Abstract
Cholangiocyte proliferation is regulated in a coordinated fashion by many neuroendocrine factors through autocrine and paracrine mechanisms. The renin-angiotensin system (RAS) is known to play a role in the activation of hepatic stellate cells and blocking the RAS attenuates hepatic fibrosis. We investigated the role of the RAS during extrahepatic cholestasis induced by bile duct ligation (BDL). In this study, we used normal and BDL rats that were treated with control, angiotensin II (ANG II), or losartan for 2 wk. In vitro studies were performed in a primary rat cholangiocyte cell line (NRIC). The expression of renin, angiotensin-converting enzyme, angiotensinogen, and angiotensin receptor type 1 was evaluated by immunohistochemistry (IHC), real-time PCR, and FACs and found to be increased in BDL compared with normal rat. The levels of ANG II were evaluated by ELISA and found to be increased in serum and conditioned media of cholangiocytes from BDL compared with normal rats. Treatment with ANG II increased biliary mass and proliferation in both normal and BDL rats. Losartan attenuated BDL-induced biliary proliferation. In vitro, ANG II stimulated NRIC proliferation via increased intracellular cAMP levels and activation of the PKA/ERK/CREB intracellular signaling pathway. ANG II stimulated a significant increase in Sirius red staining and IHC for fibronectin that was blocked by angiotensin receptor blockade. In vitro, ANG II stimulated the gene expression of collagen 1A1, fibronectin 1, and IL-6. These results indicate that cholangiocytes express a local RAS and that ANG II plays an important role in regulating biliary proliferation and fibrosis during extraheptic cholestasis.
Keywords: angiotensin, bile duct ligation, renin-angiotensin system, cholangiocyte
cholangiocytes are epithelial cells that line the intrahepatic and extrahepatic bile ducts and are the target cell type in cholangiopathies such as primary biliary cirrhosis and primary sclerosing cholangitis (4, 61). Cholangiocytes are relatively mitotically dormant unless induced by certain pathological conditions such as bile duct ligation (BDL), partial hepatectomy, and bile acid feeding (23). Cholangiopathies are characterized by an imbalance between proliferation and death of cholangiocytes with proliferation playing a critical role for the maintenance of biliary mass and function during the progression of cholangiopathies (4, 61). Numerous studies have demonstrated that proliferating cholangiocytes develop neuroendocrine phenotypes and secrete and respond to a number of hormones, neuropeptides, and neurotransmitters that modulate proliferation in autocrine and paracrine mechanisms (5, 23, 44).
Angiotensin II (ANG II) is the primary bioactive component of the renin-angiotensin system (RAS) (17, 36). ANG II is produced from angiotensinogen (Ao) in two steps. First, renin cleaves Ao to form angiotensin I (ANG I), which is subsequently cleaved by angiotensin-converting enzyme (ACE) to form ANG II (17, 36). The RAS is well known for the role it plays in the regulation of blood pressure and fluid homeostasis (17, 36). It is not only important as a regulator of the cardiovascular system, but it also plays a critical role in regulating cell physiology such as inflammation, fibrosis, and tissue remodeling in a number of tissue types (7).
The biological actions of ANG II are mediated via two G protein-coupled receptors, angiotensin receptor type 1 (AT1) and angiotensin receptor type 2 (AT2) (17, 36). AT1 and AT2 have distinct downstream targets that counteract the physiological actions of each other (58). For example, activation of AT1 stimulates vasoconstriction and cell proliferation whereas AT2 mediates vasodilatation, inhibits cell growth, and activates apoptosis (58). It has been reported that ANG II induces signal transduction by activating mitogen-activated protein kinases (MAPKs) (ERK1/2, JNK, and p38 MAPK), which play key roles in cellular differentiation, proliferation, migration, and fibrosis (62, 64). ERK1/2-dependent signaling plays a key role in the regulation of cholangiocyte proliferation (23, 44).
Recent studies have demonstrated that the RAS plays a key role in hepatic fibrosis and the activation of hepatic stellate cells (HSC). In a fibrotic liver, the local RAS is induced, which plays a role in the activation of HSC (6–10, 41). Paizis and colleagues (51) demonstrated that ACE and AT1 gene expression was upregulated in response to BDL in total liver and especially in areas of active fibrosis. In liver samples from patients with hepatic fibrosis, the expression of AT1 was downregulated in hepatocytes, whereas expression was increased in HSC, vascular endothelium, and bile duct epithelium (51). Moreover, ANG II induces HSC proliferation via the activation of AT1 (8). Studies have also shown that ACE inhibitors or AT1 receptor blockers noticeably reduce hepatic fibrosis in rat (16, 30, 52, 54, 70, 75). Another recent study demonstrated that ANG II induces tumor progression and fibrosis in intrahepatic cholangiocarcinoma through interaction with HSC (49, 50). However, the direct effects of ANG II on cholangiocyte proliferation during extrahepatic cholestasis are not known. Therefore, in the present study, we evaluated the effect of exogenous ANG II on the regulation of cholangiocytes proliferation during BDL. We also explored the underlying signaling mechanism by which RAS regulates cholangiocytes proliferation.
MATERIALS AND METHODS
Materials.
Reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. Tissue culture reagents were purchased from Invitrogen (Carlsbad, CA). The real-time PCR primers were purchased from SA Biosciences/Qiagen (Valencia, CA). The primary antibodies for Ao, renin, ACE, AT1, and AT2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse and goat IgG Vectastain ABC kits for immunohistochemistry (IHC) were purchased from Vector Laboratory (Burlingame, CA). The cyclic adenosine monophosphate (cAMP) enzyme immunoassay (EIA) was purchased from Cayman Chemical (Ann Arbor, MI). The angiotensin II (ANG II) EIA kit was purchased from Phoenix Pharmaceuticals (Burlingame, CA). The inositol phosphate-one (IP-One) ELISA kit was purchased from Cisbio US (Bedford, MA).
Animal models.
All animal experiments were performed in accordance with protocols approved by the Scott & White and Texas A&M Health Science Center Institutional Animal Care and Use Committee. Male 344 Fischer rats (150–175 g) were purchased from Charles River (Wilmington, MA) and maintained in a temperature-controlled environment (20–22°C) with 12:12-h light-dark cycles. Animals were fed ad libitum standard rat chow and had free access to drinking water. To evaluate the in vivo effect of ANG II on cholangiocyte growth, normal and BDL (immediately after surgery) rats were treated with ANG II (50 ng·kg body wt−1·min−1) (56) or 0.9% NaCl (control) by intraperitoneal implanted osmotic minipumps for 2 wk. Normal and BDL (immediately after surgery) rats were also treated with losartan (5 mg/kg body wt intraperitoneal dissolved in saline) (35) for 2 wk. Before each experimental procedure, animals were injected with pentobarbital sodium (50 mg/kg body wt ip) following the regulations of the panel on euthanasia of the American Veterinarian Medical Association.
Purification of cholangiocytes and maintenance of biliary cell lines.
Cholangiocytes were isolated by immunoaffinity separation by using a specific monoclonal antibody (IgM, kindly provided by Dr. R. Faris, Brown University, Providence, RI) that recognizes an unidentified antigen expressed by all intrahepatic rat cholangiocytes (27). The purity (98–99%) of cholangiocytes was evaluated by γ-glutamyl transpeptidase IHC (57). Cell viability (95 to 98%) was confirmed by Trypan blue exclusion. Normal rat intrahepatic cholangiocyte lines (NRIC, displaying phenotypes similar to that of freshly isolated cholangiocytes) were developed, characterized, and maintained in culture as previously described by us (3).
Evaluation of RAS expression.
The expression of the RAS components: Ao, renin, ACE, AT1, and AT2 in purified cholangiocytes was evaluated by BD Accuri C6 Flow Cytometer (BD Biosciences, San Jose, CA). Briefly, 10,000 cells were used to determine the protein expression levels. The cells were fixed by 4% paraformaldehyde and then incubated with the selected primary antibody (pAb) followed by incubation with Molecular Probes secondary antibody (Alexa Fluor 488 or 350), corresponding to the pAb species. The labeled cells were evaluated and the fluorescence-activated cell sorting (FACS) data were analyzed by using C6Flow software (BD Biosciences, San Jose, CA).
RAS component expression in purified cholangiocytes was also confirmed by real-time PCR. Total RNA was extracted by using the RNeasy mini kit (SA Biosciences/Qiagen, Valencia, CA), according to the protocol provided by manufacturer. cDNA was prepared from total RNA by using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). SYBR Green PCR Master Mix (SA Biosciences/Qiagen) was utilized in the experimental assay with RT2 PCR rat primers designed specifically for renin (NM_012642) (11), ACE (NM_012544) (28), AT1a (NM_030985) (31, 45), AT1b (NM_031009) (31), and AT2 (NM_012494) (34) as well as for the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH, NM_017008) (65). The housekeeping gene GAPDH was used to normalize gene expression levels. Real-time PCR was performed with an ABI Prism 7900HT System (Applied Biosystems/Life Technologies, Grand Island, NY). Data were expressed as relative mRNA levels ± SE of the selected gene-to-GAPDH ratio.
The expression of renin, ACE, AT1, and AT2 was evaluated by IHC in frozen liver sections from normal and BDL rats as described (5 μm thick; n = 3 per each group of animals). Immunofluorescence for renin, ACE, AT1, and AT2 expression was performed in NRIC as described (21). Images were taken in a blinded fashion on an Olympus IX71 fluorescence microscope (Tokyo, Japan) with a DP70 digital camera.
Evaluation of proliferation of biliary and NRIC.
We evaluated the effects of in vivo administration of ANG II and losartan to normal and BDL rats on cholangiocyte proliferation by PCNA (29) and CK-19 (13) IHC as previously described (29). Proliferation assays were also performed in NRIC following in vitro treatments with ANG II (10−7 M), L-162,313 (AT1 agonist, 10−7 M) (68), CGP-42112A (AT2 agonist, 10−7 M) (15), losartan [angiotensin receptor blocker (ARB), 10−5 M] (18), H89 [cAMP-dependent protein kinase A (PKA) inhibitor, 30 μM] (22), or PD098059 (ERK inhibitor, 10 nM) (22). Cell proliferation was measured at 48 h as described (14). NRIC proliferation was also evaluated in the presence of benazepril (ACE inhibitor, 1 to 40 μM) at 48 h. NRICs were seeded (7,000 cells/well) onto 96-well plates in complete medium and allowed to adhere overnight at 37°C. Cells were then serum-starved in medium containing 0.5% FBS for 24 h, washed twice with 1 × phosphate buffered saline (PBS); 8 replicates were subsequently treated with DMSO (vehicle) or different agents. Cells were incubated for 48 h with the respective treatments, at which time 10 μl of Cell Titer 96 was added to each well. Absorbance at 490 nm was determined with a microplate reader (Spectra Max 3400, Molecular Devices, Sunnyvale, CA). Absorbance is directly correlated to the number of viable cells (43).
Evaluation of ANG II levels and evaluation of intracellular signaling mechanisms.
The concentration of ANG II in cell supernatants and plasma were measured by an ANG II EIA (Phoenix Pharmaceuticals, Burlingame, CA). NRIC (1 × 106 cells per well) were cultured in a six-well plate overnight. Then, NRICs were stimulated with vehicle (0.2% BSA) and ANG II (10−7 M) for 10 min at room temperature. cAMP levels were determined by a competitive EIA kit (Cayman) (40). Next, the effects of ANG II (10−7 M at 37°C for 90 min) on the activity of PKA were evaluated in NRIC with the PepTag assay for nonradioactive detection of PKA according to the manufacturer's protocol (Promega, Madison, WI) (6). The effect of ANG II on ERK1/2 phosphorylation was evaluated by the CASE ELISA kit (SuperArray, Frederick, MD) (67). The kit includes a complete antibody-based detection system for colorimetric quantification of the relative amount of phosphorylated protein and total ERK1/2. For the assay, NRIC were seeded into 96-well plates and were stimulated with ANG II (10−7 M), for 5, 10, 30, 60, and 180 min. In another experiment NRIC were treated with ANG II (10−7 M), ARB (10−7 M) + ANG II (10−7 M), H89 (30 μM) + ANG II (10−7 M), or ARB (10−7 M) for 10 min to evaluate the effect of inhibitors on ANG II-induced ERK1/2 phosphorylation. Detection of total and phosphorylated protein expression was determined according to the manufacturer's protocol. Finally, we measured the effect of ANG II (10−7 M at 37°C for 90 min) on the activity and phosphorylation of CREB in NRIC in the absence or presence of pretreatment with H-89 (PKA inhibitor, 10 μM). Activation and phosphorylation of CREB were evaluated by a transcription factor assay kit (Active Motif, Carlsbad, CA), which detects binding activity of phosphorylated CREB (Ser-133) (41).
Evaluation of proliferation of biliary fibrosis and profibrotic gene expression.
Liver fibrosis was evaluated by Sirius red staining for identifying interstitial collagen with red color in paraffin-embedded liver sections (4–5 μm thick, 10 different fields analyzed from each sample obtained from three different animals). Image-Pro Plus software (Media Cybernetics, Silver Spring, MD) was used for semiquantitative analysis of the staining. Briefly, a series of digital images was captured from the Sirius red-stained sections. The percentage of the fibrotic area per total liver area was calculated based on the percentage area of red color in each individual slide from each group. Fibrosis was also evaluated by IHC for fibronectin (1:100; Abcam, Cambridge, MA) as described above. Fibrotic marker expression in NRIC was also confirmed by real-time PCR as described above. RT2 PCR rat primers designed specifically for collagen 1A1 (NM_053304) (37), fibronectin 1 (FN1; NM_019143) (63), and IL-6 (NM_012589) (46) as well as for GAPDH were utilized (65). The housekeeping gene GAPDH was used to normalize gene expression levels. Real-time PCR was performed with an ABI Prism 7900HT System (Applied Biosystems/Life Technologies). Data were expressed as relative mRNA levels ± SE of the selected gene-to-GAPDH ratio.
Statistical analysis.
All data are expressed as means ± SE. Differences between groups were analyzed by the Student unpaired t-test when two groups were analyzed by Mann-Whitney U-test and Kruskal-Wallis H-test when more than two groups were analyzed, followed by an appropriate post hoc test. A value of P < 0.05 was considered significant.
RESULTS
Cholangiocytes express the RAS.
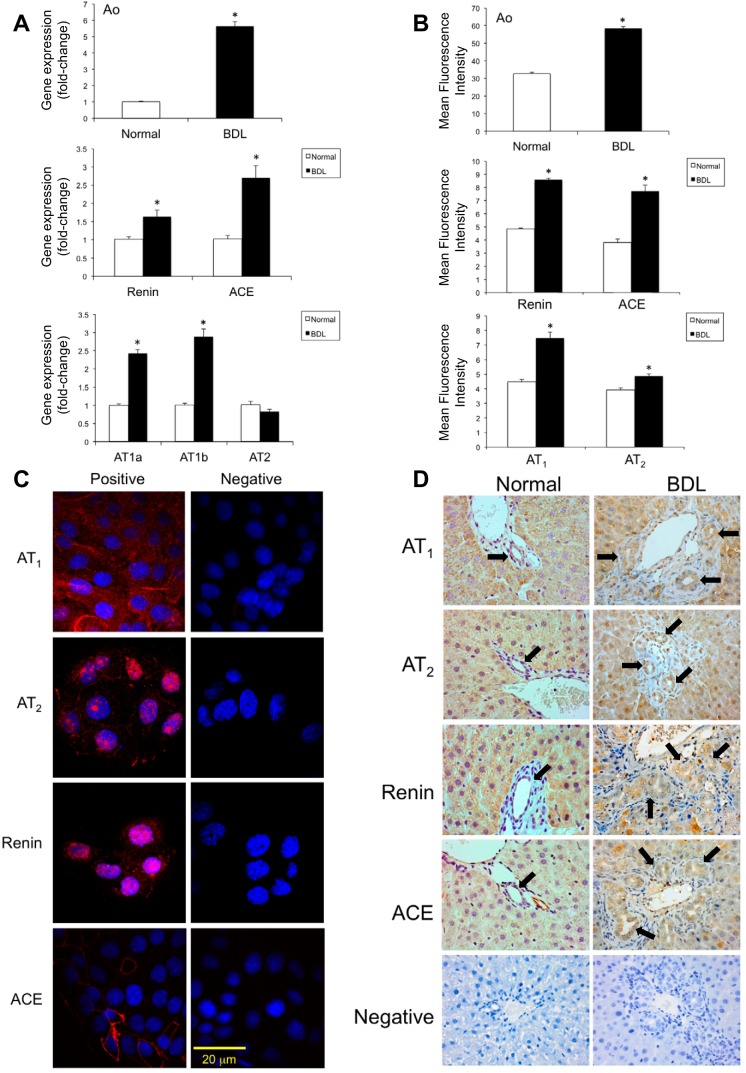
Cholangiocytes isolated from normal and BDL rats as well as NRIC expressed RAS components including Ao, renin, ACE, AT1a, and AT1b receptors (Fig. 1). There was a significant increase in the gene expression levels of Ao, renin, ACE, AT1a, and AT1b in cholangiocytes isolated from BDL compared with normal rats (Fig. 1A). Consistent with gene expression levels, there was a significant increase in the protein expression levels of Ao, renin, ACE, and AT1 in cholangiocytes isolated from BDL rats compared with normal rats as determined by FACS (Fig. 1B). No change in AT2 gene expression was observed during BDL (Fig. 1A). A small increase in AT2 protein expression was observed in BDL cholangiocytes as evaluated by FACS (Fig. 1B). The RAS components were also expressed in NRIC as evaluated by immunofluorescence (Fig. 1C). There was higher expression of RAS components in the bile ducts of BDL rat liver sections compared with normal rat as evaluated by IHC (Fig. 1D). Hepatocytes are also positive for the expression of RAS components as expected (76). As shown in Table 1, serum from BDL rats showed higher levels of ANG II compared with normal rats. Also, primary cultures of cholangiocytes from BDL rats secrete higher amounts of ANG II in the medium compared with normal cholangiocytes (Table 1). In addition, the normal cholangiocyte cell line, NRIC, secretes ANG II in the culture media (Table 1).
Fig. 1.
Expression of renin-angiotension system (RAS) components [angiotensinogen (Ao), renin, angiotensin-converting enzyme (ACE), angiotensin receptor types 1a (AT1a), 1b (AT1b), and 2 (AT2)] in liver sections and cholangiocytes isolated from normal and bile duct-ligated (BDL) rats and normal rat intrahepatic cholangiocyte lines (NRIC). RAS components were assessed by fluorescence-activated cell sorting (FACS) analysis (A) and real-time PCR (B) in cholangiocytes from normal and BDL rats. *P < 0.05 vs. normal. Data are presented as means ± SE, n = 6. Expression of RAS components in NRIC cells was evaluated by immunofluorescence (C). Expression of RAS by immunohistochemistry in normal and BDL rat liver sections (D). Original magnification ×40. Black arrows indicate positively stained bile ducts.
Table 1.
Measurement of Ang II concentration in plasma of NR and BDL rats and Ang II secretion in the supernatant of NR and BDL cholangiocytes as well as in the supernatant of NRICs
| Sample | Ang II, pg/ml |
|---|---|
| Normal serum | 110.8 ± 11.4 |
| BDL serum | 142.8 ± 6.5* |
| Normal cholangiocyte supernatant | 128.4 ± 9.5 |
| BDL cholangiocyte supernatant | 157.4 ± 6.2† |
| NRIC supernatant | 0.6 ± 0.01 |
P < 0.05 vs. normal rat (NR) plasma (n = 6); †P < 0.05 vs. normal cholangiocyte supernatant (n = 6).
Ang II, angiotensin; BDL, bile duct ligation; NRICs, normal rat intrahepatic cholangiocyte cell line.
ANG II stimulates biliary proliferation.
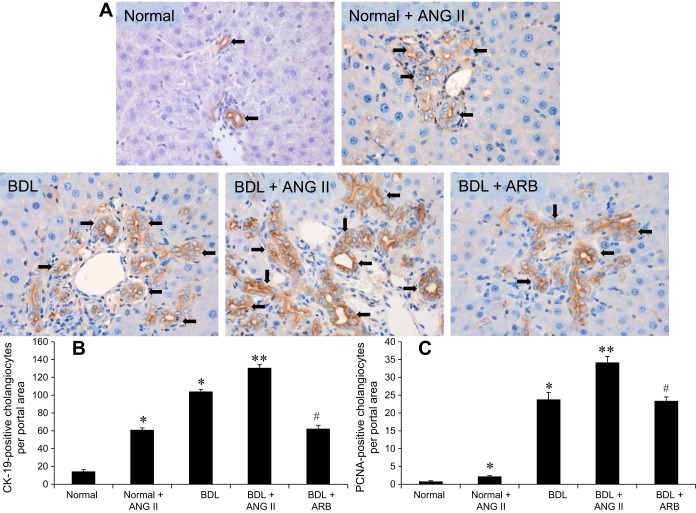
Liver sections from the BDL rats, infused with ANG II, showed higher expression of CK-19-positive cholangiocytes per portal area compared with normal control rats (Fig. 2, A and B). Similar to the increase in the number of CK-19-positive cholangiocytes per portal area (Fig. 2B), there was also a significant increase in the number of PCNA-positive cholangiocytes per portal area (Fig. 2C). The number of both CK-19- and PCNA-positive cholangiocytes per portal area was significantly reduced in BDL rats treated with the ARB (losartan) compared with BDL + ANG II-treated rats (Fig. 2, B and C).
Fig. 2.
Evaluation of CK-19 and PCNA expression in normal rat (NR) and BDL rat liver sections by immunohistochemistry. A and B: there are increased numbers of CK-19- (A and B) and PCNA-positive (C) cholangioyctes per portal area in Normal + ANG II and BDL + ANG II compared with the NR rat liver. Losartan [angiotensin receptor blocker (ARB)] treatment significantly downregulates the expression of CK-19 and PCNA in isolated cholangiocytes from BDL rats. *P < 0.05 vs. normal. **P < 0.05 vs. BDL. #P < 0.05 vs. BDL. Data are presented as means ± SE, n = 6.
As shown in Fig. 3A, the AT1 agonist induced a similar increase in NRIC proliferation compared with ANG II treatment at 48 h. However, the AT2 agonist had no effect on NRIC proliferation (Fig. 3A). ANG II-induced proliferation was inhibited by ARB (losartan) (Fig. 3A). Since cholangiocytes express RAS and secrete ANG II, we evaluated the role of ANG II in the regulation of basal biliary proliferation in NRIC treated with benazepril (ACE inhibitor). Benazepril induced a significant dose-dependent reduction in NRIC proliferation at 48 and 72 h (Fig. 3B). This was also confirmed in NRIC treated with ARB, in which a significant reduction in basal proliferation was observed at 48 h.
Fig. 3.
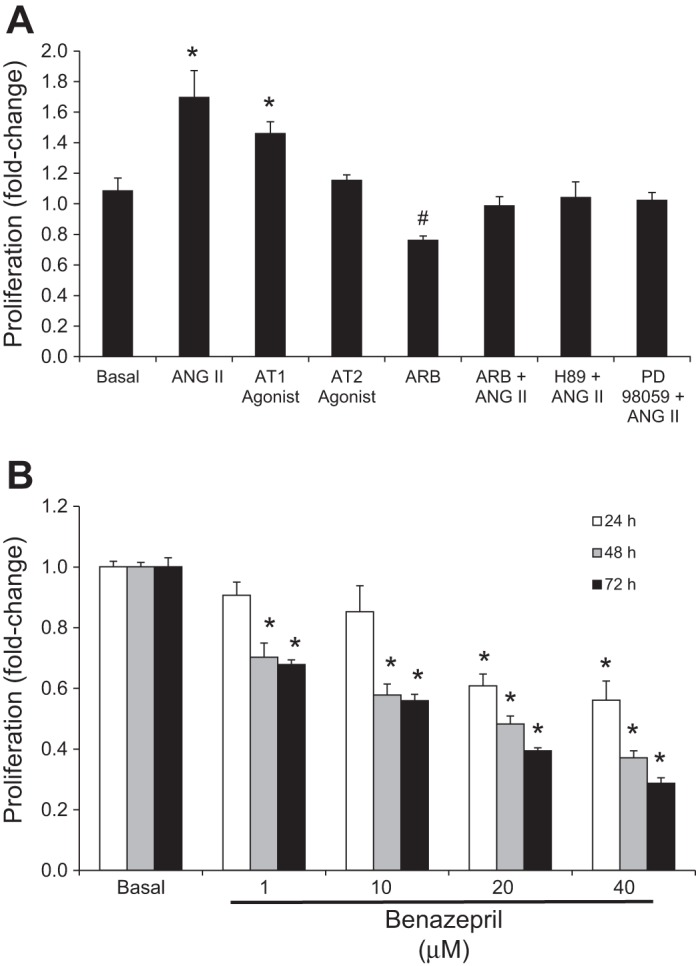
Evaluation of the effects of angiotensin II (ANG II) on the proliferation of NRICs. A: ANG II and the AT1 agonist stimulate the proliferation of NRIC at 48 h. ANG II-stimulated proliferation is blocked by ARB, H89 (PKA inhibitor), and PD98059 (ERK1/2 pathway inhibitor). ARB (losartan) decreased basal proliferation. *P < 0.05 vs. basal (control). #P < 0.05 vs. basal. Data are presented as means ± SE, n = 6. B: benazepril (ACE inhibitor) dose dependently inhibits NRIC proliferation at 48 and 72 h. *P < 0.05 vs. basal (control). Data are presented as means ± SE, n = 6.
ANG II stimulates cAMP-, PKA-, ERK1/2-, and CREB-dependent intracellular signaling mechanisms and proliferation.
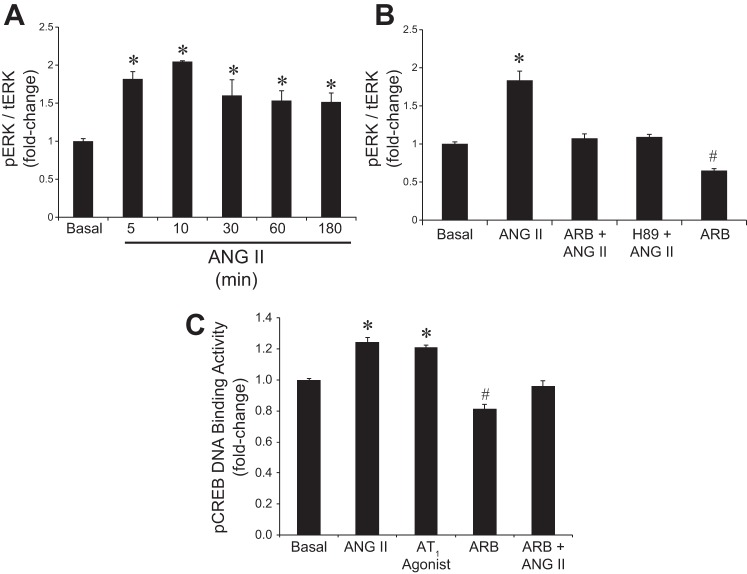
In NRIC, cAMP and inositol 1,4,5-trisphosphate (IP3) levels were evaluated to begin to elucidate ANG II mediated intracellular signaling mechanisms. ANG II caused an increase in intracellular cAMP but not IP3 levels (not shown), compared with basal values (Fig. 4A). Since ANG II stimulated intracellular cAMP levels, we measured cAMP-dependent PKA activity. There was enhanced PKA activity in NRIC treated with ANG II that was inhibited by ARB (Fig. 4B). ERK1/2 has been shown to play a key role in proproliferative signaling mechanisms in cholangiocytes (20, 22, 52). A significant activation of ERK1/2 was observed in the ANG II-stimulated NRIC in a time-dependent manner (Fig. 5A). There was higher phosphorylation of ERK1/2 in NRIC within 5–10 min of ANG II treatment and the phosphorylation levels of ERK1/2 remained unchanged up to 180 min (Fig. 5A). ANG II-induced ERK phosphorylation was attenuated both by ARB (losartan) and H89 (PKA inhibitor) (Fig. 5B). This data suggests that PKA is the upstream modulator of ERK1/2, which is supported by the inhibition of ANG II-dependent proliferation by H89 and PD98059 (ERK 1/2 pathway inhibitor) (Fig. 3A). Finally, we evaluated the effects of ANG II on downstream CREB activity, which has been shown to play an important role in modulating cholangiocyte proliferation (19, 23). Stimulation with ANG II and AT1 agonist increased pCREB DNA binding activity in NRIC, which was attenuated by ARB (losartan) (Fig. 5C). Stimulation with ARB alone also resulted in decreased pCREB DNA binding activity (Fig. 5C).
Fig. 4.
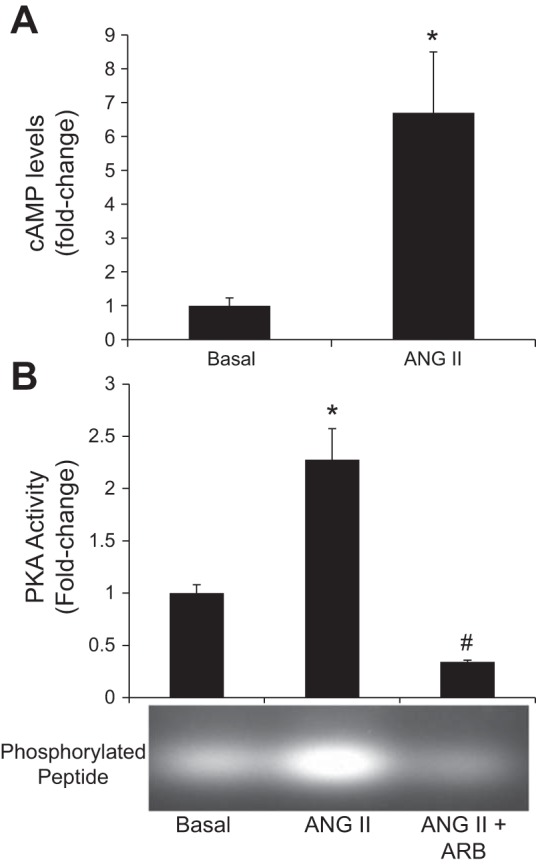
Effect of ANG II on intracellular cAMP levels and PKA activity in NRICs. A: ANG II stimulated intracellular cAMP levels in NRICs. *P < 0.05 vs. normal. Data are presented as means ± SE, n = 6. B: ANG II stimulated an increase in PKA activity compared with basal, which is prevented by ARB (losartan). *P < 0.05 vs. basal (control). #P < 0.05 vs. basal. Data are presented as means ± SE, n = 6.
Fig. 5.
Evaluation of the effects of ANG II on ERK1/2 phosphorylation and pCREB DNA binding activity. A: ANG II significantly induces the phosphorylation of ERK1/2 in NRIC in a time-dependent manner. *P < 0.05 vs. normal. Data are presented as means ± SE, n = 6. B: ANG II-mediated phosphorylation of ERK1/2 is attenuated by pretreatment with ARB (losartan) and H89 (PKA inhibitor) prior to ANG II treatment. ARB alone significantly reduced the phosphorylation of ERK1/2 compared with basal. *P < 0.05 vs. basal (control). #P < 0.05 vs. basal. Data are presented as means ± SE, n = 6. C: ANG II stimulates an increase in DNA binding activity of pCREB, which is blocked by attenuated by ARB (losartan). ARB alone significantly reduced the DNA binding activity of pCREB compared with basal. *P < 0.05 vs. basal (control). #P < 0.05 vs. basal. Data are presented as means ± SE, n = 6.
ANG II stimulates biliary fibrosis and expression of profibrotic genes.
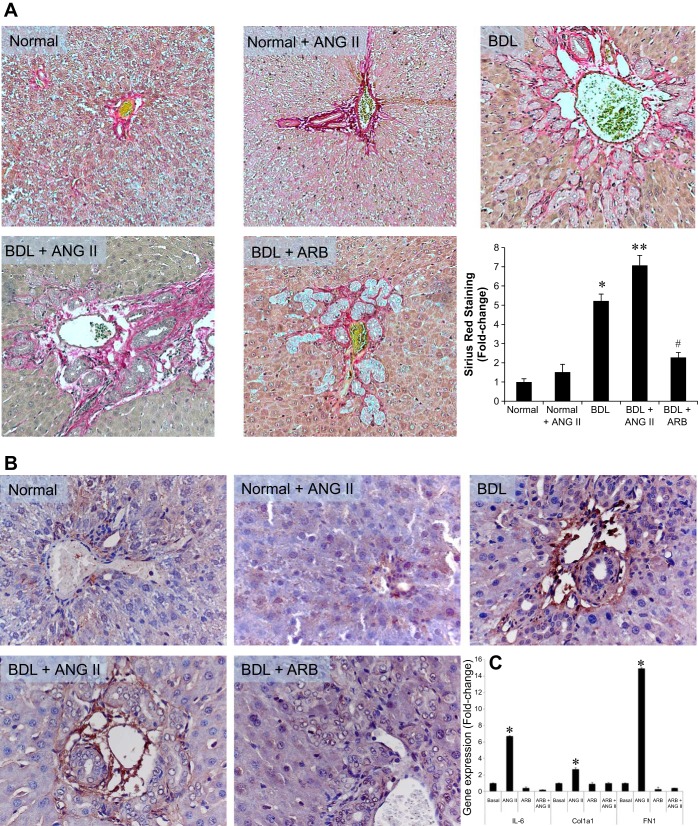
Liver sections from the BDL rats, infused with ANG II, showed significantly higher levels of Sirius red staining (i.e., fibrosis) in the portal areas compared with normal control rats (Fig. 6A). Sirius red staining was significantly reduced in BDL rats treated with the ARB (losartan) compared with BDL + ANG II-treated rats (Fig. 6A). Similar findings were observed for fibronectin staining (Fig. 6B). In vitro, ANG II stimulated increased collagen 1A1 (Col1a1), fibronectin 1 (FN1), and IL-6 gene expression in NRIC, which was blocked by ARB (Fig. 6C).
Fig. 6.
Evaluation of Sirius red staining, fibronectin expression in liver sections, and ANG II-induced IL-6 expression in NRIC. A: there is increased levels of fibrosis in the portal areas of the ANG II treated Normal + ANG II and BDL + ANG II compared with the NR rat liver. Losartan (ARB) treatment significantly downregulates fibrosis. Original magnification ×40. B: there is increased fibronectin expression in the portal areas of ANG II treated Normal + ANG II and BDL + ANG II compared with the NR rat liver. Losartan (ARB) treatment downregulates fibronectin expression. Original magnification ×40. C: in vitro, ANG II stimulated the gene expression levels of Col1a1, FN1, and IL-6, which were blocked by ARB. *P < 0.05 vs. normal. **P < 0.05 vs. BDL. #P < 0.05 vs. BDL. Data are presented as means ± SE, n = 6.
DISCUSSION
The major findings in the study relate to the expression of a local RAS by cholangiocytes and the regulation of biliary proliferation by ANG II during extrahepatic cholestasis. We found that 1) cholangiocytes express the components of the RAS and produce ANG II; 2) RAS components and ANG II levels are elevated during extrahepatic cholestasis induced by BDL; 3) administration of exogenous ANG II to normal and BDL rats increased intrahepatic bile duct mass; 4) administration of the ARB (losartan) during BDL significantly reduces intrahepatic bile duct mass; and 5) ANG II stimulates cholangiocyte proliferation via the activation of AT1 triggering cAMP-, PKA-, ERK1/2-, and pCREB-dependent signaling mechanisms. Upregulation of the RAS may play an important role in the regulation of biliary mass during extrahepatic cholestasis via autocrine and paracrine signaling mechanisms.
Previous studies have shown that RAS plays an important role in advanced liver diseases (17, 36). Several groups have evaluated the expression of RAS components in animal models of hepatic fibrosis and human tissue samples. Paizis et al. (51) demonstrated that the gene expression for ACE and AT1 were upregulated by BDL in total liver and were highly expressed in areas of active fibrosis. Similar to our findings, the biliary epithelium in tissue samples from patients with advanced liver cirrhosis expressed AT1 by IHC (59). In this study, the expression of AT1 was downregulated in hepatocytes, whereas the expression was increased in HSC, vascular endothelium, and bile duct epithelium (59).
The endocrine (or classical) RAS is known for its role in the regulation of blood pressure, electrolyte balance, and fluid homeostasis (36). Recently, studies have demonstrated the expression of all of the “classical” RAS components required for a functioning local RAS in the pancreas (38, 53). Our findings that cholangiocyte express the components necessary for the formation of ANG II, such as renin and ACE, indicate that cholangiocytes have a functional RAS. This is similar to findings observed in activated hepatic stellate cells, which have a local RAS and produce ANG II that stimulates fibrosis via activation of NADPH oxidase (77). This concept is supported by the observation in our study that cholangiocytes express the message and protein for Ao, which is the precursor for ANG II. Ao expression has also been shown in liver cells such as Kupffer cells, hepatocytes, and HSCs (42). In addition to expressing angiotensinogen, cholangiocytes also produce ANG II, which is similar to findings in activated HSCs (77). Both in vitro and in vivo, HSCs have higher expression levels of active renin and ACE and synthesis ANG II (10). Cholangiocyte production of ANG II is significantly increased during BDL in both serum and cholangiocyte supernatants. In patients with cirrhosis or chronic liver disease, there were also higher expression levels of angiotensin and plasma renin activity (69). RAS plays a key role in the alterations of portal pressure during decompensated liver cirrhosis (1). Finally, the involvement of RAS during cholestasis is supported by our finding that renin and ACE expression levels are increased in cholangiocytes during BDL. The expression of RAS in numerous liver cell types indicates that cholangiocyte proliferation may be modulated in paracrine mechanisms in addition to autocrine mechanisms. The activation of RAS in cholangiocytes may play a role in the activation of other cell types throughout the liver contributing to ANG II-induced fibrosis.
We next evaluated the effects of ANG II on biliary proliferation in both normal and cholestatic BDL rats. Chronic administration of ANG II stimulated a significant increase in biliary mass and proliferation in both normal and BDL rats, which was blocked by ARB (losartan) (AT1 inhibitor). Blockage of the RAS signaling either through ARB or ACE inhibition has been shown to attenuate fibrosis and to suppress activated HSCs and hepatic TGF-β1 expression in animal models of chronic liver injury (including CCl4 and BDL) (30, 48, 66, 72, 73, 75). In vitro, in NRIC we found that ANG II and an AT1 agonist stimulate a significant increase in proliferation, which was blocked by ARB. Interestingly, treatment of NRIC with ARB resulted in a significant reduction in basal proliferation, which supports the concept of the presence of a local RAS in cholangiocytes. We also found a significant reduction in proliferation of NRIC when treated with the ACE inhibitor benazepril. This is similar to findings reported by Li and Wanchun (39) that benazepril reduces intimal proliferation.
Next, we evaluated the intracellular signaling mechanisms regulating ANG II-induced proliferation. We found that ANG II stimulated intracellular cAMP levels but not IP3, which is in contrast to many studies (2, 12, 25). The majority of studies have show that activation of AT1 produces second messengers such as IP3, diacylglycerol, and reactive oxygen species (26). However, our data are supported by the findings that ANG II stimulates increased intracellular cAMP in glomerular epithelial cells (60). Another study has shown that that ANG II can activate cAMP formation in renal mesangial cells (55). The cAMP/PKA/ERK/CREB intracellular signaling pathway has been shown to play an important role in the regulation of biliary proliferation both in vitro and in vivo (20, 44). Therefore, we explored the downstream PKA/ERK/CREB signaling pathway related to ANG II. Similar to our previous studies, ANG II stimulated PKA activity, ERK1/2 phosphorylation, and pCREB DNA binding activity. Supporting the concept that the PKA/ERK/CREB signaling pathway plays an important role in in ANG II-stimulated biliary proliferation, inhibition of PKA or ERK1/2 signaling attenuated proliferation. The concept of the presence of a local RAS in cholangiocytes is also supported by the findings that treatment with ARB alone reduced ERK1/2 phosphorylation and pCREB DNA binding activity below basal levels.
Finally, we also evaluated the effects of chronic ANG II administration on biliary fibrosis and the expression of proproliferative/profibrotic genes. We found, similar to other studies, that ANG II stimulates a significant increase in fibrosis surrounding the portal area as well as an increase in portal fibronectin expression, which were both blocked by ARB (33, 47, 74). In a similar fashion, we found that ANG II stimulates the gene expression of collagen 1A1 and fibronectin 1 in NRIC, which was blocked by pretreatment with ARB. In vitro, we also found that ANG II stimulates a significant increase in IL-6 gene expression in cholangiocytes, which is a proproliferative cytokine that plays a key role in regulating biliary hyperplasia (71). ANG II has been shown to increase IL-6 expression levels in several cell types (24, 32). Further studies will be necessary to fully elucidate the relationship between ANG II and IL-6 on biliary proliferation and fibrosis.
The present study demonstrates that the local RAS plays an important role in the regulation of normal and cholestatic cholangiocyte proliferation and the activation of biliary fibrosis. Modulation of the RAS and the ANG II/AT1 signaling pathway in cholangiocytes may represent a novel therapeutic approach for cholangiopathies.
GRANTS
This work was supported by an NIH RO1 grant (DK-081442), VA CDA-2, and VA Merit Award to S. Glaser.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.A., M.K.M., A.K.M., M.U., M. Gergely, C.S., M. Guerrier, D.N., and D.E.D. performed experiments; S.A., M.K.M., A.K.M., M.U., M. Gergely, C.S., M. Guerrier, D.N., D.E.D., and S.G. analyzed data; S.A., D.E.D., and S.G. interpreted results of experiments; S.A., M.K.M., and S.G. prepared figures; S.A. and S.G. drafted manuscript; A.K.M., M. Guerrier, D.N., D.E.D., and S.G. edited and revised manuscript; S.G. conception and design of research; S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas. The views presented are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors thank Dr. Gianfranco Alpini for reading the manuscript and for providing the NRIC line. We also thank Anna Web for assistance with confocal imaging.
Present address for M. K. Munshi: Institute of Food and Radiation Biology, Dhaka, Bangladesh.
REFERENCES
- 1.Agasti AK, Mahajan AU, Phadke AY, Nathani PJ, Sawant P. Comparative randomized study on efficacy of losartan versus propranolol in lowering portal pressure in decompensated chronic liver disease. J Dig Dis 14: 266–271, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Alexander RW, Brock TA, Gimbrone MA Jr, Rittenhouse SE. Angiotensin increases inositol trisphosphate and calcium in vascular smooth muscle. Hypertension 7: 447–451, 1985. [PubMed] [Google Scholar]
- 3.Alpini G, Phinizy JL, Glaser S, Francis H, Benedetti A, Marucci L, LeSage G. Development and characterization of secretin-stimulated secretion of cultured rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 284: G1066–G1073, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Alpini G, Prall R, LaRusso NF. The pathobiology of biliary epithelia. In: The Liver; Biology and Pathobiology, edited by Arias I, Boyer J, Chisari F, Fausto N, Jakoby W, Schachter D, Shafritz DA. Philadelphia, PA: Lippincott Williams & Wilkins, 2001, p. 421–435. [Google Scholar]
- 5.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 132: 415–431, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bai J, Zhao Y, Wang Z, Liu C, Wang Y, Li Z. Dual-readout fluorescent assay of protein kinase activity by use of TiO-coated magnetic microspheres. Anal Chem 85: 4813–4821, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Bataller R, Gabele E, Parsons CJ, Morris T, Yang L, Schoonhoven R, Brenner DA, Rippe RA. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology 41: 1046–1055, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Bataller R, Gines P, Nicolas JM, Gorbig MN, Garcia-Ramallo E, Gasull X, Bosch J, Arroyo V, Rodes J. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology 118: 1149–1156, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Bataller R, Sancho-Bru P, Gines P, Brenner DA. Liver fibrogenesis: a new role for the renin-angiotensin system. Antioxid Redox Signal 7: 1346–1355, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Bataller R, Sancho-Bru P, Gines P, Lora JM, Al-Garawi A, Sole M, Colmenero J, Nicolas JM, Jimenez W, Weich N, Gutierrez-Ramos JC, Arroyo V, Rodes J. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology 125: 117–125, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Burnham CE, Hawelu-Johnson CL, Frank BM, Lynch KR. Molecular cloning of rat renin cDNA and its gene. Proc Natl Acad Sci USA 84: 5605–5609, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catt KJ, Carson MC, Hausdorff WP, Leach-Harper CM, Baukal AJ, Guillemette G, Balla T, Aguilera G. Angiotensin II receptors and mechanisms of action in adrenal glomerulosa cells. J Steroid Biochem 27: 915–927, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Chiu CC, Sheu JC, Chen CH, Lee CZ, Chiou LL, Chou SH, Huang GT, Lee HS. Global gene expression profiling reveals a key role of CD44 in hepatic oval-cell reaction after 2-AAF/CCl4 injury in rodents. Histochem Cell Biol 132: 479–489, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun 3: 207–212, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Cote F, Do TH, Laflamme L, Gallo JM, Gallo-Payet N. Activation of the AT(2) receptor of angiotensin II induces neurite outgrowth and cell migration in microexplant cultures of the cerebellum. J Biol Chem 274: 31686–31692, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Croquet V, Moal F, Veal N, Wang J, Oberti F, Roux J, Vuillemin E, Gallois Y, Douay O, Chappard D, Cales P. Hemodynamic and antifibrotic effects of losartan in rats with liver fibrosis and/or portal hypertension. J Hepatol 37: 773–780, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Dinh DT, Frauman AG, Johnston CI, Fabiani ME. Angiotensin receptors: distribution, signalling and function. Clin Sci (Lond) 100: 481–492, 2001. [PubMed] [Google Scholar]
- 18.Fischer JW, Stoll M, Hahn AW, Unger T. Differential regulation of thrombospondin-1 and fibronectin by angiotensin II receptor subtypes in cultured endothelial cells. Cardiovasc Res 51: 784–791, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Francis H, Glaser S, Demorrow S, Gaudio E, Ueno Y, Venter J, Dostal D, Onori P, Franchitto A, Marzioni M, Vaculin S, Vaculin B, Katki K, Stutes M, Savage J, Alpini G. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol 295: C499–C513, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francis H, Glaser S, Ueno Y, Lesage G, Marucci L, Benedetti A, Taffetani S, Marzioni M, Alvaro D, Venter J, Reichenbach R, Fava G, Phinizy JL, Alpini G. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol 41: 528–537, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Glaser S, DeMorrow S, Francis H, Ueno Y, Gaudio E, Vaculin S, Venter J, Franchitto A, Onori P, Vaculin B, Marzioni M, Wise C, Pilanthananond M, Savage J, Pierce L, Mancinelli R, Alpini G. Progesterone stimulates the proliferation of female and male cholangiocytes via autocrine/paracrine mechanisms. Am J Physiol Gastrointest Liver Physiol 295: G124–G136, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glaser S, Lam IP, Franchitto A, Gaudio E, Onori P, Chow BK, Wise C, Kopriva S, Venter J, White M, Ueno Y, Dostal D, Carpino G, Mancinelli R, Butler W, Chiasson V, DeMorrow S, Francis H, Alpini G. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology 52: 204–214, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser S, Onori P, Wise C, Yang F, Marzioni M, Alvaro D, Franchitto A, Mancinelli R, Alpini G, Munshi M, Gaudio E. Recent advances in the regulation of cholangiocyte proliferation and function during extrahepatic cholestasis. Dig Liver Dis 42: 245–252, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomolak JR, Didion SP. Angiotensin II-induced endothelial dysfunction is temporally linked with increases in interleukin-6 and vascular macrophage accumulation. Front Physiol 5: 396, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griendling KK, Tsuda T, Berk BC, Alexander RW. Angiotensin II stimulation of vascular smooth muscle cells. Secondary signalling mechanisms. Am J Hypertens 2: 659–665, 1989. [DOI] [PubMed] [Google Scholar]
- 26.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 112: 417–428, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Ishii M, Vroman B, LaRusso NF. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology 97: 1236–1247, 1989. [DOI] [PubMed] [Google Scholar]
- 28.Jacob HJ, Lindpaintner K, Lincoln SE, Kusumi K, Bunker RK, Mao YP, Ganten D, Dzau VJ, Lander ES. Genetic mapping of a gene causing hypertension in the stroke-prone spontaneously hypertensive rat. Cell 67: 213–224, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Jensen K, Afroze S, Ueno Y, Rahal K, Frenzel A, Sterling M, Guerrier M, Nizamutdinov D, Dostal DE, Meng F, Glaser SS. Chronic nicotine exposure stimulates biliary growth and fibrosis in normal rats. Dig Liver Dis 45: 754–761, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonsson JR, Clouston AD, Ando Y, Kelemen LI, Horn MJ, Adamson MD, Purdie DM, Powell EE. Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology 121: 148–155, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Kakar SS, Sellers JC, Devor DC, Musgrove LC, Neill JD. Angiotensin II type-1 receptor subtype cDNAs: differential tissue expression and hormonal regulation. Biochem Biophys Res Commun 183: 1090–1096, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Kandalam U, Sarmiento N, Haspula D, Clark MA. Angiotensin III induces signal transducer and activator of transcription 3 and interleukin-6 mRNA levels in cultured rat astrocytes. J Renin Angiotens Aldost Syst 2014June24 pii: 1470320314534509. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33.Kim MY, Baik SK, Park DH, Jang YO, Suk KT, Yea CJ, Lee IY, Kim JW, Kim HS, Kwon SO, Cho MY, Ko SB, Chang SJ, Um SH, Han KH. Angiotensin receptor blockers are superior to angiotensin-converting enzyme inhibitors in the suppression of hepatic fibrosis in a bile duct-ligated rat model. J Gastroenterol 43: 889–896, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi S, Ohnishi J, Nibu Y, Nishimatsu S, Umemura S, Ishii M, Murakami K, Miyazaki H. Cloning of the rat angiotensin II type 2 receptor gene and identification of its functional promoter region. Biochim Biophys Acta 1262: 155–158, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Kohzuki M, Yasujima M, Liu PF, Obara K, Kanazawa M, Yoshida K, Saito T, Sato T, Abe K. Cardiovascular and renal protective effects of losartan in spontaneously hypertensive rats with diabetes mellitus. Clin Exp Pharmacol Physiol Suppl 22: S366–S367, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Lavoie JL, Sigmund CD. Minireview: Overview of the renin-angiotensin system—an endocrine and paracrine system. Endocrinology 144: 2179–2183, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Leheup BP, Federspiel SJ, Guerry-Force ML, Wetherall NT, Commers PA, DiMari SJ, Haralson MA. Extracellular matrix biosynthesis by cultured fetal rat lung epithelial cells. I. Characterization of the clone and the major genetic types of collagen produced. Lab Invest 60: 791–807, 1989. [PubMed] [Google Scholar]
- 38.Leung PS. The physiology of a local renin-angiotensin system in the pancreas. J Physiol 580: 31–37, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Wanchun C. Benazepril on tissue angiotensin-converting enzyme and cellular proliferation in restenosis after experimental angioplasty. J Cardiovasc Pharmacol 30: 790–797, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Liu KC, Ge W. Evidence for gating roles of protein kinase A and protein kinase C in estradiol-induced luteinizing hormone receptor (lhcgr) expression in zebrafish ovarian follicle cells. PLoS One 8: e62524, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2: 599–609, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Morris BJ, Iwamoto HS, Reid IA. Localization of angiotensinogen in rat liver by immunocytochemistry. Endocrinology 105: 796–800, 1979. [DOI] [PubMed] [Google Scholar]
- 43.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63, 1983. [DOI] [PubMed] [Google Scholar]
- 44.Munshi MK, Priester S, Gaudio E, Yang F, Alpini G, Mancinelli R, Wise C, Meng F, Franchitto A, Onori P, Glaser SS. Regulation of biliary proliferation by neuroendocrine factors: implications for the pathogenesis of cholestatic liver diseases. Am J Pathol 178: 472–484, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature 351: 233–236, 1991. [DOI] [PubMed] [Google Scholar]
- 46.Northemann W, Braciak TA, Hattori M, Lee F, Fey GH. Structure of the rat interleukin 6 gene and its expression in macrophage-derived cells. J Biol Chem 264: 16072–16082, 1989. [PubMed] [Google Scholar]
- 47.Oakley F, Teoh V, Ching ASG, Bataller R, Colmenero J, Jonsson JR, Eliopoulos AG, Watson MR, Manas D, Mann DA. Angiotensin II activates I kappaB kinase phosphorylation of RelA at Ser 536 to promote myofibroblast survival and liver fibrosis. Gastroenterology 136: 2334–2344.e1, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Ohishi T, Saito H, Tsusaka K, Toda K, Inagaki H, Hamada Y, Kumagai N, Atsukawa K, Ishii H. Anti-fibrogenic effect of an angiotensin converting enzyme inhibitor on chronic carbon tetrachloride-induced hepatic fibrosis in rats. Hepatol Res 21: 147–158, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Okamoto K, Tajima H, Nakanuma S, Sakai S, Makino I, Kinoshita J, Hayashi H, Nakamura K, Oyama K, Nakagawara H, Fujita H, Takamura H, Ninomiya I, Kitagawa H, Fushida S, Fujimura T, Harada S, Wakayama T, Iseki S, Ohta T. Angiotensin II enhances epithelial-to-mesenchymal transition through the interaction between activated hepatic stellate cells and the stromal cell-derived factor-1/CXCR4 axis in intrahepatic cholangiocarcinoma. Int J Oncol 41: 573–582, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Okamoto K, Tajima H, Ohta T, Nakanuma S, Hayashi H, Nakagawara H, Onishi I, Takamura H, Ninomiya I, Kitagawa H, Fushida S, Tani T, Fujimura T, Kayahara M, Harada S, Wakayama T, Iseki S. Angiotensin II induces tumor progression and fibrosis in intrahepatic cholangiocarcinoma through an interaction with hepatic stellate cells. Int J Oncol 37: 1251–1259, 2010. [DOI] [PubMed] [Google Scholar]
- 51.Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, Angus PW. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology 123: 1667–1676, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Paizis G, Gilbert RE, Cooper ME, Murthi P, Schembri JM, Wu LL, Rumble JR, Kelly DJ, Tikellis C, Cox A, Smallwood RA, Angus PW. Effect of angiotensin II type 1 receptor blockade on experimental hepatic fibrogenesis. J Hepatol 35: 376–385, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Ramalho LN, Ramalho FS, Zucoloto S, Castro-e-Silva O Jr, Correa FM, Elias J Jr, Magalhaes JF. Effect of losartan, an angiotensin II antagonist, on secondary biliary cirrhosis. Hepatogastroenterology 49: 1499–1502, 2002. [PubMed] [Google Scholar]
- 55.Randolph-Habecker JR, Rahill B, Torok-Storb B, Vieira J, Kolattukudy PE, Rovin BH, Sedmak DD. The expression of the cytomegalovirus chemokine receptor homolog US28 sequesters biologically active CC chemokines and alters IL-8 production. Cytokine 19: 37–46, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 82: S12–S22, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Rutenburg AM, Kim H, Fischbein JW, Hanker JS, Wasserkrug HL, Seligman AM. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem 17: 517–526, 1969. [DOI] [PubMed] [Google Scholar]
- 58.Sadoshima J. Cytokine actions of angiotensin II. Circ Res 86: 1187–1189, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Schulte S, Oidtmann A, Kociok N, Demir M, Odenthal M, Drebber U, Dienes HP, Nierhoff D, Goeser T, Toex U, Steffen HM. Hepatocyte expression of angiotensin II type 1 receptor is downregulated in advanced human liver fibrosis. Liver Int 29: 384–391, 2009. [DOI] [PubMed] [Google Scholar]
- 60.Sharma M, Sharma R, Greene AS, McCarthy ET, Savin VJ. Documentation of angiotensin II receptors in glomerular epithelial cells. Am J Physiol Renal Physiol 274: F623–F627, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol 39: S90–S102, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Sugden PH, Clerk A. Regulation of the ERK subgroup of MAP kinase cascades through G protein-coupled receptors. Cell Signal 9: 337–351, 1997. [DOI] [PubMed] [Google Scholar]
- 63.Tamkun JW, Schwarzbauer JE, Hynes RO. A single rat fibronectin gene generates three different mRNAs by alternative splicing of a complex exon. Proc Natl Acad Sci USA 81: 5140–5144, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taniyama Y, Ushio-Fukai M, Hitomi H, Rocic P, Kingsley MJ, Pfahnl C, Weber DS, Alexander RW, Griendling KK. Role of p38 MAPK and MAPKAPK-2 in angiotensin II-induced Akt activation in vascular smooth muscle cells. Am J Physiol Cell Physiol 287: C494–C499, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Tso JY, Sun XH, Kao TH, Reece KS, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res 13: 2485–2502, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ueki M, Koda M, Yamamoto S, Matsunaga Y, Murawaki Y. Preventive and therapeutic effects of angiotensin II type 1 receptor blocker on hepatic fibrosis induced by bile duct ligation in rats. J Gastroenterol 41: 996–1004, 2006. [DOI] [PubMed] [Google Scholar]
- 67.Versteeg HH, Nijhuis E, van den Brink GR, Evertzen M, Pynaert GN, van Deventer SJ, Coffer PJ, Peppelenbosch MP. A new phosphospecific cell-based ELISA for p42/p44 mitogen-activated protein kinase (MAPK), p38 MAPK, protein kinase B and cAMP-response-element-binding protein. Biochem J 350: 717–722, 2000. [PMC free article] [PubMed] [Google Scholar]
- 68.Vianello B, Clauser E, Corvol P, Monnot C. Functional interactions of L-162,313 with angiotensin II receptor subtypes and mutants. Eur J Pharmacol 347: 113–118, 1998. [DOI] [PubMed] [Google Scholar]
- 69.Vilas-Boas WW, Ribeiro-Oliveira A Jr, Ribeiro Rda C, Vieira RL, Almeida J, Nadu AP, Simoes e Silva AC, Santos RA. Effect of propranolol on the splanchnic and peripheral renin angiotensin system in cirrhotic patients. World J Gastroenterol 14: 6824–6830, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei HS, Lu HM, Li DG, Zhan YT, Wang ZR, Huang X, Cheng JL, Xu QF. The regulatory role of AT 1 receptor on activated HSCs in hepatic fibrogenesis: effects of RAS inhibitors on hepatic fibrosis induced by CCl(4). World J Gastroenterol 6: 824–828, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiao Y, Wang J, Yan W, Zhou Y, Chen Y, Zhou K, Wen J, Wang Y, Cai W. Dysregulated miR-124 and miR-200 expression contribute to cholangiocyte proliferation in the cholestatic liver by targeting IL-6/STAT3 signalling. J Hepatol 2014October30 pii: S0168-8278(14)00799-5. doi: 10.1016/j.jhep.2014.10.033 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 72.Yoshiji H, Kuriyama S, Noguchi R, Ikenaka Y, Kitade M, Kaji K, Yoshii J, Yanase K, Yamazaki M, Asada K, Tsujimoto T, Akahane T, Uemura M, Fukui H. Angiotensin-II and vascular endothelial growth factor interaction plays an important role in rat liver fibrosis development. Hepatol Res 36: 124–129, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology 34: 745–750, 2001. [DOI] [PubMed] [Google Scholar]
- 74.Yoshiji H, Noguchi R, Ikenaka Y, Namisaki T, Kitade M, Kaji K, Shirai Y, Yoshii J, Yanase K, Yamazaki M, Tsujimoto T, Kawaratani H, Akahane T, Aihara Y, Fukui H. Losartan, an angiotensin-II type 1 receptor blocker, attenuates the liver fibrosis development of non-alcoholic steatohepatitis in the rat. BMC Res Notes 2: 70, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshiji H, Yoshii J, Ikenaka Y, Noguchi R, Tsujinoue H, Nakatani T, Imazu H, Yanase K, Kuriyama S, Fukui H. Inhibition of renin-angiotensin system attenuates liver enzyme-altered preneoplastic lesions and fibrosis development in rats. J Hepatol 37: 22–30, 2002. [DOI] [PubMed] [Google Scholar]
- 76.Zhao J, Liu J, Pang X, Wang S, Wu D, Zhang X, Feng L. Angiotensin II induces C-reactive protein expression via AT1-ROS-MAPK-NF-kappaB signal pathway in hepatocytes. Cell Physiol Biochem 32: 569–580, 2013. [DOI] [PubMed] [Google Scholar]
- 77.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol 6: 499–506, 2004. [DOI] [PubMed] [Google Scholar]