocardial infarction (MI) after coronary stenting, and whether this risk is maintained beyond the first year is uncertain.
Methods
We analyzed the long-term risks of MI or the combination of cardiovascular death and non-fatal MI in subjects participating in 4 coronary stenting trials with mandated, prospective long-term follow-up. Cox proportional hazards models were used to adjust for confounding and to generate multivariable odds ratios.
Results
1228 subjects were followed for a median of 5 years. There were 125 myocardial infarctions and 55 cardiovascular deaths. Subjects with a serum creatinine ≥1.3 mg/dL had markedly elevated risks of cardiovascular death and MI that emerged during the first year and were maintained throughout follow-up. The adjusted hazard ratio of myocardial infarction was 2.14 (p=.006) while the adjusted hazard ratio of a combined endpoint of myocardial infarction or cardiovascular death was 2.13 (p=.001). The risks were similar in subjects with moderate (serum creatinine 1.3-1.9mg/dL) or advanced chronic kidney disease.
Conclusions
The presence of even mild chronic kidney disease is associated with a high risk of cardiovascular death and myocardial infarction following coronary stenting. Further research to address the causes of the association and to define the best therapy for these patients is necessary.
Keywords: Chronic kidney disease, Cardiovascular disease, Myocardial infarction, Stent, Percutaneous coronary intervention
Introduction
More than 11%, of the U.S. population has chronic kidney disease, and more than 8 million people have moderate or greater impairment of renal function with estimated glomerular filtration rates below 60 mL/min (11). Moderate CKD has increasingly been recognized as an independent risk factor for the development of cardiovascular disease (18, 32) and is also associated with a high risk of death following myocardial infarction (1, 2, 29). The explanation for this high risk of death is uncertain, but a high frequency of standard coronary risk factors in patients with CKD, high levels of oxidative stress and inflammation and the underuse of standard cardiovascular therapies in patients with CKD have all been implicated(7, 33, 35).
While usage of cardiovascular medications and coronary revascularization are lower in patients with CKD than in patients without CKD, the extent to which this underuse is responsible for the high cardiovascular mortality remains uncertain. Among patients undergoing coronary angioplasty, for example, the presence of CKD remains associated with a high risk of all-cause death following percutaneous coronary intervention (8)—a finding that suggests that underuse of revascularization is unlikely to be solely responsible for the high mortality rates observed in patients with CKD. However, this finding must be interpreted cautiously for several reasons: First coronary angioplasty without stenting is associated with a high frequency of restenosis and is demonstrably less effective in subjects with renal failure than in those with normal renal function(3). Second, previous studies among subjects undergoing coronary revascularization have examined the association of CKD with all-cause mortality but have not specifically focused on cardiovascular mortality or MI as outcomes. Finally, prior studies generally have had limited follow-up beyond the first year following coronary intervention, thereby limiting inference on the long-terms risks associated with the presence of CKD (19). To address these limitations of the available data we pooled data from three randomized clinical trials of coronary stents and one non-randomized registry with FDA-mandated long-term follow-up. The relative hazards of non-fatal MI or a combination of non-fatal MI and cardiovascular death during follow-up were analyzed as a function of serum creatinine with adjustment for other cardiovascular risk factors.
Subjects and Methods
Subjects
The cohort consisted of participants with long-term follow-up from three randomized stent vs. stent trials (ASCENT-ACS Multilink™ stent, NIRVANA-NIR™ stent and SMART-AVE Micro II™ stent) and one non-randomized registry (AVE-GFX™ stent)(4, 5, 14, 22) that used similar inclusion and exclusion criteria. Each trial required objective evidence of coronary ischemia in the absence of recent myocardial infarction. Five-year follow-up of all Multilink and NIR patients and a randomly selected subset of 75% of Micro II and GFX patients was mandated by the FDA after conditional device approval. Consenting patients were followed annually for the occurrence of death, MI repeat revascularization and cardiac hospitalization. Baseline and follow-up data from the 1228 subjects with long-term follow-up in these four trials were pooled for this investigation.
Definitions
Deaths and cardiovascular events were adjudicated by an independent, blinded clinical events committee. Death was classified as cardiac unless non-cardiac causes were identified. Peri-procedural MI was defined as CK-MB > 3 times normal, or development of new pathologic Q waves. After this period, MI was defined as new ischemic symptoms or characteristic EKG changes with total CK >2 times normal or the development of new Q waves.
Mild to moderate renal insufficiency was defined as a baseline serum creatinine ≥1.3 mg/dL because this level reliably detects inulin clearances below 80 ml/min(12). Glomerular filtration rate was estimated according to Cockroft-Gault(10) and modified MDRD(24)equations. Formula-derived estimates of renal function were not used in the primary analysis because these formulas have not been validated in elderly and because they may not be reliable in subjects with normal renal function (25). Additionally, the choice of estimating equation can markedly alter the association of renal function with other cardiac risk factors (31) and a dichotomous categorization of renal function using estimated GFR might lead to widely discrepant results according to the arbitrary choice of estimating equation. While true GFR at any given serum creatinine can be expected to vary according to race, age, and muscle mass, a dichotomous categorization using a threshold of serum creatinine ≥1.3 mg/dL vs.<1.3 mg/dL has several advantages: First, a creatinine ≥ 1.3 mg/dL is considered abnormal in most clinical labs; Second, serum creatinine remains a more widely used criterion than estimated GFR for the identification of renal insufficiency in general practice, and this distinction is therefore clinically relevant.. Finally, an elevated creatinine level should have a high-specificity for the identification of true renal impairment in the current cohort since individuals with an elevated creatinine due to excess muscle mass should be infrequent in a middle-aged to elderly cohort with coronary artery disease.
Analysis and Statistics
Missing variables for ejection fraction (158 patients) and body mass index (4 patients) were imputed as the study median values. Heterogeneity between the four studies was evaluated for key baseline variables using chi-squared tests for dichotomous variables and Student's T-Test or Wilcoxon Rank-Sum tests for continuous variables. No significant differences were detected at the p<0.05 level. Separate analyses were conducted for the myocardial infarction and for the combined endpoint of non-fatal myocardial infarction and cardiovascular death. Event rates were determined using the Kaplan-Meier survival method, and univariate analyses of survival were conducted with the log-rank test. Cox proportional hazards regression was used to adjust for potentially confounding variables after examining the proportional hazards assumptions with the use of log-log plots and Schoenfeld residuals. Variables used in the multivariate analyses included study, age, race, body mass index, gender, diabetes, hypertension, hyperlipidemia, number of diseased vessels, left anterior descending target vessel, current smoking, prior MI, renal function, and ejection fraction. A p value <0.05 was considered statistically significant. Statistical analyses were performed using Stata for Windows version 8.0 (Stata Corporation, College Station, TX).
Results
Baseline Characteristics
Median follow-up was 1830 days with follow-up beyond 4 years in 86% of patients. Baseline characteristics of the patients are shown in Table 1. Serum creatinine was missing in 14 subjects (1.14%). 113 of the remaining subjects (9.20%) had serum creatinine ≥1.3 mg/dL. Less than 1% of subjects had a serum creatinine above 2.0 mg/dL. Subjects with elevated serum creatinine were older and more likely to be male or have hypertension. Conversely, they had higher ejection fractions and were less likely to be smokers or have an intervention on the left anterior descending artery.
Table 1.
Baseline Characteristics According to Renal Function
| Clinical Characteristic | Creatinine <1.3 N=1101 | Creatinine ≥1.3 N=113 | P Value |
|---|---|---|---|
| Age in Years (SD) | 62.1 (10.6) | 67.4 (10.2) | <0.0001 |
| Female | 359 (32.6) | 23 (20.4) | 0.008 |
| Black Race | 30 (2.72) | 6 (5.36) | 0.118 |
| Diabetes Mellitus | 230 (20.9) | 29 (25.7) | 0.242 |
| Hypertension | 607 (55.4) | 80 (70.7) | 0.001 |
| Elevated Cholesterol | 494 (45.2) | 40 (36.4) | 0.074 |
| Prior Myocardial Infarction | 349 (32.2) | 39 (36.1) | 0.404 |
| Current Smoking | 255 (23.2) | 14 (12.4) | 0.009 |
| LAD Intervention | 470 (42.7) | 34 (30.0) | 0.010 |
| Ejection Fraction (SD) | 55.8 (10.3) | 60.0 (10.6) | 0.006 |
| MDRD estimated Creatinine Clearance (SD) | 80.9 (22.3) | 43.8 (13.1) | <0.001 |
| Cockroft-Gault estimated Creatinine Clearance (SD) | 88.9 (34.6) | 53.7 (21.8) | <0.0001 |
| Number of Diseased Vessels (SD) | 1.39 (0.63) | 1.47 (0.67) | 0.221 |
Risk of Cardiac Death and MI during Follow-up
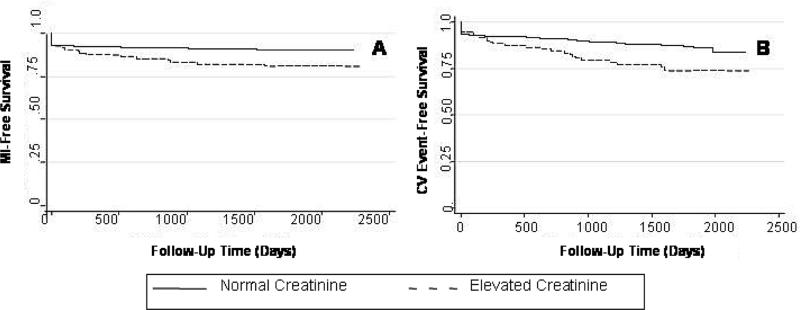
55 subjects (4.5%) died from cardiovascular causes and 125 (10.2%) subjects had an MI during the follow-up period. In 48 subjects the index procedure was complicated by a peri-procedural myocardial infarction. The risk of peri-procedural events was not different in subjects with vs. without renal impairment (HR 1.13, 95% CI: 0.45-2.84). However, the overall risk of MI or cardiac death during follow-up was significantly higher among patients with renal insufficiency than in those with normal renal function as summarized in Figure 1 and Table 2.
Figure 1.
Kaplan-Meier Plots of Event-Free Survival Stratified by Renal Function A) MI-Free Survival, P=0.0005. B) Freedom from cardiovascular death or non-fatal MI, P<0.0001.
Table 2.
Unadjusted Associations of Renal Function and Cardiovascular Outcome
| Myocardial Infarction | Cardiovascular Death or Non-Fatal MI | |||||
|---|---|---|---|---|---|---|
| Normal Creatinine | Elevated Creatinine | P Value | Normal Creatinine | Elevated Creatinine | P Value | |
| Incidence Rate | 25.56a | 50.33a | .001 | 31.14a | 65.85a | .004 |
| Hazard Ratio Elevated Creatinine (95% CI) | 1.98 (1.21-3.26) | .006 | 2.02 (1.32-3.07) | .007 | ||
| Hazard Ratio Continuous Creatinineb (95% CI) | 1.03(.0.96-1.05) | 0.09 | 1.02 (.0.99-1.05) | 0.12 | ||
Incidence rates per 1000 patient-years. b Hazard ratio per 0.1 mg/dL increase in serum creatinine
Elevated creatinine = serum creatinine>=1.3 mg/dL
MI-free survival at 5 years was 90.1% (95% CI, 88.1-91.2) in patients with non-elevated serum creatinine and 80.9% (95% CI, 71.8.-87.3) in patients with renal impairment. The relative hazard of MI during follow-up was nearly 2-fold higher in subjects with impaired renal function compared to those without renal impairment. Similar trends were apparent for the combined outcome. After 5 years, 86.8% of subjects with normal renal function (95% CI, 84.6-88.8) were alive and MI-free compared with 73.7% of subjects with renal impairment (95% CI, 63.9-81.2). There was a greater than 2-fold increase in the hazard of cardiovascular death or MI among patients with impaired renal function (Figure 1 and Table 2.). For both endpoints, each increase in serum creatinine of 0.1 mg/dL was associated with borderline significant increase in the risk of an event during follow-up (Table 2.).
Multivariate Analysis
The relationship between renal disease and cardiovascular outcomes was not significantly confounded by the differences in baseline characteristics of patients with and without renal disease (Table 3.). After adjustment for potential confounders, the hazard ratio for MI increased slightly from 1.98 to 2.14 and the hazard ratio of cardiovascular death or non-fatal MI increased from 2.02-to 2.13. Similarly, for each 0.1 mg/dL increase in serum creatinine the adjusted hazard ratio of MI (p=0.04) or cardiovascular death and non-fatal MI (p=0.06) increased by 2%. Excluding subjects with more significant renal dysfunction (serum creatinine ≥2.0) from the analysis did not significantly alter the results (data not shown).
Table 3.
Adjusted Hazard Ratios
| Adjusted Hazard Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|
| Myocardial Infarction | |||
| Impaired vs. Normal Renal Function | 2.14 | 1.28 - 3.55 | 0.006 |
| 0.1mg/dL Increase in Serum Creatinine | 1.02 | 1.00 - 1.03 | 0.04 |
| Cardiovascular Death or Myocardial Infarction | |||
| Impaired vs. Normal Renal Function | 2.13 | 1.37 - 3.30 | 0.001 |
| 0.1mg/dL Increase in Serum Creatinine | 1.02 | 0.99 - 1.03 | 0.06 |
There were no significant interactions between renal function, hyperlipidemia, diabetes, sex or race. However, there was a borderline significant interaction of renal insufficiency and hypertension on the risk of the combined outcome of cardiovascular death or non-fatal MI (p=.06). Among non-hypertensive subjects with chronic kidney disease, the hazard ratio of an event during follow-up was 1.05 (95% CI, 0.42-2.63)—a risk that was not significantly different than the risk in subjects without renal disease (p=.10). Conversely, for subjects with both hypertension and renal insufficiency, the hazard ratio was 2.88 (95% CI, 1.74-4.79), a risk significantly greater than the risk in subjects with neither of these risk factors (p<.001).
The age weight, race and estimated creatinine clearance of subjects with elevated creatinine and the absence of hypertension were examined to determine whether this interaction might be related to misclassification of renal function in young or muscular subjects with slight elevations in serum creatinine. Thirty of the 32 (87.5%) subjects with elevated creatinine but without hypertension were white. Mean age was 66.7 years and no subject was younger than 49 years old. Mean weight (SD) was 87.4 (19.4) kilograms. The mean MDRD-derived creatinine clearance for the group was 47.3 (12.0) cc/min/1.73 m2 with a maximum value of 66.6.
Discussion
We examined cardiovascular outcomes among patients undergoing coronary stenting and prospectively followed for 5 years and found that renal insufficiency is associated with a durable and markedly increased risk of myocardial infarction or cardiovascular death following elective coronary stenting. This increased risk emerges within the first year after percutaneous intervention and persists throughout follow-up. It is apparent with even minor decrements in renal function and is not attributable to the presence of a small number of subjects with severe renal dysfunction.
Chronic kidney disease appears to be a major, independent risk factor for the development of cardiovascular complications (2, 18, 21, 26-28). Although not all studies in low-risk populations have confirmed this association (13, 17, 32), in high-risk populations, there is more uniform evidence that renal impairment is associated with an increased risk of morbidity and mortality. Renal insufficiency is associated with an increased risk of death during the first year after treatment for acute coronary syndromes(1) or MI(6, 15, 16, 23, 29, 30, 34), and there is a graded increase in risk with progressive decrements in kidney function(2, 8). Effective revascularization or repair of obstructive coronary lesions might be expected to reduce the risk of MI or cardiovascular death and narrow the difference between patients with normal vs. impaired renal function and studies of outcomes in this setting can shed light on the extent to which the risks of CKD are mediated through the presence of obstructive coronary lesions.
Both Best and Gruberg have previously found renal impairment to be associated with a high risk of cardiovascular death after percutaneous coronary intervention, but both studies suffer from important limitations (8, 19). It is not clear from either report whether CKD remained associated with cardiovascular death or MI after adjustment for confounding by conditions such as diabetes or hypertension. Furthermore, both studies relied on data from hospital-based registries of coronary procedures, and they are therefore susceptible to bias from the different thresholds that exist for referral in patients with vs. without renal disease(9). Third, both studies terminated follow-up at the end of the first year and therefore provide little information on the long-terms risks associated with CKD. Finally, the patients in Best's study were generally treated with angioplasty alone, a treatment that is associated with markedly higher rates of restenosis and re-intervention than the current practice of angioplasty plus stenting.
Our results are consistent with and expand upon these studies by demonstrating that patients with CKD remain at increased risk for cardiovascular death and MI during the 5 years after coronary stenting. Further, we demonstrate that this risk is not due to confounding and can be found even in clinical trial populations with uniform indications for coronary intervention. In addition, the borderline significant interaction that we found between renal function and the presence of hypertension is intriguing. Our data does not allow us to determine whether this is a chance finding or, whether the lack of statistical significance is the consequence of a limited power to detect interactions. Further, while our results suggest that misclassification of CKD status is an unlikely explanation, we cannot rule out the possibility that hypertensive status was misclassified. Intuitively, the concept that the presence of hypertension might alter the vasculopathic or atherogenic effects of uremia is biologically plausible, and we believe that our findings merit further study in larger populations with more rigorous classification of blood pressure and GFR.
It is possible that less aggressive use of cardiovascular medications in subjects with chronic kidney disease partially explains our findings(7). We believe that a reluctance to use standard cardiovascular therapies in patients with renal failure is an unlikely explanation in a population already referred for invasive cardiovascular therapies, but we cannot rule out the possibility that there were differential thresholds for the use of medical and interventional therapies in our population. We also cannot rule out residual confounding by variables not available for our analysis such as vascular calcification, left ventricular hypertrophy and anemia (20). Each of these factors may partly explain how renal failure is related to cardiovascular diseases, but as the effect of renal failure may be partly mediated through each of these variables adjustment for them could inappropriately mask the true consequences of renal insufficiency.
In conclusion, our study demonstrates that the presence of mild to moderate renal dysfunction at the time of coronary stenting is independently associated with a doubling of the risk of myocardial infarction or cardiovascular death that persists even during prolonged follow-up. Further studies are needed to better understand the etiology of this association and to identify the optimal therapeutic approaches for these high-risk patients.
Acknowledgments
Financial Support: This work was supported by NIH grant T32 DK07199-25
References
- 1.Al Suwaidi J, Reddan DN, Williams K, Pieper KS, Harrington RA, Califf RM, Granger CB, Ohman EM, Holmes DR., Jr. Prognostic implications of abnormalities in renal function in patients with acute coronary syndromes. Circulation. 2002;106:974–980. doi: 10.1161/01.cir.0000027560.41358.b3. [DOI] [PubMed] [Google Scholar]
- 2.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 3.Azar RR, Prpic R, Ho KK, Kiernan FJ, Shubrooks SJ, Jr., Baim DS, Popma JJ, Kuntz RE, Cohen DJ. Impact of end-stage renal disease on clinical and angiographic outcomes after coronary stenting. Am J Cardiol. 2000;86:485–489. doi: 10.1016/s0002-9149(00)00998-x. [DOI] [PubMed] [Google Scholar]
- 4.Baim DS, Cutlip DE, Midei M, Linnemeier TJ, Schreiber T, Cox D, Kereiakes D, Popma JJ, Robertson L, Prince R, Lansky AJ, Ho KK, Kuntz RE. Final results of a randomized trial comparing the MULTI-LINK stent with the Palmaz-Schatz stent for narrowings in native coronary arteries. Am J Cardiol. 2001;87:157–162. doi: 10.1016/s0002-9149(00)01308-4. [DOI] [PubMed] [Google Scholar]
- 5.Baim DS, Cutlip DE, O'Shaughnessy CD, Hermiller JB, Kereiakes DJ, Giambartolomei A, Katz S, Lansky AJ, Fitzpatrick M, Popma JJ, Ho KK, Leon MB, Kuntz RE. Final results of a randomized trial comparing the NIR stent to the Palmaz-Schatz stent for narrowings in native coronary arteries. Am J Cardiol. 2001;87:152–156. doi: 10.1016/s0002-9149(00)01307-2. [DOI] [PubMed] [Google Scholar]
- 6.Beattie JN, Soman SS, Sandberg KR, Yee J, Borzak S, Garg M, McCullough PA. Determinants of mortality after myocardial infarction in patients with advanced renal dysfunction. Am J Kidney Dis. 2001;37:1191–1200. doi: 10.1053/ajkd.2001.24522. [DOI] [PubMed] [Google Scholar]
- 7.Berger AK, Duval S, Krumholz HM. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42:201–208. doi: 10.1016/s0735-1097(03)00572-2. [DOI] [PubMed] [Google Scholar]
- 8.Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, Berger PB. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002;39:1113–1119. doi: 10.1016/s0735-1097(02)01745-x. [DOI] [PubMed] [Google Scholar]
- 9.Chertow GM, Normand SL, McNeil BJ. “Renalism”: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol. 2004;15:2462–2468. doi: 10.1097/01.ASN.0000135969.33773.0B. [DOI] [PubMed] [Google Scholar]
- 10.Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 11.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 12.Couchoud C, Pozet N, Labeeuw M, Pouteil-Noble C. Screening early renal failure: cut-off values for serum creatinine as an indicator of renal impairment. Kidney Int. 1999;55:1878–1884. doi: 10.1046/j.1523-1755.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- 13.Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214–2219. doi: 10.1046/j.1523-1755.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 14.Cutlip DE, Baim DS, Ho KK, Popma JJ, Lansky AJ, Cohen DJ, Carrozza JP, Jr., Chauhan MS, Rodriguez O, Kuntz RE. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103:1967–1971. doi: 10.1161/01.cir.103.15.1967. [DOI] [PubMed] [Google Scholar]
- 15.Dixon SR, O'Neill WW, Sadeghi HM, Stone GW, Brodie B, Cox DA, Garcia E, Mattos L, Grines LL, Boura JA, Morice MC, Grines CL. Usefulness of creatinine clearance in predicting early and late death after primary angioplasty for acute myocardial infarction. Am J Cardiol. 2003;91:1454–1457. A1456. doi: 10.1016/s0002-9149(03)00396-5. [DOI] [PubMed] [Google Scholar]
- 16.Freeman RV, Mehta RH, Al Badr W, Cooper JV, Kline-Rogers E, Eagle KA. Influence of concurrent renal dysfunction on outcomes of patients with acute coronary syndromes and implications of the use of glycoprotein IIb/IIIa inhibitors. J Am Coll Cardiol. 2003;41:718–724. doi: 10.1016/s0735-1097(02)02956-x. [DOI] [PubMed] [Google Scholar]
- 17.Garg AX, Clark WF, Haynes RB, House AA. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int. 2002;61:1486–1494. doi: 10.1046/j.1523-1755.2002.00270.x. [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 19.Gruberg L, Weissman NJ, Waksman R, Laird JR, Jr., Pinnow EE, Wu H, Deible R, Kent KM, Pichard AD, Satler LF, Lindsay J., Jr. Comparison of outcomes after percutaneous coronary revascularization with stents in patients with and without mild chronic renal insufficiency. Am J Cardiol. 2002;89:54–57. doi: 10.1016/s0002-9149(01)02163-4. [DOI] [PubMed] [Google Scholar]
- 20.Gurm HS, Lincoff AM, Kleiman NS, Kereiakes DJ, Tcheng JE, Aronow HD, Askari AT, Brennan DM, Topol EJ. Double jeopardy of renal insufficiency and anemia in patients undergoing percutaneous coronary interventions. Am J Cardiol. 2004;94:30–34. doi: 10.1016/j.amjcard.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Henry RM, Kostense PJ, Bos G, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Mild renal insufficiency is associated with increased cardiovascular mortality: The Hoorn Study. Kidney Int. 2002;62:1402–1407. doi: 10.1111/j.1523-1755.2002.kid571.x. [DOI] [PubMed] [Google Scholar]
- 22.Heuser R, Lopez A, Kuntz R, Reduto L, Badger R, Coleman P, Whitlow P, Iannone LA, Safian R, Yeung A, Moses J. SMART: The microstent's ability to limit restenosis trial. Catheter Cardiovasc Interv. 2001;52:269–277. doi: 10.1002/ccd.1063. discussion 278. [DOI] [PubMed] [Google Scholar]
- 23.Langston RD, Presley R, Flanders WD, McClellan WM. Renal insufficiency and anemia are independent risk factors for death among patients with acute myocardial infarction. Kidney Int. 2003;64:1398–1405. doi: 10.1046/j.1523-1755.2003.00200.x. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 25.Lin J, Knight EL, Hogan ML, Singh AK. A Comparison of Prediction Equations for Estimating Glomerular Filtration Rate in Adults without Kidney Disease. J Am Soc Nephrol. 2003;14:2573–2580. doi: 10.1097/01.asn.0000088721.98173.4b. [DOI] [PubMed] [Google Scholar]
- 26.Manjunath G, Tighiouart H, Coresh J, Macleod B, Salem DN, Griffith JL, Levey AS, Sarnak MJ. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63:1121–1129. doi: 10.1046/j.1523-1755.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- 27.Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, Coresh J, Levey AS, Sarnak MJ. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 28.Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 29.Sadeghi HM, Stone GW, Grines CL, Mehran R, Dixon SR, Lansky AJ, Fahy M, Cox DA, Garcia E, Tcheng JE, Griffin JJ, Stuckey TD, Turco M, Carroll JD. Impact of renal insufficiency in patients undergoing primary angioplasty for acute myocardial infarction. Circulation. 2003;108:2769–2775. doi: 10.1161/01.CIR.0000103623.63687.21. [DOI] [PubMed] [Google Scholar]
- 30.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med. 2002;137:555–562. doi: 10.7326/0003-4819-137-7-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 31.Verhave JC, Gansevoort RT, Hillege HL, De Zeeuw D, Curhan GC, De Jong PE. Drawbacks of the use of indirect estimates of renal function to evaluate the effect of risk factors on renal function. J Am Soc Nephrol. 2004;15:1316–1322. [PubMed] [Google Scholar]
- 32.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 33.Winkelmayer WC, Charytan DM, Levin R, Avorn J. Poor short-term survival and low use of cardiovascular medications in elderly dialysis patients after acute myocardial infarction. Am J Kidney Dis. 2006;47:301–308. doi: 10.1053/j.ajkd.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Wright RS, Reeder GS, Herzog CA, Albright RC, Williams BA, Dvorak DL, Miller WL, Murphy JG, Kopecky SL, Jaffe AS. Acute myocardial infarction and renal dysfunction: a high-risk combination. Ann Intern Med. 2002;137:563–570. doi: 10.7326/0003-4819-137-7-200210010-00007. [DOI] [PubMed] [Google Scholar]
- 35.Zoccali C, Mallamaci F, Tripepi G. Novel cardiovascular risk factors in end-stage renal disease. J Am Soc Nephrol. 2004;15(Suppl 1):S77–80. doi: 10.1097/01.asn.0000093240.84097.fe. [DOI] [PubMed] [Google Scholar]