Abstract
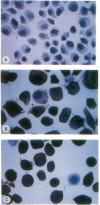
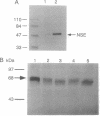
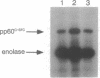
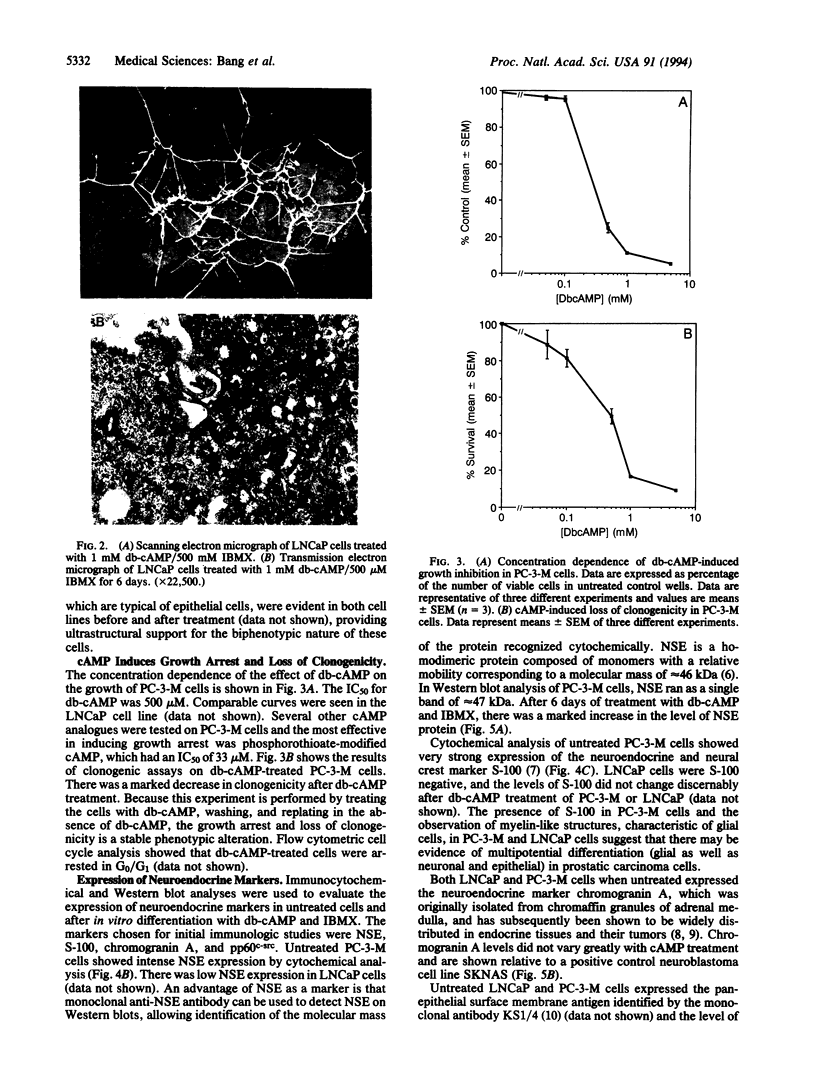
Recent clinicopathologic studies have shown that many prostatic adenocarcinomas express focal neuroendocrine differentiation and that neuroendocrine differentiation is most apparent in advanced anaplastic tumors. While studying growth-regulatory signal transduction events in human prostate carcinoma cell lines, we found that in two of four cell lines, the androgen-sensitive line LNCaP and the highly metastatic androgen-independent line PC-3-M, elevation of cAMP through addition of cAMP analogues or phosphodiesterase inhibitors induced a markedly neuronal morphology. Also in LNCaP cells ultrastructural analysis showed that cAMP induced the appearance of neurosecretory cell-like dense-core granules. Phenotypic analysis of untreated LNCaP and PC-3-M cells showed that both cell lines express markers of the neural crest including S-100, chromogranin A, pp60c-src, and neuron-specific enolase as well as the epithelial marker KS1/4 and stage-specific embryonic antigen 4. In PC-3-M cells, cAMP markedly elevated neuron-specific enolase protein and caused an increase in the specific activity of the neuroendocrine marker pp60c-src, and in both cell lines expression of KS1/4 and stage-specific embryonic antigen 4 was down-regulated. In addition to effects on lineage markers, cAMP treatment induced G1 synchronization, growth arrest, and loss of clonogenicity, indicating terminal differentiation. Our data provide direct evidence of plasticity in the lineage commitment of adenocarcinoma of the prostate. We have shown that cell-permeant cAMP analogues can induce terminal differentiation, suggesting that hydrolysis-resistant cyclic nucleotides may present an additional approach to the treatment of advanced prostate cancer.
Full text
PDF

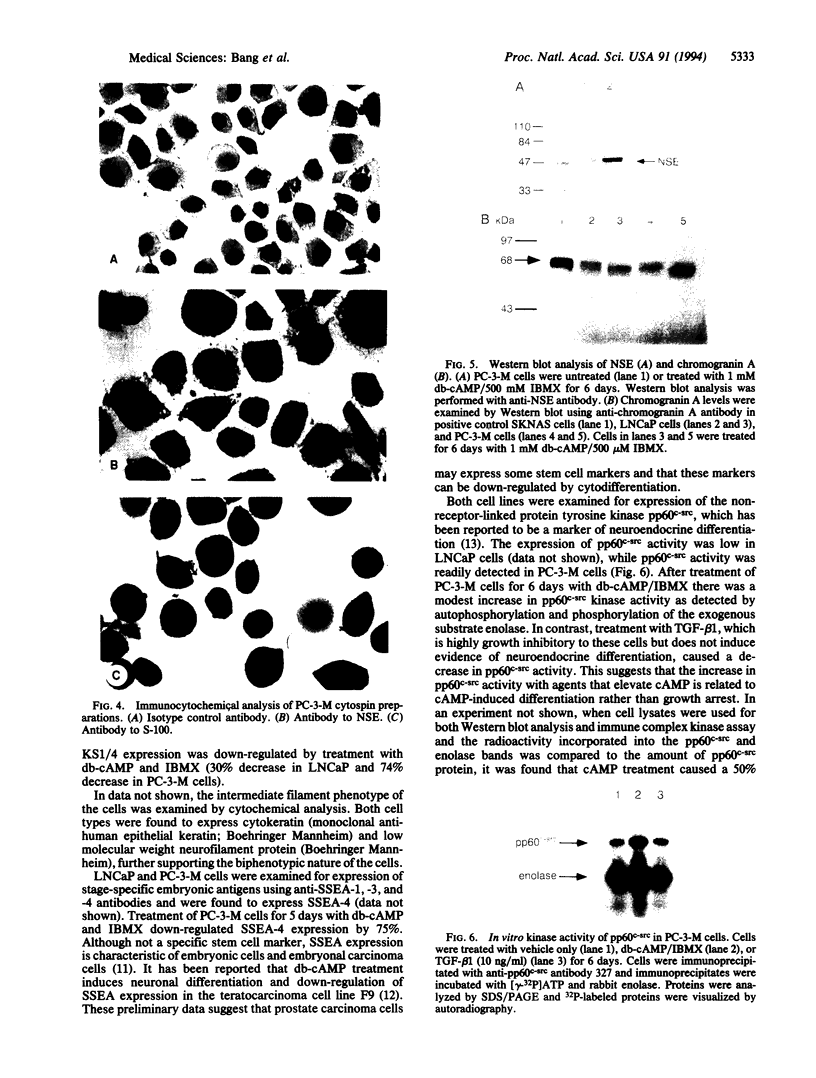

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson P. A., Falkmer S., Fält K., Grimelius L. The course of neuroendocrine differentiation in prostatic carcinomas. An immunohistochemical study testing chromogranin A as an "endocrine marker". Pathol Res Pract. 1989 Sep;185(3):373–380. doi: 10.1016/S0344-0338(89)80016-0. [DOI] [PubMed] [Google Scholar]
- Abrahamsson P. A., Wadström L. B., Alumets J., Falkmer S., Grimelius L. Peptide-hormone- and serotonin-immunoreactive tumour cells in carcinoma of the prostate. Pathol Res Pract. 1987 Jun;182(3):298–307. doi: 10.1016/S0344-0338(87)80065-1. [DOI] [PubMed] [Google Scholar]
- Andrews P. W., Gönczöl E., Plotkin S. A., Dignazio M., Oosterhuis J. W. Differentiation of TERA-2 human embryonal carcinoma cells into neurons and HCMV permissive cells. Induction by agents other than retinoic acid. Differentiation. 1986;31(2):119–126. doi: 10.1111/j.1432-0436.1986.tb00392.x. [DOI] [PubMed] [Google Scholar]
- Ashall F., Sullivan N., Puck T. T. Specificity of the cAMP-induced gene exposure reaction in CHO cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3908–3912. doi: 10.1073/pnas.85.11.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang Y. J., Kim S. J., Danielpour D., O'Reilly M. A., Kim K. Y., Myers C. E., Trepel J. B. Cyclic AMP induces transforming growth factor beta 2 gene expression and growth arrest in the human androgen-independent prostate carcinoma cell line PC-3. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3556–3560. doi: 10.1073/pnas.89.8.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsagel P. L., Victor-Kobrin C., Timblin C. R., Trepel J., Kuehl W. M. A murine cDNA encodes a pan-epithelial glycoprotein that is also expressed on plasma cells. J Immunol. 1992 Jan 15;148(2):590–596. [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Cooper M. J., Hutchins G. M., Cohen P. S., Helman L. J., Mennie R. J., Israel M. A. Human neuroblastoma tumor cell lines correspond to the arrested differentiation of chromaffin adrenal medullary neuroblasts. Cell Growth Differ. 1990 Apr;1(4):149–159. [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman L. J., Gazdar A. F., Park J. G., Cohen P. S., Cotelingam J. D., Israel M. A. Chromogranin A expression in normal and malignant human tissues. J Clin Invest. 1988 Aug;82(2):686–690. doi: 10.1172/JCI113648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadmon D., Thompson T. C., Lynch G. R., Scardino P. T. Elevated plasma chromogranin-A concentrations in prostatic carcinoma. J Urol. 1991 Aug;146(2):358–361. doi: 10.1016/s0022-5347(17)37793-5. [DOI] [PubMed] [Google Scholar]
- Kannagi R., Cochran N. A., Ishigami F., Hakomori S., Andrews P. W., Knowles B. B., Solter D. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2(12):2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski J. M., Fidler I. J., Campbell D., Xu Z. L., Kaighn M. E., Hart I. R. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984 Aug;44(8):3522–3529. [PubMed] [Google Scholar]
- Marx J. Two major signal pathways linked. Science. 1993 Nov 12;262(5136):988–990. doi: 10.1126/science.8257559. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Suzuki F., Kato K. Characterization of alpha alpha, beta beta, gamma gamma and alpha gamma human enolase isozymes, and preparation of hybrid enolases (alpha gamma, beta gamma and alpha beta) from homodimeric forms. Biochim Biophys Acta. 1983 Oct 28;748(2):278–284. doi: 10.1016/0167-4838(83)90305-9. [DOI] [PubMed] [Google Scholar]
- Simon B., Podolsky D. K., Moldenhauer G., Isselbacher K. J., Gattoni-Celli S., Brand S. J. Epithelial glycoprotein is a member of a family of epithelial cell surface antigens homologous to nidogen, a matrix adhesion protein. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2755–2759. doi: 10.1073/pnas.87.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel J. B., Colamonici O. R., Kelly K., Schwab G., Watt R. A., Sausville E. A., Jaffe E. S., Neckers L. M. Transcriptional inactivation of c-myc and the transferrin receptor in dibutyryl cyclic AMP-treated HL-60 cells. Mol Cell Biol. 1987 Jul;7(7):2644–2648. doi: 10.1128/mcb.7.7.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik L. J., Ehrenfried B., Jensen R. A. Production and characterization of monoclonal antibodies with specificity for the S100 beta polypeptide of brain S100 fractions. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6034–6038. doi: 10.1073/pnas.81.19.6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H., Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992 Aug;49(3):497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Sant'Agnese P. A. Neuroendocrine differentiation in human prostatic carcinoma. Hum Pathol. 1992 Mar;23(3):287–296. doi: 10.1016/0046-8177(92)90110-o. [DOI] [PubMed] [Google Scholar]
- di Sant'Agnese P. A., de Mesy Jensen K. L. Neuroendocrine differentiation in prostatic carcinoma. Hum Pathol. 1987 Aug;18(8):849–856. doi: 10.1016/s0046-8177(87)80060-6. [DOI] [PubMed] [Google Scholar]