Abstract
Double-chambered right ventricle (DCRV) is an uncommon congenital anomaly in which anomalous muscle bands divide the right ventricle into two chambers; a proximal high-pressure and distal low-pressure chamber. It may be associated with mid right ventricular obstruction. It is commonly associated with other congenital anomalies, most frequently perimembranous ventricular septal defect (PM-VSD). We herein present 5 adult patients with concomitant DCRV and PM-VSD who varied in their symptomatic presentations and the ways of management.
Keywords: Double chambered right ventricle, Ventricular septal defect, Echocardiography, Magnetic resonance
Introduction
Double-chambered right ventricle (DCRV) is an uncommon congenital malformation in which anomalous muscle bundles dissect the RV into two chambers. It is commonly associated with other congenital anomalies, most frequently perimembranous ventricular septal defect (PM-VSD). The majority of DCRV cases present early in life, however, infrequently it will manifest in adulthood. The nonspecific nature of the symptoms may be in part responsible for a delayed diagnosis. Surgical intervention of DCRV in adults is uncommon and long-term outcome is unclear.1),2),3),4) We herein report five patients with DCRV and PM-VSD with varying clinical presentation and different treatment strategies.
Case
Case 1
A 21-year-old female presents with progressive shortness of breath and typical exertional chest pain. Past medical history is significant for a known small PM-VSD diagnosed in childhood with no intervention required. Physical examination was notable for an ejection systolic murmur (grade IV/VI) at the left sternal border. Electrocardiogram revealed right ventricular hypertrophy (RVH) and right atrial enlargement.
Transthoracic echocardiogram (TTE) while technically challenging, revealed a small (3 mm) PM-VSD with left to right shunting. In addition, RVH and a muscular septation within the RV cavity causing obstruction with a peak gradient of 70-80 mm Hg was noted. The pulmonic valve was normal. A transesophageal echocardiogram (TEE) confirmed the TTE findings (Fig. 1A, Supplementary movie 1). Cardiac magnetic resonance (CMR) showed prominent RV outflow tract (RVOT) muscle bundles causing significant flow turbulence in systole (Fig. 2A, Supplementary movie 1).
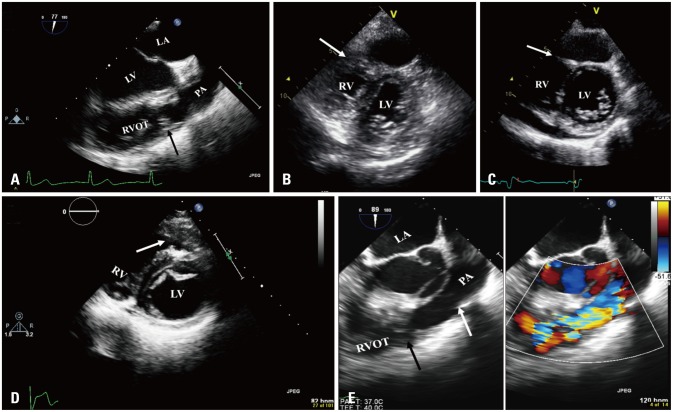
Fig. 1. A: Transesophageal echocardiogram mid esophageal view showing muscle band in the right ventricular outflow tract (arrow). B, C, and D: Transthoracic echocardiogram short axis views showing right ventricular muscle band (arrow). C: Posterior pericardial effusion was noted. E: Transesophageal echocardiogram mid esophageal view showing muscle band in the right ventricular outflow tract (left, black arrow) with color turbulence across (right). Pulmonic valve was shown (white arrow). LA: left atrium, LV: left ventricle, PA: pulmonary artery, RV: right ventricle, RVOT: right ventricular outflow tract.
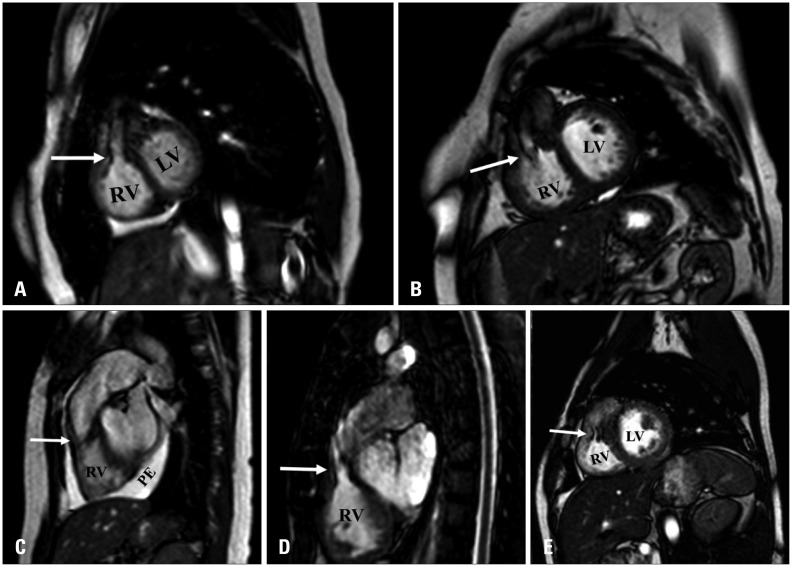
Fig. 2. A and B: Cardiac magnetic resonance: sagittal steady-state free precession views demonstrating prominent right ventricular outflow tract muscle bundles causing significant dephasing (arrow). C, D, and E: Cardiac magnetic resonance: sagittal steady-state free precession views demonstrating prominent right ventricular outflow tract muscle bundles without significant dephasing (arrow). C: Pericardial effusion was noted as well. LV: left ventricle, RV: right ventricle, PE: pericardial effusion.
The patient underwent surgery using a transatrial approach with VSD closure and resection of the anomalous muscle bands. Recovery was uneventful with no significant residual gradient across the RV on follow-up echocardiography.
Case 2
A 58-year-old female was evaluated for shortness of breath on moderate exertion. Cardiac history is that of a known small asymptomatic PM-VSD diagnosed two decades earlier. Physical examination was notable for a harsh ejection systolic murmur (grade III/VI) at the left sternal border. Electrocardiogram (ECG) revealed RVH. TTE revealed a large (14 mm) PM-VSD with left to right shunt. RVH and muscular septation of the RV causing mid-ventricular obstruction with a peak gradient of -75-80 mm Hg was noted. CMR showed prominent RVOT muscle bundles causing flow turbulence with significant obliteration in systole (area -0.6 cm2) (Fig. 1B and 2B, Supplementary movie 2). Cardiac catheterization confirmed the presence of DCRV, with an 85 mm Hg pressure gradient between the proximal and distal chambers. A decision was made to proceed with surgical repair; however, the patient declined further intervention.
Case 3
An asymptomatic 33-year-old female with a known small PM-VSD was followed up in our clinic. Past history was remarkable for a surgical closure of patent ductus arteriosus early in life. Physical examination was notable for a pansystolic murmur (grade II/VI) at the left sternal border. ECG was normal. TTE revealed a small (-3-4 mm) PM-VSD with left to right shunt and RV muscular septation causing no significant obstruction. CMR showed prominent RVOT muscle bundles causing no significant flow turbulence (Fig. 1C and 2C, Supplementary movie 3). No action was taken due to the benign nature of DCRV in this patient.
Case 4
An asymptomatic 17-year-old male, with a history of surgical closure of PM-VSD at age 12, presents for routine follow-up. Physical examination was notable for an ejection systolic murmur (grade II/VI) at the left sternal border. ECG was normal. TTE revealed a RV muscular septation without significant obstruction (peak gradient of < 20 mm Hg). No residual VSD shunt was appreciated. CMR showed prominent RVOT muscle bundles causing no flow turbulence (Fig. 1D and 2D, Supplementary movie 4). No action was taken due to the benign nature of DCRV in this patient.
Case 5
A 34-year-old male was evaluated for palpitation. Past medical history is significant for small PM-VSD years earlier with no intervention required. Physical examination was notable for an ejection systolic murmur (grade II/VI) over the left sternal border. ECG was unremarkable. TTE showed a small (4 mm) PM-VSD with left to right shunt and RV muscular septation causing no significant obstruction. TEE was confirmatory (Fig. 1E, Supplementary movie 5). CMR showed prominent RVOT muscle bundles causing no significant flow turbulence (Fig. 2E, Supplementary movie 5). No action was taken due to the benign nature of DCRV in this patient.
Discussion
DCRV is considered an acquired cardiac defect. While there may be a genetic predisposition for abnormal muscle band formation that contributes to this anomaly, it has not been clearly elucidated.1),2) The majority of DCRV cases are diagnosed and repaired early in life, however, it infrequently manifests in adulthood, because of the nonspecific nature of symptoms leading to a delayed diagnosis.3) RVOT obstruction is progressive in adults, and patients may present with effort dyspnea. In affected individuals with a significant mid RV obstruction surgical intervention of DCRV is indicated. However long-term outcome is unclear.4)
Various mechanisms of DCRV have been postulated. Superior displacement of the septal marginal trabecula (moderator band) has been proposed, particularly in association with a VSD, and flow turbulence in the RVOT.1) This flow turbulence may trigger abnormal hypertrophy of the moderator band leading to DCRV. This might elucidate the concomitant association between DCRV and VSD.5),6)
TTE is an important first line diagnostic tool in congenital heart disease, but may have limited visualization of DCRV in adults due to the retrosternal position and asymmetrical shape of the RV. TEE is an excellent supplementary tool to assist delineation of the RV abnormalities as well as assess and quantify the severity of RV cavitary obstruction. Recently, contrast computed tomography and CMR have been introduced in the identification of DCRV. Those diagnostic tools are now sufficiently mature to preclude the need for invasive testing.7),8),9)
Surgical intervention is indicated in symptomatic patients or in asymptomatic patients where the peak gradient exceed 40 mm Hg.3),9) Different approaches for resection of the anomalous muscle bands can be utilized; including a right atriotomy, a right ventriculotomy, or a combined transatrial-transpulmonary access.3),4),9) The right atriotomy and the combined transatrial-transpulmonary incision are used most commonly. Right ventriculotomy is used rarely because of the presence of RV dysfunction or it can result in ventricular arrhythmias. Nonetheless, in select cases patients with massive and prominent bundles, right ventriculotomy is still used.3),4),9)
The pentad of cases presented show the diverse expressions of DCRV from a 'forme fruste' to highly symptomatic. All cases had an associated PM-VSD with abnormal muscle bundles in the RVOT. In the first patient, the main presenting symptoms were shortness of breath and chest pain. She was initially asymptomatic with an isolated small VSD discovered early in life. However, she developed progressive symptoms with a significant gradient across the RVOT in a relatively short period of time which warranted surgical repair. The second patient presented with shortness of breath later in life. She was initially asymptomatic with an isolated VSD discovered at age of 30. Nevertheless, she developed progressive symptoms with significant gradient across the RVOT which warranted surgical repair, but declined by the patient. The third one was born with patent ductus arteriosus, treated with surgical ligation early in life, and a small VSD. She was asymptomatic and developed DCRV later in life with no significant gradient across the RVOT. The fourth patient was born with VSD which warranted surgical closure at age of 12 years. He was asymptomatic and developed DCRV after VSD closure with no significant gradient across the RVOT. The fifth patient was asymptomatic, diagnosed initially with an isolated VSD. He developed DCRV later with no significant gradient across the RVOT. This series of patients reinforces the theory that progressive obstruction in DCRV, with concomitant VSD, might be an acquired phenomenon on a background of a genetic predisposition. The triggers for phenotypic expression or progression thereof are unclear.9),10)
Supplementary movie legends
Transesophageal echocardiogram mid esophageal view (A) and cardiac magnetic resonance sagittal cine image (B) showing prominent muscle bands in the right ventricular outflow tract with significant color turbulence and dephasing respectively.
Transthoracic echocardiogram short axis view (A) and cardiac magnetic resonance sagittal cine image (B) demonstrating prominent muscle bands in the right ventricular outflow tract with significant color turbulence and dephasing respectively.
Transthoracic echocardiogram short axis view (A) and cardiac magnetic resonance sagittal cine image (B) showing prominent right ventricular muscle bands with no significant color turbulence or dephasing respectively. Posterior pericardial effusion was observed.
Transthoracic echocardiogram short axis view (A) and cardiac magnetic resonance sagittal cine image (B) revealing prominent right ventricular muscle bands with no significant color turbulence or dephasing respectively.
Transesophageal echocardiogram mid esophageal view (A) and cardiac magnetic resonance sagittal cine image (B) demonstrating prominent right ventricular muscle bands with no significant color turbulence or dephasing respectively.
References
- 1.Wong PC, Sanders SP, Jonas RA, Colan SD, Parness IA, Geva T, Van Praagh R, Spevak PJ. Pulmonary valve-moderator band distance and association with development of double-chambered right ventricle. Am J Cardiol. 1991;68:1681–1686. doi: 10.1016/0002-9149(91)90329-j. [DOI] [PubMed] [Google Scholar]
- 2.Hindle WV, Jr, Engle MA, Hagstrom JW. Anomalous right ventricular muscles: a clinicopathologic study. Am J Cardiol. 1968;21:487–495. doi: 10.1016/0002-9149(68)90280-4. [DOI] [PubMed] [Google Scholar]
- 3.McElhinney DB, Chatterjee KM, Reddy VM. Double-chambered right ventricle presenting in adulthood. Ann Thorac Surg. 2000;70:124–127. doi: 10.1016/s0003-4975(00)01320-5. [DOI] [PubMed] [Google Scholar]
- 4.Nagashima M, Tomino T, Satoh H, Nakata T, Ohtani T, Saito H. Double-chambered right ventricle in adulthood. Asian Cardiovasc Thorac Ann. 2005;13:127–130. doi: 10.1177/021849230501300206. [DOI] [PubMed] [Google Scholar]
- 5.Oliver JM, Garrido A, González A, Benito F, Mateos M, Aroca A, Sanz E. Rapid progression of midventricular obstruction in adults with double-chambered right ventricle. J Thorac Cardiovasc Surg. 2003;126:711–717. doi: 10.1016/s0022-5223(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 6.Kottayil BP, Dharan BS, Pillai VV, Panicker VT, Gopalakrishnan SK, Jayakumar K. Surgical repair of double-chambered right ventricle in adulthood. Asian Cardiovasc Thorac Ann. 2011;19:57–60. doi: 10.1177/0218492310395955. [DOI] [PubMed] [Google Scholar]
- 7.Chang RY, Kuo CH, Rim RS, Chou YS, Tsai CH. Transesophageal echocardiographic image of double-chambered right ventricle. J Am Soc Echocardiogr. 1996;9:347–352. doi: 10.1016/s0894-7317(96)90151-0. [DOI] [PubMed] [Google Scholar]
- 8.Kilner PJ, Sievers B, Meyer GP, Ho SY. Double-chambered right ventricle or sub-infundibular stenosis assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2002;4:373–379. doi: 10.1081/jcmr-120013300. [DOI] [PubMed] [Google Scholar]
- 9.Darwazah AK, Eida M, Bader V, Khalil M. Surgical management of double-chambered right ventricle in adults. Tex Heart Inst J. 2011;38:301–304. [PMC free article] [PubMed] [Google Scholar]
- 10.Pongiglione G, Freedom RM, Cook D, Rowe RD. Mechanism of acquired right ventricular outflow tract obstruction in patients with ventricular septal defect: an angiocardiographic study. Am J Cardiol. 1982;50:776–780. doi: 10.1016/0002-9149(82)91233-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transesophageal echocardiogram mid esophageal view (A) and cardiac magnetic resonance sagittal cine image (B) showing prominent muscle bands in the right ventricular outflow tract with significant color turbulence and dephasing respectively.
Transthoracic echocardiogram short axis view (A) and cardiac magnetic resonance sagittal cine image (B) demonstrating prominent muscle bands in the right ventricular outflow tract with significant color turbulence and dephasing respectively.
Transthoracic echocardiogram short axis view (A) and cardiac magnetic resonance sagittal cine image (B) showing prominent right ventricular muscle bands with no significant color turbulence or dephasing respectively. Posterior pericardial effusion was observed.
Transthoracic echocardiogram short axis view (A) and cardiac magnetic resonance sagittal cine image (B) revealing prominent right ventricular muscle bands with no significant color turbulence or dephasing respectively.
Transesophageal echocardiogram mid esophageal view (A) and cardiac magnetic resonance sagittal cine image (B) demonstrating prominent right ventricular muscle bands with no significant color turbulence or dephasing respectively.