Abstract
In the proximal tubule, axial flow (drag on brush-border microvilli) stimulates Na+ and HCO3− reabsorption by modulating both Na/H exchanger 3 (NHE3) and H-ATPase activity, a process critical to glomerulotubular balance. We have also demonstrated that blocking the angiotensin II receptor decreases baseline transport, but preserves the flow effect; dopamine leaves baseline fluxes intact, but abrogates the flow effect. In the current work, we provide evidence implicating cytosolic calcium in flow-dependent transport. Mouse proximal tubules were microperfused in vitro at perfusion rates of 5 and 20 nl/min, and reabsorption of fluid (Jv) and HCO3− (JHCO3) were measured. We examined the effect of high luminal Ca2+ (5 mM), 0 mM Ca2+, the Ca2+ chelator BAPTA-AM, the inositol 1,4,5-trisphosphate (IP3) receptor antagonist 2-aminoethoxydiphenyl borate (2-APB), and the Ca-ATPase inhibitor thapsigargin. In control tubules, increasing perfusion rate from 5 to 20 nl/min increased Jv by 62% and JHCO3 by 104%. With respect to Na+ reabsorption, high luminal Ca2+ decreased transport at low flow, but preserved the flow-induced increase; low luminal Ca2+ had little impact; both BAPTA and 2-APB had no effect on baseline flux, but abrogated the flow effect; thapsigargin decreased baseline flow, leaving the flow effect intact. With respect to HCO3− reabsorption, high luminal Ca2+ decreased transport at low flow and mildly diminished the flow-induced increase; low luminal Ca2+ had little impact; both BAPTA and 2-APB had no effect on baseline flux, but abrogated the flow effect. These data implicate IP3 receptor-mediated intracellular Ca2+ signaling as a critical step in transduction of microvillous drag to modulate Na+ and HCO3− transport.
Keywords: calcium signals, tubule transport, flow-dependent, sodium bicarbonate, IP3 receptor, extracellular Ca2+
glomerulotubular balance (GTB) was first established in the rat kidney via micropuncture (18), where variation in glomerular filtration (over a range of flows from 15 to 60 nl/min) was accompanied by a constant fractional proximal reabsorption. We have studied the mechanism of axial flow-induced changes in Na+ and HCO3− absorption by microperfusion of mouse proximal tubules in vitro under low and high flow rates. From these studies we have demonstrated that the underlying mechanism of GTB is the flow regulation of both Na/H exchanger 3 (NHE3) and H-ATPase activities. This modulation is torque dependent (bending moment at the apical membrane due to fluid flow) and requires the intact actin cytoskeleton (2). In the studies of mouse proximal tubule cells, we have shown that fluid shear stress stimulates NHE3 and H-ATPase trafficking to the apical and Na-K-ATPase to the basolateral membrane surfaces (6); this observation is supported by the mathematical model that shows both apical and basolateral transporters are regulated by flow (25). Regulatory mechanisms of the GTB, including the role of dopamine and ANG II receptors and the role of cAMP-PKA have been examined. From these studies we have demonstrated that dopamine completely blocks flow-stimulated Na+ transport and partially abolishes flow-stimulated HCO3− absorption, and the remaining portion of the increment of HCO3− reabsorption (JHCO3) by flow is blocked by the H-ATPase inhibitor bafilomycin. The effects of dopamine were completely abolished by the dopamine DA1 receptor antagonist Sch 23390 (5). We also demonstrated a dopamine-dependent decrease in the sensitivity of the renal brush border to a change in microvillous torque; a DA1 antagonist increased the transport sensitivity to torque. These results are consistent with the hypothesis that increased flow causes redistribution of NHE3 within the apical membrane, as we have reported, in the proximal tubule cells (6); this activation is essential for flow-stimulated Na+ and HCO3− absorption. The roles of angiotensin II receptors (AT1a, AT2) in flow effects in the proximal tubule were studied in wild-type (WT) and AT1a knockout (KO) mice. We found that blocking the AT1 receptor by losartan reduced absolute Na+ and HCO3− absorption at both low and high flows, but did not affect fractional flow-stimulated transport. Compared with WT control, in AT1a KO mouse tubules, 53% of flow-stimulated Na+ absorption was abolished, but flow-stimulated HCO3− absorption was retained at similar levels. The remaining flow-stimulated HCO3− absorption was eliminated by the H-ATPase inhibitor bafilomycin. Inhibition of the AT2 receptor by PD123319 increased both Na+ and HCO3− absorption, but did not affect flow-mediated fractional changes. We also found that NHE3 expression at the protein level was reduced in AT1a KO mice kidneys. These results indicate that the AT1 receptor is important in maximizing the NHE activity activated by flow; however, it is not critical for the flow-stimulated HCO3− transport, which still exists when the inhibitors are present or when the AT1a receptor is knocked out (3).
Calcium signals are the major second messenger mechanisms for primary cilium-mediated mechanosensation in Madin-Darby canine kidney (MDCK) cells (16) in cortical collecting duct (28) and in kidney epithelial cells (15). However, regulation of the flow effect by calcium signals in the proximal tubule has never been studied. In the collecting duct, flow-stimulated K+ secretion is dependent on apical calcium entry; removal of Ca2+ from the luminal perfusate prevents luminal Ca2+ entry and abrogates flow stimulation of net K+ secretion (11). It is not known whether proximal tubule cells' response to Ca2+ is similar to other cell types. In the proximal tubule, however, it is generally believed that an increase in cytosolic Ca2+ is associated with a decrease in NHE3-mediated Na+ reabsorption (23). It has been suggested that in the proximal tubule, cytosolic Ca2+ functions as a negative feedback signal at the luminal membrane to blunt Na+ entry (8, 27). In the current study, we examined the effect of low and high luminal Ca2+, the Ca2+ chelator BAPTA-AM, the inositol 1,4,5-trisphosphate (IP3) receptor antagonist 2-aminoethoxydiphenyl borate (2-APB), and the Ca-ATPase inhibitor thapsigargin on flow-stimulated Na+ and HCO3− absorption to investigate the role of calcium signals in flow-activated proximal tubule transport. We demonstrate that the IP3 receptor-mediated Ca2+ signal is crucial to flow-mediated Na+ and HCO3− absorption in proximal tubules.
METHODS
Animals.
All experiments from animal work were conducted according to an Institutional Animal Care and Use Committee-approved protocol at the Yale School of Medicine. Mice (C57BL/6) purchased from Jackson Laboratories were maintained on a normal diet and tap water until the day of the experiment and were anesthetized with intraperitoneal pentobarbital sodium (100–150 mg/kg) at the time of the experiment. Both age (5–8 wk) and gender (female) were matched among control and all experimental groups.
Microperfusion of proximal tubules.
Kidneys were removed from a mouse after it was deeply anesthetized and then sectioned into ∼1-mm-thick slices. The slices were transferred to a 4°C HEPES-buffered Ringer solution at pH of 7.4. Proximal convoluted tubules (S2 segments) were isolated by microdissection in cooled (4°C) Hanks' solution, then transferred to the stage of an inverted microscope, and cannulated using a series of concentric glass capillaries. The tubules were perfused with an ultrafiltrate-like solution as described previously (4). The bath solution contained similar electrolytes as the luminal solution, with 3 g/dl albumin added. The perfusate and bath solutions were bubbled with 95% O2-5% CO2, the pH was adjusted to 7.4, and the osmolalities to 300 mosmol/kgH2O in both solutions. All tubules were perfused at 37°C in a 1.2-ml temperature-controlled bath at a flow rate of either 5 or 20 nl/min, followed by three sample collections for measuring [3H]inulin and HCO3− concentrations (4). The order of low or high flow rate was used randomly. Bath fluid was continuously changed at a rate of 0.5 ml/min to maintain the constancy of pH and bath osmolality. Extensively dialyzed [3H]methoxy-inulin was added to the perfusate at a concentration of 30 μCi/ml as a volume marker.
BAPTA-AM (10−7 M), the IP3 receptor antagonist 2-APB (10−7 M), and the Ca-ATPase inhibitor thapsigargin (10−7 M) were added to the luminal perfusate, and all were purchased from Sigma (St. Louis, MO).
Sample measurement and analysis.
The rates of fluid (Jv) and HCO3− (JHCO3) absorption were calculated by measuring the concentrations of [3H]inulin and total CO2, as described previously (4). A constant-bore glass capillary was used to obtain precise aliquots of initial perfusates and collection of samples to be analyzed for [3H]methoxy-inulin by liquid scintillation spectroscopy. The total CO2 concentration of both initial and collected fluids was measured with a nanoflow spectrometer (WPI). The rates of net fluid and HCO3− absorption was calculated as described previously and is expressed per millimeter tubular length (4).
Na+ absorption (JNa) was calculated according to the rate of fluid absorption ([Na] * Jv), since the ratio of fluid and Na+ absorption is 1 in the proximal tubule (24). Cl− absorption (JCl) was calculated as JCl = JNa − JHCO3. The cell volume is identified with epithelial volume, and is calculated as: [π·(OD/2)2 − π·(ID/2)2 ], where ID is inner tubular diameter, and OD is outer tubule diameter (Table 1). In this estimate, it is assumed that lateral intercellular space volume is a negligible fraction of epithelial volume. In this regard, Tisher and Kokko (20) determined that in rabbit proximal tubule, the spacing between membranes of opposing cells is ∼0.03 μm at baseline, and grows or shrinks 10% with changes in transport. Thus, even with the pleated lateral membrane of proximal tubule, whose area is expanded 20-fold over a simple cylinder (26), interspace volume is estimated to be < 5% of epithelial volume.
Table 1.
Effects of low and high luminal calcium concentration, calcium chelator, Ca-ATPase inhibitor, and IP3 receptor antagonist on flow-induced changes in cell volume and torque in mouse proximal tubules
| Group | n | Vo, nl/min | L, mm | Volume, μm3 | ID, μm | OD, μm | T/Tr |
|---|---|---|---|---|---|---|---|
| Control (1.2 mM CaCl2) | 15 | 5.2 ± 0.4 | 0.82 ± 0.02 | 929.4 ± 14.7 | 11.17 ± 0.4 | 36.17 ± 0.3 | 1.00 ± 0.05 |
| 15 | 20.5 ± 0.6 | 0.82 ± 0.02 | 1,044.6 ± 41.5* | 17.29 ± 0.3 | 40.29 ± 0.7 | 1.44 ± 0.06*** | |
| 5 mM CaCl2 | 6 | 4.6 ± 0.6 | 0.92 ± 0.07 | 940.8 ± 19.7NS | 11.46 ± 0.5 | 36.46 ± 0.5 | 1.00 ± 0.09 |
| 6 | 22.7 ± 1.2 | 0.92 ± 0.07 | 890.1 ± 13.1†ns | 18.33 ± 0.4 | 38.33 ± 0.4 | 1.66 ± 0.04†*** | |
| 0 mM CaCl2+EGTA | 6 | 5.2 ± 0.4 | 1.07 ± 0.07 | 888.7 ± 7.3NS | 11.04 ± 0.5 | 35.42 ± 0.3 | 1.00 ± 0.08 |
| 6 | 21.3 ± 0.6 | 1.07 ± 0.07 | 861.8 ± 7.3 †* | 17.43 ± 0.2 | 37.43 ± 0.2 | 1.37 ± 0.02NS** | |
| BAPTA+0 mM CaCl2+EGTA | 6 | 5.3 ± 0.5 | 0.98 ± 0.04 | 949.0 ± 20.7NS | 11.67 ± 0.5 | 36.67 ± 0.5 | 1.00 ± 0.06 |
| 6 | 20.3 ± 0.8 | 0.98 ± 0.04 | 877.0 ± 8.3†** | 17.92 ± 0.3 | 37.92 ± 0.3 | 1.40 ± 0.04NS*** | |
| Thapsigargin | 6 | 5.9 ± 0.8 | 1.01 ± 0.07 | 925.7 ± 25.3NS | 11.67 ± 0.4 | 36.25 ± 0.6 | 1.00 ± 0.08 |
| 6 | 21.6 ± 1.3 | 1.01 ± 0.07 | 877.0 ± 8.3†ns | 17.92 ± 0.3 | 37.92 ± 0.3 | 1.40 ± 0.05NS** | |
| 2-APB | 7 | 5.1 ± 0.5 | 0.98 ± 0.05 | 939.7 ± 16.7NS | 11.43 ± 0.4 | 36.43 ± 0.4 | 1.00 ± 0.08 |
| 7 | 21.1 ± 1.6 | 0.98 ± 0.05 | 858.3 ± 13.4††** | 17.32 ± 0.4 | 37.32 ± 0.4 | 1.54 ± 0.07NS*** |
Values are means ± SE; n: no. of perfused tubules; Vo, original perfusion rate; L, tubular length; volume formula is π*(OD/2)2*1 − π*(ID/2)2*1; 1 indicates the length or height is 1 μm; ID, inner tubular diameter; OD, outer tubular diameter; T, total torque; Tr, torque measured at the perfusion rate of 5 nl/min. The cell-permeable calcium chelator BAPTA-AM (2 × 10−5 M), 10−7 M intracellular Ca-ATPase inhibitor thapsigargin, and 10−5 M inositol 1,4,5-trisphosphate (IP3) receptor antagonist 2-aminoethoxydiphenyl borate (2-APB) were added to the luminal perfusate, respectively. ns, Not significantly different from low flow rate in the same group.
Significant difference from low flow rates in the same group (*P < 0.05, **P < 0.01, ***P < 0.001). NS, not significantly different compared with control at the similar flow rates.
Significant difference compared with control at the similar flow rate (†P < 0.05, ††P < 0.01, †††P < 0.001).
The total torque (bending moment) T on the microvilli due to fluid flow was calculated by the equation which we have published previously (2).
Applying this formula to the experiments under consideration, variations in microvillous length, L, of 50% or less should produce inaccuracies in the torque ratio of less than 10%. That height is not measured in these experiments and is assumed constant in the torque estimates.
Statistics.
Data are presented as means ± SE. Student's t-test was used to compare control and experimental groups. ANOVA was used for comparison of several experimental groups with a control group, following by Dunnett's test. The difference between the mean values of an experimental group and a control group was considered significant if P < 0.05.
RESULTS
Impact of extracellular and intracellular Ca2+ depletion on flow-mediated tubule transport.
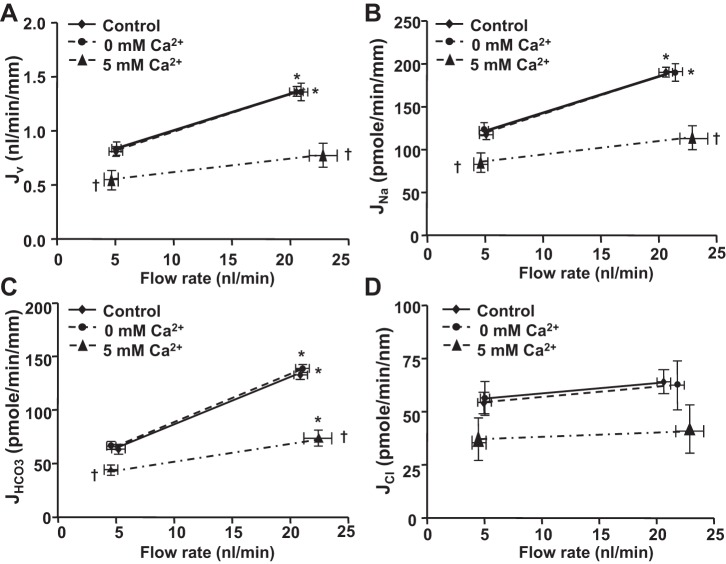
The effects of extracellular Ca2+ depletion on flow-induced changes in Na+ and HCO3− absorption were examined by microperfusion of proximal tubules with low and high flow rates. The extracellular Ca2+ depletion was achieved by using a Ca2+-free solution plus 1 mM EGTA (cell-impermeant calcium chelator) in the luminal perfusate. Under this condition, the extracellular Ca2+ in the apical side is nearly 0 mM. We have compared the flow-induced changes in fluid (Jv), Na+ (JNa), and HCO3− (JHCO3) absorption between the luminal perfusate with 1 mM Ca2+ (control group) and with 0 mM Ca2+ plus 1 mM EGTA. As we previously observed, an increase in perfusion rate from 5 to 20 nl/min increased Jv and JNa by 62% and JHCO3 by 104% in the control group (Fig. 1). Depletion of extracellular Ca2+ had no effect on Jv, JNa, or JHCO3 under either low- or high-flow conditions. The fractional changes in fluid, Na+, HCO3−, and calculated Cl− absorption (JCl) by flow are also the same in the control and extracellular Ca2+ depletion groups (Figs. 1 and 3). This finding indicates that luminal Ca2± influx does not mediate flow-stimulated proximal tubule transport.
Fig. 1.
Effects of extracellular Ca2+ depletion or high Ca2+ on flow-induced changes in fluid (A), sodium (B), bicarbonate (C), and chloride (D) absorption (Jv, JNa, JHCO3 and JCl) in mouse proximal tubules. Jv, JNa, JHCO3, and JCl were measured at low and high perfusion rates in the control (1.2 mM Ca2+), absence of Ca2+ (0 mM Ca2++EGTA), and high Ca2+ (5 mM Ca2+) group, respectively. *P < 0.05, compared with low flow rates in the same group. †P < 0.05, compared with the control at the similar flow rate.
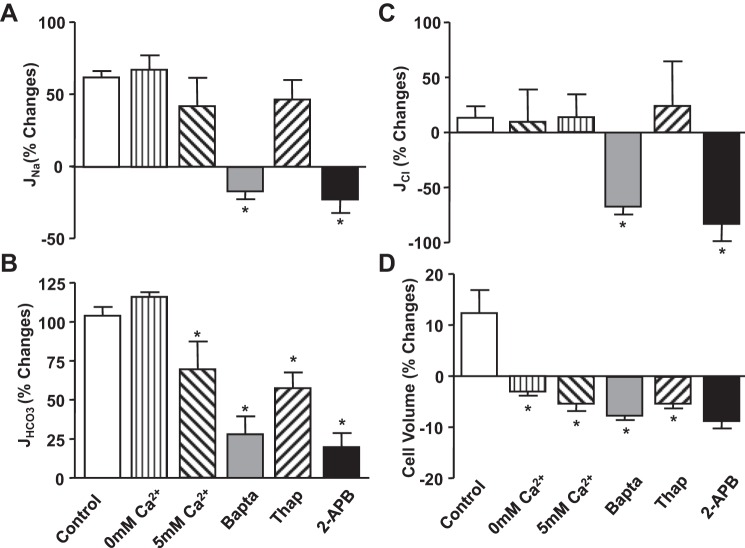
Fig. 3.
Flow-induced changes in sodium (JNa; A), bicarbonate (JHCO3; B), and chloride (JCl; C), reabsorption, and cell volume (D) under low and high perfusion rates in proximal tubules in the absence or presence of Ca2+ inhibitors and in low and high Ca2+ in the luminal perfusate. % Change is the percent differences from low flow rate of JNa, JHCO3, and JCl shown in Table 2, Table 3, and Table 4, respectively. *P < 0.05, compared with the change in control group.
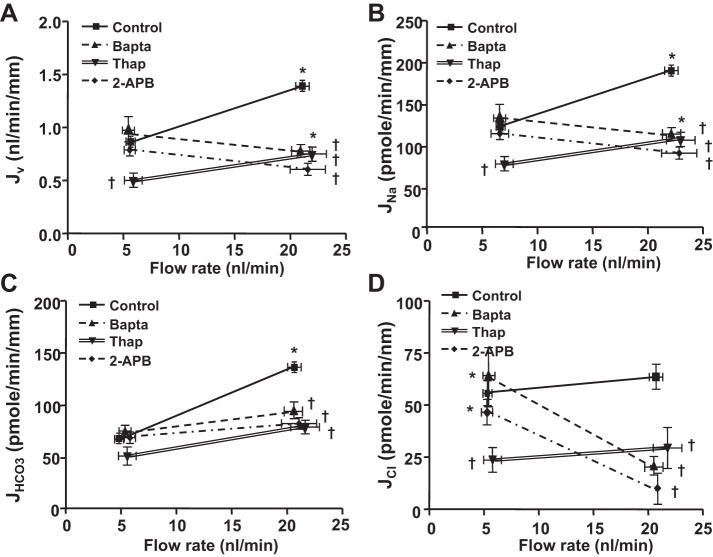
We next examined the effects of both intracellular and extracellular Ca2+ depletion on flow-induced changes in Na+ and HCO3− absorption. Complete Ca2+ depletion was achieved by using a Ca2+-free solution plus EGTA and a cell-permeant Ca2+ chelator BAPTA-AM (10−7 M) in the luminal perfusate (11). Under this condition, both extracellular and intracellular Ca2+ are depleted. We have compared the flow-induced changes in Jv, JNa, JCl, and JHCO3 between the luminal perfusate with 1 mM Ca2+ and with 0 mM Ca2+ plus EGTA and BAPTA-AM. As shown in Fig. 2, an increase in perfusion rate from 5 to 20 nl/min JNa and Jv increased by 62% and JHCO3 increased by 104% in the control group. Depletion of both extracellular and intracellular Ca2+ did not change Na+ or HCO3− absorption at the low flow rate. In contrast, flow-stimulated Na+ absorption was completely abrogated and flow-stimulated HCO3− absorption was significantly reduced in the Ca2+ depletion group compared with the control. The fractional change in JNa and Jv by flow was 62.3 and −22.7% (P < 0.001); the fractional change in JHCO3 by flow was 104 and 28% (P < 0.001) in the control and BAPTA-AM-treated group, respectively (Fig. 3, Tables 2 and 3). These findings indicate that the intracellular calcium signals are critical, but extracellular calcium has no impact on flow-mediated Na+ and HCO3− absorption in proximal tubules.
Fig. 2.
Effects of calcium inhibitors on flow-induced changes in fluid (Jv; A), sodium (JNa; B), bicarbonate (JHCO3; C), and chloride (JCl; D) absorption in proximal tubules. Jv, JNa, JHCO3, and JCl absorption were measured at low and high perfusion rates under the conditions of absence (control group) and presence of cell-permeant Ca2+ chelator BAPTA-AM (10−7 M), inositol 1,4,5-trisphosphate (IP3) receptor antagonist 2-aminoethoxydiphenyl borate (2-APB; 10−5 M), or Ca-ATPase inhibitor thapsigargin (10−7 M) in the luminal perfusate. *P < 0.05, compared with low flow rates in the same group. †P < 0.05, compared with the control at the similar flow rate.
Table 2.
Effects of low and high luminal calcium concentration, calcium chelator, Ca-ATPase inhibitor, and IP3 receptor antagonist on flow-induced changes in sodium absorption under low and high perfusion rates in mouse proximal tubules
| 5 nl/min |
20 nl/min |
||||||
|---|---|---|---|---|---|---|---|
| Group | n | JNaa, pmol·min−1·mm−1 | n | JNab, pmol·min−1·mm−1 | ΔJNa (JNab − JNaa) | ΔJNa/JNaa*100 | (ΔJNa/JNaa)/(ΔT/Tr) |
| Control (1.2 mM CaCl2) | 15 | 122.8 ± 4.13 | 15 | 199.4 ± 5.6*** | 76.5 ± 6.9 | 62.3 ± 4.5 | 1.42 ± 0.10 |
| 5 mM CaCl2 | 6 | 80.9 ± 13.6††† | 6 | 114.9 ± 15.9ns††† | 34.0 ± 20.9† | 42.0 ± 19.7NS | 0.64 ± 0.30†† |
| 0 mM CaCl2 + EGTA | 6 | 119.5 ± 8.3NS | 6 | 200.5 ± 11.8***NS | 81.0 ± 14.5NS | 67.8 ± 9.9NS | 1.83 ± 0.27NS |
| BAPTA+0 mM CaCl2+EGTA | 6 | 135.8 ± 17.4NS | 6 | 113.1 ± 8.1ns††† | −22.7 ± 19.2††† | −16.7 ± 6.0††† | −0.42 ± 0.15††† |
| Thapsigargin | 6 | 73.9 ± 9.02††† | 6 | 108.3 ± 9.95*††† | 34.4 ± 13.4†† | 46.6 ± 13.5NS | 1.17 ± 0.34NS |
| 2-APB | 7 | 115.5 ± 7.3NS | 7 | 89.9 ± 12.3ns††† | −25.6 ± 14.3††† | −22.2 ± 10.7††† | −0.41 ± 0.20††† |
Values are means ± SE; n, no. of perfused tubules; JNa, the rate of sodium reabsorption; ΔJNa, the differences in JNa between low and high perfusion rate (5 and 20 nl/min); ΔJNa/JNaa * 100, percent changes in sodium reabsorption from low flow rate. ns, Not significantly different from low flow rates in the same group.
Significant difference from low flow rates in the same group (*P < 0.05, **P < 0.01, ***P < 0.001). NS, not significantly different compared with control at the similar flow rates.
Significant difference compared with control at the similar flow rates (†P < 0.05, ††P < 0.01, †††P < 0.001).
Table 3.
Effects of low and high luminal calcium concentration, calcium chelator, Ca-ATPase inhibitor, and IP3 receptor antagonist on flow-induced changes in bicarbonate absorption under low and high perfusion rates in mouse proximal tubules
| 5 nl/min |
20 nl/min |
||||||
|---|---|---|---|---|---|---|---|
| Group | n | JHCO3a, pmol·min−1·mm−1 | n | JHCO3b, pmol·min−1·mm−1 | ΔJHCO3 (JHCO3b − JHCO3a) | ΔJHCO3/JHCO3a * 100 | (ΔJHCO3/JHCO3a)/(ΔT/Tr) |
| Control (1.2 mM CaCl2) | 15 | 66.5 ± 3.7 | 15 | 135.6 ± 3.7*** | 69.1 ± 5.3 | 103.9 ± 5.6 | 2.36 ± 0.13 |
| 5 mM CaCl2 | 6 | 43.8 ± 4.3†† | 6 | 74.2 ± 7.8**††† | 30.4 ± 8.9††† | 69.5 ± 17.8† | 1.05 ± 0.27††† |
| 0 mM CaCl2+ EGTA | 6 | 63.1 ± 2.9NS | 6 | 136.4 ± 1.9***NS | 73.2 ± 3.5NS | 116.0 ± 3.1NS | 3.13 ± 0.08††† |
| BAPTA +0 mM CaCl2+EGTA | 6 | 72.1 ± 6.4NS | 6 | 92.3 ± 8.2ns††† | 20.2 ± 10.5††† | 28.0 ± 11.4††† | 0.70 ± 0.29††† |
| Thapsigargin | 6 | 49.9 ± 8.7NS | 6 | 78.5 ± 5.1*††† | 28.6 ± 10.1††† | 57.4 ± 10.2††† | 1.44 ± 0.25††† |
| 2-APB | 7 | 68.0 ± 2.7NS | 7 | 81.4 ± 6.1ns††† | 13.4 ± 6.7††† | 19.7 ± 9.0††† | 0.36 ± 0.17††† |
Values are means ± SE; n, no. of perfused tubules; JHCO3, bicarbonate absorption; ΔJHCO3, the differences in JHCO3 between perfusion rate of 5 and 20 nl/min; ΔJHCO3/JHCO3a * 100, percent changes in bicarbonate reabsorption from low flow rate. ns, Not significantly different from low flow rates in the same group.
Significant difference from low flow rates in the same group (*P < 0.05, **P < 0.01, ***P < 0.001). NS, not significantly different compared with control at the similar flow rates.
Significant difference compared with control at the similar flow rates (†P < 0.05, ††P < 0.01, †††P < 0.001).
The effect of increased extracellular Ca2+ was also examined. As shown in Fig. 1, Tables 2 and 3, JNa, Jv, and JHCO3 was reduced significantly at both low and high flow rates when 5 mM Ca2+ was added to the lumen compared with the control group (1.2 mM Ca2+). JNa and Jv were reduced 34 and 42%, and JHCO3 was reduced by 34 and 45% at low and high flow rates, respectively. The flow-stimulated change in both JNa (P > 0.05) and JHCO3 (P < 0.05) were also reduced by the luminal high Ca2+.
Impact of the IP3 receptor and Ca-ATPase on flow-mediated tubule transport.
Since our results indicated that the intracellular Ca2+ signal is responsible for flow-stimulated Na+ and HCO3− absorption, we investigated the impact of the IP3 receptor and the Ca-ATPase (the major intracellular Ca2+ signaling pathway) on this regulation. A similar experimental procedure was used, and Na+ and HCO3− absorption were measured when tubules perfused with low or high flow rates, under the conditions with or without inhibitors of IP3 and Ca-ATPase, were compared. As shown in Figs. 2 and 3, applying the IP3 receptor inhibitor 2-APB (10−5 M) had no significant change on either Na+ or HCO3− absorption under the low flow rate. However, when the flow rate was increased from 5 to 20 nl/min, the increments in JNa and Jv were completely abolished, and the increase in JHCO3 was significantly reduced by 2-APB. Figure 3 shows the flux changes in going from a low to a high flow rate in different experimental groups. JNa increased 62% in control but decreased 22% with 2-APB; JHCO3 increased 104% in control but only increased 20% in the 2-APB group. These results indicate that the IP3 receptor-mediated Ca2+ release from the endoplasmic reticulum into the cytosol is critical for flow-stimulated proximal tubule transport.
We next examined the effect of Ca-ATPase inhibitor thapsigargin on flow-mediated tubule transport. Thapsigargin was added to the luminal perfusate at the concentration of 10−7 M, and Jv and JHCO3 were measured under the conditions of perfused tubules with low and high flow rates. As shown in Figs. 2 and 3 and Tables 2 and 3, unlike 2-APB, thapsigargin significantly reduced JNa and JHCO3 at both low and high flow rates. JNa and Jv were reduced 40 and 46%, and JHCO3 was reduced 25 and 42% at low and high flow rates, respectively, compared with the control. However, a small portion of flow-stimulated tubule transport still exists when the Ca-ATPase is inhibited. JNa and JHCO3 increased respectively by flow. These results indicated that Ca2+ recycling from the cytosol to the endoplasmic reticulum is important to maintain the maximal tubule transport activity, but is not critical for flow-mediated tubule transport.
Impact of Ca2+ signals on flow-induced changes in Cl− transport and cell volume.
The impact of Ca2+ signals on flow-induced changes in Cl− absorption were calculated according to the balance of Na+ and Cl− plus HCO3− transport under all experimental conditions (JCl = JNa − JHCO3). JNa provides a driving force for Cl− absorption and inversely JHCO3, which provides the driving force for Cl− backflux into the lumen. Data summarized in Table 4 show that increasing the flow rate from 5 to 20 nl/min did not stimulate Cl− absorption. As illustrated in Fig. 4, the fractional changes in JCl due to flow depends directly upon the change in JV (Table 5) and JNa (Table 2), and inversely upon JHCO3 (Table 3), indicating no Na+-and HCO3−-independent Cl− transport was regulated. For example, changes in extracellular Ca2+ concentration did not alter the flow-stimulated Na+ absorption, and a similar response occurred with JCl. Both BAPTA and 2-APB completely eliminated the increase in Na+ absorption by flow, but only partially reduced the change in JHCO3. Therefore, the reduction of flow-mediated JCl became more significant than JNa (Figs. 2D and 3C).
Table 4.
Effects of low and high luminal calcium concentration, calcium chelator, Ca-ATPase inhibitor, and IP3 receptor antagonist on flow-induced changes in chloride absorption under low and high perfusion rates in mouse proximal tubules
| 5 nl/min |
20 nl/min |
||||||
|---|---|---|---|---|---|---|---|
| Group | n | JCla, pmol·min−1·mm−1 | n | JClb, pmol·min−1·mm−1 | ΔJCl (JClb − JCla) | ΔJCl/JCla*100 | (ΔJCl/JCla)/(Δ T/Tr) |
| Control (1.2 mM CaCl2) | 15 | 56.3 ± 5.2 | 15 | 63.8 ± 6.0 | 7.45 ± 7.9 | 13.2 ± 10.7 | 0.30 ± 0.24 |
| 5 mM CaCl2 | 6 | 37.2 ± 10.1NS | 6 | 40.8 ± 10.9nsNS | 3.60 ± 14.8NS | 9.7 ± 29.3NS | 0.15 ± 0.44NS |
| 0 mM CaCl2+ EGTA | 6 | 56.4 ± 7.9NS | 6 | 64.1 ± 11.5nsNS | 7.74 ± 14.0NS | 13.7 ± 20.4NS | 0.37 ± 0.55NS |
| BAPTA+0 mM CaCl2 +EGTA | 6 | 63.7 ± 14.1NS | 6 | 20.8 ± 4.4*††† | −42.86 ± 14.8†† | −67.3 ± 6.9††† | −1.68 ± 0.17††† |
| Thapsigargin | 6 | 24.0 ± 5.9†† | 6 | 29.8 ± 9.8ns†† | 5.79 ± 11.4NS | 24.1 ± 40.6NS | 0.60 ± 1.02NS |
| 2-APB | 7 | 47.4 ± 6.1NS | 7 | 8.4 ± 7.5**††† | −38.98 ± 9.6†† | −82.2 ± 15.7††† | −1.52 ± 0.29††† |
Values are means ± SE. n, no. of perfused tubules; JCl, chloride absorption; ΔJCl, the differences in JCl between perfusion rates of 5 and 20 nl/min; ΔJCl/JCla*100, percent changes in chloride reabsorption from low flow rate. ns, Not significantly different from low flow rates in the same group.
Significant difference from low flow rates in the same group (*P < 0.05, **P < 0.01, ***P < 0.001). NS, not significantly different compared with control at the similar flow rates.
Significant difference compared with control at the similar flow rates (†P < 0.05, ††P < 0.01, †††P < 0.001).
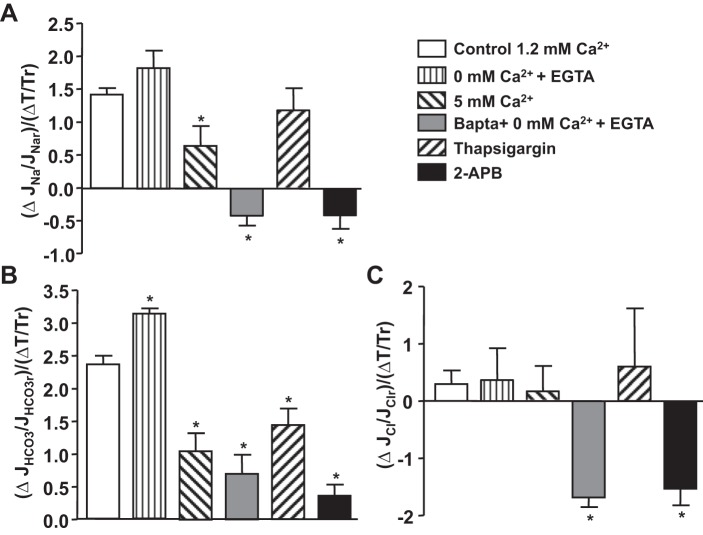
Fig. 4.
Flow-induced changes (Δ) in torque (T) and JNa, JHCO3, and JCl in proximal tubules in the absence or presence of Ca2+ inhibitors. Data are represented as the ratio of changes in ion absorption and changes in torque by flow. r, Reference volume obtained from low flow rate. *P < 0.05, compared with the change in control group.
Table 5.
Effects of low and high luminal calcium concentration, calcium chelator, Ca-ATPase inhibitor, and IP3 receptor antagonist on flow-induced changes in fluid absorption under low and high perfusion rates in mouse proximal tubules
| 5 nl/min |
20 nl/min |
||||||
|---|---|---|---|---|---|---|---|
| Group | n | JVa , pmol·min−1·mm−1 | n | JVb, pmol·min−1·mm−1 | ΔJV (JVb − JVa) | ΔJV/JVa*100 | (ΔJV/JVa)/(ΔT/Tr) |
| Control (1.2 mM CaCl2) | 15 | 0.84 ± 0.03 | 15 | 1.36 ± 0.04*** | 0.52 ± 0.05 | 62.31 ± 4.5 | 1.42 ± 0.10 |
| 5 mM CaCl2 | 6 | 0.55 ± 0.09††† | 6 | 0.78 ± 0.11ns††† | 0.23 ± 0.14† | 42.04 ± 19.7NS | 0.64 ± 0.30†† |
| 0 mM CaCl2+EGTA | 6 | 0.81 ± 0.06NS | 6 | 1.37 ± 0.08***NS | 0.55 ± 0.10NS | 67.75 ± 9.9NS | 1.83 ± 0.27NS |
| BAPTA+0 mM CaCl2+ EGTA | 6 | 0.93 ± 0.12NS | 6 | 0.77 ± 0.06ns††† | −0.15 ± 0.13††† | −16.72 ± 6.0††† | −0.42 ± 0.15††† |
| Thapsigargin | 6 | 0.50 ± 0.06††† | 6 | 0.74 ± 0.07*††† | 0.23 ± 0.09†† | −46.61 ± 13.5NS | 1.17 ± 0.34NS |
| 2-APB | 7 | 0.79 ± 0.05NS | 7 | 0.61 ± 0.08ns††† | −0.17 ± 0.09††† | −22.16 ± 10.7††† | −0.41 ± 0.20††† |
Values are means ± SE; n, no. of perfused tubules; Jv, fluid absorption; ΔJv, the differences in Jv between perfusion rate of 5 and 20 nl/min; ΔJV/JVa*100, percent changes in fluid reabsorption from low flow rate. ns, Not significantly different from low flow rates in the same group;
Significant difference from low flow rates in the same group (*P < 0.05, **P < 0.01, ***P < 0.001). NS, not significantly different compared with control at the similar flow rates.
Significant difference compared with control at the similar flow rates (†P < 0.05, ††P < 0.01, †††P < 0.001).
The flow-induced changes in cell volume are shown in Table 6 and Fig. 3D. Increasing the perfusion rate from 5 to 20 nl/min increased cell volume by 12.4% in control, suggesting a small increase in inflow over outflow until a new steady state is reached at the high-flow condition. Cell volumes were reduced by 3 and 5% by flow when extracellular Ca2+ was deleted or was increased, respectively, although there is no significant reduction of fractional Na+ absorption by flow (Fig. 4A). Both BAPTA and 2-ATB completely abrogated the flow effect on JNa but the changes in cell volume are relatively small compared with the changes in Na+ absorption. The fractional changes in JNa were −16.7 and −22.1%, and the fractional changes in cell volume were −0.42 and −0.41% by flow in the BAPTA and 2-ATB group respectively, suggesting both apical and basolateral fluid transport were regulated simultaneously.
Table 6.
Effects of low and high luminal calcium concentration, calcium chelator, Ca-ATPase inhibitor, and IP3 receptor antagonist on flow-induced changes in cell volume under low and high perfusion rates in mouse proximal tubules
| 5 nl/min |
20 nl/min |
|||||
|---|---|---|---|---|---|---|
| Group | n | Volumea, μm3 | n | Volumeb, μm3 | ΔVolume (Volumeb − Volumea) | ΔVolume/Volumea*100 |
| Control (1.2 mM CaCl2) | 15 | 929.4 ± 14.7 | 15 | 1,044.6 ± 41.5* | 115.2 ± 44.0 | 12.39 ± 4.46 |
| 5 mM CaCl2 | 6 | 940.8 ± 19.7NS | 6 | 890.1 ± 13.1ns† | −50.7 ± 23.7† | −5.39 ± 1.39† |
| 0 mM CaCl2+ EGTA | 6 | 888.7 ± 7.3NS | 6 | 861.8 ± 7.3*† | −26.9 ± 10.3NS | −3.03 ± 0.82† |
| BAPTA+0 mM CaCl2+ EGTA | 6 | 949.0 ± 20.7NS | 6 | 877.0 ± 8.3**† | −72,0 ± 22.3† | −7.59 ± 0.87†† |
| Thapsigargin | 6 | 925.7 ± 25.3NS | 6 | 877.0 ± 8.3ns† | −48.7 ± 26.6† | −5.26 ± 0.89† |
| 2-APB | 7 | 939.7 ± 16.7NS | 7 | 858.3 ± 13.4**†† | −81.3 ± 21.4†† | −8.66 ± 1.42†† |
Values are means ± SE; n, no. of perfused tubules. Volume formula is π*(OD/2)2*1 − π*(ID/2)2*1; 1 indicates the length or height is 1 μm; ΔVolume, the differences in volume between perfusion rate of 5 and 20 nl/min; ΔVolume/Volumea*100, percent changes in volume from low flow rate. ns, Not significantly different from low flow rates in the same group.
Significant difference from low flow rates in the same group (*P < 0.05, **P < 0.01, ***P < 0.001). NS, not significantly different compared with control at the similar flow rates.
Significant difference compared with control at the similar flow rates (†P < 0.05, ††P < 0.01, †††P < 0.001).
Impact of Ca2+ signals on flow-induced changes in torque and ion transport.
We investigated whether Ca2+ signals regulate the tubule sensitivity to torque and whether altered transport activity is torque dependent. The flow-induced changes in torque and in fluid, Na+, Cl−, and HCO3− transport were compared among all experimental groups. As shown in Table 1, the increased flow rate from 5 to 20 nl/min enhanced torque in control and all other experiment groups; there are no significant differences in the increment of the torque in all groups compared with the control, except the high-Ca2+ group had a higher increase of torque by flow (1.66 vs. 1.44, P < 0.05). We next examined the sensitivity of ion transport to flow, namely, the fractional change in solute flux with increased luminal flow, relative to the estimated fractional increase in microvillous torque, and this is shown in Fig. 4. In the control group, the ratio of flow-induced change in Na+ transport to torque is 1.42 for Na+ [ratio of the (ΔJNa/JNar)/(ΔT/Tr)], 2.36 for HCO3− [ratio of the (ΔJHCO3/JHCO3r)/(ΔT/Tr)], and 0.3 for Cl−. Depletion of extracellular Ca2+ (0 mM Ca2++EGTA) gave little change in the flow-sensitivity of Na+ (1.83 vs. 1.42; P > 0.05), but increased HCO3− flow sensitivity (3.13 vs. 2.36; P < 0.05); there was no change in Cl− transport (0.3 vs. 0.15; P > 0.05). High lumen Ca2+ reduced the tubule sensitivity to torque for both Na+ (55%) and HCO3− (56%), and had no change on Cl− absorption. Thapsigargin reduced the ratios of transport and torque in both JNa (P > 0.05) and JHCO3 (P < 0.05), but had no change in JCl. BAPTA and 2-APB reversed the ratios of transport and torque in JNa and JCl and extensively reduced the ratio of JHCO3.
DISCUSSION
Prior in vitro microperfusion of mouse proximal tubule has demonstrated that flow-modulated NHE3 activity is the basis for flow-dependent proximal tubule Na+ reabsorption (2). This perfusion-absorption balance is independent of neuronal and systemic hormonal regulation and requires the intact actin cytoskeleton to transmit the signal of altered axial flow sensed by brush border microvilli. Those experiments confirmed the hypothesis of Guo et al. (10) that brush border microvilli serve as the sensor for axial flow along the proximal tubule. That work provided an equation, which we have used to compare the changes in Na+ and HCO3− reabsorption to the flow-induced changes in microvillous torque (bending moment at base of microvilli), i.e., to define the sensitivity of reabsorption to flow. Ignoring small correction terms, that equation shows microvillous torque T to be proportional to Q/D2, where Q is the volume flow and D the tubule diameter. In our original studies, fractional changes in microvillous torque and Na+ and HCO3− absorption were identical, so that the sensitivity was near unity (2). Additional studies of mouse tubules in vitro have demonstrated that it is possible for hormonal or autocrine signals to effect either or both baseline transport rates and flow sensitivity. For example, with the application of dopamine, baseline Na+ and HCO3− fluxes and the flow sensitivity of each were all reduced by dopamine (5). When the tubules were treated with both dopamine and a DA1 receptor blocker, there were modest increases in baseline flows, but dramatic increases in flow sensitivity for Na+ and HCO3− (2.5 and 3.5, respectively). With respect to angiotensin, application of the AT1 inhibitor losartan halved baseline fluxes, but left flow sensitivity largely intact.
There has been a long-time interest in the role of proximal tubule cytosolic Ca2+ in modulating reabsorptive fluxes (27). In large measure, the motivation for this interest was deciphering mechanisms underlying proximal tubule cell volume homeostasis in the face of widely variable transcellular fluxes (19). The peritubular membrane of proximal tubule cells contains both a Ca2+-dependent ATPase and a 3Na+/Ca2+ exchanger (9). Either transporter, in association with a luminal entry step, could provide active reabsorption of calcium by the proximal tubule; however, the 3Na+/Ca2+ exchanger also had the potential to couple cytosolic Na+ concentrations to cytosolic Ca2+ concentrations (21). For this coupling to be homeostatic, e.g., to protect the cell against Na+ overload, increases in cytosolic Ca2+ would need to downregulate a key luminal entry step for Na+. A critical piece of this scheme was supplied by Friedman et al. (8), who studied isolated, perfused rabbit proximal convoluted tubules and demonstrated that a number of maneuvers that increased cytosolic Ca2+ all reduced the transepithelial reabsorption of Na+. Complementing this work is the observation that increased cytosolic Ca2+ is associated with a decrease in NHE3-mediated Na+ reabsorption (23). It must be acknowledged, however, that the maneuvers used by these workers were relatively severe. There is little subsequent work to suggest that small changes in cytosolic Ca2+ are important regulators of either NHE3 or the Na-K-ATPase in the range of normal variability of luminal Na+ fluxes. Most germane to the problem of coordinating entry and exit is the observation that when proximal convoluted tubules are exposed to luminal glucose and alanine, there is no significant increase in cytosolic calcium (1).
With respect to flow-dependent transport, there has been recent interest in the role of calcium, which has derived from experiments in the distal nephron. Calcium signals are the major second messenger mechanisms for primary cilium-mediated mechanosensation in MDCK cells (16), in the cortical collecting duct (28), and in kidney epithelial cells (15). In the collecting tubule, flow-stimulated K+ secretion is dependent on apical calcium entry; removal of Ca2+ from the luminal perfusate prevents luminal Ca2+ entry and abrogates flow stimulation of net K+ secretion (11). The entry of Ca2+ is believed to be associated with large membrane strains in the vicinity of the basal body where the primary cilium can rotate near its insertion at the apical surface with little bending deformation along its axoneme (17). The bending moments on the primary cilia for a realistic flow profile are calculated in Liu et al. (13), where the effects of circumferential stretch are separated from those of just flow without tubular diameter changes. Nevertheless, regulation of flow-dependent transport by calcium has never been examined in proximal tubules despite the extensive interest in the role of cytosolic Ca2+ in Na+ reabsorption already discussed. Insertion and retrieval of luminal membrane NHE3 have been most intensively studied under the condition of pressure-natriuresis (14). In this setting, the signals from the renal interstitium are most likely arachidonic acid metabolites of cytochrome P-450 (specifically, 20-hydroxyeicosatetraenoic acid). Cytosolic Ca2+ has not been implicated.
It is in this context that our present experiments were undertaken to ascertain whether a variation in cytosolic Ca2+ is instrumental in flow-dependent proximal tubule transport. The salient findings of our work are 1) under conditions in which cytosolic Ca2+ is likely to be increased (high ambient Ca2+, or Ca-ATPase inhibition with thapsigargin administration), baseline Na+ and HCO3− transport are depressed, but the flow-dependent increase is largely intact; 2) clamping cytosolic Ca2+ with a chelator (BAPTA) leaves baseline flow intact, but abrogates the flow-dependent increase; and 3) application of the IP3 receptor antagonist 2-APB also preserves baseline flow, while eliminating the flow-dependent increase. These latter two observations suggest that a local Ca2+ puff, triggered by IP3, is part of the signal transduction pathway from the force on the actin cytoskeleton to the activation of NHE3. This activation could plausibly be insertion of new transporters from vesicle reservoirs, or shuttling of NHE3 already within the luminal membrane from a basal location to a more available site. Such a role for the IP3 receptor is consistent with its function in other cells (e.g., endocrine secretion, lymphocyte function, or synaptic transmission), but has not been previously identified in kidney proximal tubule (7).
The foregoing results suggest fundamentally different mechanical activation mechanisms for the release of cytosolic Ca2+ in proximal tubule and distal nephron principal cells although both are triggered by IP3. In the distal nephron, as already noted, it is the entry of extracellular Ca2+ through strain-sensitive ion channels at the base of the primary cilia that initiates the release of Ca2+ from the endoplasmic reticulum. In neonatal principal cells, this response is depressed very likely due to the fact that the IP3 receptors and the Ca2+ channels are not fully assembled (29), although the primary cilia in neonatal animals are actually longer and thus would have a larger bending moment and experience larger membrane strains at their base (12). In proximal tubule cells, the entry of extracellular Ca2+ appears to play little if any role in the flow-dependent resorption, yet these cells also have primary cilia. The basic difference is that these primary cilia are shielded by a densely packed brush border of microvilli whose height is not much shorter that the length of fully developed primary cilia. As described in Guo et al. (10), there is virtually no flow in this layer of microvilli and therefore the torque on the primary cilia is greatly diminished. The activation of the IP3 pathway is via an actin cytoskeleton which links the cortical actin network at the apical membrane of the brush border cells with the endoplasmic reticulum, and if this is compromised there is no release of cytosolic Ca2+. In marked contrast, it is the disruption of the microtubular network in principal cells that abrogates the Ca2+ response (22).
In summary, increasing the axial flow velocity in proximal tubule exerts a force on the actin cytoskeleton of brush border microvilli. This force ultimately modulates transporter activity within both luminal and peritubular membranes, likely by increasing transporter density within each membrane. What this report adds to that picture is the finding that in this cascade of events, IP3 receptor-mediated Ca2+ release from an intracellular compartment is critical. This provides a readily testable hypothesis and raises additional questions regarding the strength and duration of the Ca2+ signal. It prompts examination of how broad the scope of the flow-effect is (i.e., which membrane transporters are impacted) and may also offer opportunities for pharmacological modulation of proximal tubule transport.
GRANTS
This investigation was supported by Public Health Service Grants RO1-DK62289 (T. Wang) and RO1-DK-29857 (A. M. Weinstein) from the National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Z.D. performed experiments; Z.D. and T.W. analyzed data; Z.D., S.W., A.M.W., and T.W. approved final version of manuscript; S.W., A.M.W., and T.W. provided conception and design of research; S.W., A.M.W., and T.W. edited and revised manuscript; A.M.W. and T.W. interpreted results of experiments; T.W. prepared figures; T.W. drafted manuscript.
ACKNOWLEDGMENTS
Present address of Z. Du: Dept. of Nephrology, Peking University Hospital, No. 5 Yiheyuan Rd., Haidian District, Beijing, China 100871.
REFERENCES
- 1.Beck JS, Breton S, Mairbaurl H, Laprade R, Giebisch G. Relationship between sodium transport and intracellular ATP in isolated perfused rabbit proximal convoluted tubule. Am J Physiol Renal Fluid Electrolyte Physiol 261: F634–F639, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Du Z, Duan Y, Yan Q, Weinstein AM, Weinbaum S, Wang T. Mechanosensory function of microvilli of the kidney proximal tubule. Proc Natl Acad Sci USA 101: 13068–13073, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Z, Wan L, Yan Q, Weinbaum S, Weinstein AM, Wang T. Regulation of glomerulotubular balance: II: impact of angiotensin II on flow-dependent transport. Am J Physiol Renal Physiol 303: F1507–F1516, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du Z, Yan Q, Duan Y, Weinbaum S, Weinstein AM, Wang T. Axial flow modulates proximal tubule NHE3 and H-ATPase activities by changing microvillus bending moments. Am J Physiol Renal Physiol 290: F289–F296, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Du Z, Yan Q, Wan L, Weinbaum S, Weinstein AM, Wang T. Regulation of glomerulotubular balance. I. Impact of dopamine on flow-dependent transport. Am J Physiol Renal Physiol 303: F386–F395, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan Y, Weinstein AM, Weinbaum S, Wang T. Shear stress-induced changes of membrane transporter localization and expression in mouse proximal tubule cells. Proc Natl Acad Sci USA 107: 21860–21865, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman PA, Figueiredo JF, Maack T, Windhager EE. Sodium-calcium interactions in the renal proximal convoluted tubule of the rabbit. Am J Physiol Renal Fluid Electrolyte Physiol 240: F558–F568, 1981. [DOI] [PubMed] [Google Scholar]
- 9.Gmaj P, Murer H, Kinne R. Calcium ion transport across plasma membranes isolated from rat kidney cortex. Biochem J 178: 549–557, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo P, Weinstein AM, Weinbaum S. A hydrodynamic mechanosensory hypothesis for brush border microvilli. Am J Physiol Renal Physiol 279: F698–F712, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Morimoto T, Woda C, Kleyman TR, Satlin LM. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol Renal Physiol 293: F227–F235, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol 289: F978–F988, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003. [DOI] [PubMed] [Google Scholar]
- 14.McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol 298: R851–R861, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens 12: 517–520, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Rydholm S, Zwartz G, Kowalewski JM, Kamali-Zare P, Frisk T, Brismar H. Mechanical properties of primary cilia regulate the response to fluid flow. Am J Physiol Renal Physiol 298: F1096–F1102, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Schnermann J, Wahl M, Liebau G, Fischbach H. Balance between tubular flow rate and net fluid reabsorption in the proximal convolution of the rat kidney. I. Dependency of reabsorptive net fluid flux upon proximal tubular surface area at spontaneous variations of filtration rate. Pflügers Arch 304: 90–103, 1968. [DOI] [PubMed] [Google Scholar]
- 19.Schultz SG. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by “flush-through.” Am J Physiol Renal Fluid Electrolyte Physiol 241: F579–F590, 1981. [DOI] [PubMed] [Google Scholar]
- 20.Tisher CC, Kokko JP. Relationship between peritubular oncotic pressure gradients and morphology in isolated proximal tubules. Kidney Int 6: 146–156, 1974. [DOI] [PubMed] [Google Scholar]
- 21.Ullrich KJ, Rumrich G, Kloss S. Active Ca2+ reabsorption in the proximal tubule of the rat kidney. Dependence on sodium- and buffer transport. Pflügers Arch 364: 223–228, 1976. [DOI] [PubMed] [Google Scholar]
- 22.Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM. Mechanotransduction in the renal tubule. Am J Physiol Renal Physiol 299: F1220–F1236, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinman EJ, Shenolikar S. Regulation of the renal brush border membrane Na+-H+ exchanger. Annu Rev Physiol 55: 289–304, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein AM, Stephenson JL. Models of coupled salt and water transport across leaky epithelia. J Membr Biol 60: 1–20, 1981. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein AM, Weinbaum S, Duan Y, Du Z, Yan Q, Wang T. Flow-dependent transport in a mathematical model of rat proximal tubule. Am J Physiol Renal Physiol 292: F1164–F1181, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Welling LW, Welling DJ. Surface areas of brush border and lateral cell walls in the rabbit proximal nephron. Kidney Int 8: 343–348, 1975. [DOI] [PubMed] [Google Scholar]
- 27.Windhager EE, Taylor A. Regulatory role of intracellular calcium ions in epithelial Na transport. Annu Rev Physiol 45: 519–532, 1983. [DOI] [PubMed] [Google Scholar]
- 28.Woda CB, Leite M Jr, Rohatgi R, Satlin LM. Effects of luminal flow and nucleotides on [Ca2+]i in rabbit cortical collecting duct. Am J Physiol Renal Physiol 283: F437–F446, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto Y, Hidaka I, Iso-o N, Komai A, Soma R, Kwak S. Noise-induced compensation for postural hypotension in primary autonomic failure. Brain Res 945: 71–78, 2002. [DOI] [PubMed] [Google Scholar]