Abstract
We describe a case of subcutaneous infection as a result of traumatic implantation caused by the fungus Lasiodiplodia theobromae. It was isolated in multiple swabs from the foot of an active healthy male. The fungus was identified by traditional mycology culture methods though this was slow with much time required for sporulation on only one of the agars used. Identification was confirmed by DNA sequencing. The patient was successfully treated with Voriconizole.
Keywords: Phaeohyphomycosis, Sporulation, Traumatic implantation, Coelmycete
1. Introduction
Lasiodoplodia theobromae is a common plant pathogen of tea and fruiting plants from the Coelomycetes family. It is a rare plant pathogen in both immunocompetent and immunocompromised hosts with a variety of infections reported including sinusitis, keratitis, pneumonia and cutaneous lesions. The fungus grew rapidly from cultures of a lesion on the foot of an active male, who had previously experienced a penetrating marine water injury. To our knowledge, there have been 3 previous cases reported in the literature of subcutaneous infections [5–7]. Whilst the fungus grows rapidly on routine media, sporulation was difficult and time consuming toachieve and DNA sequencing may be a quicker method. It is recommended that each strain of L. theobromae be tested for susceptibility as MIC data is limited [1].
2. Case
We report the case of a 59 year old male with a subcutaneous phaeohyphomycosis due to L. theobromae. The patient travelled to Kenya and spent time on the tropical island of Mombassa, which is located on Kenya's eastern coastline. The patient described a sharp lacerating injury whilst walking in shallow ocean water that he felt was likely due to a stingray barb. Following the injury, the patient elected to apply Aloe vera plant leaf to the wound.
1 month after the patient returned from his holiday (day 0), he developed a cutaneous lesion on the dorsum of his right foot (Fig. 1). There was associated soft tissue swelling but no evidence of cellulitis surrounding the lesion. An X-ray performed of his foot did not reveal any evidence of osteomyelitis, however there was significant soft tissue swelling and oedema over the dorsal aspect of the foot.
Fig. 1.
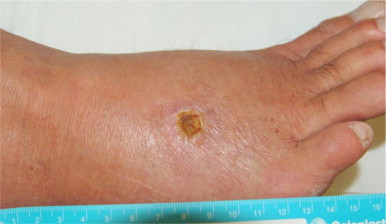
Dorsum of the right foot with presenting lesion (Day +42).
6 weeks after the initial injury (day +42), the patient underwent an excisional biopsy and swabs were taken. Histology revealed an acute inflammatory exudate overlying necrotic dermal collagen from the ulcer base. A PAS stain performed demonstrated a small amount of debris within the inflammatory exudate but no fungal hyphae were seen.
An initial wound swab received in the laboratory and grew mixed coliforms and skin flora with mixed fungal isolates. Subsequently, the wound was cleaned and a second swab was taken. Scant white blood cells were seen in the Gram stain along with fungal elements. (Fig. 2)
Fig. 2.
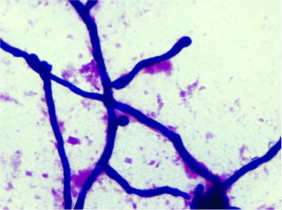
Gram stain from foot wound swab ×100 magnification (Day+45).
This swab had minimal bacterial growth and a fungal isolate, which grew on all plates.
The fungal isolate was dematiaceous and grew rapidly, quickly filling the agar plate. It grew on primary HBA bacterial plates as well as Sabourauds Dextrose Agar (SAB) after 2 days. It was subsequently subcultured onto Potato Dextrose Agar (PDA) and Cornmeal Agar (CMA) incubated at 30 °C in air. A duplicate SAB plate was incubated at room temperature (RT). There was no growth on SAB agar containing cycloheximide (SABCCG). Subcultures were initially white, fluffy growth with grey and black areas turning black and woolly over time with a black reverse.
After 9 days of incubation (day +51), the fungus had completely filled the petri dish. Microscopy was performed using a sticky tape lactophenol blue preparation and septate hyphae were noted. No sporulation was observed.
Plates were examined twice weekly for 4 weeks. SAB and PD incubated at 30 °C and SAB incubated at RT failed to observe sporulation. However, at 4 weeks CMA incubated at 30 °C did sporulate.
Following 30 days (day +72), tease mounts and sticky-tape lactophenol blue wet mounts from CMA showed small hyaline conidia 2–3 um×5 um long.
Young conidiogenous cells are initially hyaline and cylindrical developing to ovoidal and ellipsoidal cells that can be hyaline and dermatiaceous 3–4 um wide×4–6 um long. (Fig. 3)
Fig. 3.
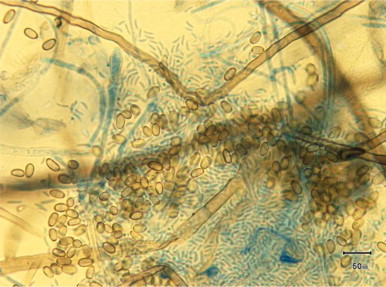
Lactophenol Blue preparation of culture ×100 magnification at 30 days of growth (Day +72).
Older conidia become 10×20 um2 single celled thick walled conidia which then develop further into 2-celled subovoid to ellipsoidal brown-black thick-walled mature conidia with longitudinal striations. (Fig. 4)
Fig. 4.
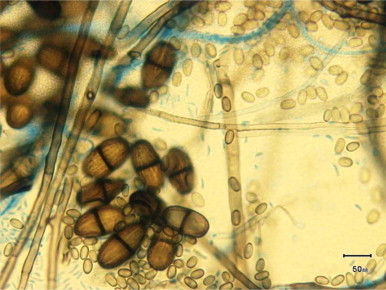
Lactophenol Blue preparation of culture ×100 magnification of mature conidia (Day+72).
It was these typical mature brown striated two-celled conidia that led to the fungus being subsequently identified as L. theobromae.
The isolate was also sent to the Medical Mycology Unit at Royal Womens and Childrens Hospital in Adelaide and identified as L. theobromae by DNA sequencing the ITS (internal transcribed spacer)region with a connote code as 0019107H15UM.
After confirming the fungal isolate as L. theobromae, he was commenced on oral voriconazole. Following a loading dose, he was maintained on a dose of 200 mg twice daily. Levels were monitored and maintained between 1 and 5 mg/L. This therapy resulted in resolution the lesion. (Fig. 5) No sign of recurrence was seen after the patient ceased treatment following a 3 month course.
Fig. 5.
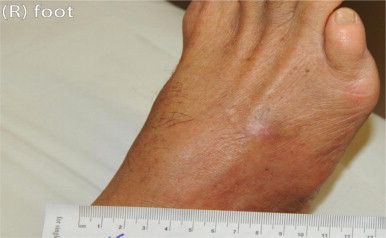
Dorsum of right foot after resolution of the lesion (Day+164).
3. Discussion
L. theobromae is a common plant pathogen of tea and fruiting plants from the Coelomycetes family. That is, it is an asexual fungus that produces conidia in fruiting bodies known as conidiomata.
It is mainly confined to tropical and subtropical climates specifically an area 40° north to 40° south of the equator [1]. The fungus affects roots, stems, leaves & fruit causing dry rot of many plant species. It is entirely plausible that the Aloe Vera plant the patient used to treat himself with may have been contaminated by L. theobromae. Therefore, the application of the plant may have introduced the fungus into the wound and even encouraged the growth of the fungus due to vitamins contained in the plant [4] and done little to help heal the injury.
Human infections are rare, but there are case reports involving both immunocompetent and immunosuppressed patients. To our knowledge, there have been 3 previous cases reported in the literature of subcutaneous infections [5–7]. These cases were managed with surgical debridement and did not require specific antifungal therapy. In contrast, in this patient the lesion resolved by treatment with oral Voriconazole.
The low number of cutaneous cases in the literature is in contrast to cases of fungal keratitis in tropical countries where L. theobromae is reported to be the cause of 6% of these cases [8]. In addition, there have been reported cases of sinusitis and pneumonia in recent years [9,10]. In the three previous reports of cutaneous infection the mode of acquisition of infection was unknown but speculated to be by direct inoculation. Since the conidia are not free, unlike Aspergillus sp., they are not usually air-borne organisms and therefore not likely to be acquired by inhalation.
There were some difficulties in gaining sporulation in the routine laboratory. There was only sporulation on CMA at 30 °C in the dark and it took 30 days. There was no sporulation on PDA without supplements or in light at room temperature. SAB and PDA at 30 °C also produced atypical microscopic characteristics that confused initial identification process. This phenomenon is described by Sutton [2].
Studies by Saha et el have shown that L. theobromae sporulates best at 28 °C in light after 25 days [3]. They also showed that the organism sporulated much better in media containing root extract supplemented media, though it doesn’t require the supplement for growth [3]. Oatmeal agar has proven a useful tool in aiding sporulation of the Coelomycetes family [2]. These supplements and agars were unavailable to our laboratory and hence a considerable amount of time was required to progress with identification by routine methods.
Treatment of infection due to L. theobromae has varied in the literature based on type of infection. Keratitis, corneal ulcers and endophthalmitis have been treated with a mixture of surgical debridement as well as topical and parental antifungals. Previous cases of subcutaneous infections were managed with surgical debridement and did not require specific antifungal therapy. In our case, the patient was treated with voriconazole therapy on the basis of a microbiologic confirmation of infection and clinical evidence of ongoing infection.
In conclusion, L. theobromae is increasing in incidence in opportunistic human infections and, as in our case, as a result of traumatic implantation. It is difficult to identify due to slow and misleading sporulation on synthetic media. Successful treatment occurred with Voriconizole with the patient making a full recovery.
Conflict of interest
There are no conflicts of interest to declare.
Acknowledgements
Coastal Pathology, Buderim, Queensland. QML Laboratories, Brisbane, Queensland for Histology. Mycology Reference Laboratory, South Australia. Study, Education and Research Committee.
References
- 1.D. Ellis, S. David, H. Alexiou, R. Handke, R. Bartley, Medical Mycology, Mycology Unit, Women’s and Children’s Hospital, School of Molecular and Biomedical Science, University of Adelaide, Adelaide, Australia, 2007.
- 2.Sutton Deanna A. Coelomycetous fungi in human disease. A review: clinical entities pathogenesis, identification and therapy. Rev. Iberoam. Micol. 1999;16:171–179. [PubMed] [Google Scholar]
- 3.Saha A., Mandal P., Dasgupta S., Saha D. Influence of culture media and environmental factors on mycelial growth and sporulation of Lasiodiplodia theobromae. J. Environ. Biol. 2008;3 (407–140) [PubMed] [Google Scholar]
- 4.Ezeibekwe I.O., Opara M.I., Mbagwu F.N. Antifungal effect of aloe-vera gel on fungal organisms associated with yam (Dioscorea rotundata, Poir) J. Mol. Genet. 2009;1(1):11–17. [Google Scholar]
- 5.Summerbell R.C., Krajden S., Levine R., Fuksa M. Subcutaneous phaeohyphomycosis caused by Lasiodiplodia theobromae and successfully treated surgically. Med. Mycol. 2004;42(6):543–547. doi: 10.1080/13693780400005916. [DOI] [PubMed] [Google Scholar]
- 6.Maslen M.M., Collis T., Stuart R. Lasiodiplodia theobromae isolated from a subcutaneous abscess in a Cambodian immigrant to Australia. J. Med. Vet. Mycol. 1996;34(4):279–283. doi: 10.1080/02681219680000471. [DOI] [PubMed] [Google Scholar]
- 7.Punithalingam Sphaeropsidales in culture from humans. Nova Hedwig. 1979;31(119–158):119–158. [Google Scholar]
- 8.Srinivasan M., Gonzales C.A., George C. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br. J. Ophthalmol. 1997;81(11):965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kindo A.J., Pramod C., Anita S., Mohanty S. Maxillary sinusitis caused by Lasiodiplodia theobromae. Indian J. Med. Microbiol. 2010;28(2):167–169. doi: 10.4103/0255-0857.62499. [DOI] [PubMed] [Google Scholar]
- 10.Woo P.C., Lau S.K., Ngan A.H., Tse H., Tung E.T., Yuen K.Y. Lasiodiplodia theobromae pneumonia in a liver transplant recipient. J. Clin. Microbiol. 2008;46(1):380–384. doi: 10.1128/JCM.01137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


