Abstract
Purpose
Assessing the frequency and evaluating the efficacy and safety of Neodymium:Yttrium Aluminum Garnet (Nd:YAG) Laser goniopuncture (LGP) following nonpenetrating deep sclerectomy (NPDS).
Design
Retrospective cohort study.
Patients and methods
We retrospectively reviewed the outcome of 197 eyes of 153 patients with open angle glaucoma who underwent either NPDS or NPDS combined with cataract extraction between January 2005 and September 2010 at King Abdulaziz University Hospital (KAUH). Both demographic and clinical data were retrieved and analysed.
Results
Goniopuncture (GP) was needed in 48 (24.4%) of the eyes which had NPDS or NPDS with cataract extraction after a mean post operative interval of 9.78 (±11.16) months. The mean IOP had significantly decreased from 23.3 (±5.9) mmHg prior to Nd:YAG LGP procedure to 14.6 (±4.4) mmHg at the last post-procedure assessment. At the last follow-up; Nd:YAG LGP was successful in controlling IOP in 27 eyes (56.3%). Mean Nd:YAG LGP failure time was 6.04 (±5.80) months. Young age (<50 years) (p = 0.001); type of glaucoma (secondary versus primary open angle, p = 0.0258) and the use of drainage implant (p = 0.038) were the identified predicting factors for the need of Nd:YAG LGP. Complications following Nd:YAG LGP occurred in 5 eyes (iris touch to TDM (4.2%), Hyphema (2.1%), hypotony maculopathy (2.1%) and choroidal detachment (2.1%).
Conclusions
LGP is an efficient IOP lowering procedure after NPDS, when it is indicated. It is a simple and noninvasive procedure. However, certain precautions should be taken to avoid complications.
Keywords: Goniopuncture, Nd:YAG, Deep Sclerectomy, Glaucoma
Introduction
Trabeculectomy is a penetrating filtration surgery widely used for the surgical treatment of medically uncontrolled glaucoma. The penetrating nature of this procedure may result in several operative and postoperative complications.1 In an attempt to lower the incidence of such complications, non-penetrating glaucoma surgery (NPGS) was developed. This procedure is designed to avoid full thickness penetration into the anterior chamber and consequently, minimize the risk of complications commonly encountered with the standard trabeculectomy. The NPGS was described by Krasnov in the late 1960s.2 He suggested deroofing Schlemm’s canal (SC) aiming to lower the intraocular pressure (IOP). However, the effect of such procedure was relatively short, and required a long learning curve. In addition, classic trabeculectomy was introduced in the same era and it was easier to perform, had higher efficacy and longer longevity. As a result, the popularity of NPGS was quite limited. In the 1980s, NPGS started to gain popularity again, and different techniques with several modifications were described.3–10
Nonpenetrating deep sclerectomy (NPDS) is one of the NPGS procedures, commonly used for surgical management of open angle glaucoma. In NPDS, removal of a deep scleral flap leads to the formation of an empty scleral space known as ‘the decompression space’, where the aqueous humour gets collected before its drainage.11 The literature reports that the aqueous outflow resistance is mainly located in the Trabecular Meshwork (TM), with approximately 75% of this resistance both in normal and abnormal glaucomatous eyes occurring in the juxtacanalicular TM and the inner wall of SC.12 Therefore, NPDS was developed to address this resistance through deroofing SC and peeling its floor, together with the juxtacanalicular TM and part of the corneoscleral layers of TM. As a consequence, aqueous drainage occurs at the level of the posterior trabeculum through the remaining corneoscleral TM and the intact uveal part of the TM (Trabeculo-Descemet’s Membrane, TDM).3,13,14 Physiologically, the TDM acts as an outflow resistance site, allowing gradual decrease of IOP which in turn precludes the sudden hypotony that usually occurs after trabeculectomy.15,16 Several routes have been suggested for the aqueous drainage from the decompression space. One of the potential routes is the subconjunctival space as demonstrated by the presence of a filtering bleb in the majority of successful cases.13,17 Other suggested routes are drainage through the suprachoroidal space and through a transcleral pathway. Furthermore, aqueous may enter the SC to be drained by the episcleral venous plexus.13 To facilitate IOP lowering efficacy, space-maintaining devices in NPDS were introduced. These devices are used to reduce scar formation and to keep the decompression space open during the time of maximal healing, which consequently enhances the drainage process. Nowadays, different absorbable and nonabsorbable; expensive and low cost; animal-, chemical-based and synthetic space-maintaining devices are being used.13 Examples of the commonly used devices are: collagen implants (STAAR® Surgical Company, California, USA), reticulated hyaluronic acid implant (SKGel™, Corneal Laboratories, Paris, France), nonabsorbable hydrophilic acrylic implant (T-flux®, loltech, La Rochelle, France), and viscoelastic implant (e.g. Healaflow, Anteis S.A., Geneva, Switzerland).
In some patients, the TDM may demonstrate an increased resistance to aqueous outflow either in the early or late post operative period causing elevation of IOP.14 The insufficient passage of aqueous humour through the TDM is usually due to fibrosis developed as a part of the TDM healing process or due to excessive deposition of debris and or pigments (Fig. 1). Goniopuncture is effective when underfiltration is due to poor TDM functionality and not due to other causes such as poor dissection plane, excessive bleb fibrosis or other more serious causes.13 Additionally, it is considered as a minor follow up procedure equivalent to postoperative scleral flap suture lysis after trabeculectomy.
Figure 1.

Gonioscopic view of the trabeculo-Descemet’s membrane with clear deposition of pigments and debris indicating the area of filtration.
Laser goniopuncture (LGP) converts deep sclerectomy from being a nonpenetrating procedure to a penetrating one, but with fewer complications compared to penetrating glaucoma surgery.
In the present study, we assessed the frequency, efficacy and safety of Nd:YAG LGP following NPDS performed at glaucoma service unit, King Abdul-Aziz University Hospital (KAUH), King Saud University, Riyadh, Kingdom of Saudi Arabia.
Materials and methods
In the current retrospective cohort study, 153 charts of patients who underwent a NPDS or NPDS with cataract extraction for medically uncontrolled primary or secondary open-angle glaucoma between January 2005 and September 2010 were reviewed. Our exclusion criteria included congenital glaucoma cases. However, cases with previous ocular surgery such as filtering procedure or cataract extraction were not excluded. Indications for surgery were uncontrolled glaucoma, which was defined as an intraocular pressure (>21 mmHg) with maximum tolerated anti-glaucoma medication, progressive glaucomatous visual field loss and/or progressive optic disk cupping. Mitomycin C was used in all the cases. Steps of our NPDS technique were as follows: after a fixation suture (a 4–0 silk suture to the superior rectus muscle or a 6–0 vicryl suture to the superior cornea) had been placed, a fornix-based conjunctival flap was fashioned, and a 5 × 5 mm one-third sclera thickness superficial flap was created, which extended 1.5 mm into the clear cornea. A sponge soaked in Mitomycin C solution 0.2 mg/ml was placed under the superficial scleral flap and Tenon’s capsule for 2 min, then thorough irrigation with 20 cm3 balanced salt solution was performed to wash the surgical site. A 4 × 4 deep scleral flap was created leaving only a very thin layer (50–70 μm) of scleral tissue over the uvea. Dissection was carried out from the posterior part of the flap and extended anteriorly to deroof SC spontaneously. Dissection continued anteriorly to create the TDM. The floor of SC was peeled off with fine-toothed forceps (Fig. 2) and the deep flap was excised.
Figure 2.
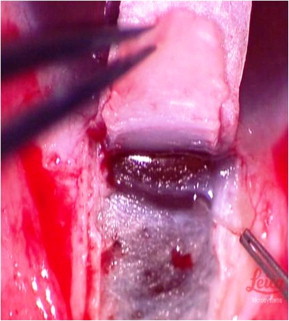
Peeling of the floor of the Schlemm’s canal with fine-toothed forceps.
If an implant was used, it was then placed in the floor of the excised deep flap (Fig. 3). The superficial scleral flap was then secured with 10–0 nylon sutures at the posterior corners. The conjunctival flap was closed in a watertight fashion. An Implant drainage device, such as T-flux® (loltech, La Rochelle, France), SKGel™ (Corneal Laboratories, Paris, France) or Healaflow was used in the majority of cases. Post operatively, all patients received topical steroid in a tapering dose and topical antibiotic eye drop.
Figure 3.
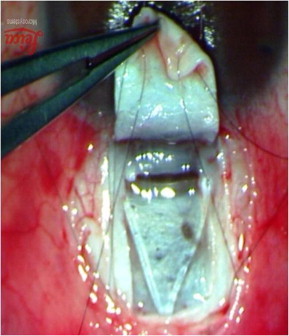
SK-GEL™ placed in the floor of the deep flap.
LGP was performed when the target IOP range for each patient was not achieved because of insufficient filtration through the TDM. For example in our glaucoma division, the target IOP at which LGP was performed depends on the type of glaucoma as well as the status of optic nerve head. The target IOP for normal tension glaucoma and those patients with advanced glaucomatous disk damage (C/D ⩾ 0.8) is <15 mmHg. In patients with less severe glaucomatous, disk damage, the target IOP is <21 mmHg. It was performed using the gonioscopy contact lens. The laser aiming beam was precisely aimed just anterior to the slightly pigmented part of the TDM to avoid a prolapse of the iris into the goniopuncture site. Using the free-running Q-switched mode with energy adjusted to 3–4 mJ, a few shots were usually enough to induce microperforation in the TDM. Following the procedure; topical 1% prednisolone acetate eye drops were prescribed for few days (3 days to one week) to quiet the eye. However, more frequent and longer duration of treatment with prednisolone acetate eye drops was used in uveitic glaucoma cases.
For each patient, the following data were collected: age; gender; type of open-angle glaucoma; the eye to undergo surgery; preoperative visual acuity and IOP; number of preoperative antiglaucoma medications; optic nerve cup-to-disk ratio; previous ocular surgeries (if any); type of implant drainage device used; intraoperative and postoperative complications. Additionally, duration of the post operative follow-up; visual acuity; IOP and number of anti-glaucoma medications used at the last follow-up visit were recorded.
If LGP was performed; the following data were noted: the interval between NPDS and LGP, preLGP IOP, and (1–2 weeks) postLGP IOP as well as at the subsequent follow-up visits which varied depending on the IOP level, and at the last visit. Complications following LGP were recorded. If the IOP failed to drop to target levels, glaucoma medications were added and the time of medication addition was obtained. In addition, the need for any further surgical interventions; including filtering procedures after Nd:YAG LGP, was noted.
Statistical methods
Data were collected in a questionnaire form. All records with missing data, inconvenient conflictions and/or outlier results were removed. Data were then entered into a specifically designed database using Microsoft Access 2007® where necessary data management was done. Data analysis was conducted using SPSS® version 19.0 (IBM Inc., Chicago, Illinois), MedCalc® 11.6 (MedCalc Software bvba, Mariakerke, Belgium) and StatsDirect® statistical software, version 2.7.2 (StatsDirect Ltd., Cheshire, UK).
Descriptive statistics were calculated to both numerical and categorical data. Inferential analysis was conducted to compare means for pre and post intervention indices using Student’s paired T test, while Chi2 was conducted to investigate the association between other variables. P-value < 0.05 was considered significant. Survival analysis using Kaplan Meier survival curve was done to estimate the mean and median survival function of cases post operatively.
Definition of success: In this study, complete success was defined as: achieving a post intervention IOP <21 mmHg based on the patient’s pre-intervention status without any anti-glaucoma medication. Qualified success was defined as: achieving a post intervention IOP <21 mmHg with the help of anti-glaucoma medication. Cases which developed uncontrolled IOP >21 mmHg with medication and/or were in the need for further surgical interventions were considered as “failed”.
Results
In the current study, NPDS was performed to 213 eyes of 158 patients with or without cataract extraction from January 2005 to September 2010 at the King Abdul Aziz University Hospital (KAUH). After data management, 6 eyes of 5 patients were excluded from this cohort and the analysis was eventually performed to 197 eyes of 153 patients. Patients were in the mean age of 44.9 (±20.2) years, and an equivalent male to female ratio (76; 49.7% to 77; 50.3% respectively). The majority of cases were unilateral (109; 71.2%) presenting with moderate mean (±SD) level of IOP (25.5 (±10.2)) mmHg under a mean number of antiglaucoma medications (2.9 (±0.9)) and within a mean cup to disk ratio of 0.8 (±0.2). Previous ocular surgeries were done to 13; 6.6%. Combined NPDS/phacoemulsification procedures were done to 34 (17.3%) of them, while, the most prevalently used implant was SK-Gel in 59 (29.9%) eyes and the operated eyes were followed up to a mean (±SD) follow up duration of 25.4 (±22.3) months. Details on demographic and clinical indices at presentation are demonstrated in Table 1.
Table 1.
Sample distributed by demographic and clinical data at presentation.
| Characteristic/indicator | Category | No. (%) | Mean (±SD) |
|---|---|---|---|
| Age (years) | 44.9 (20.2) | ||
| Gender | |||
| Male | 76 (49.7) | ||
| Female | 77 (50.3) | ||
| Laterality | |||
| Unilateral | 109 (71.2) | ||
| Bilateral | 44 (28.8) | ||
| Type of surgery (eyes) | |||
| NPDS (only) | 163 (82.7) | ||
| Combined NPDS/Phaco | 34 (17.3) | ||
| Type of implant | |||
| None | 31 (15.7%) | ||
| Healaflow | 52 (26.5%) | ||
| SK-Gel | 59 (29.9%) | ||
| T-Flux | 55 (27.9%) | ||
| Preoperative IOP | 25.5 (10.2) | ||
| Preoperative anti-glaucoma medications | 2.9 (0.9) | ||
| Preoperative CDR | 0.8 (0.2) | ||
| History of previous ocular surgery | 13 (6.6%) | ||
NPDS: Non Penetrating Deep Sclerectomy, Phaco: Phacoemulsification, SD: Standard Deviation, IOP: Intraocular Pressure SD: Standard deviation; NPDS: Non penetrating deep Sclerectomy; Phaco: Phacoemulsification; IOP: Intra-ocular pressure; CDR: Cup/Disk ratio.
Out of the 197 eyes, nearly one-forth (48 eyes; 24.4%) of 43 patients needed LGP. Within this specific group of patients, the mean (±SD) age was 34.9 (±18.4) years, mean preoperative IOP was 28.8 (±12.3) mmHg, CDR: 0.7 (±0.3) and anti-glaucoma medications: 3.3 (±0.8). The majority of such glaucoma cases were diagnosed as: uveitic OAG (21; 43.8%) followed by primary OAG (15; 31.3%), juvenile (5; 10.2%), Pseudo-exfoliation (2; 4.2%), Steroid induced (2; 4.2%), in addition to a single case (2.1%) of each of the following diagnoses; angle recession, normal tension and combined mechanism glaucoma. Previous ocular surgeries were done in 6 (12.5%) cases. The majority of surgeries were done with SK-Gel implant (18; 37.5%), while combined NPDS/phaco procedure was done in 2 (4.2%) eyes). LGP was performed as the initiative procedure if the target IOP was not achieved after excluding adherence of the iris to the TDMW. The mean duration between conduct of NPDS and the need for Nd:YAG LGP was 9.78 (± 11.16) months while these cases were followed up for mean follow-up of 16.7 (±16) ranging from 3 to 48 months after the LGP. Prior to LGP, the Mean IOP was 23.3 (±5.9) mmHg which significantly decreased to 14.6 (±4.4) mmHg at the last follow-up after the procedure (p < 0.001), and, the mean number of anti-glaucoma medications has significantly decreased from a pre LGP value of 3.3 (±0.8) to post LGP value of 0.54 (±0.9); p < 0.001.
At the last follow up visit, Nd:YAG LGP was successful in controlling the IOP in 27 eyes (56.3%); whereas 21 eyes (43.7%) needed further management to lower IOP; 11 (22.9%) were conveniently managed by anti-glaucoma medications, and 10 (20.8%) required surgical interventions. In our sample, 10 eyes of 8 patients (2 bilateral) were diagnosed as normal tension glaucoma with a pre intervention IOP of 16.4 (2.5) on anti-glaucoma medications. The mean post-intervention IOP of those patients was significantly reduced to 12.5 (2.6), and they were categorized as a complete success. Only one case (unilateral, male, age 32 years) needed Goniopuncture after 3 months of follow up, where the IOP raised to 20 mmHg on one medication. In the post LGP assessment, the patient’s eye was quiet and stable with a final IOP of 15 mmHg (CDR: 0.6; VA: 20/20).
Moreover, Kaplan Meier Survival analysis yielded that: the estimated mean survival time after Nd:YAG LGP is 35.6 (±4.1) months (95% CI: 27.755–43.479), median survival time is: Median 43.48 (10.27) (22.870–63.130) (Fig. 4). Post Nd: YAG LGP intervention, 5 (10.5%) eyes developed complications where, iris to TDM was detected in 2 (4.2%) cases, and hyphema, hypotony maculopathy, choroidal detachment in one (2.1%) case each.
Figure 4.
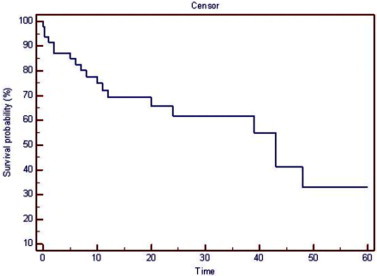
Probability of Nd:YAG LGP success.
Conduct of univariate analysis to assess potential risk factors for the subsequent need of post NPDS Nd: YAG LGP showed that: three predicting factors were found to be statistically significant, those are; age (<50 years, p = 0.001); type of glaucoma (secondary, p = 0.026), and the use of drainage implants (p = 0.038). Further in-depth analysis to different types of secondary open angle glaucoma, uveitic open angle glaucoma was the type significantly associated with the later need of Nd:YAG LGP (p = 0.0003) (Table 2).
Table 2.
Predicting factors for the need of Nd:YAG LGP.
| Characteristic | Category | Nd:YAG LGP |
Total | P value | |
|---|---|---|---|---|---|
| No. | (%) | ||||
| Age | |||||
| <50 | 36 | (33.3%) | 108 | 0.001⁎ | |
| ⩾50 | 12 | (13.5%) | 89 | ||
| Pre op. IOP | |||||
| <21 | 17 | (20.7%) | 82 | 0.316 | |
| ⩾21 | 31 | (27.0%) | 115 | ||
| No. of pre Op. medications | |||||
| <2 | 2 | (12.5%) | 16 | 0.366 | |
| ⩾2 | 46 | (25.6%) | 180 | ||
| Type of glaucoma | |||||
| Primary | 15 | (16.9%) | 89 | ||
| Secondary | 33 | (30.6%) | 108 | 0.026⁎ | |
| Early post op. hypotony (<6) | |||||
| Yes | 1 | (20.0%) | 5 | 0.890 | |
| No | 47 | (24.5%) | 192 | ||
| NPDS combined with phaco. | |||||
| Yes | 2 | (5.9%) | 34 | ||
| No | 46 | (28.2%) | 163 | 0.006⁎ | |
| Use of implant | |||||
| Yes | 45 | (27.1%) | 166 | 0.038⁎ | |
| No | 3 | (9.7%) | 31 | ||
Nd:YAG LGP: Neodymium: Yttrium Aluminum Garnet Laser goniopuncture; Pre Op.: Preoperative; IOP: Intraocular Pressure; Post Op.: Postoperative; NPDS: Non Penetrating Deep Sclerectomy; Phaco: Phacoemulsification.
Statistically significant at p< 0.05, 95% confidence interval.
Discussion
Nonpenetrating glaucoma surgery (NPGS) has been recently modified to improve the safety of filtering procedures through avoiding full thickness penetration into the anterior chamber. The goal of different NPGS procedures is to reduce IOP by overcoming the outflow resistance which is mainly attributed to the inner wall of Schlemm’s canal and the adjacent trabeculum.18 In NPDS, a physiologic membrane consisting of the rest of trabecular tissue and the Descemet’s membrane, this membrane is called the Trabeculo-Descemet’s Membrane (TDM). It dramatically increases facility of outflow but also offers sufficient resistance to prevent the sudden globe decompression that commonly occurs after penetrating glaucoma surgeries.19
When the filtration through the membrane is considered to be insufficient resulting in elevation of IOP, a goniopuncture intervention can be performed with Nd:YAG laser to create a hole in the TDM. This transforms a nonpenetrating filtering procedure into a penetrating one. Nevertheless, such transformation does not compromise on the high safety profile of NPDS afforded by the lack of intraoperative penetration.
In the current study, LGP was performed in 24.4% of eyes that either had NPDS or NPDS with cataract extraction. The mean (±SD) IOP prior to Nd:YAG LGP was 23.3 (±5.9) mmHg which decreased to 14.6 (±4.4) mmHg at the post procedure last follow-up assessment. The mean interval between surgery and LGP was 9.78 (±11.16) months while the mean follow up after LGP was 16.7 (±16) months. Mermoud et al. performed LGP in 41% of total number of studied patients.14 The mean time duration between surgery and LGP was 9.9 (±1.2) months. Moreover, the mean IOP was reduced from a pre LGP value of 22.2 (±7.0) mmHg to 12.5 (±5.8) mmHg after laser intervention. More recently, Anand and Pilling reported a success rate of LGP of 67.0%,20 in their study, the IOP was reduced from 19.6 (±4.6) mmHg to 14.3 (±6.7) mmHg after two years follow up. A meticulous review of the literature shows a lot of variation in the reported rates of LGP ranging from 4.6% to 81%. Findings from these different studies are briefed in Table 3.
Table 3.
Laser goniopuncture rates for deep sclerectomy.
| Author | Procedure | n | Mean LGP rate (%) | Mean LGP timing (months) | Mean preLGP IOP (mmHg) | Mean postLGP IOP (mmHg) | Reduction in IOP |
|---|---|---|---|---|---|---|---|
| Khairy et al.18 | DS | 43 | 4.6 | NA | NA | NA | NA |
| El Sayyad et al.32 | DS | 39 | 10.3 | 9.6 | NA | NA | 7.4 mmHg |
| Shaarawy33 | DS | 52 | 40 | 23.7 | 23.6 | 16.7 | 29% |
| Wishart et al.34 | DS | 52 | 19 | 20.6 | 33.5 | 20.6 | 38.5% |
| Devloo et al.25 | DS | 69 | 30 | NA | 23.75 | 16 | 29.42% |
| Shaarawy & Mermoud26 | DS | 26 | 46 | 13.2 | 19.5 | 14.7 | NA |
| Mousa11 | DS + implant | 20 | 45 | NA | NA | NA | NA |
| Bissig et al.15 | DS + implant | 105 | 59.8 | 29 | 19.8 | 12 | 39.4% |
| Mermoud et al.14 | DS + implant | 100 | 41 | 9.9 | 22.2 | 12.5 | NA |
| Shaarawy19 | DS + implant | 105 | 51 | 21 | 20 | 11 | 45% |
| Jehn et al.35 | DS + implant | 27 | 30 | NA | NA | NA | NA |
| Devloo et al.25 | DS + implant | 24 | 50 | NA | 25.63 | 15.75 | 37.03% |
| Shaarawy & Mermoud26 | DS + implant | 26 | 46 | 14.5 | 22 | 7.3 | NA |
| Ates et al.36 | DS + implant | 54 | 5.5 | NA | NA | NA | NA |
| Drolsum37 | DS + implant | 29 | 44.8 | NA | NA | NA | NA |
| Ravinet et al.38 | DS + implant | 22 | 50 | 10 | NA | NA | 5.1 mmHg |
| Hamel et al.39 | DS + implant | 21 | 71.4 | NA | 20.3 | 11.5 | 43.3% |
| Lachkar et al.27 | DS + implant | 157 | 47.3 | NA | NA | NA | NA |
| Ates et al.40 | DS + implant | 23 | 13 | NA | NA | NA | NA |
| Mermoud et al.41 | DS + implant | 44 | 23 | 9.1 | 21.0 | 12.0 | NA |
| Wevill et al.42 | DS + implant | 17 | 22 | 1 | NA | NA | NA |
| Anand and Atherley43 | DS + implant | 19 | 81 | NA | NA | NA | NA |
| Anand and Pilling20 | DS + implant ± MMC | 258 | 67 | 10.3 | 19.6 | 14.3 | NA |
| Ollikainen et al.24 | DS + implant + MMC | 31a | 29 | NA | 24.4 | 12.9 | NA |
| Ollikainen et al.24 | DS + implant + MMC | 37b | 55.6 | NA | 26.2 | 10.6 | |
| Anand & Atherley43 | DS + implant + MMC | 52 | 45 | NA | NA | NA | NA |
| Mendrinos et al.44 | DS + implant ± MMC | 22 | 63.6 | 16.6 | 21.8 | 9 | NA |
| Lachkar et al.27 | DS + 5-FU | 90 | 46.9 | NA | NA | NA | NA |
n: number of eyes in the study; LGP: Laser goniopuncture; IOP: Intraocular pressure; DS: Deep Sclerectomy; MMC: Mitomycin C.
Eyes with primary open angle glaucoma.
Eyes with pseudoexfoliative glaucoma.
In a critical evaluation study of nonpenetrating glaucoma surgery21, Sarodia et al. reported that success rates of NPDS seem to be lower when LGP rate is low. Consequently, the frequency of LGP increased as there is an increasing evidence on its IOP lowering effect.
In the current study, 24.4% of our cases needed LGP after a mean follow up of 25.03 (±22.03) months, with a success rate of 56.3%. It has been noticed that the need for LGP increases with longer follow up after deep sclerectomy to achieve a within target IOP range.20 Montanes et al. showed that LGP rate increased from 17.6% during the first year to 69.6% with a follow up longer than 2 years.22
This study has identified four predicting factors for the subsequent need of LGP. One of the factors was being at younger age (<50 years). Younger individuals have a stronger healing process which may lead to earlier fibrosis of the TDM and consequently, the later need of LGP.23 In contrast to our finding, Mermoud et al.14 did not find patients’ age as a risk for the need of Nd:YAG LGP. Meanwhile, among our series, the mean age (34.88 ± 18.35 years) was younger than their mean age (67.8 ± 12.7 years). Furthermore, none of their patients were diagnosed to have uveitic open angle glaucoma.
Another risk factor was the diagnosis of secondary open angle glaucoma (p = 0.026); of which, specifically, uveitic open angle glaucoma was significantly associated with the need of Nd:YAG LGP (p = 0.0003). Mermoud et al.14 showed a tendency for an increased need for goniopuncture in patient with pseudoexfoliative and primary open angle glaucomas in pseudophakia. Ollikainen et al.24 showed that Nd:YAG LGP was performed more often postoperatively in pseudoexfoliation eyes than in their primary open angle glaucoma counterparts (p = 0.047).
In our study, the third predicting factor was the use of drainage implants (p = 0.038). In a study by Devloo et al.25, reported that Nd:YAG LGP was performed more frequently and earlier in patients who had NPDS with autologous scleral implant than those who had NPDS only (50% and 30%, respectively). However, the difference was not statistically significant. They attributed this finding to a possible implant-induced fibrosis. Additionally, Sanchez et al.5 found an equal rate of Nd:YAG LGP in patients who had NPDS alone and those who had NPDS with collagen device (15% in each group). Similar findings were reported by Al-Shaarawy and Mermoud.26 Lachkar et al.27 reported a Nd:YAG LGP rate of 47.3% in patients who underwent NPDS with collagen device, and 46.9% in patients who had NPDS with 5-FU. A relatively recent meta-analysis by Hondur et al.28 showed that the rate of Nd:YAG LGP after NPDS ranged between 4.6% and 40%; while after NPDS with implant, it ranged between 5.5% and 81%. Fourth, of the 34 patients who had NPDS with cataract extraction in our series, only 2 patients (5.9%) needed Nd:YAG LGP later, whereas Nd:YAG LGP was performed in 28.2% of those who had NPDS alone. The difference was statistically significant (p = 0.006). This finding may imply that combining cataract extraction with NPDS is a ‘protective’ factor against the subsequent need of Nd:YAG LGP. It was reported earlier that cataract extraction without filtering surgery may result in a small but significant decrease of IOP in glaucoma patients, glaucoma suspects, and normal patients.28
Several mechanisms for the reduction of IOP after phacoemulsification have been proposed. The anterior chamber is visibly deeper in a unilateral pseudophakic eye compared with the fellow phakic eye. This postoperative increase in angle width with its potential effect on the trabecular meshwork may lead to increased aqueous outflow and consequently to IOP reduction. Another possible mechanism for IOP reduction may be traction on the ciliary body via the zonules in the presence of a contracting capsule. Such traction could possibly lead to decreased aqueous secretion and hence, lower IOP. The increase in prostaglandin F2 levels postoperatively may increase uveoscleral outflow and again lower IOP.28 Another possible mechanism of less need for LGP after combined DS + Phaco + MC IOL is possibility of spontaneous rupture of TDW during irrigation aspiration of viscolelastic material at the end of procedure where most surgeons follow.
D’Eliseo et al.29 compared NPDS with implant and combined glaucoma surgery (NPDS and phacoemulsification). They reported that: as the angle widens with lens extraction, phacoemulsification seems to lower the risk of iris adherence at the TDM, thus decreasing the risk of internal filtration block in patients who had NPDS with phacoemulsification.
The most common complication following LGP in the current study was iris touch or adherence to the TDM in two eyes (4.2%). In one eye it resulted in a rise of IOP, necessitating the need for Nd:YAG laser treatment to release the touch. The TDM remained free of any recurrent touch throughout the follow up period and IOP was within the target range. A similar complication was reported by Kim et al.30
Iris adherence to the TDM can occur without a previous Nd:YAG LGP (Fig. 5). Other common iris-related complications after LGP are iris incarceration and iris prolapse in the Trabeculo–Descemet’s Window created by the LGP20,24,25,31 Vuori reported a spontaneous iris prolapse after LGP in three patients out of 31 patients who underwent LGP. In two patients, the decrease in IOP following LGP was substantial, which may have contributed to the prolapse.31 A recent study showed a 2.0% incidence of iris prolapse.24 Iris incarceration can be easily overlooked because it is not necessarily accompanied by pupil deformation.25 Therefore, it is advised to do regular gonioscopy at each follow up visit to check for iris synechiae and incarceration.
Figure 5.

Iris adherence to most of trabeculo-Descemet’s membranes (arrow), discovered on routine gonioscopic examination. No previous goniopuncture had been performed, and intraocular pressure was well controlled without medications.
Other reported complications following LGP may include: Hyphema,20 choroidal detachment,14,20,24 hypotony, hypotony maculopathy, blebitis and delayed bleb leak.20 In the present study, choroidal detachment occurred in one eye (2.1%) and hypotony maculopathy in another eye (2.1%). In both cases, LGP was performed few days after NPDS and the drop in IOP was substantial. Similarly, Mermoud et al.,14 reported 2 cases of choroidal detachment. All of the cases had a substantial drop in IOP following LGP.
Those findings highlight the fact that avoiding early LGP and lowering highly elevated IOP with medications prior to the procedure should be considered before deciding on performing LGP.
In conclusion, NPDS is a safe filtering procedure; however, insufficient filtration through TDM may occur with time. Nd:YAG LGP creates an opening in the TDM; which enables aqueous humour filtration from the anterior chamber to the scleral decompression space. It is a simple, effective and noninvasive procedure, equivalent to postoperative scleral flap suture lysis after trabeculectomy. However, it is not a complication-free procedure.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgement
We would like to gratefully acknowledge the full support of the Glaucoma Research Chair, King Saud University, which facilitated the fulfillment of the current study in its final shape. The author would also like to express deep thanks and appreciation to Ms. Priscilla W. Gikandi, Research Assistant, Research Unit, Department of Ophthalmology, King Saud University for her outstanding efforts in data management, reviewing, editing, and finalization of the manuscript.
References
- 1.Watson P.G., Jakeman C., Ozturk M. The complications of trabeculectomy (a 20-year follow-up) Eye (Lond) 1990;4(Pt 3):425–438. doi: 10.1038/eye.1990.54. [DOI] [PubMed] [Google Scholar]
- 2.Krasnov M.M. Externalization of Schlemm’s canal (sinusotomy) in glaucoma. Br J Ophthalmol. 1968;52:157–161. doi: 10.1136/bjo.52.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmerman T.J., Kooner K.S., Ford V.J. Trabeculectomy vs. nonpenetrating trabeculectomy: a retrospective study of two procedures in phakic patients with glaucoma. Ophthalmic Surg. 1984;15:734–740. [PubMed] [Google Scholar]
- 4.Stegmann R., Pienaar A., Miller D. Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refract Surg. 1999;25:316–322. doi: 10.1016/s0886-3350(99)80078-9. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez E., Schnyder C.C., Mermoud A. Deep sclerectomy: results with and without collagen implant. Int Ophthalmol. 1997;20:157–162. doi: 10.1007/BF00212963. [DOI] [PubMed] [Google Scholar]
- 6.Mermoud A. Sinusotomy and deep sclerectomy. Eye (Lond) 2000;14(Pt 3B):531–535. doi: 10.1038/eye.2000.140. [DOI] [PubMed] [Google Scholar]
- 7.Krasnov M.M. Symposium: microsurgery of the outflow channels. Sinusotomy. Foundations, results, prospects. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:368–374. [PubMed] [Google Scholar]
- 8.Fydorov S.N. Nonpenetrating deep sclerectomy in open angle glaucoma. Eye Microsurg. 1989;1:52–55. [Google Scholar]
- 9.Demailly P., Lavat P., Kretz G. Non-penetrating deep sclerectomy (NPDS) with or without collagen device (CD) in primary open-angle glaucoma: middle-term retrospective study. Int Ophthalmol. 1996;20:131–140. doi: 10.1007/BF00212959. [DOI] [PubMed] [Google Scholar]
- 10.Arenas E. Trabeculectomy ab-externo. Highlights Ophthalmol. 1991;19:60–65. [Google Scholar]
- 11.Mousa A.S. Preliminary evaluation of nonpenetrating deep sclerectomy with autologous scleral implant in open-angle glaucoma. Eye (Lond) 2007;21:1234–1238. doi: 10.1038/sj.eye.6702571. [DOI] [PubMed] [Google Scholar]
- 12.Grant W.M. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- 13.Al Obeidan S. Nonpenetrating deep sclerectomy. Expert Rev Ophthalmol. 2009;4:299–315. [Google Scholar]
- 14.Mermoud A., Karlen M.E., Schnyder C.C. Nd:Yag goniopuncture after deep sclerectomy with collagen implant. Ophthalmic Surg Lasers. 1999;30:120–125. [PubMed] [Google Scholar]
- 15.Bissig A., Rivier D., Zaninetti M. Ten years follow-up after deep sclerectomy with collagen implant. J Glaucoma. 2008;17:680–686. doi: 10.1097/IJG.0b013e318182ed9e. [DOI] [PubMed] [Google Scholar]
- 16.Mermoud A. Deep sclerectomy: surgical technique. J Fr Ophtalmol. 1999;22:781–786. [PubMed] [Google Scholar]
- 17.Kozobolis V.P., Christodoulakis E.V., Tzanakis N. Primary deep sclerectomy versus primary deep sclerectomy with the use of mitomycin C in primary open-angle glaucoma. J Glaucoma. 2002;11:287–293. doi: 10.1097/00061198-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Khairy H.A., Green F.D., Nassar M.K. Control of intraocular pressure after deep sclerectomy. Eye (Lond) 2006;20:336–340. doi: 10.1038/sj.eye.6701878. [DOI] [PubMed] [Google Scholar]
- 19.Shaarawy T., Mansouri K., Schnyder C. Long-term results of deep sclerectomy with collagen implant. J Cataract Refract Surg. 2004;30:1225–1231. doi: 10.1016/j.jcrs.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 20.Anand N., Pilling R. Nd:YAG laser goniopuncture after deep sclerectomy: outcomes. Acta Ophthalmol. 2010;88:110–115. doi: 10.1111/j.1755-3768.2008.01494.x. [DOI] [PubMed] [Google Scholar]
- 21.Sarodia U., Shaarawy T., Barton K. Nonpenetrating glaucoma surgery: a critical evaluation. Curr Opin Ophthamol. 2007;18:152–158. doi: 10.1097/ICU.0b013e328091c1ae. [DOI] [PubMed] [Google Scholar]
- 22.Moreno-Montanes J., Rebolleda G., Munoz-Negrete F.J. Prognostic value of gonioscopy after deep sclerectomy. Eur J Ophthalmol. 2007;17:702–708. doi: 10.1177/112067210701700503. [DOI] [PubMed] [Google Scholar]
- 23.Sandford-Smith J. third ed. F A Thorpe; 2004. Eye surgery in hot climates. [chapter 6, p. 207–8] [Google Scholar]
- 24.Ollikainen M., Puustjarvi T., Rekonen P. Mitomycin-C-augmented deep sclerectomy in patients with primary open-angle glaucoma and exfoliation glaucoma: a 1-year prospective study. Acta Ophthalmol. 2010;88:20–26. doi: 10.1111/j.1755-3768.2009.01509.x. [DOI] [PubMed] [Google Scholar]
- 25.Devloo S., Deghislage C., von Malderen L. Non-penetrating deep sclerectomy without or with autologus scleral implant in open-angle glaucoma: medium-term results. Graef’s Arch Clin Exp Ophthalmol. 2005;243:1206–1212. doi: 10.1007/s00417-005-0020-9. [DOI] [PubMed] [Google Scholar]
- 26.Shaarawy T., Mermoud A. Deep sclerectomy in one eye vs deep sclerectomy with collagen implant in the contralateral eye of the same patient: long-term follow-up. Eye (Lond) 2005;19:298–302. doi: 10.1038/sj.eye.6701469. [DOI] [PubMed] [Google Scholar]
- 27.Lachkar Y., Neverauskiene J., Jeanteur-Lunel M.N. Nonpenetrating deep sclerectomy: a 6-year retrospective study. Eur J Ophthalmol. 2004;14:26–36. doi: 10.1177/112067210401400105. [DOI] [PubMed] [Google Scholar]
- 28.Hondur A., Onol M., Hasanreisoglu B. Nonpenetrating glaucoma surgery: meta-analysis of recent results. J Glaucoma. 2008;17:139–146. doi: 10.1097/IJG.0b013e31814b98f7. [DOI] [PubMed] [Google Scholar]
- 29.D’Eliseo D., Pastena B., Longanesi L. Comparison of deep sclerectomy with implant and combined glaucoma surgery. Ophthalmologica. 2003;217:208–211. doi: 10.1159/000068974. [DOI] [PubMed] [Google Scholar]
- 30.Kim C.Y., Hong Y.J., Seong G.J. Iris synechia after laser goniopuncture in a patient having deep sclerectomy with a collagen implant. J Cataract Refract Surg. 2002;28:900–902. doi: 10.1016/s0886-3350(01)01307-4. [DOI] [PubMed] [Google Scholar]
- 31.Vuori M.L. Complications of Neodymium:YAG laser goniopuncture after deep sclerectomy. Acta Ophthalmol Scand. 2003;81:573–576. doi: 10.1046/j.1600-0420.2003.0154.x. [DOI] [PubMed] [Google Scholar]
- 32.El Sayyad F., Helal M., El-Kholify H. Nonpenetrating deep sclerectomy versus trabeculectomy in bilateral primary open-angle glaucoma. Ophthalmology. 2000;107:1671–1674. doi: 10.1016/s0161-6420(00)00263-3. [DOI] [PubMed] [Google Scholar]
- 33.Shaarawy T., Nguyen C., Schnyder C. Comparative study between deep sclerectomy with and without collagen implant: long term follow up. Br J Ophthalmol. 2004;88:95–98. doi: 10.1136/bjo.88.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wishart P.K., Wishart M.S., Porooshani H. Viscocanalostomy and deep sclerectomy for the surgical treatment of glaucoma: a longterm follow-up. Acta Ophthalmol Scand. 2003;81:343–348. doi: 10.1034/j.1600-0420.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 35.Jehn A.B., Bohnke M., Mojon D.S. Deep sclerectomy with collagen implant: initial experience. Ophthalmologica. 2002;216:235–238. doi: 10.1159/000063846. [DOI] [PubMed] [Google Scholar]
- 36.Ates H., Andac K., Uretmen O. Non-penetrating deep sclerectomy and collagen implant surgery in glaucoma patients with advanced field loss. Int Ophthalmol. 2001;23:123–128. doi: 10.1023/a:1010745204480. [DOI] [PubMed] [Google Scholar]
- 37.Drolsum L. Deep sclerectomy in patients with capsular glaucoma. Acta Ophthalmol Scand. 2003;81:567–572. doi: 10.1111/j.1395-3907.2003.00186.x. [DOI] [PubMed] [Google Scholar]
- 38.Ravinet E., Bovey E., Mermoud A. T-Flux implant versus Healon GV in deep sclerectomy. J Glaucoma. 2004;13:46–50. doi: 10.1097/00061198-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Hamel M., Shaarawy T., Mermoud A. Deep sclerectomy with collagen implant in patients with glaucoma and high myopia. J Cataract Refract Surg. 2001;27:1410–1417. doi: 10.1016/s0886-3350(01)00959-2. [DOI] [PubMed] [Google Scholar]
- 40.Ates H., Uretmen O., Andac K. Deep sclerectomy with a nonabsorbable implant (T-Flux): preliminary results. Can J Ophthalmol. 2003;38:482–488. doi: 10.1016/s0008-4182(03)80027-3. [DOI] [PubMed] [Google Scholar]
- 41.Mermoud A., Schnyder C.C., Sickenberg M. Comparison of deep sclerectomy with collagen implant and trabeculectomy in open-angle glaucoma. J Cataract Refract Surg. 1999;25:323–331. doi: 10.1016/s0886-3350(99)80079-0. [DOI] [PubMed] [Google Scholar]
- 42.Wevill M.T., Meyer D., Van Aswegen E. A pilot study of deep sclerectomy with implantation of chromic suture material as a collagen implant: medium-term results. Eye (Lond) 2005;19:549–554. doi: 10.1038/sj.eye.6701541. [DOI] [PubMed] [Google Scholar]
- 43.Anand N., Atherley C. Deep sclerectomy augmented with mitomycin C. Eye (Lond) 2005;19:442–450. doi: 10.1038/sj.eye.6701403. [DOI] [PubMed] [Google Scholar]
- 44.Mendrinos E., Mansouri K., Mermoud A. Long-term results of deep sclerectomy with collagen implant in exfoliative glaucoma. J Glaucoma. 2009;18:361–367. doi: 10.1097/IJG.0b013e3181879e4e. [DOI] [PubMed] [Google Scholar]


