Abstract
While elective total hip arthroplasty (THA) for end-stage osteoarthritis (OA) improves pain, mobility function, and quality of life in most cases, a large proportion of patients suffer persistent muscle atrophy, pain, and mobility impairment. Extensive skeletal muscle damage is unavoidable in these surgical procedures, and it stands to reason that poor recovery and long-term mobility impairment among some individuals after THA is linked to failed muscle regeneration and regrowth following surgery and that local muscle inflammation susceptibility (MuIS) is a major contributing factor. Here we present results of two integrated studies. In study 1, we compared muscle inflammation and protein metabolism signaling in elective THA (n = 15) vs. hip fracture/trauma (HFX; n = 11) vs. nonsurgical controls (CON; n = 19). In study 2, we compared two subgroups of THA patients dichotomized into MuIS(+) (n = 7) or MuIS(−) (n = 7) based on muscle expression of TNF-like weak inducer of apoptosis (TWEAK) receptor (Fn14). As expected, HFX demonstrated overt systemic and local muscle inflammation and hypermetabolism. By contrast, no systemic inflammation was detected in elective THA patients; however, local muscle inflammation in the perioperative limb was profound in MuIS(+) and was accompanied by suppressed muscle protein synthesis compared with MuIS(−). Muscle from the contralateral limb of MuIS(+) was unaffected, providing evidence of a true inflammation susceptibility localized to the muscle surrounding the hip with end-stage OA. We suggest MuIS status assessed at the time of surgery may be a useful prognostic index for muscle recovery potential and could therefore provide the basis for a personalized approach to postsurgery rehabilitation.
Keywords: total hip arthroplasty, muscle atrophy, inflammation, muscle protein metabolism, muscle regeneration
elective total joint arthroplasty is the last resort for end-stage osteoarthritis (OA) and, in most cases, total hip or knee arthroplasty (THA or TKA) significantly improves pain, mobility function, and quality of life (7); however, numerous THA/TKA patients suffer persistent muscle atrophy (22, 24), reduced muscle strength (14, 23), pain (27–29, 31), and mobility impairment (6, 28, 30). In fact, epidemiologic findings from our group reveal a troubling prevalence of significant mobility limitation (35%) and frank disability (13%) 5 yr postsurgery (30). Given that THA/TKA volumes are increasing exponentially (32) with over 1.1 million in the U.S. in 2009 (12), refractory mobility impairment is a major public health problem that is not understood.
Extensive skeletal muscle damage is unavoidable in these surgical procedures, and it stands to reason that successful muscle regeneration postsurgery is necessary to fully restore muscle function. Regeneration of damaged muscle requires a complex integration of coordinated processes involving acute inflammation, infiltration of immune cells (i.e., macrophages), proteolysis, protein synthesis, and the activation of resident muscle stem cells (i.e., satellite cells) (4, 25). Chronic, hyperinflammation is a well-known inhibitor of muscle regeneration; thus elevated inflammatory signaling in the musculature surrounding and supporting a diseased joint would be expected to impair regeneration after arthroplasty. For example, chronic, local muscle infusion of IL-6 induces atrophy (11) and while some TNF-α signaling appears to promote the initiation of muscle regeneration (5), chronic hyperactivity of the major transcription factor activated by TNF-α, nuclear factor-κB (NF-κB), leads to impaired muscle regeneration (1) and atrophy in mice driven in part by the E3 ubiquitin ligase MuRF1(3). Furthermore, TNF-like weak inducer of apoptosis (TWEAK) impairs myogenesis (9) and induces muscle atrophy (15), also via NF-κB, by binding to its receptor, TWEAK-R [aka fibroblast growth factor-inducible 14 (Fn14)], and degrading the inhibitor of κB (IκB) via the E3 ubiquitin ligase TNF receptor-associated factor 6 (TRAF6).
In a recent aging study, we identified and described a novel state of muscle inflammation susceptibility (MuIS), defined as a heightened basal state of proinflammatory signaling in skeletal muscle independent of circulating cytokine levels, as demonstrated by hyperactivity of the tumor necrosis factor-α (TNF-α), TWEAK, and/or interleukin (IL)-6 signaling pathways in muscle tissue. Additionally, satellite cells isolated from muscles of older individuals displaying MuIS [i.e., MuIS(+)] were found to have elevated basal proinflammatory signaling in vitro, along with cytokine hypersensitivity and impaired myogenic potential compared with satellite cells isolated from MuIS(−) individuals, indicative of a true cellular phenotype beyond the niche. In a follow-up K-means cluster analysis, we identified adults of all ages who shared the MuIS(+) phenotype (unpublished observations). We therefore suspect MuIS status may distinguish those who experience a poor regenerative response after surgery independent of age.
It is our overarching hypothesis that poor recovery and long-term mobility impairment among some individuals after THA or TKA is linked to failed muscle regeneration and regrowth following surgery and that local MuIS is a major contributing factor. The primary objective of this initial investigation was to fully characterize the inflammatory burden in skeletal muscle of hip surgery patients and to test whether MuIS status in elective THA patients can discriminate muscle anabolic potential. If so, MuIS status at the time of surgery may prove (in future studies) to be a promising biological, prognostic index that could predict long-term muscle regrowth potential and identify patients in need of a targeted, proanabolic rehabilitation program.
Here we present results of two coordinated studies. In study 1, we compared local skeletal muscle inflammation and systemic inflammation across three groups: elective THA patients with end-stage OA vs. trauma victims with hip fracture (denoted HFX) vs. healthy, nonsurgical controls (CON). We recruited the HFX patients specifically for the purpose of defining the most extreme state of muscle inflammation, which enabled us to better interpret findings in elective THA patients classified as MuIS(+) vs. MuIS(−) in study 2. Comparisons included markers of systemic inflammation (serum cytokines), muscle tissue gene expression and protein cell signaling for proinflammatory and muscle protein turnover pathways, and rates of muscle protein synthesis and breakdown. Among THA and HFX, comparisons were made between perioperative samples of the major hip extensor (gluteus maximus) on the surgical side and vastus lateralis muscle samples from the contralateral thigh. Results were compared with vastus lateralis muscle tissue samples from CON. In study 2 the THA patients from study 1 were dichotomized into two groups, MuIS(+) vs. MuIS(−), based on the inflammatory status of the skeletal muscle surrounding and supporting the diseased hip (i.e., perioperative gluteus maximus muscle). Differences between MuIS(+) and MuIS(−) in both ipsilateral (surgical) and contralateral muscles were then tested: 1) rates of muscle protein synthesis; 2) cell signaling pathways that regulate inflammation and protein turnover; and 3) associated transcriptional activity.
METHODS
Subjects
THA patients were recruited from the University of Arkansas for Medical Sciences (UAMS) Orthopedic Clinic once scheduled for elective THA (n = 10), resurfacing (n = 4), or THA revision (n = 1). For this study, we collapsed all three of these elective surgery indications into a single group, noted as THA (n = 15). For contrast purposes, 11 trauma patients with hip fracture (8 motor vehicle accident victims, 3 falls from height) undergoing emergency hip surgery were recruited from the UAMS Trauma Service, noted throughout as HFX. Subjects were excluded if they were taking insulin, thiazolidinedione drugs, or metformin; had a history of chronic renal insufficiency/disease or liver disease; had uncontrolled hypertension at the time of presurgical screening; had any history of hypo- or hypercoagulation disorders including the taking of Coumadin; had a history of atrial fibrillation, angina, or congestive heart failure; had recently (6 mo or less) been treated for cancer other than basal cell carcinoma; or if they were pregnant. UAMS Institutional Review Board-approved informed consent was obtained by the study nurse after the study was described and discussed in detail. Coded, deidentified muscle and serum samples from the UAMS hip surgery patients were sent to and analyzed in the Core Muscle Research Laboratory serving both the Birmingham Veterans Affairs Medical Center and University of Alabama at Birmingham (UAB). For a nonsurgical comparison, muscle and serum samples, along with muscle mass and strength data, from 19 healthy subjects similar in age, gender, and body mass index (BMI) to the elective THA patients were used as controls (CON). These controls were drawn from the Core Muscle Research Laboratory's de-identified human tissue and data bank.
Lower Limb Muscle Mass
Muscle mass was determined by dual-energy X-ray absorptiometry (DXA) before surgery in THA patients to compare surgical vs. contralateral lower limb muscle mass using a Hologic Discovery QDR DXA scanner (Hologic, Bedford, MA) according to manufacturer's instructions.
Serum Cytokine Analysis
Circulating concentrations of proinflammatory cytokines IL-6, IL-1β, IL-8, and TNF-α were assessed using ELISA on 28 subjects (10 THA, 9 HFX, and 9 CON) with MS2400 Human Pro-Inflammatory 4-Plex II Ultra-Sensitive Kits (Meso Scale Discovery, Gaithersburg, MD) and standard procedures. Samples were measured in triplicate, and demonstrated coefficient of variations of 3.05, 5.47, and 6.76% for IL-6, IL-8, and TNF-α, respectively. IL-1β levels were not sufficient to meet the minimum level of detection in some samples.
Muscle Tissue Collection and Muscle Protein Metabolism Measurements
For THA and HFX patients, a 1-h perioperative metabolic study was conducted to determine the fractional synthetic and breakdown rates (FSR/FBR) of skeletal muscle (gluteus maximus) protein in the surgical limb. For THA subjects only, FSR was also assessed in the contralateral thigh (vastus lateralis) before discharge. These contralateral muscle samples from THA patients were collected using our standard percutaneous needle biopsy procedure in the morning, fasted state under local anesthetic (1% lidocaine) with a 5-mm Bergstrom-type biopsy needle under suction as previously described (2). Any visible fat or connective tissues were dissected from muscle samples at the bedside. Identical procedures were used at UAB to collect the stored vastus lateralis samples of CON subjects. All muscle tissue samples were weighed, divided, and snap frozen (25–35 mg/tube) in liquid nitrogen and stored at −80°C.
The method of determining FSR and FBR has been validated and described in detail (34). Briefly, this method involves a stable isotopic pulse tracer injection and the measurement of enrichment in arterial blood and muscle. Calculations of FSR are based on the precursor-product principle by calculating the rate of tracer incorporation from the muscle intracellular free pool to the protein bound pool. Calculations of FBR are also based on the precursor-product principle; however, the precursor is now the unlabeled protein bound amino acid (phenylalanine), and the product the arterial dilution of a second tracer ([15N]phenylalanine). Patients were anesthetized using a standardized anesthesia protocol of propofol, isoflurane, and an opioid of choice as deemed necessary by the anesthesiologist. Blood samples were drawn from an existing arterial line. Isotope infusions were given via an existing peripheral catheter separate from the one used for blood draws. A background blood sample was drawn before the isotope was injected. Once the surgeon had the subject prepped and draped for surgery, the stable isotope ring-[13C6-]phenylalanine (35 μmol/kg) was given by an intravenous push and the study timer was started. Blood samples (∼5 ml) were drawn at ∼5, 10, 15, 20, 30, 35, 40, 50, and 60 min on the timer (exact times were recorded). Samples of the gluteus maximus muscle (∼100–200 mg) were taken from the surgical site 5 and 60 min after tracer injection, subsequently cleaned of visible fat, and snap frozen in liquid nitrogen. To measure FBR, a second tracer, [15N]phenylalanine (35 μmol/kg), was injected at the 30-min time point and arterial blood was sampled as described above.
Before discharge from the hospital following THA surgery, patients were studied to determine 24-h FSR. Briefly, ring-[2H5]phenylalanine infusion was started and the first muscle biopsy was taken from the vastus lateralis of the contralateral (nonsurgical) leg ∼2 h later. The following morning, after an overnight fast of 10 h, a second biopsy was taken from a different incision site in the same leg ∼21–22 h after the first biopsy.
Blood and muscle samples were analyzed for isotope enrichment using methods previously described (8). Briefly, upon thawing, biopsy samples were precipitated with 800 μl of 14% perchloroacetic acid. Tissue was homogenized and centrifuged, and tissue free amino acids (labeled phenylalanine) were extracted from the supernatant by cation exchange chromatography (Dowex AG 50W-8X; 100- to 200-mesh H+ form; Bio-Rad Laboratories, Richmond, CA) and dried under vacuum (Savant Industries, Farmingdale, NY). The remaining muscle pellet was washed and dried and hydrolyzed in 6 N HCl at 50°C for 24 h. Muscle free and protein-bound ring-[13C6]phenylalanine enrichment was determined using the tert-butyldimethylsilyl derivative and GCMS (HP model 5973/5; Agilent Technologies) with electron impact ionization and selective ion monitoring for ions 234, 239, and 240.
Muscle Protein and RNA Isolation
As we have described in more detail elsewhere (19, 21), muscle samples (∼30 mg) were homogenized after a 15-min preincubation in 6 μl/mg muscle of ice-cold lysis buffer with protease and phosphatase inhibitors and then centrifuged at 15,000 g for 40 min at 4°C. The supernatant was stored at −80°C until assayed for protein content using the bicinchoninic acid technique with BSA as a standard. Total RNA was isolated and further purified from frozen muscle samples (∼30 mg) using Tri-Reagent (Molecular Research Center, Cincinnati, OH) and RNeasy Mini Kits (Qiagen, Valencia, CA), respectively, following the manufacturer's instructions. RNA quantity and quality were determined using a spectrophotometer (NanoDrop ND-1000; ThermoScientific, Rockford, IL).
Quantitative PCR
Muscle transcript levels for 10 genes involved in proinflammatory, proteolytic, and anabolic processes were quantified via quantitative (q)RT-PCR using Taqman gene expression assays (Applied Biosystems, Foster City, CA) as we have described previously (21, 33). Briefly, cDNA was synthesized via reverse transcription using the SuperScript VILO cDNA synthesis kit (Invitrogen, Carlsbad, CA). Specific mRNAs of interest assayed via qPCR included the following: IL-6 (Hs00985639_m1); IL-6 receptor (Hs00794121_m1); TNF-α (Hs00174128_m1); TNF receptor 1A (Hs00533560_m1); TWEAK (Hs00356411_m1); TWEAK receptor (Hs0017993_m1); atrogin-1 (Hs01041408_m1); MuRF1 (Hs00822397_m1); IGF-I (Hs01547656_m1); and IGF-I receptor (IGF1R, HS00609566_m1). GAPDH (Hs02758991_g1) expression served as internal control. All samples were run in triplicate. Relative amounts of target mRNA were determined using the comparative threshold cycle method using StepOne software version 2.2.2 (Applied Biosystems). All results are expressed as the relative fold difference compared with the nonsurgical controls.
Immunoblotting
Immunoblotting was performed as we have detailed elsewhere (2, 20, 21) on 15 THA (muscle samples from both surgical and contralateral legs), 11 HFX, and 19 CON. Twenty-five micrograms of skeletal muscle mixed protein lysate were resolved on 4–12% SDS-PAGE gels (Invitrogen) and transferred to PVDF membranes. Protein cell signaling analysis included the proinflammatory pathways of interest (TWEAK/NF-κB and IL-6/STAT3), along with key regulators of translation initiation/protein synthesis (p70S6K1 and 4EBP1) and protein catabolism (FOXO3a). Primary antibodies against TWEAK-R/Fn14 (no. 4403), TRAF6 (no. 8028), p-NF-κB p65Ser536 (no. 3033), NF-κB p50 (no. 3035), p-S6K1Ser421/Thr424 (no. 9204), p-4EBP1Thr37/46 (no. 9459), STAT3 (no. 4904), p-STAT3Ser727 (no. 9136), p-STAT3Tyr705 (no. 9138), p-FOXO3aSer253 (no. 9466), and FOXO3aThr32 (no. 9464) were purchased from Cell Signaling Technologies (Danvers, MA) and used at 1:1,000 dilution in either 5% goat serum (monoclonal antibodies) or 2% milk/2% BSA (polyclonal antibodies). Horseradish peroxidase-conjugated secondary antibody (Pierce, ThermoScientific) was used at 1:50,000 (wt/vol) followed by chemiluminescent detection in a Bio-Rad (Hercules, CA) ChemiDoc imaging system with band densitometry performed using Bio-Rad Quantity One (version 4.5.1).
Statistical Analysis
Study 1.
Among perioperative THA, HFX, and CON, group differences in muscle protein signaling, gene expression, and serum cytokine levels were tested by one-way, between-groups ANOVA. For measures that were collected only on two groups (e.g., THA and CON), tests of between-groups differences were made by independent t-tests. Within THA, surgical and contralateral limbs were tested for differences in DXA-derived muscle mass by a paired t-test.
Study 2.
We conducted a secondary analysis in THA patients to explore the impact of MuIS status. THA patients were dichotomized into MuIS(+) (n = 7) and MuIS(−) (n = 7) based on gene expression of TWEAK-R in perioperative gluteus maximus muscle from the diseased hip. There was no bias among surgical indications or procedures in the subjects dichotomized as MuIS(+) (4 THA, 2 resurfacing, and 1 revision) vs. MuIS(−) (5 THA, 2 resurfacing). Two-tailed, independent t-tests were used to assess differences between MuIS(+) and MuIS(−) in serum cytokines, muscle protein signaling and gene expression, and muscle protein synthesis. For all tests, Fisher's least significant difference post hoc analysis was performed where appropriate. Results are expressed as means ± SE. An alpha level of P < 0.05 was considered statistically significant.
RESULTS
Study 1 Results
Subject characteristics.
Descriptive characteristics of the three groups (CON, THA, and HFX) are shown in Table 1. HFX patients were significantly younger, but no other group differences were noted. Among THA, muscle mass of the limb undergoing surgery was lower compared with the contralateral limb (P < 0.05), indicating muscle atrophy associated with the disease process and/or reduced weight-bearing activity/loading.
Table 1.
Descriptive characteristics for the 3 groups in study 1
| Elective THA |
|||||
|---|---|---|---|---|---|
| Variable | Control | Total | Surgical | Contralateral | HFX |
| Age, yr | 54.6 ± 2.4 | 55.2 ± 1.9 | 44.1 ± 4.1† | ||
| Gender | 12M, 7F | 9M, 6F | 7M, 4F | ||
| BMI, kg/m2 | 25.6 ± 0.7 | 26.1 ± 1.2 | 30.5 ± 1.9 | ||
| Lower limb muscle mass, g | 8,554 ± 616* | 9,174 ± 680 | |||
Values are means ± SE.
THA, total hip arthroplasty; HFX, hip fracture; BMI, body mass index; M, male; F, female.
Different from contralateral, P < 0.05.
Different from all other groups, P < 0.05.
Systemic inflammation.
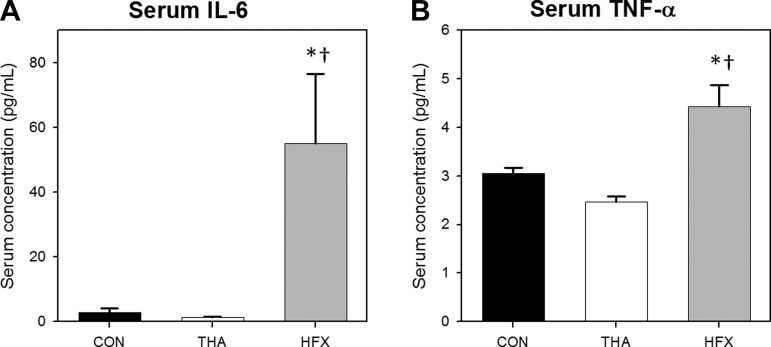
Marked systemic inflammation was noted in HFX as expected. Significant group effects (P < 0.01) for serum concentrations of IL-6 and TNF-α were driven by substantial hyperinflammation in HFX. For example, serum IL-6 concentration in HFX was 40-fold higher than THA and 20-fold higher than CON (P < 0.01; Fig. 1A), while circulating TNF-α concentration in HFX was 79% higher than THA and 45% higher than CON (P < 0.01; Fig. 1B). IL-8 levels were not elevated in HFX (10.9 ± 1.7 pg/ml) compared with CON (9.7 ± 1.4 pg/ml) and, surprisingly, serum IL-8 was low (P < 0.05) in THA (4.9 ± 1.0 pg/ml) compared with the other two groups (not shown). Because IL-1β levels were not sufficient to meet the minimum level of detection in several samples, complete data were not available. Overall, we found no indications of heightened systemic inflammation in THA vs. CON.
Fig. 1.
Indexes of systemic inflammation. Serum IL-6 (A) and TNF-α (B) concentrations in controls (CON) vs. patients undergoing elective total hip arthroplasty (THA) vs. trauma/hip fracture (HFX) victims. Values are means ± SE. *Different from CON, P < 0.05. †Different from THA, P < 0.05.
Skeletal muscle inflammation.
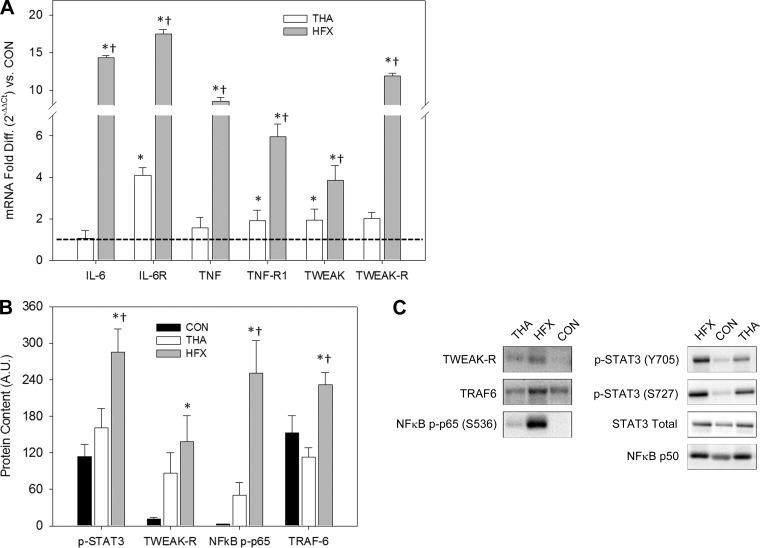
The major hip extensor (gluteus maximus) surrounding the fractured hip in HFX at the time of emergency surgery was severely inflamed, as indicated by markedly elevated expression of proinflammatory genes and concordant elevations in proinflammatory signaling. In many cases, perioperative gluteus maximus samples from THA were also characterized by hyperinflammation, albeit to a lesser degree than HFX. Results for each of the inflammatory pathways studied are summarized in Fig. 2 and described here.
Fig. 2.
Inflammatory profile of muscle surrounding surgical hip. A: muscle gene expression in patients undergoing elective THA vs. HFX victims of 3 proinflammatory cytokines [IL-6, TNF-α, and TNF-like weak inducer of apoptosis receptor (TWEAK-R)] and their receptors relative to the CON group (dotted line). Values are means ± SD. *Different from CON, P < 0.05. †Different from THA, P < 0.05. B: muscle protein signaling results. S727 phosphorylation of STAT3 is plotted. A.U., arbitrary units. Values are means ± SE. *Different from CON, P < 0.05. †Different from THA, P < 0.05. C: representative immunoblots.
IL-6 PATHWAY.
Main group effects (P < 0.001) for the skeletal muscle expression of IL-6 and its receptor were driven by HFX. Muscle IL-6 mRNA was 14-fold higher in HFX vs. CON and THA (P < 0.001), while levels in THA and CON were not different (Fig. 2A). IL-6 receptor mRNA was >17-fold higher in HFX vs. CON and 4-fold higher in THA vs. CON (P < 0.00; Fig. 2A). The greater than fourfold difference between HFX and THA was also significant (P < 0.001). Regarding IL-6/STAT3 signaling, STAT3 (S727) phosphorylation in HFX was 150% higher than CON and 78% higher than THA (P < 0.05; Fig. 2, B and C); however, total STAT3 protein and STAT3 phosphorylation at Y705 were not significantly elevated in HFX (Fig. 2C).
TNF-α AND TWEAK PATHWAYS.
Main group effects (P < 0.001) were found for all gene transcripts assayed with the highest levels of expression noted in HFX. TNF-α mRNA levels were more than eightfold higher (P < 0.001; Fig. 2A) in HFX vs. both THA and CON (which were not different). Compared with CON, TNF-α receptor (TNF-R1) expression was sixfold higher in HFX and twofold higher in THA (P < 0.001; Fig. 2A). The threefold difference in TNF-α receptor expression between HFX and THA was also significant (P < 0.001). Similarly, muscle TWEAK mRNA levels were significantly different (P < 0.01) among all groups (Fig. 2A): nearly fourfold higher in HFX and twofold higher in THA vs. CON. TWEAK mRNA was also higher in HFX compared with THA. TWEAK-R expression was 12-fold higher in HFX vs. CON (P < 0.001; Fig. 2A) and nearly 6-fold higher in HFX vs. THA (P < 0.001), while levels did not differ between THA and CON. Regarding TWEAK/TRAF6/NF-κB signaling, protein levels were remarkably elevated (P < 0.05) in HFX compared with both CON and THA: TWEAK-R was 12-fold higher in HFX vs. CON; TRAF6 levels in HFX were 52% greater than CON and 105% greater than THA (Fig. 2, B and C); and the phosphorylation state of NF-κB p65 (S536) was 83-fold higher than CON and 5-fold higher than THA (Fig. 2, B and C). NF-κB p50 levels were not different among groups (Fig. 2C).
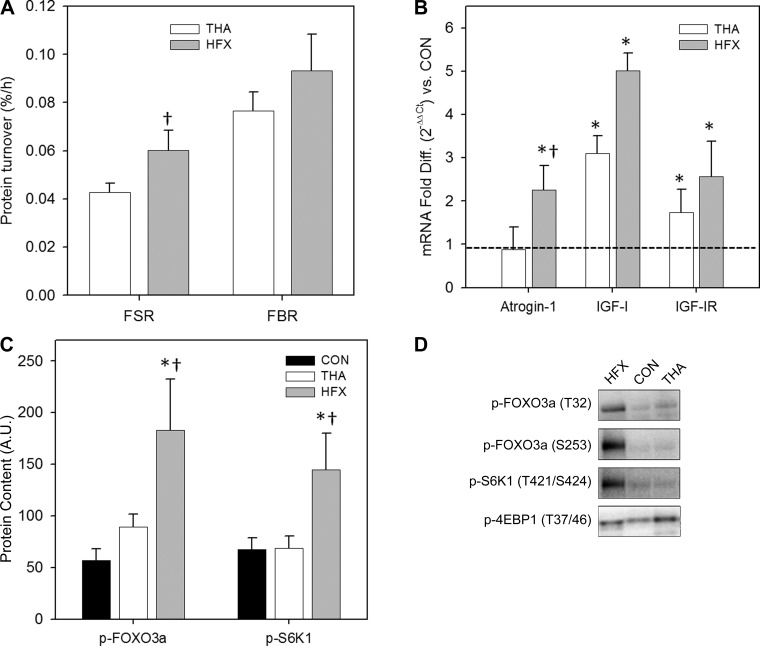
Skeletal muscle protein turnover and associated regulation.
Results of muscle protein metabolism and putative regulatory pathways are shown in Fig. 3. The mixed muscle protein FSR was higher in HFX vs. THA at the time of surgery (P < 0.05), while FBR did not differ between groups (Fig. 3A). Both groups were in negative protein balance, reflective of the fasted state and likely exacerbated by stress. Muscle protein metabolism studies via tracer infusions were not performed in CON. Regarding the expression of genes whose protein products modulate protein turnover, we analyzed transcript levels of the ubiquitin E3 ligases MuRF1 and atrogin-1, as well as the anabolic IGF-I and its primary receptor (IGF1R), and main group effects (P < 0.005) were found for three of four transcripts (atrogin-1, IGF-I, and IGF1R; Fig. 3B). Atrogin-1 mRNA was more than twofold higher in HFX vs. THA and CON (P < 0.01), while MuRF1 expression did not differ between groups (not shown). Compared with CON, IGF-I expression was threefold higher in THA (P < 0.005) and fivefold higher in HFX (P < 0.001). Also relative to CON, IGF1R expression was 1.7-fold higher in THA (P < 0.05) and 2.6-fold higher in HFX (P < 0.001). We also noted significant group differences in protein turnover signaling (Fig. 3C). The phosphorylation state of FOXO3a was highest in HFX at both phosphorylation sites assessed (T32 phosphorylation shown). FOXO3a phosphorylation was threefold higher in HFX vs. CON at both sites (P < 0.001) and, in HFX vs. THA, T32 phosphorylation was 106% higher (P < 0.05) and S253 phosphorylation tended to be higher (80%, P = 0.054). S6K1 phosphorylation (S421/T424) was also highest in HFX (113% higher than both CON and THA; P < 0.01). No group differences in 4EBP1 phosphorylation were found.
Fig. 3.
Muscle protein metabolism and putative regulators in muscle surrounding surgical hip. A: muscle protein fractional synthesis (FSR) and breakdown (FBR) rates in patients undergoing elective THA vs. HFX victims. †Different from THA, P < 0.05. B: muscle gene expression in THA vs. HFX of atrogin-1, IGF-I, and IGF-I receptor relative to the control (CON) group (dotted line). *Different from CON, P < 0.05. †Different from THA, P < 0.05. C: muscle protein signaling results. T32 phosphorylation of FOXO3a is plotted. All values are means ± SE. *Different from CON, P < 0.05. †Different from THA, P < 0.05. D: representative immunoblots.
Effects of fracture/trauma on muscle inflammation are systemic.
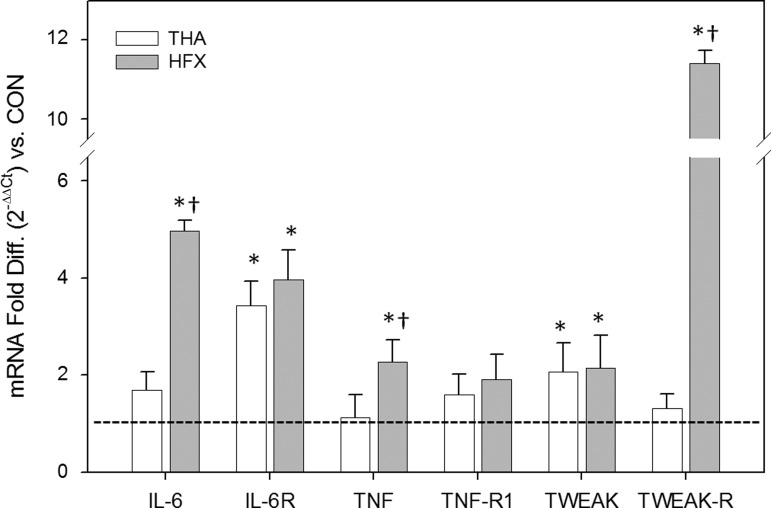
In addition to the perioperative muscle samples collected from the gluteal muscle of the surgical hip, in both HFX and THA patients we biopsied the vastus lateralis of the contralateral thigh just before discharge. Among HFX, this contralateral muscle showed elevated proinflammatory gene expression compared with CON, indicating a systemic inflammatory state (for at least a few days) in addition to the local trauma (Fig. 4). Proinflammatory gene expression was heightened in the contralateral thigh of HFX for IL-6 (5-fold), IL-6R (4-fold), TNF-α (2.3-fold), TWEAK (2.2-fold), and TWEAK-R (11-fold; P < 0.05). By contrast, among THA, heightened proinflammatory gene expression in the contralateral muscle (vs. CON) was noted only for IL-6R (3.4-fold) and TWEAK (2.1-fold) (P < 0.05), suggesting the inflammatory burden was largely localized to the ipsilateral limb (muscles surrounding the diseased hip). Furthermore, for three target genes (IL-6, TNF-α, and TWEAK-R), contralateral muscle expression was greater in HFX vs. THA (P < 0.05). Levels of gene expression for atrogin-1, MuRF1, IGF-I, and IGFR1 in the contralateral muscle did not differ among HFX, THA, and CON (not shown), suggesting the hypermetabolic effects were localized to the injured muscle.
Fig. 4.
Proinflammatory gene expression profile of contralateral muscle in patients undergoing elective THA vs. HFX victims relative to the CON group (dotted line). Values are means ± SD. *Different from CON, P < 0.05. †Different from THA, P < 0.05.
Study 2 Results
Differentiating MuIS(+) from MuIS(−) among elective THA patients.
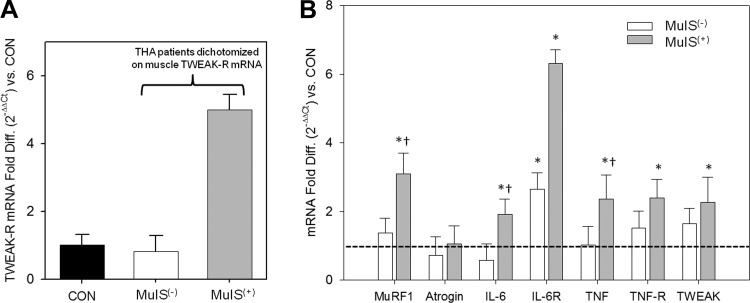
We dichotomized the elective THA patients into MuIS(+) and MuIS(−) based on TWEAK-R [aka fibroblast growth factor inducible-14 (Fn14)] gene expression in the muscle tissue surrounding the diseased hip. TWEAK-R gene expression averaged fivefold higher in the seven elective THA patients with the highest levels, which we identified as the MuIS(+) group, while TWEAK-R expression in the MuIS(−) group did not differ from CON (Fig. 5A). We verified an elevated proinflammatory and proteolytic gene expression profile in the gluteus muscle surrounding the diseased hip in the MuIS(+) group (Fig. 5B). In MuIS(+) vs. CON, IL-6 mRNA was twofold higher, IL-6R expression was elevated sixfold, transcript levels for TNF, TNF-R, and TWEAK were ∼2.5 fold higher, and MuRF1 expression was >3-fold higher (P < 0.05). Among MuIS(−) THA patients, only IL-6R expression was higher than CON (∼2.7-fold, P < 0.05).
Fig. 5.
Inflammatory profile of muscle surrounding surgical hip in THA patients classified as muscle inflammation susceptibility positive vs. negative [MuIS(−) vs. MuIS(+)]. A: THA patients dichotomized into MuIS(−) vs. MuIS(+) based on TWEAK-R gene expression. TWEAK-R expression was 5-fold higher in MuIS(+) than both CON and MuIS(−). B: muscle expression profile of proteolytic (MuRF1 and atrogin-1) and inflammatory (IL-6, IL-6R, TNF, TNF-R, and TWEAK) genes in MuIS(−) and MuIS(+) relative to the CON group (dotted line). Values are means ± SD. *Different from CON, P < 0.05. †Different from MuIS(−), P < 0.05.
Among elective THA patients, MuIS is a local, nonsystemic phenomenon.
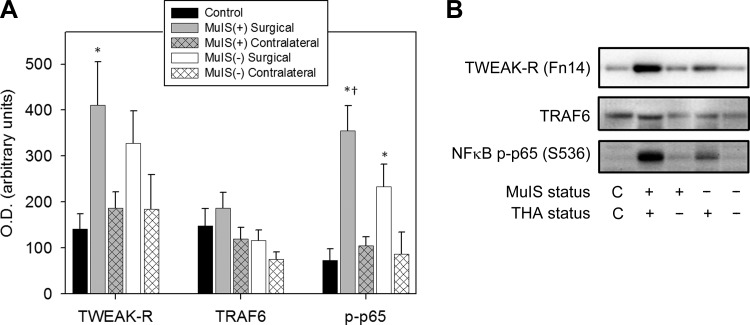
The impact of MuIS was largely localized to the muscles of the diseased hip. No differences in systemic inflammation (serum cytokines) were seen between MuIS(+) and MuIS(−) groups (Table 2), and in the contralateral thigh of MuIS(+) only IL-6R (+5-fold) and TWEAK (+3-fold) mRNAs were elevated vs. CON (not shown). Follow-up immunoblotting revealed that TWEAK-related protein cell signaling was elevated in MuIS(+) vs. MuIS(−) and CON but only in the muscle surrounding the diseased hip (Fig. 6, A and B). Among MuIS(+), muscle TWEAK-R protein was elevated 190% on the surgical side compared with CON (P < 0.05), but not significantly different from CON on the contralateral side. Compared with CON, the phosphorylation of NF-κB p65 (S536) was fivefold higher in MuIS(+) on the surgical side (P < 0.05) but not different from CON in the contralateral muscle. While p-NF-κB p65 (S536) was also elevated on the surgical side in MuIS(−) vs. CON (P < 0.05), the degree of phosphorylation in MuIS(+) was significantly greater than in MuIS(−) (P < 0.05). TRAF6 protein levels did not differ among groups or between limbs.
Table 2.
Descriptive characteristics and serum cytokines THA patients dichotomized as MuIS(+) or MuIS(−) based on muscle TWEAK-R gene expression in study 2
| Variable | MuIS(−) (n = 7) | MuIS(+) (n = 7) |
|---|---|---|
| Age, yr | 52.0 ± 1.8 | 58.9 ± 3.1 |
| BMI, kg/m2 | 26.4 ± 1.3 | 26.9 ± 2.0 |
| Serum IL-6, pg/ml | 1.32 ± 0.44 | 1.41 ± 0.23 |
| Serum IL-8, pg/ml | 4.45 ± 1.22 | 5.39 ± 1.67 |
| Serum TNF-α, pg/ml | 2.55 ± 0.15 | 2.38 ± 0.15 |
Values are means ± SE.
TWEAK-R, TNF-like weak inducer of apoptosis receptor; MuIS, muscle inflammation susceptibility. No differences between groups.
Fig. 6.
Inflammatory TWEAK signaling in muscle surrounding surgical hip compared with contralateral limb in MuIS(+) vs. MuIS(−) THA patients. A: protein levels of TWEAK-R, TRAF6, and the phosphorylation of NF-κB p65 (S536). All values are means ± SE. *Different from CON, P < 0.05. †Different from MuIS(−), P < 0.05. B: representative immunoblots.
Skeletal muscle protein synthesis.
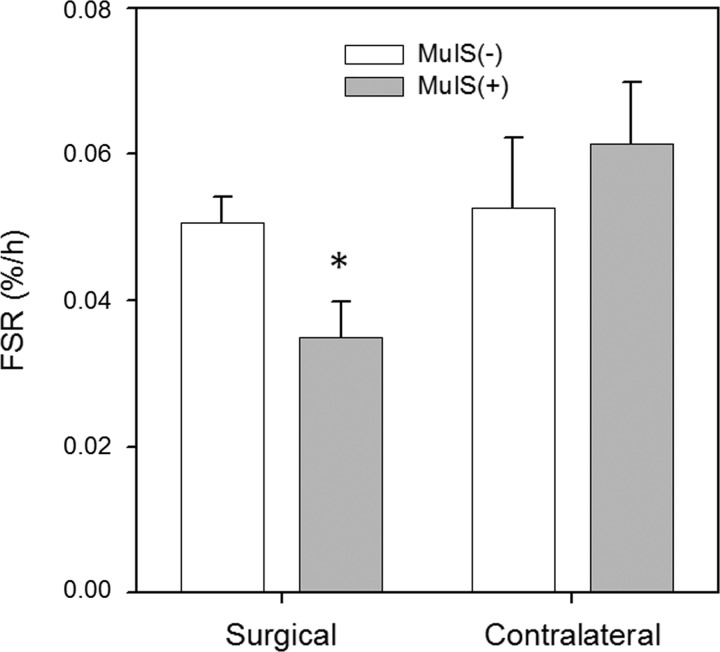
MuIS(+) patients displayed a markedly depressed rate of mixed muscle protein synthesis in the muscle tissue surrounding the diseased hip at the time of THA surgery compared with MuIS(−) patients (−32%; P < 0.05) (Fig. 7). By contrast, muscle protein synthesis rates in the contralateral limbs did not differ between MuIS(+) and MuIS(−) patients. Direct, within-subjects comparisons of surgical vs. contralateral FSR could not be made because two different FSR assessment protocols were used (surgical = 1-h fasting pulse tracer protocol; contralateral = 24-h FSR protocol, which included meals).
Fig. 7.
Muscle protein synthesis rates in muscle surrounding the surgical hip and in the contralateral limb of MuIS(+) vs. MuIS(−) THA patients. Values are means ± SE. *Different from MuIS(−), P < 0.05.
DISCUSSION
This is the first study to fully characterize the inflammatory burden and protein metabolism profile in skeletal muscle of hip surgery patients and to examine whether MuIS status in elective THA patients can discriminate muscle anabolic potential. The HFX patients experienced blunt force trauma resulting in hip fracture and a host of other injuries and, as expected, they displayed overt systemic inflammation along with markedly elevated muscle proinflammatory gene expression (up to +17-fold) and protein signaling (up to +83-fold) profiles at the site of injury. Muscle surrounding the fractured hip was also found to be hypermetabolic, a well-known consequence of acute trauma (10). This heightened state of muscle protein turnover was accompanied by elevated expression of genes and protein signaling involved in anabolism and proteolysis. Interestingly, concurrent with hyperphosphorylation of S6K1, which promotes protein synthesis, we noted hyperphosphorylation of FOXO3a. The FOXOs promote the transcription of atrogenes (atrogin-1, MuRF1) and subsequent proteolysis via the ubiquitin-proteasome pathway (35). Phosphorylation inhibits nuclear translocation and therefore squelches FOXO3a activity. The data therefore suggest a negative feedback response in HFX in an attempt to counteract the upregulated proteolysis.
The overt hyperinflammatory state of HFX muscle resulted at least in part from systemic inflammation, and this is supported by upregulation of all proinflammatory genes in muscle from the contralateral limb. While most of these genes were overexpressed at much lower levels in the contralateral vs. injured limb muscles, TWEAK-R was equally induced in both limbs (∼12-fold). This suggests transcriptional regulation of TWEAK-R is highly sensitive to stress, which led us to focus on its expression as the biomarker for MuIS status in the elective THA patients. In stark contrast to HFX, we found no indications of systemic inflammation in MuIS(+) THA patients, indicating the heightened skeletal muscle inflammatory burden in perioperative muscle among MuIS(+) was not driven by circulating cytokines. This is supported by results in muscle from the contralateral limb showing a limited induction of only two proinflammatory genes, no elevation of TWEAK-R/NF-κB signaling, and a normal rate of muscle protein synthesis.
Heightened inflammatory cytokine expression has been noted in the vastus lateralis of knee OA patients (16). However, in this prior work, differences between OA patients and matched controls were relatively modest and variability among OA patients was fairly high for some transcripts (e.g., TNF-α mRNA). It is possible, if not likely, that some of the OA patients in the report by Levinger et al. (16) expressed a high muscle inflammatory burden [i.e., MuIS(+)] while others remained normal. Dichotomization in the current design enabled us to reveal a phenotype that may be particularly susceptible to a chronic inflammatory burden in skeletal muscle supporting the diseased joint. It is important to point out that, when analyzed as a single group, the current results in elective THA were in many cases not different from CON. However, when dichotomized by TWEAK-R expression, several key differences between MuIS(+) and CON and/or MuIS(−) were revealed. For example, only MuIS(+) demonstrated heightened expression of all inflammatory genes in muscle of the surgical limb, with coordinate elevations in TWEAK-R signaling and suppressed muscle protein synthesis (i.e., FSR). The potential impact of these stark MuIS(+) vs. MuIS(−) differences on postsurgery recovery could be profound.
Hyperactivity of NF-κB, the major downstream transcription factor of both TNF-α and TWEAK signaling, has previously been shown to reduce myogenic activity and skeletal muscle regeneration by multiple mechanisms (reviewed in Ref. 1) and induce muscle atrophy (reviewed in Refs. 13, 17) in part via MuRF1(3), while NF-κB inhibition can enhance myogenesis and improve muscle regeneration in vivo (18). Here we show the phosphorylation (activation) state of the NF-κB p65 subunit was elevated fivefold in the perioperative muscle of MuIS(+) patients with a coordinate and nearly twofold elevation of TWEAK-R protein. Such heightened TWEAK signaling would certainly be expected to impair myofiber regeneration potential following surgery in the MuIS(+) group. That the aberrant signaling was found only in the muscle surrounding the diseased hip (i.e., not in contralateral muscle) suggests a local muscle hypersensitivity to an inflamed OA joint among MuIS(+) individuals. A logical follow-up would be to use -omics approaches to explore molecular determinants of MuIS. Because the transcriptional regulation of, for example, TWEAK-R is incompletely understood, a muscle molecular profile may shed some light on how and why MuIS develops in some individuals but not others despite the presence of end-stage OA in both groups.
It is noteworthy that THA patients in this study were consuming various individually prescribed combinations of analgesics, muscle relaxants, glucocorticoids (dexamethasone), and/or nonsteroidal antiinflammatory drugs before and after surgery; however, there was no distinct bias in the medication regimens of those classified as MuIS(+) vs. MuIS(−). While the medication regimens of these subjects may have helped attenuate systemic inflammation, the drugs apparently had little to no effect on attenuating intracellular inflammatory signaling within the muscle of the diseased hip in the MuIS(+) group.
The findings should be interpreted in the context of a few study limitations. A small sample size did not allow us to adjust for differences in age/gender/BMI. Our THA cohort was a bit younger than that reported frequently, which indicates that the present findings are applicable to younger patients; whether these can be generalized to older patients undergoing THA remains to be determined. Direct, within-subjects comparisons of surgical vs. contralateral FSR could not be made because two different FSR assessment protocols were used. FSR was determined by the 1-h pulse tracer method in the surgical limb perioperatively in the fasted state, while FSR in the contralateral limb was assessed just before discharge via a 24-h protocol. Despite limitations, the findings of these novel experiments may offer some biological insight toward understanding a profoundly important clinical problem. Epidemiologic findings indicate as many as 35% of elective THA patients experience persistent muscle atrophy (22, 24) and mobility impairment (6, 30) even several years postsurgery, and age >70 yr, female gender, BMI ≥30, and depression are each associated with increased relative risk (26, 30). Presumably the long-term mobility impairment results at least in part from impaired muscle regeneration and regrowth following THA surgery.
In summary, the results suggest MuIS status at the time of surgery may be a powerful determinant of recovery potential independent of age and BMI. While MuIS(+) and MuIS(−) groups did not differ in age or BMI and both groups were nonobese and well below 70 yr of age, they displayed marked differences in perioperative muscle protein synthesis and muscle inflammatory burden. Clearly the concept of MuIS is real. It is measurable and, in the diseased limb, reflects an intracellular proinflammatory and proteolytic state that approaches the acute hyperinflammatory state following hip fracture/trauma. Certainly long-term study is needed, but it is attractive to speculate that perioperative MuIS status may be a useful prognostic indicator of long-term muscle regrowth potential. We suspect MuIS(+) patients may be in need of a rehabilitation program that is more intensive or more promyogenic than usual care to overcome MuIS. If this proves to be the case, identifying MuIS(+) patients at the time of surgery may enable clinicians to make an informed decision regarding postsurgery rehabilitation, essentially facilitating a personalized rehabilitation medicine approach.
GRANTS
The research was supported in part by National Institutes of Health Grants R01-AR-052293 (to A. A. Ferrando) and P30-AG-028718 (to A. A. Ferrando), a Veterans Affairs Merit Review Award (to M. M. Bamman), and the UAB Center for Exercise Medicine Core Muscle Research Laboratory (to M. M. Bamman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.M.B., A.A.F., and J.A.S. conception and design of research; M.M.B., A.A.F., M.J.S., N.A.K., K.L.C., and J.R.T. analyzed data; M.M.B., A.A.F., R.P.E., M.J.S., N.A.K., J.M.G., K.L.C., and J.A.S. interpreted results of experiments; M.M.B., A.A.F., M.J.S., N.A.K., K.L.C., and J.R.T. prepared figures; M.M.B., A.A.F., M.J.S., and N.A.K. drafted manuscript; M.M.B., A.A.F., R.P.E., M.J.S., N.A.K., J.M.G., K.L.C., and J.A.S. edited and revised manuscript; M.M.B., A.A.F., R.P.E., M.J.S., N.A.K., J.M.G., K.L.C., J.R.T., and J.A.S. approved final version of manuscript; R.P.E., M.J.S., N.A.K., J.M.G., K.L.C., and J.R.T. performed experiments.
ACKNOWLEDGMENTS
We sincerely thank the participants for the effort and invaluable contributions to this work.
REFERENCES
- 1.Bakkar N, Guttridge DC. NF-kappaB signaling: a tale of two pathways in skeletal myogenesis. Physiol Rev 90: 495–511, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, Allman RM. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol 97: 1329–1337, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Cai D, Frantz JD, Tawa NE Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Chazaud B, Brigitte M, Yacoub-Youssef H, Arnold L, Gherardi R, Sonnet C, Lafuste P, Chretien F. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev 37: 18–22, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Chen SE, Jin B, Li YP. TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK. Am J Physiol Cell Physiol 292: C1660–C1671, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dechartres A, Boutron I, Nizard R, Poiraudeau S, Roy C, Ravaud JF, Ravaud P. Evolution of disability in adults with hip arthroplasty: a national longitudinal study. Arthritis Rheum 57: 364–371, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am 86-A: 963–974, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, McComb A, Symons TB, Wolfe RR, Evans W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr 29: 18–23, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Girgenrath M, Weng S, Kostek CA, Browning B, Wang M, Brown SA, Winkles JA, Michaelson JS, Allaire N, Schneider P, Scott ML, Hsu YM, Yagita H, Flavell RA, Miller JB, Burkly LC, Zheng TS. TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. EMBO J 25: 5826–5839, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiths RD, Hinds CJ, Little RA. Manipulating the metabolic response to injury. Br Med Bull 55: 181–195, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol 98: 911–917, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Healthcare Cost and Utilization Project (HCUP). HCUP Facts and Figures 2009. Section 3: Inpatient Hospital Stays by Procedure. Exhibit 3.1 Most Frequent All-listed Procedures. Rockville, MD: Agency for Healthcare Research and Quality, 2009. [Google Scholar]
- 13.Jackman RW, Cornwell EW, Wu CL, Kandarian SC. NF-kappaB signaling pathway and transcriptional regulation in skeletal muscle atrophy. Exp Physiol 98: 19–24, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen C, Aagaard P, Overgaard S. Recovery in mechanical muscle strength following resurfacing vs standard total hip arthroplasty–a randomised clinical trial. Osteoarthritis Cartilage 19: 1108–1116, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Bhatnagar S, Paul PK. TWEAK and TRAF6 regulate skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care 15: 233–239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levinger I, Levinger P, Trenerry MK, Feller JA, Bartlett JR, Bergman N, McKenna MJ, Cameron-Smith D. Increased inflammatory cytokine expression in the vastus lateralis of patients with knee osteoarthritis. Arthritis Rheum 63: 1343–1348, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med (Berl) 86: 1113–1126, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu A, Proto JD, Guo L, Tang Y, Lavasani M, Tilstra JS, Niedernhofer LJ, Wang B, Guttridge DC, Robbins PD, Huard J. NF-kappaB negatively impacts the myogenic potential of muscle-derived stem cells. Mol Ther 20: 661–668, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayhew DL, Hornberger TA, Lincoln HC, Bamman MM. Eukaryotic initiation factor 2B epsilon induces cap-dependent translation and skeletal muscle hypertrophy. J Physiol 589: 3023–3037, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merritt EK, Cross JM, Bamman MM. Inflammatory and protein metabolism signaling responses in human skeletal muscle after burn injury. J Burn Care Res 33: 291–297, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, Craig Tuggle S, Kosek DJ, Kim JS, Bamman MM. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol (1985) 115: 937–948, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasch A, Bystrom AH, Dalen N, Martinez-Carranza N, Berg HE. Persisting muscle atrophy two years after replacement of the hip. J Bone Joint Surg Br 91: 583–588, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Rasch A, Dalen N, Berg HE. Muscle strength, gait, and balance in 20 patients with hip osteoarthritis followed for 2 years after THA. Acta Orthop 81: 183–188, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reardon K, Galea M, Dennett X, Choong P, Byrne E. Quadriceps muscle wasting persists 5 months after total hip arthroplasty for osteoarthritis of the hip: a pilot study. Intern Med J 31: 7–14, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 139: 2845–2856, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Singh JA, Lewallen D. Age, gender, obesity, and depression are associated with patient-related pain and function outcome after revision total hip arthroplasty. Clin Rheumatol 28: 1419–1430, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh JA, Lewallen D. Predictors of pain and use of pain medications following primary Total Hip Arthroplasty (THA): 5,707 THAs at 2-years and 3,289 THAs at 5-years. BMC Musculoskelet Disord 11: 90, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh JA, Lewallen DG. Ipsilateral lower extremity joint involvement increases the risk of poor pain and function outcomes after hip or knee arthroplasty. BMC Med 11: 144, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh JA, Lewallen DG. Medical and psychological comorbidity predicts poor pain outcomes after total knee arthroplasty. Rheumatology (Oxford) 52: 916–923, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh JA, Lewallen DG. Predictors of activity limitation and dependence on walking aids after primary total hip arthroplasty. J Am Geriatr Soc 58: 2387–2393, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh JA, Lewallen DG. Predictors of use of pain medications for persistent knee pain after primary Total Knee Arthroplasty: a cohort study using an institutional joint registry. Arthritis Res Ther 14: R248, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh JA, Vessely MB, Harmsen WS, Schleck CD, Melton LJ 3rd Kurland RL, Berry DJ. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc 85: 898–904, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thalacker-Mercer A, Stec M, Cui X, Cross J, Windham ST, Bamman MM. Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiol Genomics 45: 499–507, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang XJ, Chinkes DL, Wolfe RR. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am J Physiol Endocrinol Metab 283: E753–E764, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Zheng B, Ohkawa S, Li H, Roberts-Wilson TK, Price SR. FOXO3a mediates signaling crosstalk that coordinates ubiquitin and atrogin-1/MAFbx expression during glucocorticoid-induced skeletal muscle atrophy. FASEB J 24: 2660–2669, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]