Abstract
Short bowel syndrome (SBS) is a devastating condition in which insufficient small intestinal surface area results in malnutrition and dependence on intravenous parenteral nutrition. There is an increasing incidence of SBS, particularly in premature babies and newborns with congenital intestinal anomalies. Tissue-engineered small intestine (TESI) offers a therapeutic alternative to the current standard treatment, intestinal transplantation, and has the potential to solve its biggest challenges, namely donor shortage and life-long immunosuppression. We have previously demonstrated that TESI can be generated from mouse and human small intestine and histologically replicates key components of native intestine. We hypothesized that TESI also recapitulates native small intestine function. Organoid units were generated from mouse or human donor intestine and implanted into genetically identical or immunodeficient host mice. After 4 wk, TESI was harvested and either fixed and paraffin embedded or immediately subjected to assays to illustrate function. We demonstrated that both mouse and human tissue-engineered small intestine grew into an appropriately polarized sphere of intact epithelium facing a lumen, contiguous with supporting mesenchyme, muscle, and stem/progenitor cells. The epithelium demonstrated major ultrastructural components, including tight junctions and microvilli, transporters, and functional brush-border and digestive enzymes. This study demonstrates that tissue-engineered small intestine possesses a well-differentiated epithelium with intact ion transporters/channels, functional brush-border enzymes, and similar ultrastructural components to native tissue, including progenitor cells, whether derived from mouse or human cells.
Keywords: tissue engineering, regenerative medicine, intestinal failure, intestinal stem cell, short bowel syndrome
patients with intestinal failure (IF) lose nutritional autonomy secondary to a broad range of metabolic and physiological disturbances, including fluid, nutrient, and weight loss secondary to insufficient intestinal surface area (10). Short bowel syndrome (SBS) is a major cause of IF and generally occurs with loss of ∼50–75% of small intestinal length (33). Congenital anomalies and perinatal disorders of the small intestine, such as gastroschisis, intestinal atresia, malrotation with volvulus, and necrotizing enterocolitis, may lead to SBS in children (32), whereas trauma and intestinal ischemia from fixation within an internal hernia following gastric bypass surgery are leading causes of SBS in adults. A 2004 study found an incidence of 22.1/1,000 neonatal intensive care unit admissions and a population estimate of 24.5/100,000 live births (35). The incidence of SBS is increasing, and morbidity and cost remain high, with a 30% 5-yr mortality rate and a mean annual combined inpatient and outpatient cost of $505,250 per pediatric patient for the first year of treatment; cost of care correlated strongly with decreased predicted small bowel length (31).
Tissue-engineered small intestine (TESI) is a promising alternative to permanently treat SBS. Intestinal transplantation has many limitations, including donor shortage and the need for life-long immunosuppression. TESI derived from autologous organ-specific stem cells is a possible therapy for tissue replacement following intestinal injury, resection, or congenital absence. TESI was first generated from syngeneic tissue in a rat model, transplanting organoid units (OU) into the momentum on a polymer scaffold (5). OUs are multicellular cell clusters derived from donor intestine and contain both mesenchymal and epithelial cells (9). The resulting engineered intestine is morphologically similar to native intestine with a muscle layer containing nerves supporting a differentiated epithelium (15, 27). Human intestine has the same regenerative potential. We previously demonstrated that TESI can be generated from human small intestine donor tissue implanted into immunocompromised host mice (2, 21).
However, in these initial pilot studies, only gross identification of the key components of the intestine were identified. In mouse and human TESI (mTESI/hTESI), there are typical columnar epithelial cells lining a lumen and in some regions forming primitive villus-like projections and crypt-like invaginations. A muscle layer supports the epithelium at the basolateral side opposite the lumen. Typical intracellular vacuoles can also be seen in secretory cells of the epithelium, representative of goblet cells. We previously published the identification of four terminally differentiated epithelial cell types in both mouse and human-derived TESI, including lysozyme-positive Paneth cells located at the crypt base, mucin-2 (Muc2)-positive goblet cells, apical villin-positive enterocytes, and enteroendocrine cells as identified by chromogranin A staining (21, 27).
More stringent indications of function have not been demonstrated beyond a pilot surgical replacement study in the rat (15). For clinical relevance, TESI must demonstrate intact barrier function as well as digestive and absorptive capabilities.
Previously, we determined that TESI could be an effective salvage strategy in a Lewis rat SBS model. Following >80% small bowel resection, TESI was connected to the shortened proximal intestine and resulted in an improvement in weight gain (15). Tissue-engineered colon has been shown to demonstrate basic in vivo physiological functions, such as ion transport and water, bile acid, and short-chain fatty acid absorption in the rat (12, 13), and tissue-engineered esophagus anastomosed to the distal esophagus as an onlay patch or interposition graft allowed normal deglutition in rats and conducted luminal content on fluoroscopic examination (11). The size of the engineered gastrointestinal tract constructs generated in the rat allow for anastomosis and in vivo investigations. However, the two key models for developing TESI into a future human therapy are mTESI and hTESI grown in the mouse model. The mouse model allows for implantation of transgenic tissue to evaluate key morphogens and mechanisms. In the hTESI model, transplanted human cells grow in an immunocompromised host mouse, allowing quantification and qualification of the process and resulting tissue growth to evolve a human clinical trial. In both of these models, a functional profile was largely unexplored. Because we cannot connect mTESI to the native gastrointestinal tract as in the rat and because we had only a few examples of hTESI on our pilot report that human cells would grow in this model, we sought to define whether TESI of either mouse or human donor origin possesses further evidence of the required machinery for correct in vivo function, including intact tight junctions necessary for normal barrier function, correctly positioned ion and nutrient transporters, and functional brush-border enzymes necessary for digestion and absorption. Furthermore, because mouse models of human processes are sometimes inadequate, we sought to determine whether mTESI is highly similar to the TESI derived from human cells.
MATERIALS AND METHODS
OU production and implantation.
All animal experiments were carried out after protocol approval by the IACUC. Human small intestinal samples were collected under a CHLA IRB-approved protocol. Human ileum or mouse full-length small intestine tissue was washed in 4°C Hank's balanced salt solution; tissue digestion, OU preparation, polymer preparation, and implantation were then carried out using the established protocol previously validated and published by our group (3, 21, 27). Host animals were C57BL/6 for mouse OU implantation and adult Non-Obese Diabetic/Severe Combined ImmunoDeficiency (NOD/SCID) gamma mice (The Jackson Laboratories, Sacramento, CA) for human OU implantation. NOD/SCID mice were irradiated (350 CGy) immediately before implantation. All mice were treated with sulfamethoxazole/trimethoprim (Septra) and antibiotic (Hi-Tech Pharmaceuticals, Norcross, GA) in sterile drinking water for the entire duration of implantation. Mice were killed after 4 wk by CO2 euthanasia. For the time-course experiment, mice were killed, and TESI was harvested at 1, 2, and 4 wk after implantation.
Electron microscopy.
Following harvest, TESI was cut in half and fixed with 2% glutaraldehyde in 0.1 M phosphate buffer and then postfixed with 1% osmium tetroxide in 0.1 M phosphate buffer, followed by a series of alcohol dehydrations. After they were embedded in epoxy resin and polymerized at 60°C for 72 h, thick (1 μm) sections were cut and stained for review by a pathologist to determine the areas of interest in the samples. Ultra-thin sections (90 nm) were then cut onto grids and stained with uranyl acetate solution and lead stain solution for 14 s in a microwave. Ultrastructural images were analyzed by transmission electron microscope (Morgagni 268).
Immunostaining.
Five-micron sections of 10% formalin-fixed, paraffin-embedded mTESI and hTESI were stained. C57BL/6 mouse jejunum and human native small intestine obtained from healthy ends of surgical specimens from donor subjects served as controls. After deparaffinization and rehydration, antigens were exposed through heat-induced epitope retrieval for 10 min in 10 mM Na+ citrate (pH = 6.0). The slides were incubated overnight at 4°C with the following primary antibodies diluted at 1/100 in blocking solution: Muc2 (Santa Cruz Biotechnology, Santa Cruz, CA), lysozyme (Dako Cytomation, Carpenteria, CA), Na+/H+ exchanger (NHE3, Ab1381; Novus, Littleton, CO), cystic fibrosis transmembrane conductance regulator (CFTR; Ab769 from CF consortium; Millipore, Billerica, MA), aquaporin 7 (chicken polyclonal; Abcam, Cambridge, MA), NHE1 (Ab1950), Cdc42 (Cell Signaling Technologies, Danvers, MA), Na+/K+-ATPase (a5; University of Iowa Developmental Hybridoma), actin (Sigma, St. Louis, MO), sucrase isomaltase (SI) (A-17, Santa Cruz Biotechnology), zonula occludens-1 (ZO-1; Zymed, San Francisco, CA), enhanced green fluorescence protein (Abcam), and Ki-67 (Santa Cruz Biotechnology). Slides were then washed twice in 1× PBS with 0.1% Tween and incubated at room temperature with Alexa Fluor (488 or 568)-conjugated secondary antibodies (anti-mouse, anti-rabbit, or anti-chicken) as well as Hoechst 33342 dye (Invitrogen, Carlsbad, CA) or DAPI (Vector Laboratories, Burlingame, CA) for nuclear counterstain. Slides were mounted with Fluorogel anti-fade mounting media (Electron Microscopy Sciences, Fort Washington, PA), and images were obtained on a Zeiss 510 META confocal microscope.
Alcian blue staining.
Tissue sections were deparaffinized and dehydrated in ethanol, incubated with Alcian blue (Sigma) for 10 min, and counterstained with nuclear fast red (Fluka Analytical, St. Gallen, Switzerland). Images were captured on an upright light microscope (Leica Microsystems, Buffalo Grove, IL).
Histological staining.
Cross-sections (5 μm) were stained with hematoxylin and eosin according to standard procedures.
Immunohistochemistry staining.
Immunohistochemistry was carried out on paraffin sections using sodium-glucose cotransporter 1 (SGLT-1) antibody (1/100, Abcam) with horseradish peroxidase reporter, as described in the manufacturer's immunohistochemistry protocol (EnVision Dual Link System-HRP, DAB+, Dako Cytomation).
In vitro disaccharidase enzyme assay.
Three mTESI and three hTESI were harvested and immediately homogenized in 1× PBS. The supernatant was separated into aliquots. Enzymatic activity of maltase and SI was assessed by measuring the amount of glucose released after hydrolysis of the complex sugars maltose and sucrose, respectively, following a 60-min incubation at 37°C. Negative control values were measured before incubation and by omission of tissue homogenate. Homogenized adult mouse C57BL/6 jejunum and human ileum served as positive controls. A standard curve was made using a glucose oxidase kit (Sigma), and the amount of glucose liberated from TESI and control specimens was measured using the Dahlquist method (6).
Alkaline phosphatase enzymatic reaction.
Tissues were deparaffinized and washed in 1× PBS (2 times for 10 min each) and blocked. Sections were incubated in Vector Red Alkaline Phosphatase Substrate (Vector Laboratories) in 100 mM Tris·HCl, pH 8.5, with 0.1% Tween-20 for 30 min at room temperature. Sections were washed in 1× PBS (2 times for 10 min each), rinsed with water, mounted, and coverslipped for imaging by confocal microscopy, as described above. Mouse jejunum and human ileum served as controls.
Statistics.
Samples were run in triplicate for the disaccharide assay experiment. Liberated glucose was calculated by plotting the average for each sample on the standard curve. Because of the small sample size, we report each individual result without statistical interpretation.
RESULTS
Ultrastructural elements of an intact and well-polarized gut barrier are present in TESI epithelium.
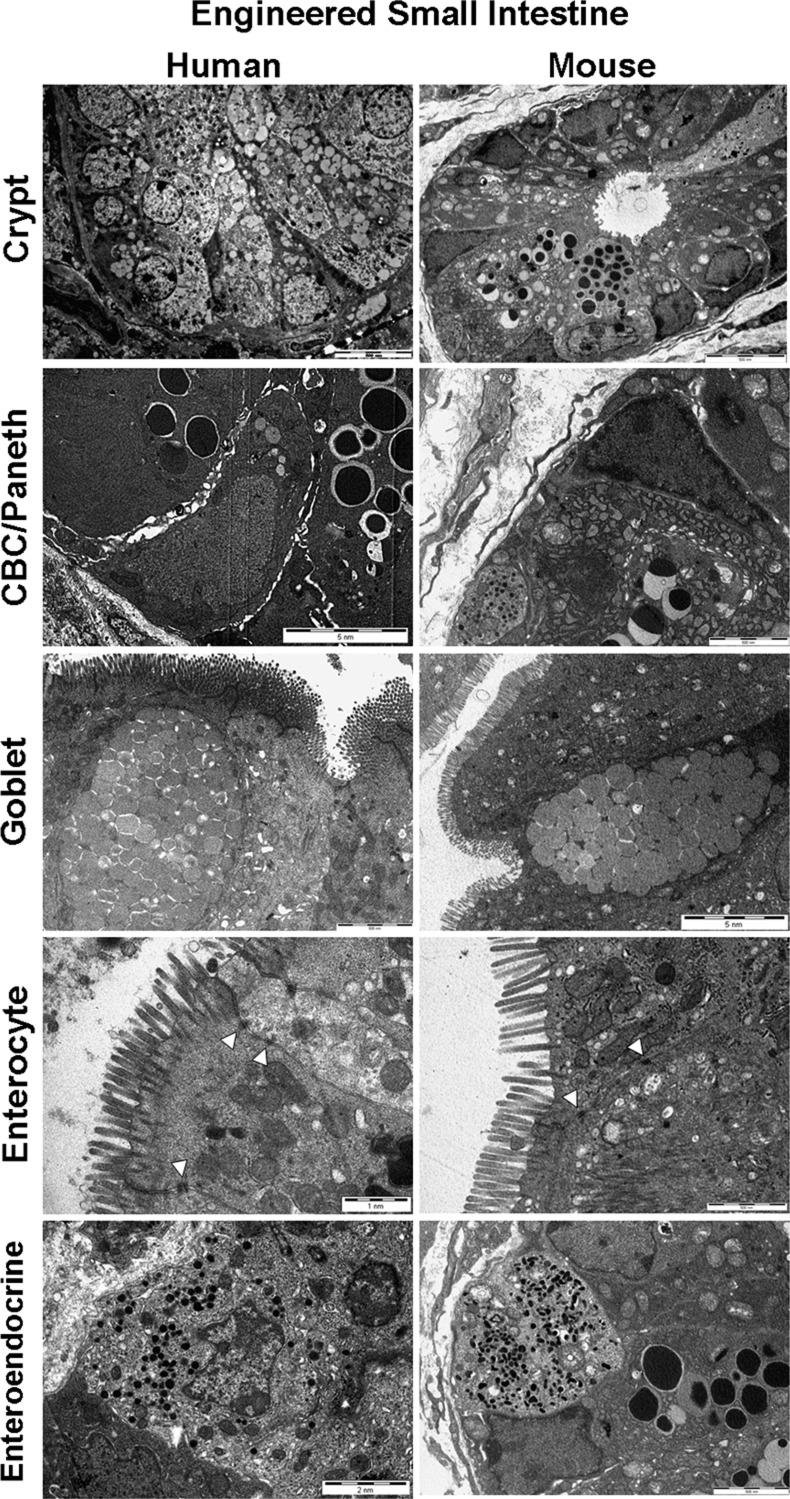
Transmission electron microscopy (TEM) defined the ultrastructure of the TESI epithelium in detail. Comparisons were made to native mouse and human small intestine controls. In both native epithelium and TESI, a low-power view identifies a crypt structure with thin triangular crypt-based columnar cells (CBCs) adjacent to Paneth cells containing abundant secretory granules. These triangular cells are the major intestinal progenitor cell or intercalated cell first described by Bjerknes and Cheng (4), now more commonly known as CBCs (1). Thin, spindle-shaped cells, likely representing intestinal subepithelial myofibroblasts, were found in close apposition to the basement membrane. Absorptive enterocytes were well polarized, with densely packed apical microvilli facing the luminal compartment. Mucus-packed goblet cells are identified in all samples, as are enteroendocrine cells exhibiting stored secretory products in cytoplasmic granules. TESI epithelial cells exhibited intact tight junctions bridging complex intercalated intercellular spaces, as well as typical basolateral cell membrane folds known to increase surface area for basolateral transport between enterocytes and the bloodstream (Fig. 1).
Fig. 1.
Tissue-engineered small intestine (TESI) recapitulates the ultrastructure of native intestine. Transmission electron microscopy (TEM) on a low-power field shows a crypt-like structure in both human and mouse-derived TESI. Within these crypts, dense vesicles within supporting Paneth cells are noted flanking characteristic triangular-shaped crypt-base columnar cells (CBCs), an intestinal epithelial progenitor cell. There are goblet cells containing mucus and absorptive polarized enterocytes bearing apical microvilli facing the luminal compartment. Enteroendocrine cells are notable for smaller cytoplasmic granules containing secretory products than the larger vesicles demonstrated in the Paneth cells. TESI epithelial cells exhibited intact tight junctions bridging complex intercalated intercellular spaces (arrows). Scale bars as indicated.
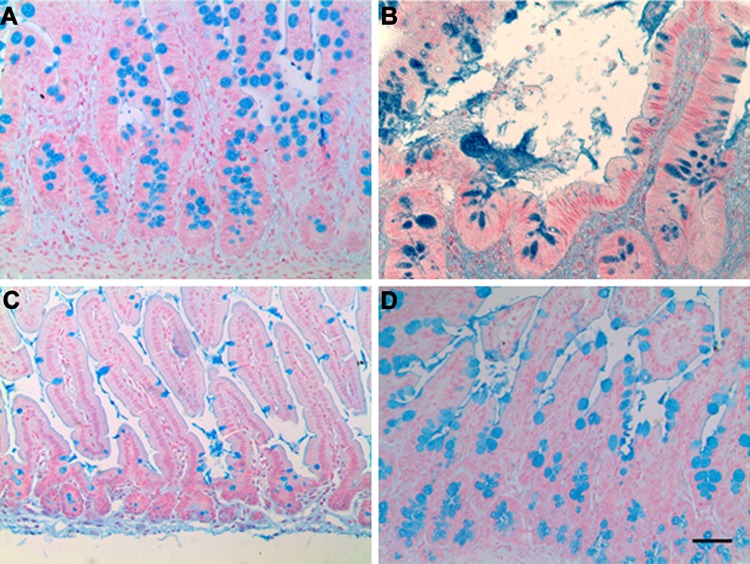
Alcian Blue staining confirmed the presence of goblet cells with abundant vacuoles containing mucus (Fig. 2), and blue staining in the lumen highlighted active secretion.
Fig. 2.
Goblet cells are abundant in TESI. Goblet cells are identified by Alcian blue staining throughout the epithelium of human TESI (hTESI) (B) and mouse TESI (mTESI) (D). Goblet cell location and appearance are similar to native human ileum (A) and mouse jejunum (C) controls. Scale bar = 50 μm.
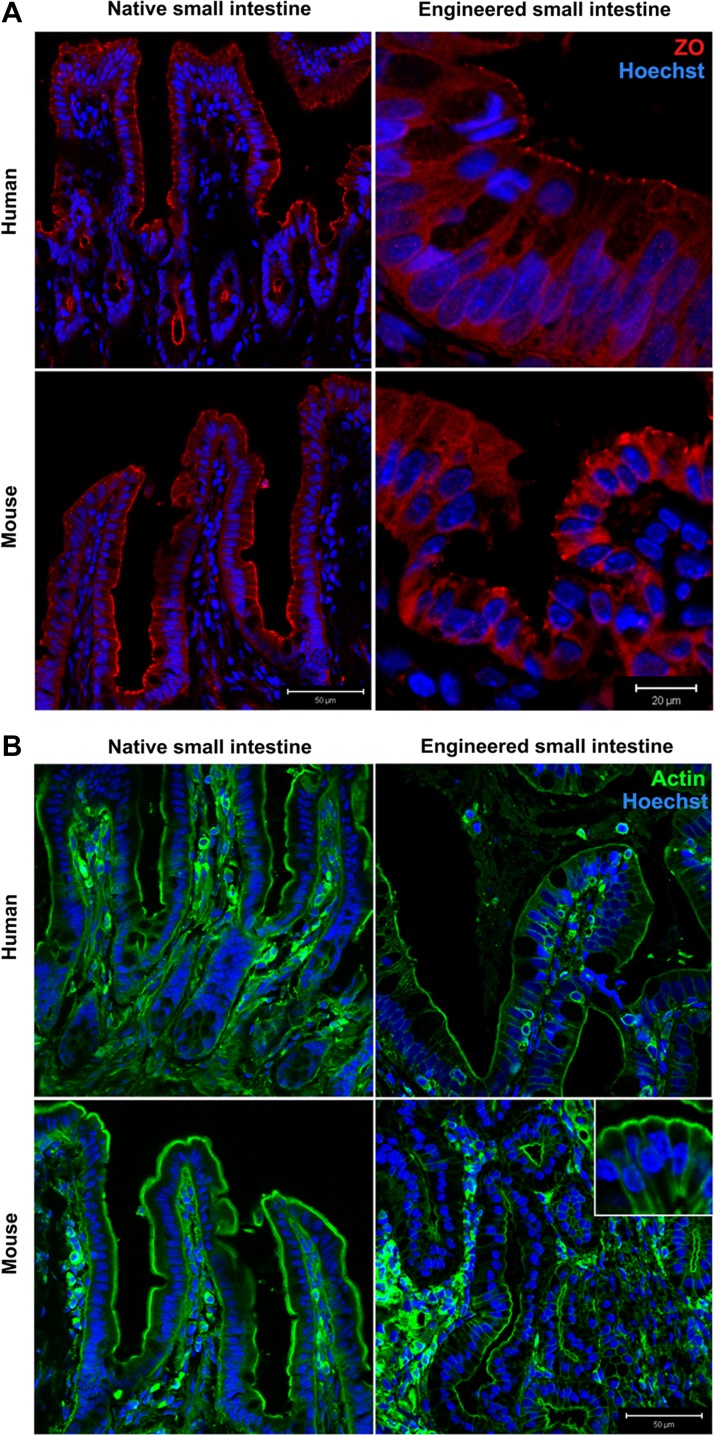
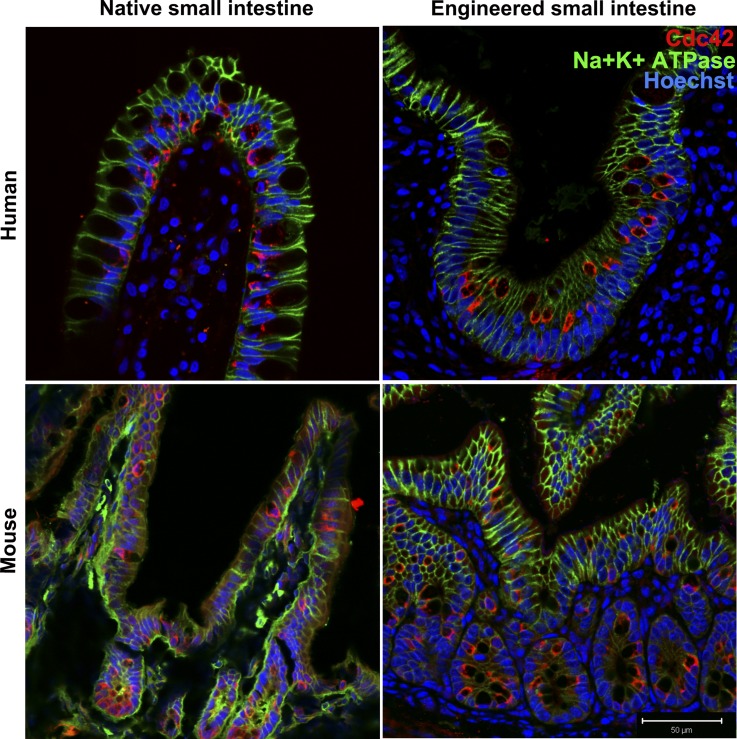
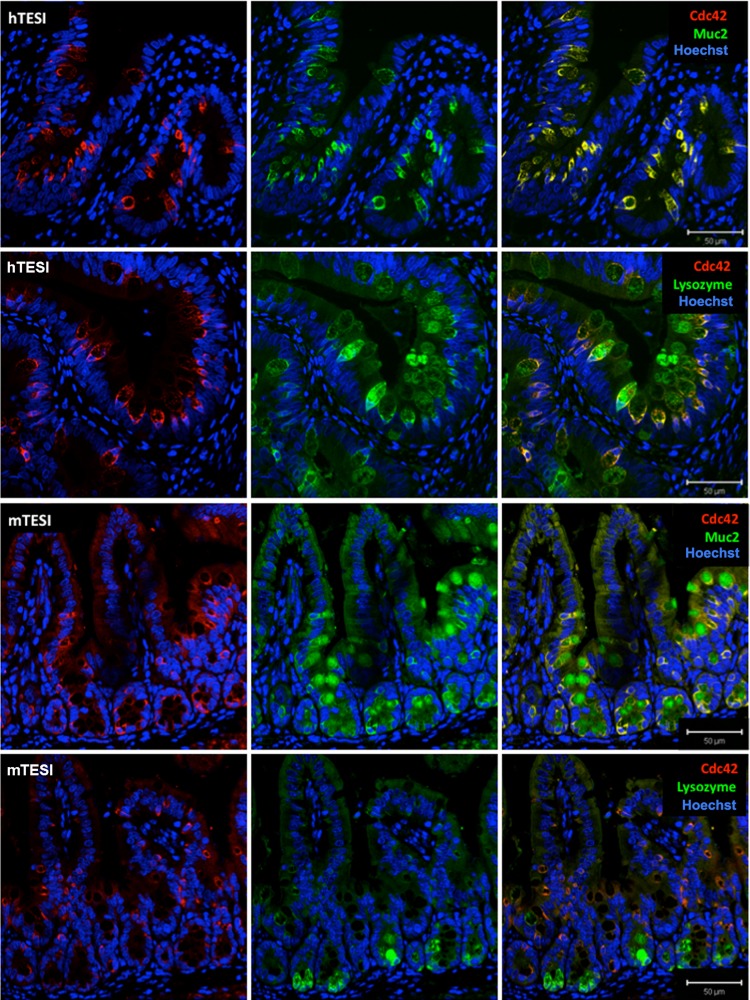
Immunofluorescent confocal microscopy confirmed that mTESI and hTESI epithelium is well polarized, expressing tight junction marker ZO-1 (Fig. 3A). Actin, a cytoskeletal protein important for maintenance of cell structure and cell-cell contact through interaction with membrane-bound proteins present in adherens junctions and desmosomes, demonstrates identical staining in TESI compared with native mouse and human intestine (Fig. 3B). The small Rho-GTPase, Cdc42, regulates small intestinal barrier function and epithelial cell polarity, proliferation, and differentiation. We observed that, although the brush border also stained positive for Cdc42 (Fig. 4), there was a predilection for cells of the secretory lineage in both native human and mouse ileum and in human-and mouse-derived TESI. To confirm this, we costained Cdc42 with either lysozyme or Muc2 in TESI to confirm the expression of Cdc42 in Paneth and goblet cells, respectively. Cdc42 colocalized strongly with Muc2+ cells in both mTESI and hTESI. This was also true of Cdc42 and lysozyme in hTESI; however, fewer lysozyme+ cells colocalized with Cdc42 in mTESI (Fig. 5).
Fig. 3.
Cytoskeletal elements are intact in TESI. A: punctate areas at the apical junction between epithelial cells demonstrate immunofluorescent staining for zonula occludens (ZO)-1. B: actin localizes to cell membranes. Blue indicates nuclear Hoechst dye. Scale bars as indicated.
Fig. 4.
Presence of Cdc42 and Na+/K+ ATPase demonstrate TESI polarity. Cdc42 (red) localizes faintly to the brush border and primarily to secretory cells in TESI epithelium as in controls. Na+/K+ATPase (green) localizes to the basolateral membranes throughout. Blue = nuclear Hoechst dye. Scale bar = 50 μm.
Fig. 5.
Cdc42 colocalizes with secretory cell types in TESI. Overlapping Cdc42 stains with mucin 2 (Muc2) and lysozyme in mTESI (top and top, middle) and hTESI (bottom, middle and bottom) demonstrate colocalization with secretory cell types. Cdc42 and lysozyme colocalization is noted in fewer cells in mTESI. Scale bar = 50 μm.
TESI expresses the major Na+ absorptive and Cl− secretory transporters.
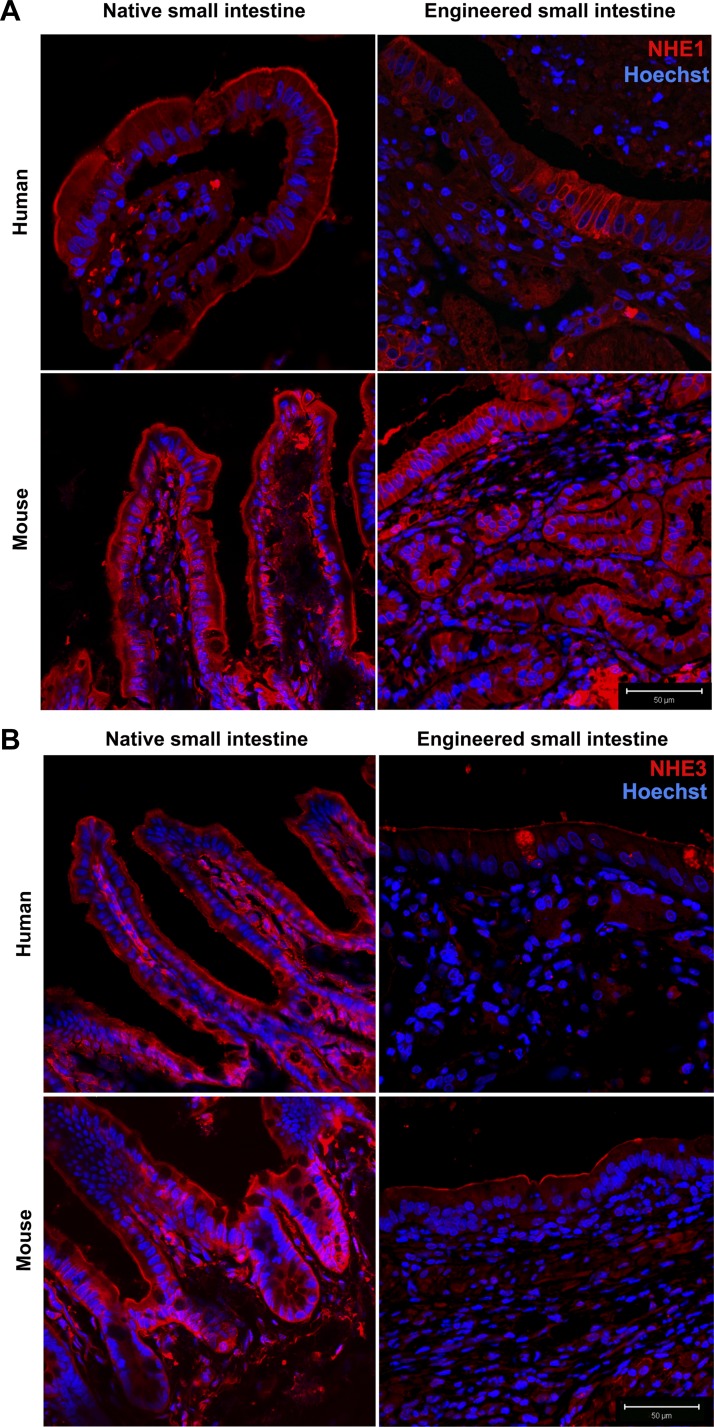
Na+/K+-ATPase, the major ion transporter responsible for regulating the intracellular Na+ gradient necessary for the luminal absorption of glucose, amino acids, and other nutrients that are coupled to sodium absorption, is exclusively localized to the basolateral membrane in TESI and native intestine (Fig. 4). TESI also expresses two major isoforms of the NHE family, NHE1 and NHE3. NHE1, the major NHE isoform responsible for regulating intracellular pH, is normally expressed in the basolateral membrane of enterocytes uniformly throughout the small intestine and colon (8) but has also been demonstrated on both apical and basolateral surfaces of polarized epithelia in culture (24). In both mTESI and hTESI, NHE1 is expressed in both the apical and basolateral membranes (Fig. 6A). NHE3 is the major brush-border ion transporter responsible for electroneutral Na+ absorption in the small intestine (37) and accounts for most of the inhibition of Na+ absorption that occurs in diarrheal diseases (8). Intense staining of NHE3 was observed at the apical membrane in the ileum of both human and mouse ileum (Fig. 6B). In TESI, NHE3 appears to be less intense compared with mouse and human control ileum but remains localized to the apical membrane.
Fig. 6.
Major intestinal isoforms of the sodium/hydrogen exchanger (NHE), including NHE1 and NHE3 are present on the epithelial membrane of TESI. NHE1 (A) stains both apical and basolateral membranes, whereas NHE3 (B) stains the apical membrane more specifically. Blue = nuclear Hoechst dye. Scale bar = 50 μm.
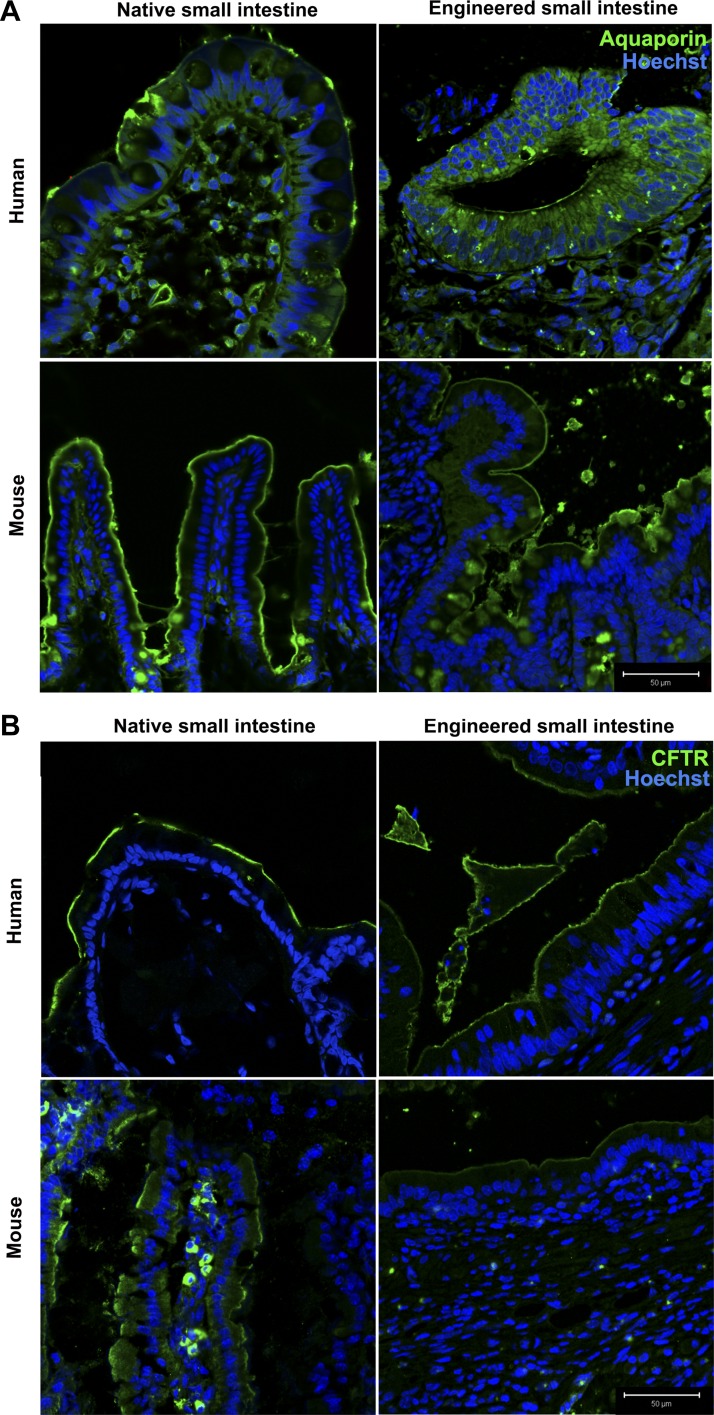
The CFTR is the apical Cl− channel most responsible for water secretion into the gut lumen during normal digestive physiology and the massive water loss in some enterotoxigenic diarrheas (38). In TESI, CFTR localizes to the apical membrane as is expected in native intestine (Fig. 7B). Aquaporins are another family of membrane channels important for transcellular water movement. Topographic studies in rat and human intestines demonstrate that aquaporin 7 is more strongly expressed in distal small intestine and colon than in the proximal small intestine, in the villi more so than the crypts, more strongly apically than intracellularly, and is absent basolaterally (19). In TESI, aquaporin 7 localizes to the apical brush border as in control mouse and human ileum (Fig. 7A).
Fig. 7.
Water channels and chloride transporter controlling transcellular water transport are present in TESI. A: aquaporin shows apical specificity in TESI as in native intestine. B: cystic fibrosis transmembrane conductance regulator (CFTR) localizes to the brush border in TESI and controls. Blue = nuclear Hoechst dye. Scale bar = 50 μm.
TESI exhibits absorptive and digestive capacity ex vivo.
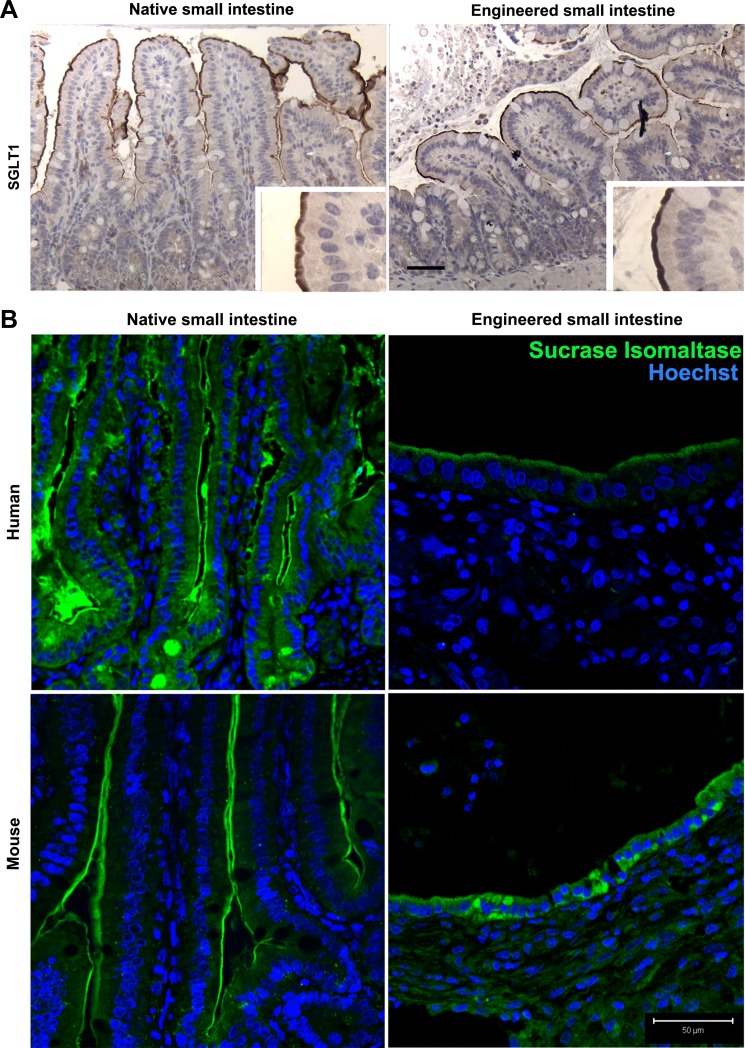
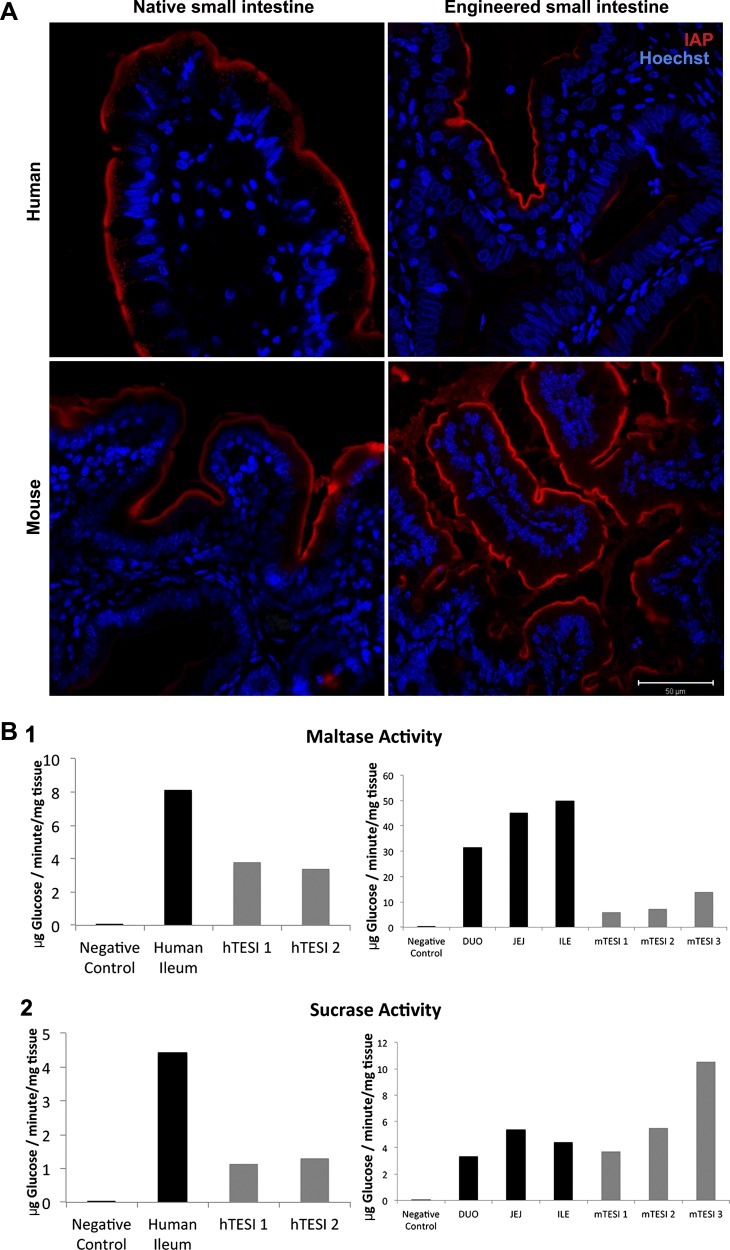
The apical membrane glucose transporter, SGLT-1, is expressed in mTESI, as determined by immunohistochemistry (Fig. 8A). The dimeric brush-border enzyme, SI, was also observed in mouse- and human-derived TESI by immunofluorescence (Fig. 8B). We investigated both maltase and SI enzyme activity in a functional assay performed on three samples each of mTESI and hTESI. Following TESI homogenization, the complex sugars maltose and sucrose were broken down to yield the simple sugar, glucose. The disaccharidases, maltase and sucrose, were active in both mouse- and human-derived TESI although, in these samples that do not receive luminal flow, activity was decreased compared with mouse jejunum and human ileum control tissue (Fig. 9B). Intestinal alkaline phosphatase (IAP) was present at the apical brush border in both mTESI and hTESI, as demonstrated by the strong signal from the fluorescent byproduct imaged after incubation with the enzymatic substrate (Fig. 9A).
Fig. 8.
TESI possesses membrane components necessary for complex sugar breakdown and absorption. A: immunohistochemistry demonstrates strong apical staining for the sodium glucose transporter (SGLT-1) in mouse native jejunum and slightly less intense but properly localized staining in mTESI. B: dimeric brush-border enzyme sucrase isomaltase also showed slightly less intense staining in hTESI and mTESI compared with controls. Blue = nuclear Hoechst dye. Scale bar = 50 μm.
Fig. 9.
Functional brush-border enzymes are present in TESI epithelium. A: intestinal alkaline phosphatase (IAP) is active at the brush border of TESI and control intestine, as indicated by the red fluorescent byproduct of a completed enzymatic reaction carried out by IAP. Blue = nuclear Hoechst dye. Scale bar = 50 μm. B: functional membrane disaccharidases are present on the brush border of TESI. The amount of glucose released after hydrolysis of the complex sugars sucrose and maltose is shown. Sucrase was measured following incubation with TESI homogenate (B2). Homogenized adult C57BL/6 jejunum (JEJ) and human ileum (ILE) served as positive controls. Control tissue homogenate incubated without added complex sugar served as negative control (blank). 3 mTESI and 3 hTESI were studied (sequentially numbered TESI represent individual TESI samples). Both maltase (B1) and sucrase (B2) were active in mTESI and hTESI although only at a fraction of the activity of native mouse and human intestine. Maltase activity was higher than sucrase in both hTESI and mTESI.
Rapidly dividing label-retaining cells are present in TESI.
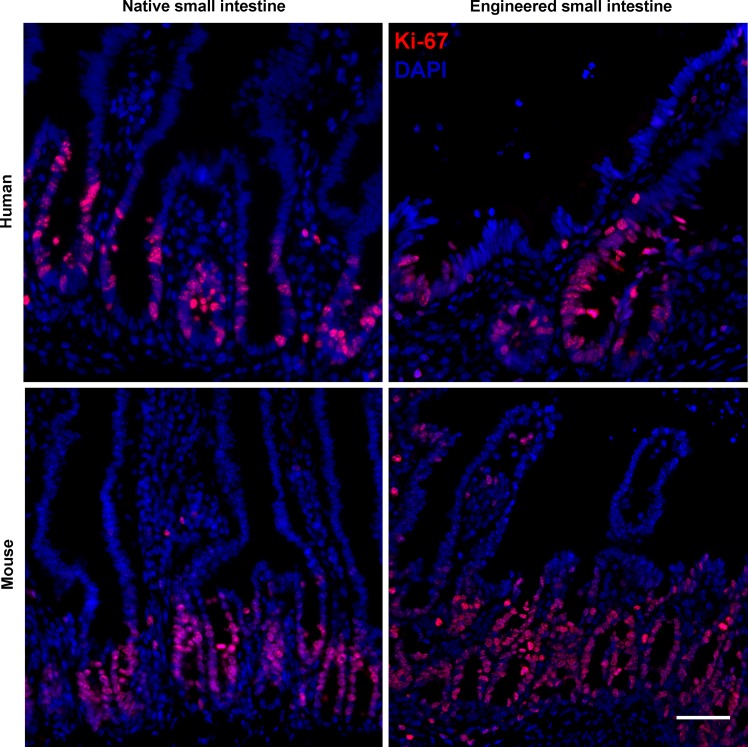
hTESI and mTESI sections stained for Ki-67 in both epithelial and mesenchymal cells (Fig. 10). The proliferating cells displayed a similar pattern to the location of the presumed progenitors, primarily staining in the crypts and extending up into the transit-amplifying region.
Fig. 10.
Rapidly dividing label-retaining cells are present in TESI. Ki-67 localizes rapidly dividing cells in hTESI epithelium and less so in mesenchyme. These cells appear to be concentrated in the forming crypt-like structure. Ki-67 more reliably stains crypt structures in mTESI, with interspersed mesenchymal cells. Human ileum and mouse jejunum controls demonstrate that Ki-67 is localized in crypt epithelium. Scale bar = 50 μm.
Apical proteins gain expression at later time points in TESI.
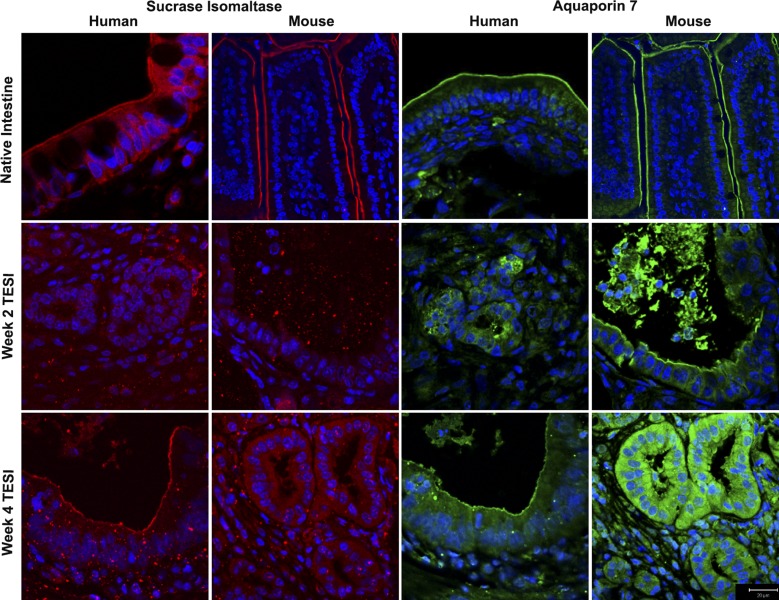
At week 1, the cells in TESI were not yet organized into congruous epithelial sheets; therefore, no mTESI or hTESI explanted at week 1 stained positive for aquaporin 7 or SI (data not shown). By week 2, aquaporin 7 stained strongly at the apical membrane of hTESI and mTESI, and staining remained present at week 4. At week 2, hTESI and mTESI did not demonstrate positive SI staining at the brush border. Positive staining was noted in all by week 4 (Fig. 11).
Fig. 11.
Apical proteins gain expression at different time points in TESI. At week 2, there is no apical staining of sucrase isomaltase in TESI. By week 4, it is strongly expressed. Aquaporin 7 stained strongly at the apical membrane of TESI by week 2 and remained present at week 4. Blue = nuclear Hoechst dye. Scale bar = 20 μm.
DISCUSSION
TESI, whether derived from murine or human cells, is highly similar to the native donor tissue in these ultrastructural and functional investigations. These results indicate the possibility of exploiting this model to identify morphogens for improved TESI growth and in the investigation of stem/progenitor cell growth. Results from murine-derived TESI may correlate with outcomes of experiments performed with human-derived TESI, allowing useful pilot studies before advancing to experiments that require a more precious resource. Unlike nonautologous models or systems that do not contain mesenchymal elements, TESI could be a clinically useful future therapy that recapitulates key components of native intestine, including all barrier functions and, importantly, maltase, sucrase, and alkaline phosphatase function. We previously published the successful salvage of short bowel syndrome after anastomosis to TESI in rats (15), but ultrastructural data and absorptive function were not directly demonstrated. To validate the correct formation of TESI from human tissue in an immunocompromised mouse model (21), these data were necessary. However, the ultrastructure and function of hTESI and mTESI had to be determined ex vivo because mice are not eligible for repeat survival surgery to connect the engineered intestine. The small size of the TESI also precludes additional in vivo evaluation of the engineered intestine including luminal injection.
By TEM, both mTESI and hTESI epithelia are well polarized and terminally differentiated. We demonstrated the presence of crypts surrounding a lumen, containing Paneth cells adjacent to CBC stem/progenitor cells, a key relationship demonstrated by Sato et al. in the native intestinal crypt (30). In this close association, the Paneth cell demonstrates the capacity to secrete bacteriocidal products as well as to express essential signals for stem-cell maintenance to the interspersed CBC, including Wnt3, TGF-α, EGF, and Dll4 (30). TEM also demonstrates enteroendocrine cells, Goblet cells, and enterocytes bearing microvilli. In addition, supporting structures necessary for the physical properties of an intact gut barrier such as tight junctions were demonstrated in TESI by both TEM and immunofluorescence microscopy.
Muc2+ goblet cells were present in mTESI and hTESI, and luminal mucin was also present in both, suggesting active secretory function. The correct localization of each membrane and ion transporter we investigated confirmed that the epithelium of TESI is well polarized and has the potential to facilitate ion fluxes critical to secretory and absorptive functions of the intestinal epithelium. Immunofluorescent staining localized ZO-1 to the apicolateral tight junctions (Fig. 3A), villin along the apical surface, and Na+/K+-ATPase (Fig. 4) at the basolateral membrane of the enterocytes in TESI. Actin is a cytoskeletal component of most eukaryotic cells. In the intestinal epithelium, its interactions with apical microvilli, basolateral adherens, and tight junctions are important in maintenance of cell size and shape (7); alterations in these interactions may contribute to the pathophysiology of diarrheal diseases (18). Actin localized strongly to the epithelium of both mTESI and hTESI (Fig. 3B).
Of particular interest was the presence and localization of the small Rho-GTPase, Cdc42, a so-called “molecular switch” for functions such as polarity and vesicle trafficking (16). Cdc42 facilitates vesicle exocytosis by modulating vesicle production, trafficking, and the number of granules entering exocytosis (29). Disruption of Cdc42 affects Paneth cell differentiation and stem/progenitor cell migration (23). Our data identify Cdc42 colocalization with goblet and Paneth cells in TESI, and control intestine staining is congruent. To our knowledge, this association is yet unreported in human intestine. It is known that conditional ablation of Cdc42 in mice leads to the formation of large intracellular vacuolar structures called microvillus inclusion bodies, a phenotype analogous to the devastating human illness microvillus inclusion disease (MVID). (25) Characterized by malabsorption and secretory diarrhea, MVID is a known cause of IF. In addition to exocytosis, Rho GTPases can regulate endocytosis; this is exploited by toxins such as Shiga toxin, which employs macropinocytosis for enterocyte invasion, in addition to microtubule-assisted transport, another Cdc42-dependent cellular function (17). The fact that some cells in mTESI do coexpress lysozyme/Cdc42 and there are no differences in polarity that we have observed suggest that there is enough Cdc42 for normal intestinal epithelial cell development. There is a slightly decreased colocalization of Cdc42 and lysozyme in mTESI compared with hTESI. Given the reported involvement of Cdc42 in intestinal stem cell migration, cell polarity, and protein and microvesicle trafficking (23), it is not surprising that Cdc42 is highly expressed in secretory cell types although its role in these cells in TESI is as yet undetermined. Costaining the intestinal stem cell marker Lgr5 in mTESI and Cdc42 did not demonstrate overlapping cells in our experiment (data not shown). Given the many different roles Cdc42 plays in intestinal homeostasis, normal expression in TESI strongly supports our hypothesis that techniques used to develop TESI lead to the generation of a normal and functional intestinal epithelium.
Channels known as aquaporins facilitate transcellular water transport in the small bowel (19), and transepithelial water absorption is dependent on NHEs. CFTR is another protein that maintains the balance between salt and water movement into and out of cells. Aquaporin 7, CFTR, NHE1, and NHE3 localized to the epithelial cell membranes, demonstrating that the mTESI and hTESI epithelia are well polarized and express the major small intestinal ion and water transporters and channels necessary for maintaining proper intestinal homeostasis (Figs. 6 and 7).
Perhaps the most important function of any intestinal replacement is the ability to break down and absorb nutrients to maximize intestinal absorption of nutrition. Disaccharides are the most basic form of digestible carbohydrates, and the small intestine is necessary for their absorption. There is a variable number of hexose transporters throughout the gastrointestinal tract, and the SGLT-1 is specific to the luminal surface of both the small intestine and colon (36). In one of the first investigations of the functionality of TESI, SGLT-1 expression and topography were studied in engineered intestine generated in the rat. Although mRNA expression of SGLT-1 was similar to native jejunum after TESI was in continuity with luminal contents, TESI epithelium not in contact with luminal flow had very low SGLT-1 mRNA expression (34). In the current study, SGLT-1 localized to the luminal surface of TESI in an identical pattern to that seen in control mouse jejunum (Fig. 8A). The dimeric enzyme SI also localized to the brush border of TESI (Fig. 8B). In a functional assay, the disaccharidases maltase and sucrase were active in both mouse- and human-derived TESI although at lower levels than control native intestine (Fig. 9B). Disaccharidase activity in the absence of luminal content was blunted compared with control, an expected finding. In the case of dimeric enzymes such as SI, decreased activity may be due to the absence of activating proteases such as trypsin, which are secreted by the pancreas and available only in the lumen of intestine in continuity. Both mTESI and hTESI showed strong IAP activity, equivalent to controls (Fig. 9A) (20). IAP has several important roles in the intestine. By limiting transepithelial passage of bacteria and dephosphorylating bacterial lipopolysaccharide, IAP contributes to maintaining gut barrier integrity. Although IAP has no direct role in phosphate absorption, it may increase digestibility of certain foods by catalyzing the hydrolysis of multiple phosphorylated compounds and lysing phosphate esters that do not readily penetrate cell membranes.
In control human ileum and mouse jejunum, Ki-67-positive cells are restricted to the crypt bases where progenitor cells are known to populate. However, in TESI, the rapidly dividing cells labeled by Ki-67 are found both in the epithelium and the mesenchyme, suggesting that multiple progenitors are continuing to divide in the developing TESI construct (Fig. 10).
We have previously published a time course showing incomplete development of TESI before week 4 (27). Although all examined functional and structural proteins were present in both mTESI and hTESI by week 4, SI was not present in week 2 hTESI or mTESI (Fig. 11). Aquaporin 7 was present in both weeks 2 and 4 hTESI and mTESI; in this case, membrane integrity and water channel transport function were present before digestive capacity (Fig. 11).
TESI derived from both mouse and human cells is surprisingly intact after 4 wk of growth, with many of the key functional components demanded for eventual therapeutic application or modeling human disease. There are limitations of this model, owing to the small size of the animal for repeat survival surgery and the size of the engineered construct. These limitations precluded classically reported investigations such as the determination of tensile strength and transepithelial resistance and Ussing chamber experiments, as previously performed in rat tissue-engineered colon (5). Larger animal models will be necessary to identify the further facets of TESI function, including motility. Application of this method to larger animal models will likely yield TESI large enough for anastomosis, as previously published in a proof-of-concept experiment in a large animal model where neonatal Yorkshire swine were anesthetized and underwent partial small bowel resection with immediate OU preparation and reimplantation during a single operation (26). In vivo functionality of the anastomosed TESI segment will be a crucial step toward application of this regenerative medicine technology to infants and children with IF.
Every functional study reported here yielded identical results in both mouse- and human-derived TESI, confirming the regenerative potential of postnatal human intestinal stem cells and indicating that the study of hTESI in the mouse model is a useful pretranslational step in which the cellular and molecular mechanisms can be dissected in aid of the goal of future human therapy.
GRANTS
This work was supported by CIRM RN2 00946-1, CIRM RN3 06425 (T. Grikscheit), NIH/NIDDK:K01-DK080930 (N. Zachos), and R03-DK091482 (N. Zachos).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.N.G., G.M.S., F.G.S., J.R.H., H.S., and N.C.Z. performed experiments; C.N.G., G.M.S., F.G.S., J.R.H., D.E.L., A.L.S., E.R.B., H.S., N.C.Z., and T.C.G. analyzed data; C.N.G., G.M.S., F.G.S., J.R.H., D.E.L., A.L.S., E.R.B., H.S., N.C.Z., and T.C.G. interpreted results of experiments; C.N.G., G.M.S., J.R.H., N.C.Z., and T.C.G. prepared figures; C.N.G., G.M.S., F.G.S., and T.C.G. drafted manuscript; C.N.G., G.M.S., N.C.Z., and T.C.G. edited and revised manuscript; C.N.G., G.M.S., N.C.Z., and T.C.G. approved final version of manuscript; F.G.S. and T.C.G. conception and design of research.
ACKNOWLEDGMENTS
We thank The Johns Hopkins Ross Confocal Facility and The Kudsi Imaging Core of the Hopkins Conte Digestive Disease Basic and Translational Research Core Center; we thank The Department of Pathology, Children's Hospital Los Angeles.
REFERENCES
- 1.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Barthel ER, Levin DE, Speer AL, Sala FG, Torashima Y, Hou X, Grikscheit TC. Human tissue-engineered colon forms from postnatal progenitor cells: an in vivo murine model. Regen Med 7: 807–818, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Barthel ER, Speer AL, Levin DE, Sala FG, Hou X, Torashima Y, Wigfall CM, Grikscheit TC. Tissue engineering of the intestine in a murine model. J Vis Exp 70: e4279, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng H, Merzel J, Leblond CP. Renewal of Paneth cells in the small intestine of the mouse. Am J Anat 126: 507–525, 1969. [DOI] [PubMed] [Google Scholar]
- 5.Choi RS, Riegler M, Pothoulakis C, Kim BS, Mooney D, Vacanti M, Vacanti JP. Studies of brush border enzymes, basement membrane components, and electrophysiology of tissue-engineered neointestine. J Pediatr Surg 33: 991–996; discussion 996–997, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem 22: 99–107, 1968. [DOI] [PubMed] [Google Scholar]
- 7.Drenckhahn D, Gröschel-Stewart U. Localization of myosin, actin, and tropomyosin in rat intestinal epithelium: immunohistochemical studies at the light and electron microscope levels. J Cell Biol 86: 475–482, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudeja PK, Rao DD, Syed I, Joshi V, Dahdal RY, Gardner C, Risk MC, Schmidt L, Bavishi D, Kim KE, Harig JM, Goldstein JL, Layden TJ, Ramaswamy K. Intestinal distribution of human Na+/H+ exchanger isoforms NHE-1, NHE-2, and NHE-3 mRNA. Am J Physiol Gastrointest Liver Physiol 271: G483–G493, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Evans GS, Flint N, Somers AS, Eyden B, Potten CS. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci 101: 219–231, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Fishbein TM. Intestinal transplantation. N Engl J Med 361: 998–1008, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Grikscheit T, Ochoa ER, Srinivasan A, Gaissert H, Vacanti JP. Tissue-engineered esophagus: experimental substitution by onlay patch or interposition. J Thorac Cardiovasc Surg 126: 537–544, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Grikscheit TC, Ochoa ER, Ramsanahie A, Alsberg E, Mooney D, Whang EE, Vacanti JP. Tissue-engineered large intestine resembles native colon with appropriate in vitro physiology and architecture. Ann Surg 238: 35–41, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grikscheit TC, Ogilvie JB, Ochoa ER, Alsberg E, Mooney D, Vacanti JP. Tissue-engineered colon exhibits function in vivo. Surgery 132: 200–204, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Grikscheit TC, Siddique A, Ochoa ER, Srinivasan A, Alsberg E, Hodin RA, Vacanti JP. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann Surg 240: 748–754, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris KP, Tepass U. Cdc42 and vesicle trafficking in polarized cells. Traffic 11: 1272–1279, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Hehnly H, Longhini KM, Chen JL, Stamnes M. Retrograde Shiga toxin trafficking is regulated by ARHGAP21 and Cdc42. Mol Biol Cell 20: 4303–4312, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koshy SS, Montrose MH, Sears CL. Human intestinal epithelial cells swell and demonstrate actin rearrangement in response to the metalloprotease toxin of Bacteroides fragilis. Infect Immun 64: 5022–5028, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laforenza U. Water channel proteins in the gastrointestinal tract. Mol Aspects Med 33: 642–650, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Lallès JP. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev 68: 323–332, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Levin DE, Barthel ER, Speer AL, Sala FG, Hou X, Torashima Y, Grikscheit TC. Human tissue-engineered small intestine forms from postnatal progenitor cells. J Pediatr Surg 48: 129–137, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Melendez J, Liu M, Sampson L, Akunuru S, Han X, Vallance J, Witte D, Shroyer N, Zheng Y. Cdc42 coordinates proliferation, polarity, migration, and differentiation of small intestinal epithelial cells in mice. Gastroenterology 145: 808–819, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noel J, Roux D, Pouysségur J. Differential localization of Na+/H+ exchanger isoforms (NHE1 and NHE3) in polarized epithelial cell lines. J Cell Sci 109: 929–939, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Sakamori R, Das S, Yu S, Feng S, Stypulkowski E, Guan Y, Douard V, Tang W, Ferraris RP, Harada A, Brakebusch C, Guo W, Gao N. Cdc42 and Rab8a are critical for intestinal stem cell division, survival, and differentiation in mice. J Clin Invest 122: 1052–1065, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sala FG, Kunisaki SM, Ochoa ER, Vacanti J, Grikscheit TC. Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J Surg Res 156: 205–212, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Sala FG, Matthews JA, Speer AL, Torashima Y, Barthel ER, Grikscheit TC. A multicellular approach forms a significant amount of tissue-engineered small intestine in the mouse. Tissue Eng Part A 17: 1841–1850, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato M, Kitaguchi T, Numano R, Ikematsu K, Kakeyama M, Murata M, Sato K, Tsuboi T. The small GTPase Cdc42 modulates the number of exocytosis-competent dense-core vesicles in PC12 cells. Biochem Biophys Res Commun 420: 417–421, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spencer AU, Kovacevich D, McKinney-Barnett M, Hair D, Canham J, Maksym C, Teitelbaum DH. Pediatric short-bowel syndrome: the cost of comprehensive care. Am J Clin Nutr 88: 1552–1559, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Squires RH, Duggan C, Teitelbaum DH, Wales PW, Balint J, Venick R, Rhee S, Sudan D, Mercer D, Martinez JA, Carter BA, Soden J, Horslen S, Rudolph JA, Kocoshis S, Superina R, Lawlor S, Haller T, Kurs-Lasky M, Belle SH, Pediatric Intestinal Failure Consortium. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr 161: 723–728, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stollman NH, Neustater BR, Rogers AI. Short-bowel syndrome. Gastroenterologist 4: 118–128, 1996. [PubMed] [Google Scholar]
- 34.Tavakkolizadeh A, Berger UV, Stephen AE, Kim BS, Mooney D, Hediger MA, Ashley SW, Vacanti JP, Whang EE. Tissue-engineered neomucosa: morphology, enterocyte dynamics, and SGLT1 expression topography. Transplantation 75: 181–185, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Wales PW, de Silva N, Kim J, Lecce L, To T, Moore A. Neonatal short bowel syndrome: population-based estimates of incidence and mortality rates. J Pediatr Surg 39: 690–695, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Yoshikawa T, Inoue R, Matsumoto M, Yajima T, Ushida K, Iwanaga T. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem Cell Biol 135: 183–194, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Mannan I, Schulz S, Parkinson SJ, Alekseev AE, Gomez LA, Terzic A, Waldman SA. Interruption of transmembrane signaling as a novel antisecretory strategy to treat enterotoxigenic diarrhea. FASEB J 13: 913–922, 1999. [DOI] [PubMed] [Google Scholar]