Abstract
This study aimed to investigate thrombin-activatable fibrinolysis inhibitor (TAFI) Thr325Ile polymorphism and TAFI antigen (Ag) levels in breast cancer (BC) in the Egyptian population to clarify their role in relation to BC. A group of 300 females was recruited in this study; of these 150 unrelated patients with different stages of BC and 150 age-matched healthy controls. Plasma TAFI Ag was measured by ELISA and TAFI Thr325Ile (rs1926447) polymorphism was genotyped using TaqMan single nucleotide polymorphism (SNP) genotyping assay. The results showed the genotypes of the minor allele; Thr/Ile (CT) and Ile/Ile (TT) were significantly more frequent in patients compared to control group (50.0% and 22.0% vs. 42.0% and 13.3%, respectively) and were also associated with BC susceptibility [OR = 1.9 and 2.6; 95% CI: (1.1–3.3) and (1.3–5.5), respectively P = 0.01]. Ile325 allele carriers were more frequent in cases than in controls (47.0% vs. 34.0%) [OR = 1.7, (95% CI = 1.2–2.4), P = 0.001]. However, TAFI Thr325Ile polymorphism was not associated with BC stage or other clincopathological characteristics. TAFI Ag levels were correlated with advanced stages of BC, poor prognosis and risk of recurrence (P = 0.02, P = 0.04 and P < 0.001, respectively) and Thr325Ile SNP was significantly correlated with TAFI antigen levels with the C/C genotype corresponding to the highest and the T/T genotype to the lowest TAFI antigen levels (P < 0.001) in the study groups. In conclusion, this study showed for the first time that TAFI Thr325Ile polymorphism could have a contribution to BC susceptibility in our population. Furthermore, high TAFI plasma levels may serve as a predictor of poor prognosis in patients with BC.
Abbreviations: BC, breast cancer; TAFI, thrombin-activatable fibrinolysis inhibitor; Ag, antigen; TAFIa, activated TAFI; SNP, single nucleotide polymorphism; DIC, disseminated intravascular coagulopathy; VTE, vascular thromboembolic events; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; NPI, nottingham prognostic index; IHPI, immunohistochemical prognostic index; ELISA, enzyme linked immunosorbent assay; NPP, normal pooled plasma; WBC, white blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PT, prothrombin time; APTT, activated partial thromboplastin time
Keywords: Thrombin-activatable fibrinolysis inhibitor, Breast cancer, Thr325Ile polymorphism, Egyptian
Highlights
-
•
TAFI Thr325Ile polymorphism associated with breast cancer in studied population
-
•
TAFI Thr325Ile polymorphism was significantly correlated with TAFI antigen levels.
-
•
High TAFI antigen levels were correlated with advanced stages of breast cancer.
-
•
High TAFI plasma level could be used to recognize tumor prognosis and risk of recurrence.
Introduction
Breast cancer (BC) is the most common cancer of women worldwide (Ferlay et al., 2010). In Egypt, the disease accounts for 37% of womens cancer with an incidence rate of 49.6/100,000 (Hussein et al., 2013) and it is predicted to be a significant increase in BC caseloads from 2009 to 2015 among women aged 30–49 years (Hirko et al., 2013). It has been estimated that hypercoagulation state accounts for a significant percentage of mortality and morbidity in cancer patients (Caine et al., 2002). This state has been linked to a chronic low-grade disseminated intravascular coagulopathy (DIC), with vascular thromboembolic events (VTE) occurring both alone and in the presence of DIC (Dipasco et al., 2011).
As a potent inhibitor of fibrinolysis, thrombin-activatable fibrinolysis inhibitor (TAFI) has been isolated and characterized from human plasma. This zymogen can be activated by thrombin, plasmin or the thrombin thrombomodulin complex of the carboxypeptidase B-like enzyme, TAFIa (activated TAFI). When exposed to a fibrin clot, TAFIa catalyzes the removal of carboxyl-terminal lysines, thereby diminishing the cofactor activity for plasminogen activation. Less efficient plasminogen activation on the fibrin clot corresponds to prolongation of fibrinolysis, and TAFIa can serve as a potent antifibrinolytic enzyme by this way (Schneider et al., 2002). Non-modifiable factors such as polymorphisms in the genes encoding these proteins that may affect their structure, concentration, or function, might have an influence in the balance between coagulation and fibrinolysis and should be investigated to help identify a subset of patients at higher risk. Several single nucleotide polymorphisms (SNPs) of the TAFI gene have been identified (Brouwers, 2001). This gene is located on chromosome 13q14.11, spanning ~ 48 kb and consisting of 11 exons (Boffa et al., 1999). Plasma TAFI levels or function has been related to these polymorphisms, of which is 1040 C/T SNP as described in the NCBI SNP database (rs1926447) in the coding region that results in the conversion of a Threonine codon (ACU) to an Isolucine codon (AUU) at a position 325 (Thr325Ile) is of particular interest since it has been shown that the presence of an Ile325 residue has a positive influence on both TAFIa activity and stability in vitro resulting in increased antifibrinolytic activity (Schneider et al., 2002). Although several reports have been published on the relationship between TAFI and deep vein thrombosis (Martini et al., 2006), DIC (Watanabe et al., 2001), ischemic stroke (Eicheinger et al., 2004, Leebeek et al., 2005), coronary artery disease (Kamal et al., 2011, Ta'ssies et al., 2009, Tregouet et al., 2009), and diabetes mellitus (Akinci, 2012), the potential role of TAFI in tumor development remains controversial.
Apparently, any disturbance in TAFI levels or its activity may contribute to increased rates of thrombotic complications in cancer patients (Chengwei et al., 2013). To the best of our knowledge, this is the first work on relation between TAFI polymorphism and BC in our population (Caucasian ancestry). Here, we investigated the associations of Thr325Ile TAFI polymorphism and its plasma level with BC and its progress. In combination with other genetic and non-genetic data, this could be helpful in refining a prognostic profile for cancer patients and identify those who are prone to thromboembolic events.
Subjects and methods
Participants
Patients
One hundred and fifty newly diagnosed female patients (mean age; 52.1 ± 13.8 years) with pathologically proven BC were recruited between 2011 and 2013 from the Oncology Diagnostic Unit of Suez Canal University Hospital. BC patients were further stratified into two subgroups; those in early stages with no distant metastases (stages I and II; n = 80, mean age 50.1 ± 8.6 years) and a group in late stages of BC with invasive behavior and distant metastases (stages III and IV; n = 70, mean age 52.4 ± 12.5). BC was diagnosed based on the pathological examination and the relevant stage was assigned according to the TNM classification of the Sixth Edition of the American Joint Committee on Cancer (AJCC) Manual for Staging of Cancer (Singletary and Connolly, 2006) and graded by the Nottingham grading system (Rakha et al., 2008). There was no pre-surgical treatment of chemotherapy, radiotherapy, endocrine therapy or biotherapy. All relevant clinical and pathological information was retrieved from their files, including tumor size, type, grade and stage at the time of diagnosis, presence of lymph node metastases, estrogen receptors (ER), progesterone receptors (PR) and human epidermal growth factor receptor 2 (HER2) expression in BC tissue. Receptor status was used to divide breast cancer into four biological tumor subtypes; Luminal A, Luminal B (triple positive), Basal-like (triple negative) and HER2+ subsets (Lowery et al., 2012). A scoring assessment of prognosis for each patient was performed using both the Nottingham Prognostic Index (NPI) and Immunohistochemical Prognostic Index (IHPI) to predict the overall and disease free survival. The former index is based on 3 prognostic indicators; tumor size, histological grade, and lymph-node status; patients with NPI of ≤ 3.4 have a good prognosis, 3.4 > NPI ≤ 5.4 is moderate, and of > 5.4 has a poor prognosis, with 5-year survival rates of 85%, 70%, and 50%, respectively (Galea et al., 1992, Homa et al., 2005). The IHPI was used as a complementary index to the NPI, based on the three receptor status; ER, PR and HER2. It was calculated as follows: one point will be assigned for each positive value for HER2 and zero points will be given for negative values; one point will be given for any negative value of ER or PR and zero points will be given when positive. Tumors were ranked from 0 to 4 points using the arithmetic sum of the three individual immunohistochemical factors, and grouped into good prognosis (0–1 points), moderate prognosis (2 points), or poor prognosis (3 points) (Guerra et al., 2003). The risk of recurrence of each patient was estimated according to the European Society of Medical Oncology (ESMO) Clinical Recommendations for follow-up of primary breast cancer, and it was classified into three categories; low, intermediate and high risk of recurrence (Kataja and Castiglione, 2009). The clinicopathological features of BC patients were presented in Table 1.
Table 1.
The clinicopathological features of breast cancer patients.
| Patient characteristics | Early stages BC patients (n = 80) | Late stages BC patients (n = 70) |
|---|---|---|
| Menopausal state | ||
| Premenopausal | 33 (41.2) | 25 (35.7) |
| Postmenopausal | 47 (58.8) | 45 (64.3) |
| First degree family history | ||
| Negative | 75 (93.8) | 65 (92.9) |
| Positive | 5 (6.2) | 5 (7.1) |
| Histological type of breast cancer | ||
| Duct carcinoma | 80 (100) | 30 (42.9) |
| Lobular carcinoma | 0 (0.0) | 23 (32.8) |
| Mixed duct and lobular | 0 (0.0) | 10 (14.3) |
| Poorly differentiated tumor | 0 (0.0) | 7 (10.0) |
| Biological type of breast cancer | ||
| Luminal A | 43 (53.8) | 27 (38.6) |
| Luminal B | 3 (3.7) | 7 (10.0) |
| Basal-like | 34 (42.5) | 18 (25.7) |
| HER2+ | 0 (0.0) | 18 (25.7) |
| Tumor grade | ||
| GI | 12 (15.0) | 0 (0) |
| GII | 65 (81.3) | 30 (42.9) |
| GIII | 3 (3.7) | 40 (57.1) |
| Tumor stage | ||
| I | 10 (12.5) | 0 (0.0) |
| IIA | 45 (56.2) | 0 (0.0) |
| IIB | 25 (31.3) | 0 (0.0) |
| IIIA | 0 (0.0) | 32 (45.7) |
| IIIC | 0 (0.0) | 3 (4.3) |
| IV | 0 (0.0) | 35 (50) |
| Lymph node involvement | ||
| Negative | 55 (68.8) | |
| Positive | 25 (31.2) | 70 (100) |
| Estrogen receptors | ||
| Negative | 33 (41.2) | 36 (51.4) |
| Positive | 47 (58.8) | 34 (48.6) |
| Progesterone receptors | ||
| Negative | 35 (43.7) | 35 (50) |
| Positive | 45 (56.3) | 35 (50) |
| HER 2 expression | ||
| Score 0 | 77 (96.3) | 45 (64.3) |
| Score 1 | 3 (3.7) | 25 (35.7) |
| NPI | ||
| Good prognosis | 37 (46.3) | 0 (0.0) |
| Moderate prognosis | 43 (53.7) | 27 (38.6) |
| Poor prognosis | 0 (0.0) | 43 (61.4) |
| IHPI | ||
| Good prognosis | 45 (56.3) | 35 (50) |
| Moderate prognosis | 35 (43.7) | 17 (24.3) |
| Bad prognosis | 0 (0.0) | 18 (25.7) |
| Risk of recurrence | ||
| Intermediate | 72 (90) | 28 (40) |
| High | 8 (10) | 42 (60) |
Values in parentheses indicate percentage; BC, breast cancer; n, number; HER2, human epidermal growth factor receptor 2; NPI, Nottingham prognostic index; IHPI, immunohistochemical prognostic index.
Controls
The control group included 150 unrelated apparently healthy women (mean age 50.2 ± 4.2 years), not suffering from any disease interfering with the study and had no family history of BC or any other neoplastic diseases. They were recruited from blood bank donors.
The study was conducted in accordance with the guidelines in the Declaration of Helsinki and was approved by the Ethical Committee of the Faculty of Medicine, Suez Canal University. An informed consent was obtained from all participants.
Blood sample collection
From patient and control group venous blood samples were collected under a complete aseptic condition on citrate (3.8% trisodium citrate, 0.129 mmol/l), centrifuged at 2500 g for 30 min at 4 °C and platelet-poor plasma was divided into aliquots and kept frozen at − 80 °C until analysis (within 6 months). At the same time when blood samples were taken, whole blood cell count, liver function tests, renal function tests and coagulation parameters were also detected. For genotyping studies, samples were drawn in trisodium EDTA (1 mg/ml) tubes.
Laboratory methods
Quantification of TAFI Ag level was performed with a commercially available Zymutest TAFI Ag enzyme linked immunosorbent assay (ELISA) kit (Hyphen BioMed, France), which contained a capture antibody (monoclonal antibody specific for human TAFI) and a detecting antibody (polyclonal labeled antibody). The intra and interassay coefficients of variability were 2.8% and 1.9%; respectively. Results were expressed as percentage of normal pooled plasma (NPP) from 30 healthy volunteers from the blood bank.
White blood cell count (WBC) was detected by an automated cell counter (Cell Dyne-2700, Abbott Lab., USA). Liver and renal function tests, including serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), albumin, creatinine, and blood urea nitrogen were routinely determined using CX-9 Beckman (Coulter., France) with the company's original kits. The coagulation parameters, including prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen and D-dimer (Latex enhanced immune turbidity method) were assayed using CA-1500 system blood coagulation analyzer (Sysmex, Japan).
Genotyping
Genomic DNA was extracted from peripheral blood leukocytes using the Wizard® Genomic DNA Purification Kit (Promega, Madison, Wisconsin, USA), quantified by absorbance at 260 nm using Nanodrop-1000 spectrophotometer (NanoDrop Tech., Wilmington, USA) and stored at − 80 °C. TAFI Thr325Ile SNP (rs1926447) was genotyped using a real-time PCR allelic discrimination TaqMan assay (Applied Biosystems, California, USA) on StepOne™ Real-Time PCR System (Applied Biosystems, USA) as described in detail previously (Kamal et al., 2011). Genotyping was performed blinded to case/control status. Ten percent of the randomly selected samples were regenotyped in separate runs to exclude the possibility of false genotype calls with 100% concordance.
Statistical analysis
Numerical values were analyzed by Student's t-test and ANOVA followed by Newman–Keuls post hoc test for inter-sample comparisons. In the case of non parametric data, Mann–Whitney U or Kruskal–Wallis tests for comparing 2 or > 2 categories; respectively, were used followed by Tukey multiple comparison test. Comparison of allelic and genotype frequencies between groups and association of polymorphism with quantitative traits were examined for statistical significance using standard Pearson chi-square test (χ2) or Fisher's exact test when appropriate. The odds ratio (OR) was calculated by means of logistic regression and the confidence interval (CI) was calculated at the 95% level. Data were analyzed by Texasoft WINKS 4.651 software (Texas, USA). Statistical significance was assumed for P values less than 0.05. The appropriate sample size and power calculations were carried out using Quanto software package version 1.2.4 (University of Southern California, http://hydra.usc.edu/gxe/). Calculations showed that the sample size, together with the specified study design, allele frequencies and allowable error rates; can give as high as 80% power and can detect a variant allele frequency of at least 0.05. Consistency of genotype frequencies with Hardy–Weinberg equilibrium was tested on a contingency table of observed and expected genotype frequencies using the Markov simulation-based goodness of fit.
Results
Demographic and clinical characterization of the studied groups
Some demographic and basic laboratory parameters of patients and controls are shown in Table 2. Patients and controls were matched as regards age and BMI. However, as compared with normal subjects, patients had significantly higher plasma fibrinogen levels (2.5 ± 1.2 vs. 3.9 ± 1. 6, respectively; P < 0.001), D-dimer levels (151.0 ± 81.1 vs. 549.5 ± 101.2, respectively; P = 0.007) and TAFI levels (91.1 ± 19.2 vs. 101.3 ± 24.4, respectively; P = 0.004). No significant difference in TAFI antigen levels was observed in relation to the plasma fibrinogen levels and D-dimer values.
Table 2.
Some demographic and laboratory data of study subjects.
| Parameter | Controls (n = 150) (mean ± SD) |
Patients (n = 150) (mean ± SD) |
P value |
|---|---|---|---|
| Age (years) | 50.2 ± 4.2 | 52.1 ± 3.8 | 0.223 |
| BMI (kg/m2) | 26.3 ± 3.2 | 27.1 ± 3.5 | 0.275 |
| White blood cell (X103/µl) | 6.7 ± 1.6 | 6.5 ± 1.8 | 0.152 |
| ALT (U/l) | 29.7 ± 3.1 | 31.4 ± 2.9 | 0.417 |
| AST (U/l) | 27.2 ± 7.1 | 28.7 ± 6.8 | 0.599 |
| Albumin (g/l) | 44.0 ± 5.8 | 43.9 ± 6.1 | 0.539 |
| Serum creatinine (μmol/l) | 84.5 ± 11.9 | 85.3 ± 11.1 | 0.397 |
| Blood urea nitrogen (mmol/l) | 6.3 ± 2.0 | 6.8 ± 2.3 | 0.089 |
| PT (s) | 10.7 ± 2.4 | 11.1 ± 2.1 | 0.104 |
| APTT (s) | 31.4 ± 4.5 | 30.1 ± 4.8 | 0.432 |
| Fibrinogen (g/l) | 2.5 ± 1.2 | 3.9 ± 1.6 | 0.000⁎ |
| D-Dimer (ng/ml) | 151.0 ± 81.1 | 549.5 ± 101.2 | 0.007⁎ |
| TAFI Ag (%) | 91.1 ± 19.2 | 101.3 ± 24.4 | 0.004⁎ |
n; number, BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PT, prothrombin time; APTT, activated partial thromboplastin time; TAFI Ag, thrombin-activatable fibrinolysis inhibitor antigen.
P < 0.05.
Genotype and allele frequencies among studied groups
The genotype and allele distributions for the TAFI Thr325Ile polymorphism of the study groups were shown in Table 3. The genotype distribution of both studied groups fits the Hardy–Weinberg equilibrium estimated with a χ2 test (P > 0.05). The genotypes carrying the high risk allele [Thr/Ile (CT) and Ile/Ile (TT)] were significantly more frequent in patients compared to control group (50.0% and 22.0% vs. 42.0% and 13.0%, respectively) and were also associated with developing BC in the studied patients (OR: 2.08; [95% CI: 1.3–3.5]; P = 0.002, Table 3). BC patients were stratified into those in early stages and those in late stages. When compared with controls, there was statistically significant difference only between early BC group and controls regarding the different TAFI Thr325Ile genotypes and alleles frequencies (Table 3). However, there was no statistically significant difference in genotype and allele distribution between early stage and late stage BC patients (P > 0.05).
Table 3.
Genotype and allele frequencies of TAFI Thr325Ile polymorphism in studied subjects.
| TAFI Genotypes | Controls (n = 150) |
BC patients (n = 150) |
Odds ratio (95% CI) |
P a value | Early stage BC patients (n = 80) |
Odds ratio (95% CI) |
P a value | Late stage BC patients (n = 70) |
Odds ratio (95% CI) | P a value |
|---|---|---|---|---|---|---|---|---|---|---|
| No (%) | No (%) | No (%) | No (%) | |||||||
| Thr/Thr (CC) | 67 (44.7) | 42 (28.0) | 1(Ref.) | 17 (21.2) | 1(Ref.) | 25 (35.7) | 1(Ref.) | |||
| Thr/Ile (CT) | 63 (42.0) | 75 (50.0) | 1.9 (1.1–3.3) | 0.01⁎ | 43 (53.8) | 2.7 (1.4–5.2) | 0.003⁎ | 32 (45.7) | 1.4 (0.7–2.5) | 0.334 |
| Ile/Ile (TT) | 20 (13.3) | 33 (22.0) | 2.6 (1.3–5.5) | 0.01⁎ | 20 (25.0) | 3.9 (1.7–8.9) | < 0.001⁎ | 13 (18.6) | 1.7 (0.8–4.0) | 0.191 |
| Thr/Ile (CT) + Ile/Ile (TT) | 83 (55.3) | 108 (72.0) | 2.08 (1.3–3.5) | 0.002⁎ | 63 (78.8) | 2.9 (1.6–5.6) | < 0.001⁎ | 45 (64.3) | 1.5 (0.8–2.6) | 0.210 |
| TAFI alleles | ||||||||||
| C | 197 (66) | 159 (53) | 1.7 (1.2–2.4) | 0.001⁎ | 77 (48.1) | 2.1 (1.4–3.0) | < 0.001⁎ | 82 (58.6) | 1.4 (0.9–2.0) | 0.151 |
| T | 103 (34) | 141 (47) | 83 (51.9) | 58 (41.4) |
The TAFI genotype's reference group was CC and the TAFI allele's reference group was allele C. TAFI, thrombin-activatable fibrinolysis inhibitor; N, number; Ref., reference; *Chi-square test is statistically significant at confidence interval (CI) 95%; a When compared with controls. P value was statistically non-significant when early stage BC patients compared to late stage group by the genotype and allele frequencies.
TAFI Thr325Ile genotypes and Ag levels among study groups
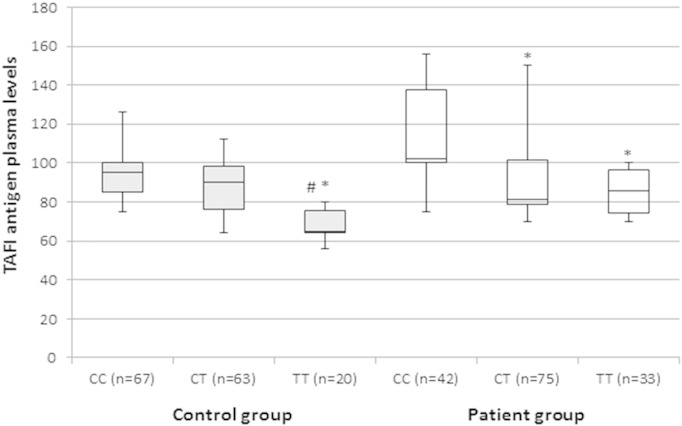
TAFI Thr325Ile polymorphism was significantly correlated with TAFI antigen levels with the C/C genotype corresponding to the highest and the T/T genotype with the lowest TAFI antigen levels in each group (overall P < 0.001, Fig. 1). Individuals with TT genotype, in addition, showed a significant decrease in plasma TAFI levels as compared with those having CT genotypes in each studied group.
Fig. 1.
Correlation between TAFI antigen plasma levels and different TAFI Thr325Ile genotypes among study groups. Box and Whisker plot of the TAFI (thrombin-activatable fibrinolysis inhibitor) Ag levels; expressed as percentage of normal pooled plasma, among control (gray box) and patient (white box) groups. The median and interquartile range (25th–75th percentile), are shown as solid horizontal lines. Comparisons were performed by Kruskal–Wallis test followed by Tukey multiple comparison test for each group. *, indicates significant difference compared to CC carriers in the same group at P < 0.05. #, indicates significant difference compared to CT carriers in the same group at P < 0.05.
The relation of TAFI Thr325Ile polymorphism and Ag levels to clinicopathological parameters of patient group
No significant differences in the genotype distributions were found in the different subgroup analyses (including tumor stage, prognosis and risk of recurrence, etc.) for the TAFI Thr325Ile polymorphism (Supplementary Table 1). However, Patients presented with advanced tumor stage (III and IV) and poorly differentiated tumor, had significantly higher levels of plasma TAFI Ag (P = 0.02 and P = 0.01, respectively). Regarding the receptor status, higher TAFI Ag levels were significantly associated with HER2+ tumors in comparison with other biological sub-types like luminal A, B and basal-like tumors (P = 0.01). Higher levels of TAFI Ag were also observed in patients with poor prognosis with 5-year survival rates of 50% (either estimated with NPI or IHPI), and those with higher recurrence risk > 50% (P = 0.04 and P < 0.001, respectively; Table 4).
Table 4.
The relationship between plasma TAFI levels among different TAFI genotypes and different clinicopathological parameters of breast cancer patients.
| Patient characteristics |
TAFI level by Thr325Ile genotypes |
||
|---|---|---|---|
| CC |
CT + TT |
Total |
|
| (n = 42) | (n = 108) | (n = 150) | |
| Menopausal state | |||
| Premenopausal | 100 (75–126) | 80 (71–126) | 58 |
| Postmenopausal | 130 (86–156) | 84 (70–150) | 92 |
| First degree family history | |||
| Negative | 102 (75–156) | 86 (70–150) | 140 |
| Positive | 0 | 79.5 (78–93) | 10 |
| Histological type of Breast cancer* | |||
| Duct carcinoma | 102 (86–156) | 79 (70–124) | 110 |
| Lobular carcinoma | 115 (75–156) | 100 (75–175) | 23 |
| Mixed duct and lobular | 94 (86–102) | 120 (86–125) | 10 |
| Poorly differentiated tumor | 143 (130–155) | 120 (100–140) | 7 |
| Biological sub-type of Breast cancer* | |||
| Luminal A | 100 (75–156) | 79.5 (71–124) | 70 |
| Luminal B | 0 | 89.5 (76–120) | 10 |
| Basal-like | 110 (86–156) | 84 (70–107) | 52 |
| HER2+ | 137 (130–145) | 126 (79–150) | 18 |
| Tumor grade | |||
| GI | 0 (0) | 82 (75–100) | 12 |
| GII | 101 (75–156) | 80 (70–124) | 95 |
| GIII | 137 (86–156) | 100 (75–150) | 43 |
| Tumor stage* | |||
| Early (I, IIA and IIB) | 102 (100–126) | 79 (70–124) | 80 |
| Late (IIIA, IIIC and IV) | 115 (75–156) | 95 (71–150) | 70 |
| NPI* | |||
| Good prognosis | 105 (101–110) | 80 (70–100) | 37 |
| Moderate prognosis | 100 (75–156) | 79 (70–124) | 70 |
| Poor prognosis | 137 (86–156) | 100 (75–150) | 43 |
| IHPI* | |||
| Good prognosis | 100 (75–156) | 80 (71–124) | 80 |
| Moderate prognosis | 110 (86–156) | 84 (70–107) | 52 |
| Bad prognosis | 138 (130–145) | 126 (79–150) | 18 |
| Risk of recurrence** | |||
| Intermediate | 130 (100–126) | 100 (79–150) | 100 |
| High | 115 (75–156) | 79 (70–124) | 50 |
TAFI (thrombin-activatable fibrinolysis inhibitor) Ag levels were expressed as percentage of normal pooled plasma (NPP); data are presented as median (range); HER2, human epidermal growth factor receptor 2; NPI, Nottingham Prognostic Index; IHPI, Immunohistochemical Prognostic Index. Comparisons were performed by Kruskal–Wallis test followed by Tukey multiple comparison test for each group in case of more than two subgroup analysis. *, indicates a statistically significant difference P < 0.05. **, indicates statistically significantly difference P < 0.001.
Discussion
Malignant transformation, the net outcome of oncogene activation and suppressor gene inactivation, also induces the genes which control hemostasis as activation of coagulation confers a selective survival advantage on tumor cells (Boccaccio and Comoglio, 2009).
TAFI is a potent direct modulator of fibrinolysis and indirect regulator of plasmin formation and as such could possibly participate in events leading to tumor development and metastasis (Bouma and Meijers, 2003). The contribution of TAFI polymorphisms to cancer development has not been examined so far.
In the present study, we found that TAFI Thr325Ile polymorphism was associated with BC, with the genotypes carrying the T allele [Thr/Ile (CT) and Ile/Ile (TT)] occurring more frequently in patients with BC (OR = 2.08, 95% CI 1.3–3.5 for combined genotypes; P = 0.002). When patients stratified into early stage BC and a late one, significant association was more evident with the former subgroup. In the literature, the reasons for an association with early-stage cancer than late-stage one are unknown. However, it is possible that some unknown genetic factors may contribute to these different propensities with BC stages (Han et al., 2004). Some of the genes, in addition, show age-specific effects. These can confer relative risks that decrease with age (Easton, 1999). As our late stage BC patients were older than early stage one, this assumption will need further studies to clarify.
This finding was in agreement with Chengwei et al. (2013) who reported similar results in their Chinese Han patients. Otherwise, up to our knowledge, no data are available on the frequency of this variant in other populations of BC patients. In contrast, Vairaktaris et al. (2007), observed lack of association between this polymorphism and their 150 European patients with oral squamous cell carcinoma, but this is not necessarily in contradiction to our results, as the etiology of malignant diseases is multifaceted and differs strongly between different cancers.
Regarding the effect of TAFI Thr325Ile polymorphism genotypes on TAFI Ag concentration, it was similar in our cases and controls. It was significantly correlated with TAFI antigen levels with the CC genotype corresponding to the highest and the TT genotype to the lowest TAFI antigen levels. This is in accordance with that plasma TAFI concentration is almost entirely genetically determined (Henry et al., 2001) with a low contribution of measured environmental factors (Frère et al., 2006). However, we should mention that great care should be taken with the interpretation and comparison of current reported results as the commercially available ELISA kit used in TAFI estimation is genotype artifact-dependent as shown in Heylen et al. (2011). The measurement of 325Thr is higher than 325Ile genotypes, due to the specificity of monoclonal antibody, but as the results presented here show higher levels related to 325Ile genotype in patients, this finding is very reasonable, and even it can be underestimated.
Breast cancer patients showed a significant increase in their plasma TAFI levels, when compared with the control group; contributing to the fibrinolytic deficiency in these patients and reflecting their thrombotic state (Chengwei et al., 2013, Florian-Kujawski et al., 2004, Kaftan et al., 2011). This finding runs in-line with disease progression, as the fibrin accumulation in the tumor could increase endothelial motility, worsen angiogenesis, and contribute to an increased risk of thrombosis in breast cancer patients (Byrne et al., 2007, Dirix et al., 2002). Otherwise, this finding disagreed with Kotschy et al. (2010), with partial explanation for this discrepancy could be related to difference in study population ethnicity (affect gene-gene and gene-environment interactions) and the methods used to measure plasma TAFI, if we excluded from the first the confounder effect of the very small sample size of this study (27 patients with colon cancer, 37 women with BC and 20 healthy persons).
The D-dimer and fibrinogen levels of the patient group in this study were also statistically significantly higher than that of the control group which likely reflects the biology of the underlying tumor (Knowlson et al., 2010). D-dimer assessment constitutes a first attempt to consider a product of fibrin degradation as a specific marker related to the extent of disease in human breast cancer (Benoy et al., 2005). Plasma D-dimer levels have been correlated with an enhanced progression of the disease and a reduced overall survival in metastatic breast cancer (Dirix et al., 2002). Its level reflects the hypercoagulability and the fibrinolytic system activity and was significantly increased with fibrinogen levels in newly diagnosed breast cancer patients compared with controls in many previous studies (Batschauer et al., 2010, Yigit et al., 2008) and in patients with invasive carcinoma compared with those patients with either benign breast disease or carcinoma-in-situ (Blackwell et al., 2000).
The association of higher plasma TAFI levels and plasma TAFI lowering alleles (TT) in BC patients than controls; a finding that is opposite to what might be expected, could be attributed to that TAFIa has no known physiologic inhibitors and consequently, its primary regulatory mechanism involves its intrinsic thermal instability. The rate of TAFI activation and stability of the active form function in maintaining its concentration above the threshold value required to down-regulate fibrinolysis (Foley et al., 2013). In the case of the Ile-325 variant of TAFI, changing the Thr residue which is hydrophilic in nature to a hydrophobic Ile residue may diminish the effect of the favorable entropic change that accompanies the inactivation process contributing to TAFIa stabilization. Activated TAFI–Thr325, in addition, has a normal in vitro half-life of 8 min (at 37 °C), whereas activated TAFI–Ile325 has a half-life of about 16 min and a 60% greater antifibrinolytic activity compared to TAFI–Thr325. Possibly, this increased stability and half-life of TAFIa compensates for the effect of TAFI lowering alleles (Kamal et al., 2011). We should consider also that under physiological conditions, liver cells are the main source of TAFI production and during inflammation TAFI acts as an acute reactant protein. In addition to hepatic cells, adipocytes, endothelial and epithelial cells may also produce and secrete TAFI in vitro. Inflammatory cytokines released by malignant cells, in addition, may stimulate the production and secretion of TAFI from liver or vascular endothelial cells and thus augment its systemic circulation (Hataji et al., 2004).
As the risk of VTE is higher in patients with distant metastases (Dickmann et al., 2013) and increases with advancing stages of BC (Amer, 2013, Chew et al., 2006), we evaluated TAFI Thr325Ile polymorphism and TAFI level at various stages of BC and correlated with other clinicopathological parameters to clarify their roles in relation to BC. There was lack of association between TAFI Thr325Ile polymorphism and all evaluated parameters as tumor type, grade, stage and receptor status of breast tissue (P > 0.05). However, BC patients with advanced state (those who have an advanced tumor stage and poorly differentiated type of cancer), bad prognosis and a higher rate of recurrence showed significantly higher plasma TAFI Ag levels. This could be explained by that excessive host fibrin formation as a result of increasing TAFI levels, favors tumor growth, progression, angiogenesis and hematogenous metastasis (Falanga and Rickles, 1999), and contributes to an increased risk of thrombosis in patients with breast cancer (Dirix et al., 2002). As a selective advantage for cancer cells, fibrin provides a scaffold for anchorage and invasion promoting invasive growth (Boccaccio and Comoglio, 2009).
As a limitation of our study should be considered is that identifying a significant association of genetic variation with diseases as BC may need a larger sample size. Our relatively small sample size has an advantage in the low age of participants as breast cancer risk has also been dependent on age (Hirko et al., 2013) and could have an impact on SNP analysis. The present study, in addition, fulfills most of the criteria of a good genetic association study (Hattersley and McCarthy, 2005) and the first one relates this polymorphism to BC in our region.
In conclusion, TAFI Thr325Ile SNP was associated with BC susceptibility in Egyptian females. The current results supported the relation between this polymorphism and plasma TAFI levels. Moreover, TAFI levels may be closely related to tumor progression and may serve as a predictor of poor prognosis in patients with BC. Many further studies, including follow-up one of our patients and controls on larger cohorts will be required to evaluate the prognosis and outcome related to the studied SNP and its level as well as to examine the efficacy of preventive measures in BC patients with a high TAFI plasma level before any immediate rush to use this information to give individual predictions of prognosis. Future studies, in addition, are necessary to elucidate whether TAFI levels (measured by highly specific genotype artifact-independent assay) could be one of the potential markers of tumor response to treatment.
The following is the supplementary data related to this article.
TAFI Thr325Ile polymorphism and clinicopathological parameters of breast cancer patients.
Funding
None.
References
- Akinci B. Role of thrombin activatable fibrinolysis inhibitor in endocrine and cardiovascular disorders: an update. Recent. Patents Endocr. Metab. Immune. Drug Discov. 2012;6(3):210–217. doi: 10.2174/187221412802481748. [DOI] [PubMed] [Google Scholar]
- Amer M.H. Cancer-associated thrombosis: clinical presentation and survival. Cancer Manag. Res. 2013;5:165–178. doi: 10.2147/CMAR.S47094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschauer A.P. D-dimer as a possible prognostic marker of operable hormone receptor-negative breast cancer. Ann. Oncol. 2010;21(6):1267–1272. doi: 10.1093/annonc/mdp474. [DOI] [PubMed] [Google Scholar]
- Benoy I.H. Relative microvessel area of the primary tumor, and not lymph node status, predicts the presence of bone marrow micrometastases detected by reverse transcriptase polymerase chain reaction in patients with clinically non-metastatic breast cancer. Breast Cancer Res. 2005;7:R210–R219. doi: 10.1186/bcr980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell K. Plasma D-dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status. J. Clin. Oncol. 2000;18(3):600–608. doi: 10.1200/JCO.2000.18.3.600. [DOI] [PubMed] [Google Scholar]
- Boccaccio C., Comoglio P.M. Genetic link between cancer and thrombosis. J. Clin. Oncol. 2009;27:4827–4833. doi: 10.1200/JCO.2009.22.7199. [DOI] [PubMed] [Google Scholar]
- Boffa M., Reid T., Joo E., Nesheim M., Koschinsky M. Characterization of the gene encoding human TAFI (thrombin-activable fibrinolysis inhibitor; plasma procarboxypeptidase B) Biochemistry. 1999;38:6547–6558. doi: 10.1021/bi990229v. [DOI] [PubMed] [Google Scholar]
- Bouma B.N., Meijers J.C. Thrombin-activatable fibrinolysis inhibitor (TAFI, plasma procarboxypeptidase B, procarboxypeptidase R, procarboxypeptidase U) J. Thromb. Haemost. 2003;1(7):1566–1574. doi: 10.1046/j.1538-7836.2003.00329.x. [DOI] [PubMed] [Google Scholar]
- Brouwers G.-J. A novel, possibly functional, single nucleotide polymorphism in the coding region of the thrombin-activatable fibrinolysis inhibitor (TAFI) gene is also associated with TAFI levels. Blood. 2001;98:1992–1993. doi: 10.1182/blood.v98.6.1992. [DOI] [PubMed] [Google Scholar]
- Byrne G.J., McDowell G., Agarawal R., Sinha G., Kumar S., Bundred N.J. Serum vascular endothelial growth factor in breast cancer. Anticancer Res. 2007;27:3481–3488. [PubMed] [Google Scholar]
- Caine G.J., Stonelake P.S., Lip G.Y., Kehoe S.T. Hypercoagulable state of malignancy. Neoplasia. 2002;4(6):465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chengwei X. Plasma thrombin-activatable fibrinolysis inhibitor levels and its Thr325Ile polymorphism in breast cancer. Blood Coagul. Fibrinolysis. 2013;24(7):698–703. doi: 10.1097/MBC.0b013e3283610381. [DOI] [PubMed] [Google Scholar]
- Chew H.K., Wun T., Harvey D.J., Zhou H., White R.H. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J. Clin. Oncol. 2006;25(1):70–76. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- Dickmann B. Regional lymph node metastases are a strong risk factor for venous thromboembolism: results from the Vienna Cancer and Thrombosis Study. Haematologica. 2013;98(8):1309–1314. doi: 10.3324/haematol.2012.073338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipasco P.J., Misra S., Koniaris L.G., Moffat F.L. Thrombophilic state in cancer, part I: biology, incidence, and risk factors. J. Surg. Oncol. 2011;104(3):316–322. doi: 10.1002/jso.21925. [DOI] [PubMed] [Google Scholar]
- Dirix L.Y. Plasma fibrin D-dimer levels correlate with tumour volume, progression rate and survival in patients with metastatic breast cancer. Br. J. Cancer. 2002;86:389–395. doi: 10.1038/sj.bjc.6600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton D.F. How many more breast cancer predisposition genes are there? Breast Cancer Res. 1999;1:14–17. doi: 10.1186/bcr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicheinger S. Thrombin-activatable fibrinolysis inhibitor and the risk for recurrent venous thromboembolism. Blood. 2004;103:3773–3776. doi: 10.1182/blood-2003-10-3422. [DOI] [PubMed] [Google Scholar]
- Falanga A., Rickles F.R. Pathophysiology of the thrombophilic state in the cancer patient. Semin. Thromb. Hemost. 1999;25(2):173–182. doi: 10.1055/s-2007-994919. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Héry C., Autier P., Sankaranarayanan R. Global burden of breast cancer. In: Li C., editor. Breast Cancer Epidemiology. Springer; New York, NY: 2010. pp. 1–19. [Google Scholar]
- Florian-Kujawski M.R. 2004 ASCO Annu. Meet. Proc. Post-Meeting Edition. Vol. 22. 2004. Role of PAI-1 and TAFI in the mediation of fibrinolytic deficit in cancer patients; p. 9727. (J. Clin. Oncol.). (Suppl.) [Google Scholar]
- Foley J.H., Kim P.Y., Mutch N.J., Gils A. Insights into thrombin activatable fibrinolysis inhibitor function and regulation. J. Thromb. Haemost. 2013;11(Suppl. 1):306–315. doi: 10.1111/jth.12216. [DOI] [PubMed] [Google Scholar]
- Frère C. Fine mapping of quantitative trait nucleotides underlying thrombin-activatable fibrinolysis inhibitor antigen levels by a transethnic study. Blood. 2006;108:1562–1568. doi: 10.1182/blood-2006-01-008094. [DOI] [PubMed] [Google Scholar]
- Galea M., Blamey R., Elston C., Ellis I. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res. Treat. 1992;22(3):207–219. doi: 10.1007/BF01840834. [DOI] [PubMed] [Google Scholar]
- Guerra I., Algorta J., Díaz de Otazu R., Pelayo A., Fariña J. Immunohistochemical prognostic index for breast cancer in young women. Mol. Pathol. 2003;56(6):323–327. doi: 10.1136/mp.56.6.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W. Associations between breast cancer susceptibility gene polymorphisms and clinicopathological features. Clin. Cancer Res. 2004;10:124–130. doi: 10.1158/1078-0432.ccr-0834-3. [DOI] [PubMed] [Google Scholar]
- Hataji O. Increased circulating levels of thrombin-activatable fibrinolysis inhibitor in lung cancer patients. Am. J. Hematol. 2004;76(3):214–219. doi: 10.1002/ajh.20079. [DOI] [PubMed] [Google Scholar]
- Hattersley A.T., McCarthy M.I. What makes a good genetic association study? Lancet. 2005;366(9493):1315–1323. doi: 10.1016/S0140-6736(05)67531-9. [DOI] [PubMed] [Google Scholar]
- Henry M. Identification of polymorphisms in the promoter and the 3/ region of the TAFI gene: evidence that plasma antigen levels are strongly genetically controlled. Blood. 2001;97:2053–2058. doi: 10.1182/blood.v97.7.2053. [DOI] [PubMed] [Google Scholar]
- Heylen E., Willemse J.L., Hendriks D.F. Comparative study of commercially available procarboxypeptidase U (thrombin-activatable fibrinolysis inhibitor) assays. J. Thromb. Haemost. 2011;9:1407–1409. doi: 10.1111/j.1538-7836.2011.04325.x. [DOI] [PubMed] [Google Scholar]
- Hirko K.A. Trends in Breast Cancer Incidence Rates by Age and Stage at Diagnosis in Gharbiah, Egypt, over 10 Years (1999–2008) J. Cancer Epidemiol. 2013;2013 doi: 10.1155/2013/916394. (7 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa O. Prognostic factors in breast cancer: the value of the Nottingham prognostic index for patients treated in a single institution. Surg. Today. 2005;35:907–911. doi: 10.1007/s00595-005-3056-x. [DOI] [PubMed] [Google Scholar]
- Hussein O. Hormone receptors and age distribution in breast cancer patients at a university hospital in Northern Egypt. Breast Cancer Basic Clin. Res. 2013;7:51–57. doi: 10.4137/BCBCR.S12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaftan O., Kasapoglu B., Koroglu M., Kosar A., Yalcin S.K. Thrombinactivatable fibrinolysis inhibitor in breast cancer patients. Med. Princ. Pract. 2011;20:332–335. doi: 10.1159/000324547. [DOI] [PubMed] [Google Scholar]
- Kamal H.M., Ahmed A.S., Fawzy M.S., Mohamed F.A., Elbaz A.A. Plasma thrombin-activatable fibrinolysis inhibitor levels and Thr325Ile polymorphisms as a risk marker of myocardial infarction in Egyptian patients. Acta Cardiol. 2011;66:483–488. doi: 10.1080/ac.66.4.2126597. [DOI] [PubMed] [Google Scholar]
- Kataja V., Castiglione M. Primary breast cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2009;20(4):iv10–iv14. doi: 10.1093/annonc/mdp114. [DOI] [PubMed] [Google Scholar]
- Knowlson L., Bacchu S., Paneesha S., McManus A., Randall K., Rose P. Elevated D-dimers are also a marker of underlying malignancy and increased mortality in the absence of venous thromboembolism. J. Clin. Pathol. 2010;63(9):818–822. doi: 10.1136/jcp.2010.076349. [DOI] [PubMed] [Google Scholar]
- Kotschy M., Witkiewicz W., Freier B., Dubis J., Kotschy D. Thrombin activatable fibrinolysis inhibitor (TAFI) in blood of patients with colon and breast cancer. Contemp. Oncol. 2010;14(4):248–252. [Google Scholar]
- Leebeek F.W. High functional levels of thrombin activatable fibrinolysis are associated with increased risk of first ischemic stroke. J. Thromb. Haemost. 2005;3:2211–2218. doi: 10.1111/j.1538-7836.2005.01484.x. [DOI] [PubMed] [Google Scholar]
- Lowery A.J., Kell M.R., Glynn R.W., Kerin M.J., Sweeney K.J. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res. Treat. 2012;133(3):831–841. doi: 10.1007/s10549-011-1891-6. [DOI] [PubMed] [Google Scholar]
- Martini C.H. The effect of genetic variants in the thrombin activatable fibrinolysis inhibitor (TAFI) gene on TAFI-antigen levels, clot lysis time and the risk of venous thrombosis. Br. J. Haematol. 2006;134(1):92–94. doi: 10.1111/j.1365-2141.2006.06117.x. [DOI] [PubMed] [Google Scholar]
- Quanto Software http://hydra.usc.edu/gxe/ (Last accessed April 1st 2014)
- Rakha E.A. Prognostic significance of Nottingham Histologic Grade in invasive breast carcinoma. J. Clin. Oncol. 2008;26(19):3153–3158. doi: 10.1200/JCO.2007.15.5986. [DOI] [PubMed] [Google Scholar]
- Schneider M., Boffa M., Stewart R., Rahman M., Koschinsky M., Nesheim M. Two naturally occurring variants of TAFI (Thr-325 and Ile-325) differ substantially with respect to thermal stability and antifibrinolytic activity of the enzyme. J. Biol. Chem. 2002;277:1021–1030. doi: 10.1074/jbc.M104444200. [DOI] [PubMed] [Google Scholar]
- Singletary S.E., Connolly J.L. breast cancer staging: working with the sixth edition of the AJCC cancer staging manual. CA Cancer J. Clin. 2006;56:37–47. doi: 10.3322/canjclin.56.1.37. [DOI] [PubMed] [Google Scholar]
- Ta'ssies D. Thrombin-activatable fibrinolysis inhibitor genetic polymorphisms as markers of the type of acute coronary syndrome. Thromb. Res. 2009;124:614–618. doi: 10.1016/j.thromres.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Tregouet D.A. Activated thrombin-activatable fibrinolysis inhibitor levels are associated with the risk of cardiovascular death in patients with coronary heart disease: the AtheroGene study. J. Thromb. Haemost. 2009;7(1):49–57. doi: 10.1111/j.1538-7836.2008.03221.x. [DOI] [PubMed] [Google Scholar]
- Vairaktaris E. The 1040C/T polymorphism influencing thermal stability and activity of thrombin activatable fibrinolysis inhibitor is associated with risk for oral cancer. Am. J. Hematol. 2007;82(11):1010–1012. doi: 10.1002/ajh.20985. [DOI] [PubMed] [Google Scholar]
- Watanabe R. Activity and antigen levels of thrombin activatable fibrinolysis inhibitor in plasma of patients with disseminated intravascular coagulation. Thromb. Res. 2001;104(1):1–6. doi: 10.1016/s0049-3848(01)00331-0. [DOI] [PubMed] [Google Scholar]
- Yigit E., Gönüllü G., Yücel İ., Turgut M., Erdem D., Çakar B. Relation between hemostatic parameters and prognostic/predictive factors in breast cancer. Eur. J. Intern. Med. 2008;19(8):602–607. doi: 10.1016/j.ejim.2007.06.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TAFI Thr325Ile polymorphism and clinicopathological parameters of breast cancer patients.