Abstract
Intestinal epithelial cell renewal relies on the right balance of epithelial cell migration, proliferation, differentiation, and apoptosis. Intestinal epithelial cells consist of absorptive and secretory lineage. The latter is comprised of goblet, Paneth, and enteroendocrine cells. Fibroblast growth factor 10 (FGF10) plays a central role in epithelial cell proliferation, survival, and differentiation in several organs. The expression pattern of FGF10 and its receptors in both human and mouse intestine and their role in small intestine have yet to be investigated. First, we analyzed the expression of FGF10, FGFR1, and FGFR2, in the human ileum and throughout the adult mouse small intestine. We found that FGF10, FGFR1b, and FGFR2b are expressed in the human ileum as well as in the mouse small intestine. We then used transgenic mouse models to overexpress Fgf10 and a soluble form of Fgfr2b, to study the impact of gain or loss of Fgf signaling in the adult small intestine. We demonstrated that overexpression of Fgf10 in vivo and in vitro induces goblet cell differentiation while decreasing Paneth cells. Moreover, FGF10 decreases stem cell markers such as Lgr5, Lrig1, Hopx, Ascl2, and Sox9. FGF10 inhibited Hes1 expression in vitro, suggesting that FGF10 induces goblet cell differentiation likely through the inhibition of Notch signaling. Interestingly, Fgf10 overexpression for 3 days in vivo and in vitro increased the number of Mmp7/Muc2 double-positive cells, suggesting that goblet cells replace Paneth cells. Further studies are needed to determine the mechanism by which Fgf10 alters cell differentiation in the small intestine.
Keywords: Fgf10, Fgfr2b, small intestine, differentiation
the intestine is lined by a single layer of epithelial cells that allows the exchange of nutrients but also functions as a physical barrier against bacteria and ingested toxins. The intestinal epithelium self-renews continuously while maintaining this barrier. This renewal relies on the right balance of cell proliferation, migration, differentiation, and apoptosis. Stem cells near the base of the crypt of Lieberkühn divide, feeding into a transit amplifying (TA) population. Differentiating cells migrate out of the TA compartment either into the villi or the base of the crypt. Fully mature intestinal epithelial cells belong to either the absorptive or the secretory cell lineage. Secretory cell types are goblet, Paneth, tuft, and enteroendocrine (27). Secretory lineages arise from progenitors expressing Atonal Homolog 1 gene (Atoh1, also called Math1) (37, 44, 48). The notch effector, hairy/enhancer of split 1 (Hes1), deletion of which results in excessive formation of all three secretory cells types, inhibits Atoh1 expression (20, 40, 44). Within the secretory lineage, enteroendocrine cell fate specification depends on the expression of Neurogenin 3 (Neurog 3) (19, 26); Paneth cell differentiation and maturation rely on the expression of SRY-box containing gene (Sox9) and Wnt signaling via Frizzled 5 (1, 27), and differentiation of goblet cells requires Kruppel like factor 4 (Klf4) (22). SAM pointed domain containing Ets transcription factor (Spdef) regulates both goblet and Paneth cell differentiation. Deletion of Spdef severely disrupts the maturation of goblet and Paneth cells (13), whereas overexpression of Spdef in mice increases goblet cell differentiation and decreases Paneth cells, enterocytes, and enteroendocrine cells (28).
Fibroblast growth factor 10 (FGF10), one of 22 members of the FGF family, is known to play a central role in cell proliferation and/or differentiation of the epithelium in several organs (2, 34, 39, 46). During development of the gastrointestinal tract, Fgf10 is expressed in the mesenchyme of the stomach, duodenum, cecum, and colon (4, 9, 33) and is critical for the development of these organs (4, 29, 33, 41, 42). The loss of Fgf10 in mice results in duodenal, cecal, and colonic atresia (8, 10, 11, 21). We recently showed that Fgf10 expression is induced in the ileum of mice during gut adaptation (41). Moreover, Fgf10 overexpression promotes the formation of tissue-engineered small intestine (42). However, to date, the impact of gain or loss of Fgf10 signaling on adult mouse small intestine has not been investigated.
In this study, we analyzed the expression of FGF10, its receptors FGFR1 and FGFR2, as well as other FGFR2 ligands in the human ileum and the three segments of the adult mouse small intestine (duodenum, jejunum, and ileum). We showed that FGF10, FGFR1b, and FGFR2b are expressed in the human ileum. In the mouse intestine, Fgf10 is expressed in the duodenum, whereas Fgfr1 and Fgfr2 are expressed throughout the intestine. Furthermore, we demonstrated that overexpression of Fgf10 both in vivo and in vitro induced goblet cell differentiation and reduced Paneth cells, whereas sequestering Fgfr2b ligands with a soluble receptor did not affect intestinal differentiation. Moreover, FGF10 decreases stem cell markers such as Lgr5, Lrig1, Hopx, Ascl2, and Sox9 in ileal enteroids cultured in vitro. FGF10 inhibited Hes1 expression in the enteroids, suggesting that FGF10 induces goblet cell differentiation likely through the inhibition of Notch signaling. Interestingly, Fgf10 overexpression in vivo increased the number of goblet cells in the crypt compartment. Furthermore, we showed that Fgf10 overexpression for 3 days in vivo and in vitro increased the number of Mmp7/Muc2 double-positive cells. Taken together, these results suggest that goblet cells replace Paneth cells following Fgf10 overexpression. We demonstrated that Fgf10 plays an important role in intestinal cell differentiation. Further studies are needed to determine the mechanism(s) by which Fgf10 alters cell differentiation in the small intestine.
MATERIALS AND METHODS
Human subjects.
Fresh human tissue was obtained from patients 3 mo–18 yr old, admitted for surgery at Children's Hospital Los Angeles under an IRB-approved protocol to collect waste tissue derived from surgeries that is not needed for pathological diagnosis. Families signed consent for the tissue collection and demographic, and curated medical history data are available through the protocol. The indications for surgery for these patients did not include primary intestinal disease.
Mice.
All the mice were housed in the Animal Care facility of the Saban Research Institute, Children's Hospital Los Angeles. The Institutional Animal Care and Use Committee approved all animal protocols used in this study in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health. The approval identification number for Children's Hospital Los Angeles is AAALAC A3276-01. CD1 wild-type mice were purchased from the Charles Rivers Laboratory and C57Bl/6 mice from the Jackson Laboratory.
Generation of mutant mice.
For this study we used mice that allow ubiquitous, inducible, and reversible overexpression of Fgf10 as well as a soluble form of Fgfr2b (sFgfr2b) as previously described (6, 14, 30, 32). Briefly, mice (CD1 mixed background) expressing rtTA under the ubiquitous Rosa26 promoter were crossed with lines harboring tet(O)sFgfr2b or tet(O)Fgf10 to obtain animals carrying both transgenes [R26rtTA/+; tet(O)sFgfr2b/+ and R26rtTA/+; tet(O)Fgf10] and single-transgene littermate controls (all mice carried only a single copy of a given transgenic allele). Tet promoter-induced Fgf10 overexpression was achieved by feeding doxycycline-containing food (rodent diet with 0.0625% doxycycline, Harlan Teklad TD01306) to double-transgenic adult (4-wk-old) mice [R26rtTA/+; tet(O)Fgf10/+] for a period of 10 days; sFgfr2b induction was achieved by feeding doxycycline to double-transgenic adult (4-wk-old) mice for 1 or 3 mo. Double transgenics without doxycycline or single transgenics with or without doxycycline were used as controls as described in results. At the end of the doxycycline treatment, mice were euthanized and the small intestines were harvested and separated in segments (duodenum, jejunum, and ileum). Tissues were either fixed in formalin or frozen in liquid nitrogen for RNA extraction.
β-Galactosidase staining.
Mlcv1v-nLacZ-24 or Fgf10LacZ reporter mouse was used to investigate the spatiotemporal expression of Fgf10 throughout the small intestine (23, 24). Small intestines were collected from 4-wk-old Fgf10LacZ animals and stained for β-galactosidase activity as previously described (38). Briefly, samples were shortly fixed in 4% paraformaldehyde (PFA), then stained in a LacZ solution containing a final concentration of 2 mg/ml of X-gal overnight at 37°C (RPI). These samples were then fixed, paraffin embedded, sectioned at 5 μm, and mounted on slides. Slides were deparaffinized, rehydrated in an ethanol gradient, counterstained with nuclear fast red, dehydrated, cleared with HistoChoice, and mounted with Permount (Fisher Scientific, Fair Lawn, NJ).
Immunohistochemistry.
All three segments of the small intestine (duodenum, jejunum, and ileum) were collected, fixed, and paraffin embedded for histological analyses. The samples were sectioned at 5 μm, then deparaffinized and rehydrated. The slides were stained with hematoxylin and eosin to examine their histology. The depth of the crypts and height of the villi were measured by use of ImageJ software (National Institutes of Health, Bethesda, MD). Alcian blue staining was used to visualize goblet cells. Immunohistochemistry (IHC) for FGFR1 and FGFR2 was performed with use of the following antibodies: rabbit anti-FGFR1 [1:100, Flg (C-15) Santa Cruz], rabbit anti-FGFR2 [1:200, Bek (C-17) Santa Cruz], and anti-rabbit IgG (1:100, Santa Cruz). For these antibodies, an antigen retrieval step was performed by boiling the slides in a microwave for 12 min in Tris-EDTA (10 mM Tris base, 1 mM EDTA solution, 0.05% Tween 20, pH = 9.0). To confirm the stainings, we also used rabbit anti-FGFR1 (1:100, Abcam ab63601) and rabbit anti-FGFR2 (1:100, Abcam ab10648) with antigen retrieval steps as recommended by the manufacturer. For the remaining IHC and immunofluorescence (IF) stainings, the slides were boiled in the microwave for 12 min in 10 mM sodium-citrate buffer, pH = 6.0. Cell proliferation was assessed by IHC using mouse anti-proliferating cell nuclear antigen (PCNA) (1:100, Vector Laboratories) and IF staining for phospho-histone H3 (Phh3) (1:100, Cell Signaling). The staining was visualized by using Dako Cytomation kit following the manufacturer's instructions. Slides were dehydrated and mounted with xylene mounting medium. IF staining was performed to identify Paneth cells (anti-lysozyme 1:100, Dako) and enteroendocrine cells [rabbit anti-chromogranin A (CGA) 1:100, Abcam]. Cell death was assessed by use of rabbit anti-caspase 3 (active) (1:500, R&D Systems). Cy3-conjugated secondary antibodies were used for the IF stainings. Slides were counterstained with DAPI (1:500, Life Technologies) and mounted with ProLong Diamond Antifade Mountant (Life Technologies). Images were acquired by using a camera attached to an upright fluorescent microscope (Leica DM5500).
Cell counting and quantification.
Epithelial nuclei, lysozyme, and CGA-stained cells were counted semiautomatically by use of MetaMorph 7.7.3.0 software (Molecular Devices, Sunnyvale, CA). First, the blue (DAPI) channel was processed as follows: background was subtracted by manual thresholding, intensity was normalized by dividing by a 20×20 low-pass filtered copy of the channel, and the normalized nuclei were smoothed with a 5×5 median filter to preserve edges. A binary image of the smoothed nuclei was created by thresholding automatically by using the isodata histogram algorithm under visual inspection to manually adjust the threshold value if needed. Overlapping binary nuclei were separated by Watershed segmentation with FoveaPro 3.0 software (Reindeer Graphics, Asheville, NC). To count lysozyme and CGA-positive cells, the red (Cy3) channel was processed in the same manner as the blue channel except that the thresholding step employed the “legacy heuristic” algorithm and an additional step to fill all dark holes (where nuclei didn't show in blue) was added just prior to the segmentation.
The total number of PCNA, Alcian blue, lysozyme, and CGA-positive cells and the total number of epithelial cells and/or epithelial cells per crypt were counted separately for three randomly selected high-power fields (×20 magnification) per sample per mouse and averaged, with six mice in every experimental set. The total number of cell type-positive cells per the total number of epithelial cells, or percent cell type positive was calculated for each sample. Results were reported as percent ± SE.
Mouse crypt cultures.
Mouse crypt culture were isolated and grown from wild-type mouse ileum as previously described (35). Briefly, 6 cm of mouse ileum were isolated, washed, and treated with 2 mM cold EDTA for 30 min at 4°C. The suspension is transferred into sucrose-sorbitol buffer, gently shaken until the crypts start separating, and strained through 70-μm filters. After washing and centrifugation, the crypt units were embedded in Matrigel containing EGF (50 ng/ml), Noggin (10 ng/ml), and R-Spondin (500 ng/ml) and incubated at 37°C to allow the polymerization of the Matrigel. Culture medium containing DMEM/F12, l-glutamine, penicillin/streptomycin, HEPES (10 mM), N2, and B27 supplement was added to the Matrigel. The crypts were grown for 7 days then passaged by gently dissociating the Matrigel and breaking down the enteroids, then plated and cultured for 3 days before treatment for 4 days with 200 ng/ml of human recombinant FGF10.
Crypts from Rosa26rtTA, Tet(O)Fgf10 animals were isolated as described above, replacing 1 mM cold EDTA with 3 mM EDTA. Enteroids were treated with 2 μg of doxycycline for 72 h, after which the enteroids from the same experiment were pooled and split for RNA extraction and histology. RNA was extracted for gene expression analyses.
Enteroid histology.
Enteroids fixed in 4% PFA were then embedded in HistoGel, dehydrated through ethanol gradient, embedded in paraffin, and sectioned for histology. IF staining was performed with anti-MMP7 antibody (1:100, Vanderbilt University), anti-Muc-2 (1:100, Santa Cruz), anti-lysozyme (1:100, Dako), anti-Phh3 (1:100, Cell Signaling), and anti-E-cadherin (1:200, BD Biosciences). Slides were visualized under a Leica DM5500. Cells were counted in two images per sample in four independent experiments from four independent animals at ×20 magnification and results are reported as percentage of positive cells to the total number of cells (nuclei).
Whole mount mouse enteroid staining.
The mouse enteroid cultures in polymerized Matrigel were rinsed with PBS and fixed for 30 min in 2% PFA. The fixation was then quenched with 50 mM NH4Cl for 30 min at 4°C. Crypts were permeabilized with 0.05% Triton X-100 in PBS for 30 min. Blocking was achieved by using 3% BSA in PBS for at least 1 h at room temperature. Cell proliferation was assessed by use of phospho-histone H3 antibody (1:100, Cell Signaling), in which the cultures were incubated overnight at 4°C. The staining was visualized with Alexa Fluor 647 donkey anti-rabbit (1:100, Life Technologies), in which the cultures were incubated overnight at 4°C. Nuclei were stained with 2 μg/ml Hoechst 33342 fluorescent stain (Thermo Scientific) for 20 min. Cultures are ultimately suspended in PBS when imaging.
Z-stack images were acquired with an LSM 700 confocal system mounted on an AxioObserver Z.1 inverted microscope equipped with a ×20/0.8 Plan-APOCHROMAT lens and controlled by ZEN 2009 software (Carl Zeiss Microimaging, Thornwood, NY). Fluorescence images of DAPI and Alexa Fluor 647 were obtained by using excitation laser lines of 405 and 639 nm and emission filters of short-pass 490 nm and long-pass 640 nm, respectively. The z-slice interval was 5 μm with the confocal pinhole set to 1 Airy unit for Alexa Fluor 647. A z-correction factor of 1.33 was used to obtain more accurate z scaling. If needed, the laser transmission percentage and/or detector gain were increased linearly with depth to obtain more uniform brightness (z-brightness correction). To measure the total visualized volume of each specimen the DAPI and Alexa Fluor 647 channels were first combined by using the Image Calculator MAX function of Fiji ImageJ software (36). Then volume measurements were made with Imaris software by creating Surface objects using manual thresholding (Bitplane, Zurich, Switzerland).
RT-PCR and qRT-PCR analyses.
RNA was isolated from adult wild-type (C57Bl/6) mouse small intestines (duodenum, jejunum, and ileum) (n = 3) and embryonic day 14.5 (E14.5) wild-type whole mouse embryo (n = 3). One microgram of RNA was used for cDNA synthesis using Tetro cDNA Synthesis Kit (Bioline). RT-PCR reactions were performed by following the manufacturer's instructions with either the Taq PCR Master Mix (Qiagen, Valencia, CA) and or the primers previously described and detailed hereafter for human primers and in Table 1 for mouse primers (38). Human primers used are as follows: FGF1 [Left (L): CCTCGGCCTACAAGCTCTTT; Right (R): AGAGGAGTTTGGGCTTCTTGTA], FGF3 (L: TGGAGAACAGCGCCTACAGT; R: GGAGAAGAGACCCCTGATGG), FGF7 (L: GCGCTCACACACAGAGAGAA; R: CAGCAGAATCATAATTGTTTCCAT), FGF10 (L: GAAGGAGAACTGCCCGTACA; R: GGCAACAACTCCGATTTCTACT), FGF22 (L: TTCTCCTCCACTCACTTCTTCC; R: CACGTGTACAGAGCGGATCTC), FGFR1b (L: GCATTCGGGGATTAATAGCTC; R: CCACAGGTCTGGTGACAGTG), FGFR2b (L: GCACAAGCTGACCAAACGTA; R: CTGGACTCAGCCGAAACTGT), and CDH1 (L: TGGAGGAATTCTTGCTTTGC; R: CGCTCTCCTCCGAAGAAAC).
Table 1.
Primer sequences and probe numbers used in qRT-PCR analyses of mouse gene expression
| Gene | Probe | Primer Orientation | Sequence (5′-3′) |
|---|---|---|---|
| Ascl2 | 17 | Right | AGGTCCACCAGGAGTCACC |
| Left | GAGAGCTAAGCCCGATGGA | ||
| Atoh1 | 69 | Right | CTCTTCTGCAAGGTCTGATTTTT |
| Left | TGCGATCTCCGAGTGAGAG | ||
| Axin2 | 50 | Right | TGCCAGTTTCTTTGGCTCTT |
| Left | CTGCTGGTCAGGCAGGAG | ||
| Fgf10 | 80 | Right | AACAACTCCGATTTCCACTGA |
| Left | CGGGACCAAGAATGAAGACT | ||
| s Fgfr2b | 108 | Right | GAAGGAGATCACGGCTTCC |
| Left | AGACAGATGATACTTCTGGGACTGT | ||
| Hes1 | 20 | Right | CCATGATAGGCTTTGATGACTTT |
| Left | TGCCAGCTGATATAATGGAGAA | ||
| Hopx | 68 | Right | GCGCTGCTTAAACCATTTCT |
| Left | ACCACGCTGTGCCTCATC | ||
| Klf4 | 62 | Right | GAGTTCCTCACGCCAACG |
| Left | CGGGAAGGGAGAAGACACT | ||
| Lgr5 | 60 | Right | CAGCCAGCTACCAAATAGGTG |
| Left | CTTCACTCGGTGCAGTGCT | ||
| Lrig1 | 89 | Right | CAGGTGGCTGTCACAAAGG |
| Left | TGCAAAGATGAAGAACCTTAAAGA | ||
| Lysozyme | 46 | Right | CACCACCCTCTTTGCACATT |
| Left | TGGGATCAATTGCAGTGCT | ||
| Muc2 | 76 | Right | CGTGTCATATTTGCACCTCTTG |
| Left | GTCAGAGACGCCCTTTGC | ||
| Sox9 | 75 | Right | TCCACGAAGGGTCTCTTCTC |
| Left | CAGCAAGACTCTGGGCAAG | ||
| Spdef | 22 | Right | GACGAGTCCACCTCACCATC |
| Left | CCCACCTGGACATCTGGA |
β-Galactosidase staining of whole mount mouse enteroid culture.
Enteroids from Axin2-LacZ animals were isolated as previously described, by use of 2 mM EDTA. Enteroids were treated with 200 ng/ml FGF10 for 48 h, with media change and FGF10 supplementation occurring at 24 h. Mouse enteroid cultures were stained for β-galactosidase activity while in Matrigel. Samples were briefly washed in PBS and fixed in 2% PFA (2 min). They were then momentarily washed in LacZ buffer and stained in a LacZ solution containing 2 mg/ml of X-gal overnight at 37°C (RPI).
Statistical analysis.
Statistical analyses were performed with GraphPad Prism software. Paired t-tests compared the results from the enteroids cultures. Data are presented as average values ± SE. The results were considered significant if P < 0.05.
RESULTS
Expression of FGF10 and its receptors FGFR1 and FGFR2 in human ileum.
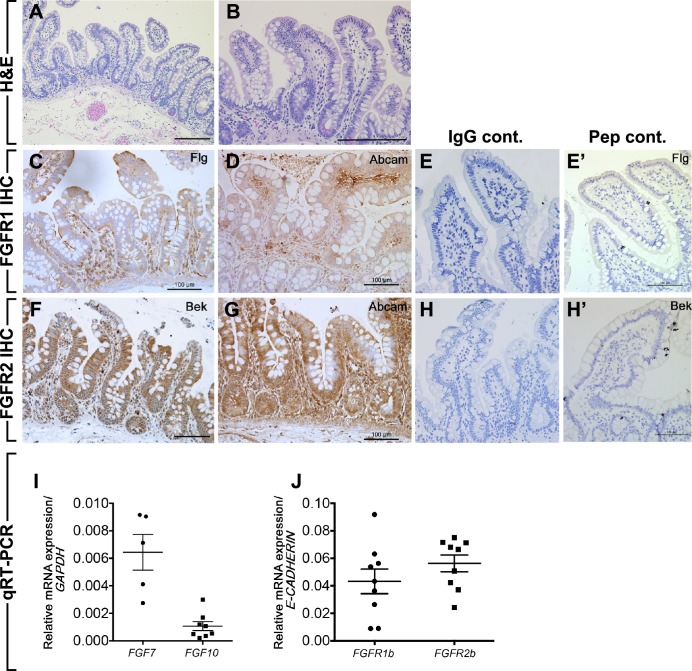
Little is known about the expression of FGFs in human intestine. We first sought to study the expression of FGFR1, FGFR2, and FGF10 family members (FGFR2b ligands), FGF1, FGF3, FGF7 (also known as keratinocyte growth factor or KGF), FGF10, and FGF22, in healthy human ileum tissue obtained from patients aged 3 mo–18 yr old who required surgery for various indications that did not include primary intestinal disease. In most cases, these patients required short-term intestinal diversion 6+ wk prior to tissue collection and were assessed as healthy and nutritionally replete at the time of surgery to reconnect the intestine. Hematoxylin and eosin staining of human ileum show normal crypt and villi (Fig. 1, A and B). Staining by IHC, using Flg (C-15) antibody, demonstrated that FGFR1 is expressed in human ileum throughout the length of the crypt and villi (Fig. 1C). This distribution was confirmed by using a second FGFR1 antibody from Abcam (Fig. 1D). The use of matching rabbit IgG as primary antibody did not show any nonspecific staining (Fig. 1E). Moreover, the Flg C-15 blocking peptide competed away binding, supporting the specificity of this antibody (Fig. 1E′). FGFR2 staining, using Bek (C17) antibody, was also seen throughout the epithelium of the ileum and to a lesser extent in the mesenchyme (Fig. 1F). This pattern was confirmed by use of a second FGFR2 antibody from Abcam (Fig. 1G). Matched IgG primary and peptide competition staining confirmed the specificity of staining (Fig. 1, H and H′).
Fig. 1.
Expression of FGFR1, FGFR2, and their ligands in human ileum. A and B: hematoxylin and eosin (H&E) staining of normal human ileum showing crypt and villus structures (A) and higher magnification of A (B). C and D: immunohistochemistry (IHC) using anti-FGFR1 antibody from Santa Cruz (Flg) (C) and anti-FGFR1 from Abcam (D) on human ileum. E: negative control (Cont.) using matching anti-IgG. E′: negative control using the competition peptide (Pep) for Flg antibody. F and G: IHC using anti-FGFR2 antibody from Santa Cruz (Bek) (F) and anti-FGFR2 antibody from Abcam (G) on human ileum. H: negative control using matching anti-IgG. H′: negative control using the competition peptide for Bek antibody. I and J: qRT-PCR for FGFs ligands (I, n = 5 at least), FGFR1b, and FGFR2b (J, n = 9) on human ileum. Scale bars are 100 μm.
To determine the expression of the ligands of FGFR1 and FGFR2 in the human ileum, we performed qRT-PCR on the same samples. We detected FGF7 and FGF10 in the human ileum but not FGF1, FGF3, and FGF22 (Fig. 1I). We defined the threshold for positive expression at or before 35 RT-PCR cycles. Similar to protein expression, both FGFR1b and FGFR2b receptor genes were expressed in the human ileum as shown (using E-cadherin as reference, since b isoforms of these receptors are expressed exclusively in the epithelium) (Fig. 1J).
Expression of Fgf10 family members and Fgf10 receptors in the adult mouse small intestine.
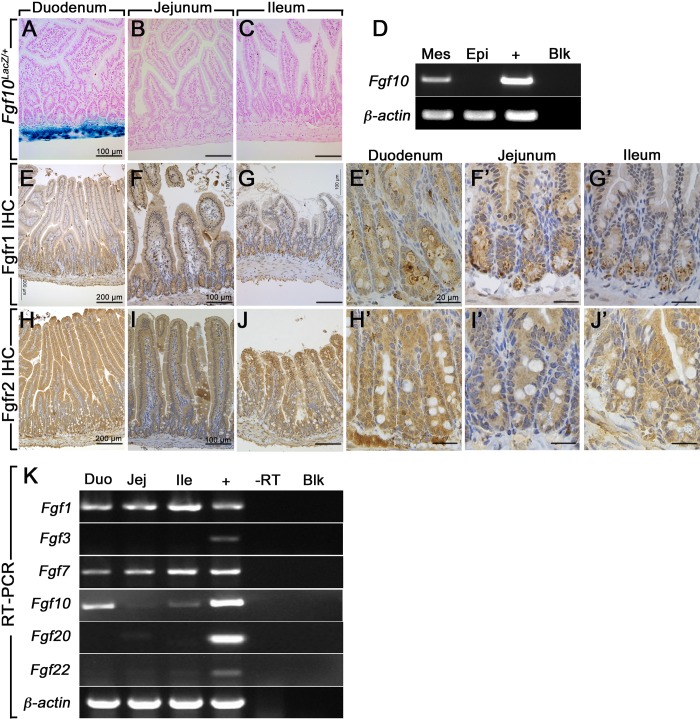
Next, we aimed to assess the expression of the Fgf10 family members, as well as Fgf10 receptors Fgfr1 and Fgfr2, in the three segments of the adult mouse small intestine. Analyzing the Fgf10LacZ reporter mouse, we found that Fgf10 is strongly expressed in the mesenchymal compartment of adult mouse duodenum, but not detected in the jejunum or the ileum (Fig. 2A, n = 3). Fgf10+/+ (LacZ-negative) control littermates did not show any LacZ staining (n = 3). To confirm that Fgf10 was expressed only in the mesenchyme, we separated epithelium from the rest of the duodenal mouse tissue using EDTA and confirmed by RT-PCR that Fgf10 is absent from the epithelial fraction as shown in Fig. 2D. Immunohistochemical analyses using antibodies against the receptors Fgfr1 and Fgfr2 (detecting both isoforms b and c) revealed that Fgfr1 (Fig. 2, E–G) and Fgfr2 (Fig. 2, H–J) are expressed throughout the adult mouse small intestine (n = 3). Negative controls with IgG and secondary antibodies alone did not show any staining (data not shown). Higher magnifications of the crypts containing the stem cell niche showed expression in the crypts of both Fgfr1 (Fig. 2E′–G′) and Fgfr2 (Fig. 2, H′–J′). Using RT-PCR, we showed that Fgf1, Fgf7, and Fgf10 were expressed in all three segments (duodenum, jejunum, and ileum) of the adult mouse small intestine as shown in Fig. 2D, whereas Fgf3, Fgf20, and Fgf22 were not detected (Fig. 2D). Importantly, Fgf10 seems to be expressed at higher levels in the duodenum compared with the jejunum and ileum, which likely explains the lack of detection of LacZ activity in the jejunum and ileum of the Fgf10LacZ animals. E14.5 whole wild-type embryo was used as a positive control for the various gene expressions. Furthermore, we did not detect any nonspecific bands for any of the genes tested.
Fig. 2.
Expression of FGFR1, FGFR2, and FGFR2b ligands in the small intestine. A–C: LacZ (blue) and nuclear red (pink) staining on tissue sections from duodenum (A), jejunum, (B) and ileum (C) of adult Fgf10-LacZ mice. D: RT-PCR for Fgf10 and β-actin on separated epithelial and mesenchymal fractions from adult mouse duodenum (n = 3). E–G: IHC staining for Fgfr1 on mouse adult duodenum (E), jejunum, (F) and ileum (G). E′–G“: higher magnifications of crypts from panels E, F and G. H–J: IHC staining for Fgfr1 on mouse adult duodenum (H), jejunum, (I) and ileum (J). H′–J′: higher magnifications of crypts from H, I and J. K: qRT-PCR for Fgf10 family members on adult mouse duodenum (Duo), ileum (Ile), and jejunum (Jej) and embryonic day 14.5 whole mouse embryo (positive control). Negative controls did not show any bands; n = 3. To show the entire villus height, the duodenum sections are shown at lower magnifications than the jejunum and ileum panels. Scale bars are 100 μm in A–C, F, G, I, and J; 200 μm in E–H; and 20 μm in E′–G′ and H′–J′.
Fgf10 overexpression increased crypt depth and villus height in the mouse small intestine.
To investigate the impact of Fgf10 signaling on the adult small intestine independently from development, we generated inducible gain- and loss-of-function mouse models. First, we crossed CMV-Cre animals with Rosa26-rtTA to generate an ubiquitous expression of the rtTA promoter. Then, we crossed these animals with a tet(O)Fgf10 promoter, allowing overexpression of mouse Fgf10 under the conditional control of the Rosa26-rtTA promoter, hereafter referred to as Rosa26rtTA/+; tet(O)Fgf10/+. Fgf10 overexpression was achieved by feeding 4-wk-old mice a doxycycline-containing diet for 10 days. Single-transgenic littermates exposed to doxycycline (Control Dox) or double-transgenic heterozygous mice on normal diet (Control no Dox) served as controls.
To investigate the effects of Fgf signaling loss, we crossed the CMV-Cre;Rosa26-rtTA animals with mice harboring a tet(O)sFgfr2b promoter to generate mice overexpressing a soluble form of the mouse Fgfr2b receptor under the conditional control of the Rosa26-rtTA promoter similar to the Fgf10 overexpressing mice, hereafter referred to as Rosa26rtTA/+; tet(O)sFgfr2b/+. In these mice, exogenous sFgfr2b acts as a decoy receptor, and upon exposure to doxycycline it binds all Fgfr2b ligands available in the gut, thus preventing their action. These mice were exposed to doxycycline food starting at 4 wk of age for a period of 1 mo. This mouse model has been previously validated during embryonic development, when ubiquitous overexpression of sFgfr2b phenocopies Fgf10 null mice (7). Single-transgenic animals treated with doxycycline or double-transgenic animals not exposed to doxycycline were identical to wild-type controls (31). The mice from both strains were monitored for body weight changes during the treatment period. The overexpression of Fgf10 for 10 days and the overexpression of sFgfr2b for 1 or 3 mo did not affect the body weight of the mutant mice compared with the controls (data not shown).
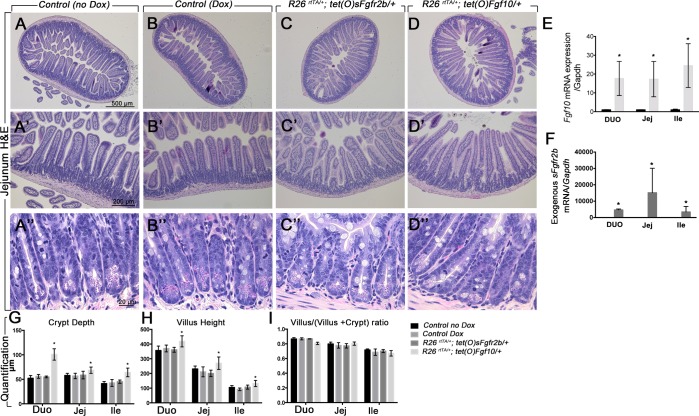
Histological analyses were performed on the Rosa26rtTA/+; tet(O)Fgf10/+, Rosa26rtTA/+; tet(O)sFgfr2b/+, and control littermates treated or not with doxycycline. Hematoxylin and eosin staining did not show any macroscopic differences in the gross histology of the jejunum between Rosa26rtTA/+; tet(O)sFgfr2b/+ and controls (Fig. 3, A–C, 3, A′–C′, and 3, A″–C″), whereas an altered histology was observed in the Rosa26rtTA/+; tet(O)Fgf10/+ compared with the controls (Fig. 3, D, D′, D″ vs. A and B, A′–B′, A″–B″). There was no difference in the control littermates between single-transgenic mice on doxycycline food for 10 days or 1 mo or double-transgenic animals on regular diet (B–B″ vs. A–A″). The mice overexpressing Fgf10 displayed longer villi and deeper crypts (Fig. 3, D–D″). To validate the animal models, we assessed the expression of Fgf10 by qRT-PCR and showed a robust increase in Fgf10 expression in the animals harboring Rosa26rtTA and tet(O)Fgf10 transgenes (Fig. 3E). Fgf10 expression was very low in the jejunum and ileum of controls treated or not with doxycycline and undetectable in the mutants overexpressing sFgfr2b. Moreover, after 10 days of doxycycline treatment, the mutant mice developed a wet hair appearance and swelling of the eyelids and the tongue as previously reported (38).
Fig. 3.
Increased villus height in the duodenum and jejunum after Fgf10 overexpression. A–D: hematoxylin and eosin staining of jejunum sections of control not treated with doxycycline (Dox; A, A′, A″), Dox-treated controls (B, B′, B″), Rosa26rtTA/−; tet(o)sFgfr2b/+ (C, C′, C″), and Rosa26rtTA/−; tet(o)Fgf10/+ animals (D, D′, D″). A′–A″, B′–B″, C′–C″, D′–D″ are higher magnifications of A, B, C, and D, respectively. E: qRT-PCR validating the expression of Fgf10 in the controls vs. Rosa26rtTA/−; tet(o)Fgf10/+ animals (n = 6). E: qRT-PCR showing the expression of exogenous sFgfr2b transcripts in the controls vs. Rosa26rtTA/−; tet(o)sFgfr2b/+ animals (n = 6). G: quantification of the crypt depths. H: quantification of the villus height. I: quantification of the ratio villus/(villus + crypt). N = 6 for each group, *P ≤ 0.05. Scale bars are 500 μm in A–D, 200 μm in A′–D′, and 20 μm in A″–D″.
We also assessed the expression of the exogenous sFgfr2b transgene and demonstrated that it is highly expressed in all the animals harboring Rosa26rtTA and sFgfr2b transgenes but not detectable in any of the other groups (Fig. 3F). Both Fgf10 and sFgfr2b expression were similar in the controls treated or not with doxycycline.
We next measured the crypt depth (Fig. 3G), the villus height (Fig. 3H), and the ratio villus/(villus + crypt) (Fig. 3I) in the three segments of the intestine of the animals overexpressing Fgf10 and sFgfr2b and in the controls. Crypt depth was significantly increased in the duodenum, jejunum, and ileum of the animals overexpressing Fgf10 but not in the animals overexpressing sFgfr2b (Fig. 3G). Villus height was significantly increased in the duodenum, jejunum, and ileum of the animals overexpressing Fgf10, with no change in the animals overexpressing sFgfr2b compared with littermate controls (Fig. 3H). The ratio villus/(villus + crypt) was unchanged between the controls and the animals overexpressing Fgf10 or sFgfr2b (Fig. 3I; n = 6; P = 0.4 for duodenum, P = 0.5 for jejunum, and P = 0.8 for ileum). In addition, crypt fission was not affected in the mutants overexpressing Fgf10 (Fig. 3D″) or sFgfr2b (Fig. 3C″) compared with controls treated or not with doxycycline (Fig. 3, A″ and B″), as shown in the high-power field of crypts.
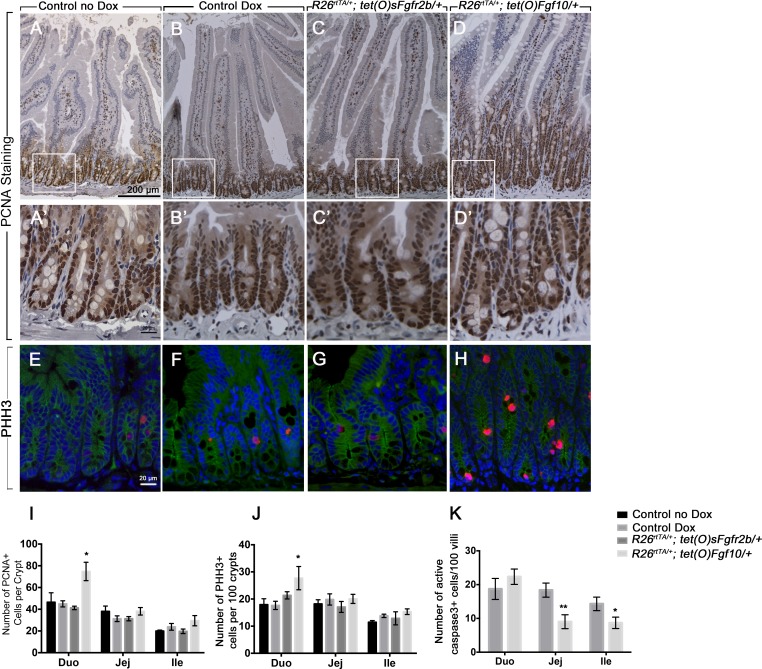
Fgf10 overexpression increases cell proliferation in the duodenum and decreases cell death in the jejunum and ileum.
FGF10 is known to promote cell proliferation during the development of the gastrointestinal tract, as well as during gut adaptation (12, 33, 39, 41). Therefore, we assessed the impact of Fgf10 signaling on intestinal epithelial cell proliferation. PCNA staining (Fig. 4, A–D) on intestinal sections and quantification of the number of PCNA-positive cells per crypt (Fig. 4I) showed a significant increase in cell proliferation in the duodenum of the mice overexpressing Fgf10 compared with controls treated or not with doxycycline (Fig. 4, D and D′ vs. A and A′ and B and B′; n = 6, P = 0.002), whereas no significant change in cell proliferation was observed in the jejunum or ileum (Fig. 4I; n = 6; P = 0.11 and 0.323, respectively). Similar results were obtained by using another cell proliferation marker, Phh3 (Fig. 4, E–H). Quantification of the Phh3-positive cells per crypt showed a significant increase in the duodenum of the animals overexpressing Fgf10 (Fig. 4J, n = 6, P = 0.035), but not in the jejunum or ileum (n = 6, P = 0.9 and 0.25, respectively). However, overexpression of sFgfr2b did not affect cell proliferation in any of the intestinal segments studied as shown by PCNA staining in Fig. 4, C and C′ vs. B and B′ and A and A′ and by Phh3 staining shown in Fig. 4, G vs. E and F; quantification shown in Fig. 4J. Since proliferation was only increased in the duodenum, we assessed cell death as a possible explanation for the increase in crypt depth and villus height in the jejunum and ileum of the Fgf10-overexpressing animals. We performed active (cleaved) caspase 3 IHC on doxycycline-treated controls and the animals overexpressing Fgf10 and counted the number of active caspase-3-positive cells per 100 villi. We observed no change in cell death in the duodenum of the animals overexpressing Fgf10 compared with controls, whereas there was a significant decrease in cell death in the jejunum and ileum of these animals compared with controls (Fig. 4K) (n = 5, P = 0.005 and 0.045, respectively, for jejunum and ileum).
Fig. 4.
Increased cell proliferation in the duodenum of mutants overexpressing Fgf10. A–D: IHC for PCNA on duodenum sections from controls not treated with Dox (A), Dox-treated controls (B), Rosa26rtTA/−; tet(o)sFgfr2b/+ (C), and Rosa26rtTA/−; tet(o)Fgf10/+ animals (D). A′, B′, C′, and D′ are higher magnifications of the inset in A, B, C, and D, respectively. E–H: immunofluorescence (IF) staining for phospho-histone H3 (Phh3) on duodenum sections from controls not treated with Dox (E), Dox-treated controls (F), Rosa26rtTA/−; tet(o)sFgfr2b/+ (G), and Rosa26rtTA/−; tet(o)Fgf10/+ animals (H). I: quantification of cell proliferation in the crypts as the percentage of the number of PCNA+ positive to the number of total cells per crypt. J: quantification of the number of Phh3+ cells per 100 crypts. K: quantification of cell death in the villi as the number of active caspase 3 + cells per 100 villi. The results are expressed as means ± SE of at least 5 independent animals per group. *P ≤ 0.05; **P ≤ 0.01. Scale bars are 200 μm in A–D, 20 μm in A′–D′, and E–H.
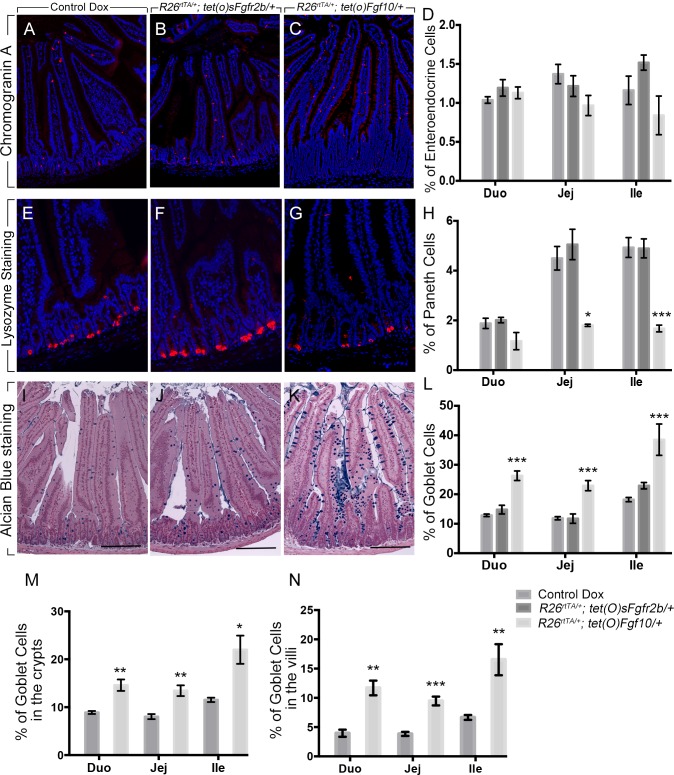
Fgf10 overexpression increases goblet cells and decreases Paneth cells in vivo.
Next, we aimed to determine the effect of Fgf10 on epithelial cell differentiation in the mouse adult small intestine. We performed immunofluorescent staining for CGA (a marker of enteroendocrine cells) and lysozyme (a marker of Paneth cells), as well as Alcian blue staining to assess goblet cell differentiation, on tissue sections from mice overexpressing Fgf10 and sFgfr2b and from doxycycline-treated controls. There was no change in CGA staining in the jejunum between the animals overexpressing Fgf10 and sFgfr2b and the controls (Fig. 5, C, B, and A, respectively). The quantification of the cells stained for CGA did not show any difference in the mice overexpressing Fgf10 or soluble Fgfr2b (Fig. 5D). Lysozyme staining, used to label Paneth cells, demonstrated decreased staining in the small intestinal segments of the mice overexpressing Fgf10 (Fig. 5G, jejunum, shown) compared with doxycycline-treated controls (Fig. 5E). Quantification of the number of Paneth cells in all three segments of the mouse intestine (duodenum, jejunum, and ileum) confirmed a significant decrease in the number of Paneth cells in the jejunum and ileum of the mice overexpressing Fgf10 (n = 6, P = 0.02 and 0.0008, respectively) but not in the duodenum (P = 0.099) (Fig. 5H). In contrast, no change in the number of Paneth cells was observed in the duodenum, jejunum, or ileum of the animals overexpressing soluble Fgfr2b (Fig. 5, F and H). Alcian blue staining showed increased goblet cells in the jejunum of the mice overexpressing Fgf10 (Fig. 5K) compared with doxycycline-treated controls (Fig. 5I). No change in Alcian blue staining was observed in the jejunum of the mice overexpressing sFgfr2b (Fig. 5J) compared with doxycycline-treated controls (Fig. 5I). Quantification of the percentage of goblet cells compared with the total number of epithelial cells showed significant increase in the duodenum, jejunum, and ileum (n = 6; P = 0.0003, P = 0.0005, and P = 0.0002, respectively) in the mice overexpressing Fgf10 compared with doxycycline-treated controls (Fig. 5L). No change was observed in the mice overexpressing sFgfr2b (Fig. 5L). Taking into consideration the distribution of goblet cells along the crypt/villus axis, we investigated whether there was an increase in goblet cells in both crypt and villus compartments of the mice overexpressing Fgf10. The number of goblet cells was significantly higher in the crypts (Fig. 5M) of the mice overexpressing Fgf10 compared with doxycycline-treated controls in the duodenum, jejunum, and ileum (Fig. 5M, n = 6, P = 0.005, 0.002, and 0.01, respectively). In addition, the number of goblet cells was significantly higher in the villi (Fig. 5N) of the mice overexpressing Fgf10 compared with doxycycline-treated controls in the duodenum, jejunum, and ileum (Fig. 5N, n = 6, P = 0.001, 0.0001, and 0.008, respectively).
Fig. 5.
Fgf10 overexpression results in increased goblet cells and decreased Paneth cells. A–C: chromogranin A IF staining in the jejunum of Dox-treated control (A), Rosa26rtTA/−; tet(o)sFgfr2b/+ (B), and Rosa26rtTA/−; tet(o)Fgf10/+ animals (C). D: quantification of the number of chromogranin A-positive cells per total number of epithelial cells. E–G: lysozyme staining (red) a marker of Paneth cells in the jejunum of Dox-treated control (E), Rosa26rtTA/−; tet(o)sFgfr2b/+ (F), and Rosa26rtTA/−; tet(o)Fgf10/+ (G). H: quantification of the number of lysozyme-positive cells per total number of epithelial cells per crypt. I–K: Alcian blue staining to label goblet cells in the jejunum of Dox-treated control (I), Rosa26rtTA/−; tet(o)sFgfr2b/+ (J), and Rosa26rtTA/−; tet(o)Fgf10/+ animals (K). L: quantification of the number of goblet cells per total number of epithelial cells. M: quantification of the number of goblet cells per total number of epithelial cells in the crypts of control Dox and Rosa26rtTA/−; tet(o)Fgf10/+. N: quantification of the number of goblet cells per total number of epithelial cells in the villi of Dox-treated control and Rosa26rtTA/−; tet(o)Fgf10/+. The results in D, H, L, M, and N are expressed as means ± SE of 6 independent animals per group. *P ≤ 0.05; **P ≤ 0.01; ***P < 0.0001. Scale bars are 100 μm.
FGF10 induces goblet cell differentiation in intestinal enteroids.
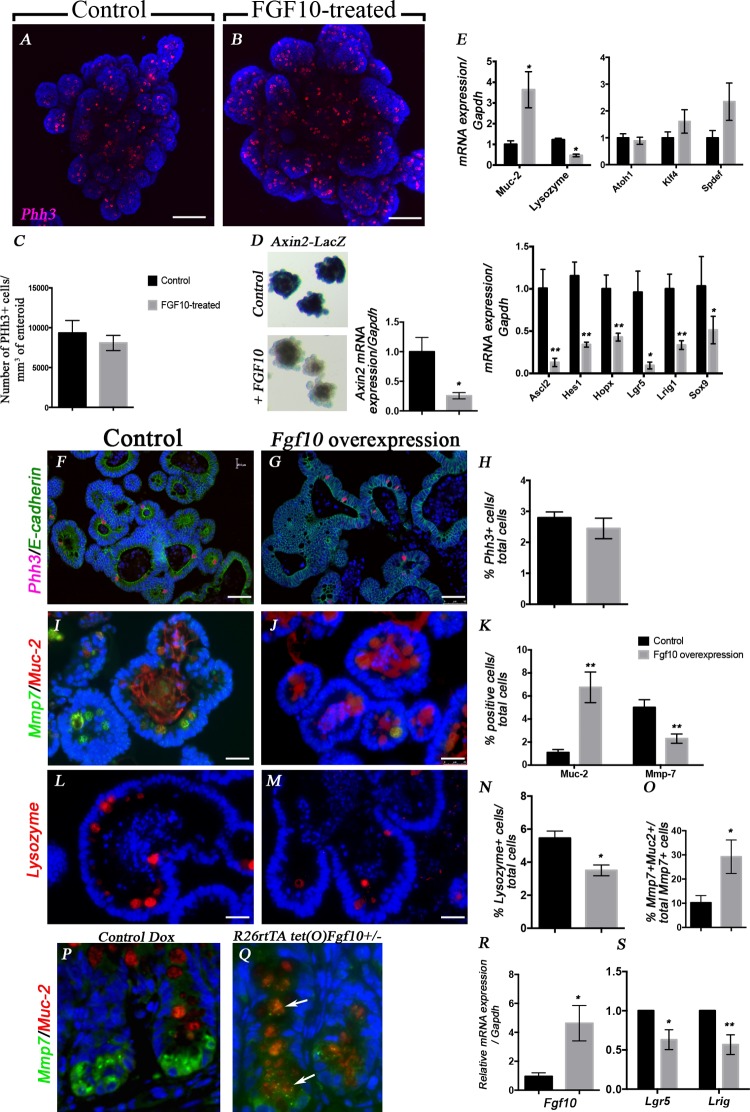
Since the overexpression of Fgf10 was achieved in a ubiquitous manner, we next asked whether Fgf10 could act directly on the epithelium to control intestinal cell proliferation and/or differentiation. For that purpose, we used an epithelial-only culture system (35), in which crypts from mouse intestine were embedded in Matrigel and grown in the presence of a mixture of growth factors (EGF, Noggin, R-Spondin), with or without recombinant human FGF10 (200 ng/ml). After 4 days in culture in the presence or absence of rhFGF10, the cultures were fixed, immunostained for Phh3 to assess epithelial proliferation, and imaged as described in materials and methods to obtain a 3D reconstruction of each crypt bud (Fig. 6, A and B). In addition, we quantified of the number of Phh3-positive cells normalized to enteroid volume. We did not observe any difference in the proliferation in the presence or absence of FGF10 (Fig. 6C).
Fig. 6.
FGF10 acts directly on the epithelium to induce goblet cells and decrease Paneth cells and stem cell markers. A and B: 3D images of whole mount IF staining of Phh3 and Hoechst on enteroid cultures in absence (A) or presence of rhFGF10 (200 ng/ml). C: quantification of the number of Phh3-positive cells per mm3. Results are expressed as means ± SE of 5 independent experiments; 3 enteroids were analyzed in each experiment. D: LacZ staining of ileal enteroids from Axin2-LacZ reporter mouse in absence (control) or presence (+FGF10) of rhFGF10 and qRT-PCR for Axin2 transcripts in wild-type enteroids treated or not with rhFGF10. E: qRT-PCR on RNA from wild-type enteroids untreated (black bars) or treated (gray bars) with 200 ng/ml of rhFGF10 for Muc-2, lysozyme, Atoh1, Klf4, Spdef, Ascl2, Hes1, Hopx, Lgr5, Lrig1, and Sox9. F and G: IF staining for Phh3 (red) and ECadherin (green) on enteroids isolated from Rosa26rtTA/−; tet(o)Fgf10/+ treated (G) or not (F) with Dox. H: quantification of the percent of Phh3+ cells per total number of DAPI. I and J: IF staining for Mmp7 (green) and Muc2 (red) on enteroids isolated from Rosa26rtTA/−; tet(O)Fgf10/+ treated (J) or not (I) with Dox. K: quantification of the percent of Muc2+ and Mmp7+ cells per total number of DAPI. L and M: IF staining for lysozyme (red) on enteroids isolated from Rosa26rtTA/−; tet(o)Fgf10/+ treated (M) or not (L) with Dox. N: quantification of the percent of lysozyme+ cells per total number of DAPI. O: quantification of the percent of double-positive cells for Mmp7 and Muc2 compared with the total number of Mmp7+ cells. P and Q: Mmp7 and Muc2 IF staining on ileum from control animals (P) and Rosa26-RtTA; tet(o)Fgf10 animals treated with Dox for 3 days. Arrows show double-positive cells. R: qRT-PCR confirming Fgf10 overexpression in the enteroids treated with Dox compared with the untreated enteroids. S: qRT-PCR to assess gene expression of Lgr5 and Lrig1 following Fgf10 overexpression. Scale bars are 100 μm in A and B, 50 μm in F and G, and 25 μm in I, J, L, and M. *P ≤ 0.05; **P ≤ 0.01.
To determine the effect of FGF10 on epithelial cell differentiation, we analyzed the gene expression of Muc-2 and lysozyme in the cultures (Fig. 6E). In the presence of rhFGF10, we found a significant increase in Muc-2 expression compared with untreated enteroids (n = 6, P = 0.01), reflecting an increase in goblet cells. In addition, there was a significant decrease in lysozyme in the FGF10-treated enteroids compared with untreated (n = 6, P = 0.014). To determine whether FGF10 affected the stem cell niche, we analyzed the expression of several genes important for the differentiation and commitment of the intestinal stem cells. There was no change in the expression of Atoh1 or Klf4 in the FGF10-treated crypts compared with controls, suggesting that FGF10 does not affect the global secretory progenitor pool. An increase in Spdef expression was observed in the FGF10-treated enteroids (Fig. 6D). Several stem cell markers were decreased in the FGF10-treated enteroids compared with control enteroids such as Lgr5 (n = 4, P = 0.028), Hopx (n = 5, P = 0.0072), Lrig1 (n = 5, 0.0032), Ascl2 (n = 5, P = 0.0033), and Sox9 (n = 4, P = 0.05). Next we sought to determine whether this effect of FGF10 in driving goblet cell differentiation is dependent on Notch signaling. Therefore, we assessed the expression of Hes1 and found a significant decrease in Hes1 expression in FGF10-treated vs. untreated enteroids (n = 4, P = 0.009). It has been reported that Paneth cell differentiation requires high Wnt activity; therefore we tested the effect of FGF10 on Wnt activation using enteroids from the ileum of Axin2-LacZ reporter mice. We showed that LacZ staining was significantly decreased in the presence of rhFGF10 (Fig. 6D). These data were confirmed by qRT-PCR, where FGF10 significantly decreased Axin2 expression (n = 5, P = 0.016).
Enteroids from the ileum of animals overexpressing Fgf10 were also cultured in the presence of doxycycline to induce Fgf10 overexpression. Treating wild-type enteroids with doxycycline did not affect cell proliferation or expression of cell differentiation markers (data not shown), consistent with previous studies (18). Immunofluorescence staining of enteroid sections cultured for 3 days in absence or presence of 2 μg of doxycycline did not show any difference in cell proliferation as shown by Phh3 staining (Fig. 6, G and H) and quantification (Fig. 6H). We performed staining for Mmp7 (Paneth cells), Muc2 (goblet cells), and lysozyme (Paneth cells) to assess cell differentiation in the enteroids following Fgf10 overexpression. We demonstrated that Fgf10 overexpression increased the number of Muc2-positive cells (Fig. 6J vs. I and K, n = 4, P = 0.003). A decrease in the number of Mmp7-positive cells (Fig. 6J vs. I and K, n = 4, P = 0.008) and lysozyme-positive cells (Fig. 6M vs. L and N, n = 4, P = 0.01) was observed compared with controls. Interestingly, in the enteroids overexpressing Fgf10, the number of cells positive for both Mmp7 and Muc-2 was increased by threefold compared with controls (Fig. 6O, n = 4, P = 0.04), suggesting that, following Fgf10 overexpression, goblet cells replace Paneth cells in the enteroids. Moreover, following 3 days of doxycycline administration in mice, we observed the presence of double-positive cells for Mmp7 and Muc2 in the ileum of animals overexpressing Fgf10 (Fig. 6Q) compared with doxycycline-treated controls (Fig. 6P). Fgf10 overexpression was confirmed by qRT-PCR, the enteroids treated with doxycycline had a fivefold increase in Fgf10 expression (Fig. 6R, n = 4, P = 0.02). Moreover, Fgf10 overexpression significantly decreased the Lgr5 and Lrig1 expression in the enteroids (Fig. 6S, n = 4, P = 0.02 and 0.008, respectively).
DISCUSSION
This study describes the effect of Fgf10 overexpression on the adult mouse small intestine. Although Fgf10 and Fgf signaling in general have been extensively studied during intestinal development, little is known their roles in adulthood. In addition, the expression of FGFR1 and FGFR2, as well as FGF ligands in the human intestine, have not been described. We demonstrated that both FGFR1 and FGFR2 are expressed in the human ileum. Previous reports have shown that several human tissues express FGFR1 and FGFR2 such as stomach, pancreas, salivary gland, and duodenum (15). Both FGFR1 and FGFR2 are expressed throughout the epithelium and the mesenchyme of the ileum. It was previously reported that FGF1 and FGF2 are widely expressed in normal adult tissue, mainly in the kidney, skin, and liver (16). Here we showed that FGF3 and FGF7 but not FGF1, 3, or 22 are expressed in human ileum. Moreover, FGFR1b and FGFR2b (b isoforms), the receptors for FGF10, are both expressed in the human ileum. To our knowledge, this is the first report of expression of FGF10 family members and FGF10 receptors in human ileum.
We have recently shown that Fgfr2b ligands, Fgf1, Fgf7, Fgf10, and Fgf22, are expressed in adult mouse stomach (38). Here, we provide evidence that Fgf1, Fgf7, and Fgf10 are expressed throughout the adult mouse intestine, whereas in Fgf10LacZ reporter β-galactosidase staining is detected only in the duodenum. This could be due to the lack of sensitivity of the reporter insertion or to a lower expression of Fgf10 in the jejunum and ileum compared with the duodenum. Our data showed that FGFR1 and FGFR2 have similar distribution in the human compared with the mouse ileum. However, Fgfr2b is considered to be the main receptor of Fgf10 given the phenotypic similarities between Fgf10-null and Fgfr2b-null mice (7, 25). The presence of FGF10 and its receptors, FGFR1b and FGFR2b in human and mouse intestine, suggests an important role for FGF10 signaling in intestinal homeostasis. Moreover, given the similar distribution of FGFR1 and FGFR2 in human and mouse, we propose that FGF10/FGFR2b signaling plays a similar and important role in small intestine both in mouse and human.
FGF10 is known as a mitogen during the development of several organs such as the trachea (34) and colon (12, 33). It also promotes cell proliferation during homeostasis in mouse mammary gland (32), incisors (31), and stomach (38). Our data suggest that Fgf10 overexpression in the mouse adult small intestine increases crypt depth and villus height in the duodenum, likely as a result of increased proliferation. In the jejunum and ileum, there was no significant difference in proliferation. However, crypt depth and villus heights were increased in both segments upon Fgf10 overexpression. This is likely due to a decrease in cell turnover in the intestine as a result of decreased cell death shown by decreased active caspase-3-positive cells in the jejunum and ileum following Fgf10 overexpression. These results are consistent with our previous findings that Fgf10 enhances epithelial cell survival during colon development (33). Other studies have shown that Fgf10 does not affect cell proliferation in embryonic intestinal development including in the duodenum (29). This discrepancy between our studies could be due to the fact that we employed a ubiquitous overexpression system and analyzed adult organs whereas Nyeng et al. (29) used a Pdx1 driver and studied organogenesis. Similar to our results, a recent study showed that FGF7 increases villus height and crypt depth. However, that was accompanied with increased proliferation in the jejunum of adult mice (5).
Fgf10 is known to play an important role in epithelial cell differentiation during organogenesis in several organ systems (2, 34, 39, 46), including the intestine (29). Nyeng et al. (29) used Pdx1 driver to overexpress Fgf10 and showed a decrease in Paneth cells, thus corroborating our results in adult mouse intestine. During embryonic development, overexpression of Fgf10 did not seem to affect goblet cell differentiation (29), whereas here we provide evidence that overexpressing Fgf10 in adult mouse intestine increases goblet cell differentiation in both crypt and villus and decreases Paneth cell numbers. In a colon epithelial cell line, Iwakiri and Podolsky (17) showed that FGF7 regulates the goblet cell silencer inhibitor and induces the differentiation of goblet cells in vitro. Although we show that activating Fgf10 signaling reduces the numbers of Paneth cells, other Fgf receptor activation such as via Fgfr3 acts in an opposite manner. Lack of Fgfr3 results in fewer Paneth cells in the mouse intestine (47). In contrast, Fgfr3 activation was shown to induce Paneth cell differentiation, either through MAPK or β-catenin activation (3). Therefore, it seems as though Fgf10 and Fgfr3 have opposing signaling activities in the small intestine.
Our data raise interesting questions about regulation of the dynamics of the goblet and Paneth cell populations in the intestine. The increase in goblet cells in the crypt along with decreased Paneth cells may reflect goblet cells arising from mature Paneth cells in the crypt compartment. This possibility is consistent with our observation of “intermediate” cells expressing both Paneth and goblet cell markers at 3 days following FGF10 induction in either enteroid culture or mice (Fig. 6), which are not observed in vivo 10 days after induction (data not shown). The possibility of this transitional cell type has previously been demonstrated, for example, in the case of altered Notch signaling (45). Alternatively, it may be that goblet cell differentiation is accelerating in concert with extrusion or death of Paneth cells. Further investigation will be needed to distinguish between these possibilities.
It is still unclear what controls the balance between goblet and Paneth cell lineage specification, although it is well established that Paneth cell formation is dependent on Wnt/β-catenin/TCF4 signaling (43). In our experiments, FGF10 decreased transcript levels of the Axin2 gene as well as LacZ expression in enteroids from Axin2-LacZ reporter mice, indicating that FGF10 inhibits Wnt signaling in these enteroids (Fig. 6). The inhibition of Wnt signaling was also accompanied by a decrease in the expression of a subset of stem cell-associated Wnt target genes such as Lgr5, Lrig1, Ascl2, and Hopx. Furthermore, FGF10 decreased Hes1 expression. These results are in accordance with published data that Notch inhibition induces goblet cell hyperplasia (45), since in many models ablation of Paneth cells results in a decrease of Lgr5-positive cells (35).
In summary, our studies implicate FGF signaling as an important regulator of the balance between the different intestinal secretory cell types. Future studies in this area are needed to elucidate how FGF10 affects these secretory lineages and what signaling mechanisms could be involved to compensate for the loss of the different stem cell markers studied here. Understanding the role of FGF signaling in this context could lead to therapeutically relevant insights for disease states associated with goblet cell hyperplasia.
GRANTS
D. Al Alam acknowledges support from the Saban Research Institute and NIH/NCATS Grant no. UL1TR000130 via USC-CTSI. S. Danopoulos is supported by a training grant from NIH/5T90DE021982-03. M. R. Frey is supported by NIH/NIDDK R01DK095004, a Senior Research Award from the Crohn's and Colitis Foundation of America, an American Cancer Society Research Scholar Award, and a Research Career Development Award from the Saban Research Institute. T. Grikscheit receives support from the California Institute for Regenerative Medicine (CIRM) RN 2 00946-1 and RN3-06425. H. R. Ford and S. Bellusci acknowledge the support of NIH/NIDDK R01HD052609 and R01AI014032 (H. R. Ford).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.A.A., F.G.S., S.G., M.R.F., H.R.F., T.C.G., and S.B. Conception and design of research; D.A.A., S.D., K.S., F.G.S., D.A., G.E.F., S.G., M.R.F., and S.B. performed experiments; D.A.A., S.D., K.S., F.G.S., D.A., G.E.F., M.R.F., T.C.G., and S.B. analyzed data; D.A.A., H.R.F., T.C.G., and S.B. interpreted results of experiments; D.A.A., S.D., F.G.S., and S.B. prepared figures; D.A.A., S.G., T.C.G., and S.B. drafted manuscript; D.A.A., S.D., G.E.F., M.R.F., H.R.F., T.C.G., and S.B. edited and revised manuscript; D.A.A., S.D., K.S., F.G.S., D.A., G.E.F., S.G., M.R.F., H.R.F., T.C.G., and S.B. approved final version of manuscript.
REFERENCES
- 1.Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol 178: 635–648, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124: 4867–4878, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Brodrick B, Vidrich A, Porter E, Bradley L, Buzan JM, Cohn SM. Fibroblast growth factor receptor-3 (FGFR-3) regulates expression of Paneth cell lineage-specific genes in intestinal epithelial cells through both TCF4/beta-catenin-dependent and -independent signaling pathways. J Biol Chem 286: 18515–18525, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns RC, Fairbanks TJ, Sala F, De Langhe S, Mailleux A, Thiery JP, Dickson C, Itoh N, Warburton D, Anderson KD, Bellusci S. Requirement for fibroblast growth factor 10 or fibroblast growth factor receptor 2-IIIb signaling for cecal development in mouse. Dev Biol 265: 61–74, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Cai YJ, Wang WS, Yang Y, Sun LH, Teitelbaum DH, Yang H. Up-regulation of intestinal epithelial cell derived IL-7 expression by keratinocyte growth factor through STAT1/IRF-1, IRF-2 pathway. PLoS One 8: e58647, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Clark JC, Tichelaar JW, Wert SE, Itoh N, Perl AK, Stahlman MT, Whitsett JA. FGF-10 disrupts lung morphogenesis and causes pulmonary adenomas in vivo. Am J Physiol Lung Cell Mol Physiol 280: L705–L715, 2001. [DOI] [PubMed] [Google Scholar]
- 7.De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 127: 483–492, 2000. [DOI] [PubMed] [Google Scholar]
- 8.El Agha E, Al Alam D, Carraro G, MacKenzie B, Goth K, De Langhe SP, Voswinckel R, Hajihosseini MK, Rehan VK, Bellusci S. Characterization of a novel fibroblast growth factor 10 (Fgf10) knock-in mouse line to target mesenchymal progenitors during embryonic development. PLoS One 7: e38452, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairbanks TJ, De Langhe S, Sala FG, Warburton D, Anderson KD, Bellusci S, Burns RC. Fibroblast growth factor 10 (Fgf10) invalidation results in anorectal malformation in mice. J Pediatr Surg 39: 360–365; discussion 360–365, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Fairbanks TJ, Kanard RC, De Langhe SP, Sala FG, Del Moral PM, Warburton D, Anderson KD, Bellusci S, Burns RC. A genetic mechanism for cecal atresia: the role of the Fgf10 signaling pathway. J Surg Res 120: 201–209, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Fairbanks TJ, Kanard RC, Del Moral PM, Sala FG, De Langhe SP, Lopez CA, Veltmaat JM, Warburton D, Anderson KD, Bellusci S, Burns RC. Colonic atresia without mesenteric vascular occlusion. The role of the fibroblast growth factor 10 signaling pathway. J Pediatr Surg 40: 390–396, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Fairbanks TJ, Sala FG, Kanard R, Curtis JL, Del Moral PM, De Langhe S, Warburton D, Anderson KD, Bellusci S, Burns RC. The fibroblast growth factor pathway serves a regulatory role in proliferation and apoptosis in the pathogenesis of intestinal atresia. J Pediatr Surg 41: 132–136; discussion 132–136, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, Peters PJ, Clevers H. The Ets-domain transcription factor Spdef promotes maturation of goblet and Paneth cells in the intestinal epithelium. Gastroenterology 137: 1333–1345e1–e3, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Hokuto I, Perl AK, Whitsett JA. Prenatal, but not postnatal, inhibition of fibroblast growth factor receptor signaling causes emphysema. J Biol Chem 278: 415–421, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J Histochem Cytochem 45: 1005–1019, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Hughes SE, Hall PA. Immunolocalization of fibroblast growth factor receptor 1 and its ligands in human tissues. Lab Invest 69: 173–182, 1993. [PubMed] [Google Scholar]
- 17.Iwakiri D, Podolsky DK. Keratinocyte growth factor promotes goblet cell differentiation through regulation of goblet cell silencer inhibitor. Gastroenterology 120: 1372–1380, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Jarde T, Evans RJ, McQuillan KL, Parry L, Feng GJ, Alvares B, Clarke AR, Dale TC. In vivo and in vitro models for the therapeutic targeting of Wnt signaling using a Tet-ODeltaN89beta-catenin system. Oncogene 32: 883–893, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J 21: 6338–6347, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet 24: 36–44, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Kanard RC, Fairbanks TJ, De Langhe SP, Sala FG, Del Moral PM, Lopez CA, Warburton D, Anderson KD, Bellusci S, Burns RC. Fibroblast growth factor-10 serves a regulatory role in duodenal development. J Pediatr Surg 40: 313–316, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 129: 2619–2628, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell 1: 435–440, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Mailleux AA, Kelly R, Veltmaat JM, De Langhe SP, Zaffran S, Thiery JP, Bellusci S. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development 132: 2157–2166, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Mailleux AA, Spencer-Dene B, Dillon C, Ndiaye D, Savona-Baron C, Itoh N, Kato S, Dickson C, Thiery JP, Bellusci S. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development 129: 53–60, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Mellitzer G, Beucher A, Lobstein V, Michel P, Robine S, Kedinger M, Gradwohl G. Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J Clin Invest 120: 1708–1721, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori-Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, de Crombrugghe B. SOX9 is required for the differentiation of Paneth cells in the intestinal epithelium. Gastroenterology 133: 539–546, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Noah TK, Kazanjian A, Whitsett J, Shroyer NF. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp Cell Res 316: 452–465, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyeng P, Bjerke MA, Norgaard GA, Qu X, Kobberup S, Jensen J. Fibroblast growth factor 10 represses premature cell differentiation during establishment of the intestinal progenitor niche. Dev Biol 349: 20–34, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsa S, Kuremoto K, Seidel K, Tabatabai R, Mackenzie B, Yamaza T, Akiyama K, Branch J, Koh CJ, Al Alam D, Klein OD, Bellusci S. Signaling by FGFR2b controls the regenerative capacity of adult mouse incisors. Development 137: 3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsa S, Kuremoto K, Seidel K, Tabatabai R, Mackenzie B, Yamaza T, Akiyama K, Branch J, Koh CJ, Al Alam D, Klein OD, Bellusci S. Signaling by FGFR2b controls the regenerative capacity of adult mouse incisors. Development 137: 3743–3752, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsa S, Ramasamy SK, De Langhe S, Gupte VV, Haigh JJ, Medina D, Bellusci S. Terminal end bud maintenance in mammary gland is dependent upon FGFR2b signaling. Dev Biol 317: 121–131, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Sala FG, Curtis JL, Veltmaat JM, Del Moral PM, Le LT, Fairbanks TJ, Warburton D, Ford H, Wang K, Burns RC, Bellusci S. Fibroblast growth factor 10 is required for survival and proliferation but not differentiation of intestinal epithelial progenitor cells during murine colon development. Dev Biol 299: 373–385, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Sala FG, Del Moral PM, Tiozzo C, Alam DA, Warburton D, Grikscheit T, Veltmaat JM, Bellusci S. FGF10 controls the patterning of the tracheal cartilage rings via Shh. Development 138: 273–282, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shroyer NF, Helmrath MA, Wang VY, Antalffy B, Henning SJ, Zoghbi HY. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology 132: 2478–2488, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Speer AL, Al Alam D, Sala FG, Ford HR, Bellusci S, Grikscheit TC. Fibroblast growth factor 10-fibroblast growth factor receptor 2b mediated signaling is not required for adult glandular stomach homeostasis. PLoS One 7: e49127, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spencer-Dene B, Sala FG, Bellusci S, Gschmeissner S, Stamp G, Dickson C. Stomach development is dependent on fibroblast growth factor 10/fibroblast growth factor receptor 2b-mediated signaling. Gastroenterology 130: 1233–1244, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki K, Fukui H, Kayahara T, Sawada M, Seno H, Hiai H, Kageyama R, Okano H, Chiba T. Hes1-deficient mice show precocious differentiation of Paneth cells in the small intestine. Biochem Biophys Res Commun 328: 348–352, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Tai CC, Curtis JL, Sala FG, Del Moral PM, Chokshi N, Kanard RJ, Al Alam D, Wang J, Burns RC, Ford HR, Grishin A, Wang KS, Bellusci S. Induction of fibroblast growth factor 10 (FGF10) in the ileal crypt epithelium after massive small bowel resection suggests a role for FGF10 in gut adaptation. Dev Dyn 238: 294–301, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Torashima Y, Levin DE, Barthel ER, Speer AL, Sala FG, Hou X, Grikscheit TC. Fgf10 overexpression enhances the formation of tissue-engineered small intestine. J Tissue Eng Regen Med. 2013March7. doi: 10.1002/term.1720 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, Taketo MM, Clevers H. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol 7: 381–386, 2005. [DOI] [PubMed] [Google Scholar]
- 44.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435: 959–963, 2005. [DOI] [PubMed] [Google Scholar]
- 45.VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud A, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 139: 488–497, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veltmaat JM, Relaix F, Le LT, Kratochwil K, Sala FG, van Veelen W, Rice R, Spencer-Dene B, Mailleux AA, Rice DP, Thiery JP, Bellusci S. Gli3-mediated somitic Fgf10 expression gradients are required for the induction and patterning of mammary epithelium along the embryonic axes. Development 133: 2325–2335, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Vidrich A, Buzan JM, Brodrick B, Ilo C, Bradley L, Fendig KS, Sturgill T, Cohn SM. Fibroblast growth factor receptor-3 regulates Paneth cell lineage allocation and accrual of epithelial stem cells during murine intestinal development. Am J Physiol Gastrointest Liver Physiol 297: G168–G178, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294: 2155–2158, 2001. [DOI] [PubMed] [Google Scholar]