Abstract
Women with polycystic ovary syndrome (PCOS) have hyperandrogenemia and increased prevalence of risk factors for cardiovascular disease, including elevated blood pressure. We recently characterized a hyperandrogenemic female rat (HAF) model of PCOS [chronic dihydrotestosterone (DHT) beginning at 4 wk of age] that exhibits similar characteristics as women with PCOS. In the present studies we tested the hypotheses that the elevated blood pressure in HAF rats is mediated in part by sympathetic activation, renal nerves, and melanocortin-4 receptor (MC4R) activation. Adrenergic blockade with terazosin and propranolol or renal denervation reduced mean arterial pressure (MAP by telemetry) in HAF rats but not controls. Hypothalamic MC4R expression was higher in HAF rats than controls, and central nervous system MC4R antagonism with SHU-9119 (1 nmol/h icv) reduced MAP in HAF rats. Taking a genetic approach, MC4R null and wild-type (WT) female rats were treated with DHT or placebo from 5 to 16 wk of age. MC4R null rats were obese and had higher MAP than WT control rats, and while DHT increased MAP in WT controls, DHT failed to further increase MAP in MC4R null rats. These data suggest that increases in MAP with chronic hyperandrogenemia in female rats are due, in part, to activation of the sympathetic nervous system, renal nerves, and MC4R and may provide novel insights into the mechanisms responsible for hypertension in women with hyperandrogenemia such as PCOS.
Keywords: hypertension, female, dihydrotestosterone, melanocortins, sympathetic nervous systen, renal denervation
polycystic ovary syndrome (PCOS) is the most common reproductive dysfunction, affecting ∼10% of reproductive age women and is characterized by hyperandrogenemia with or without cystic ovaries and oligomenorrhea (2). Women with PCOS often present with cardiovascular risk factors, such as insulin resistance, Type 2 diabetes, hyperlipidemia, and endothelial dysfunction (13). They also have an increased prevalence for elevated blood pressure (16, 18) but are not always treated with antihypertensive drugs since their blood pressure may not reach the levels required by treatment guidelines. However, an increase in mean arterial blood pressure (MAP) of even 5–10 mmHg is significant since normotensive young women typically have arterial blood pressures in the 70–80 mmHg (mean) range, and even small elevations in their blood pressure may predispose them to later cardiovascular disease and target organ damage.
The mechanisms responsible for elevated blood pressure in young women with PCOS are not clear; however, there is evidence that they have sympathetic nervous system activation (21, 24) and renal sympathetic nerve activation. In support of a role for the latter in the elevated blood pressure, radiofrequency renal nerve ablation attenuated hypertension in two women with PCOS, although the procedure was more successful in lowering blood pressure in one than in the other (21).
The mechanisms responsible for sympathetic activation in women with PCOS have not been determined. Some but not all women with PCOS are overweight or even obese (1, 18). Hall and colleagues determined that obesity-related hypertension and the concomitant sympathetic activation are mediated, in part, by the melanocortin-4-receptor (MC4R) in the brain (9, 10, 12). However, the MC4R can contribute to sympathetic activation and hypertension even if the subject is not obese, as shown by da Silva and colleagues in male SHR (8). Thus MC4R activation may play a key role in contributing to sympathetic nervous system activation and hypertension, although its importance in PCOS in still unclear.
Over the past few years, we have been studying a model of hyperandrogenemia in female rats (HAF) first described by Manneras and colleagues (19) that mimics many of the characteristics of women with PCOS (26). In this model, female normotensive rats are treated with dihydrotestosterone (DHT, 7.5 mg pellet/90 d) from the age of 3–4 wk. We found that this dose of DHT in female Sprague-Dawley (SD) rats caused an approximately three- to fourfold increase in plasma DHT, with normal to slightly decreased estradiol levels compared with placebo-treated controls. The model also exhibits many of the metabolic and cardiovascular characteristics found in women with PCOS (19, 26). For example, HAF rats are hyperphagic compared with placebo-treated controls (∼3 g of food/day) and gain weight and at 14–16 wk of age HAF rats are ∼20–30% heavier than placebo controls (26). In addition, HAF rats exhibit increases in plasma cholesterol and insulin and have abnormal oral glucose tolerance tests (19, 26). They also have ∼10 mmHg higher blood pressure than placebo controls and reductions in glomerular filtration rate (26).
In the present study we tested the hypotheses that both the sympathetic nervous system and the renal sympathetic nervous system contributes to the hypertension in our HAF rats model, and that sympathetic nervous system activation could be due to activation of MC4R.
METHODS
Animal models.
Female SD rats were obtained from the vendor (Harlan Sprague-Dawley, Indianapolis, IN) at 3 wk of age. MC4R−/− rats on a Wistar background and Wistar wild-type rats (WT) were obtained at 4–6 wk of age from the colony maintained by Drs. Frank Spradley and Joey Granger at the University of Mississippi Medical Center (22). The MC4R−/− was originally bred by Transposagen and exhibits hyperphagia, obesity, hyperinsulinemia, and elevated leptin levels. WT rats are Wistar Hannover strain.
Rats were maintained throughout the studies on standard rat chow (Harlan 8640, Harlan SD, Indianapolis, IN) and housed in temperature-controlled rooms with a constant light-dark cycle (12 h/12 h) and free access to water. At 4–6 wk of age, rats were implanted subcutaneously on the back of the neck with nonaromatizable, continuous-release DHT (7.5 mg/90 days; daily dose = 83 μg; Innovative Research, Sarasota, FL) or placebo pellets (Innovative Research) (26). Rats were allowed to age to 12–16 wk before study. These HAF rats were compared with their placebo control SD counterparts, termed “controls.” Female MC4R−/− and WT rats were also treated with DHT, and termed “DHT-treated” or “control” rats. The protocols complied with the Guidelines for the Care and Use of Laboratory Animals by the National Institutes of Health and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Measurement of adipose tissue distribution by computed tomography.
At 14–16 wk of age, rats (n = 4/group) were individually placed in the prone position inside cardboard boxes and imaged in the prone position using a Siemens Sensation 64 CT scanner. The field of view was made as small as possible in the X–Y direction (∼10 cm) and included the whole body (nose through the hindfeet) in the Z direction. A modification of a clinical abdominal protocol was selected and included the following parameters: helical mode, pitch 1.0, 120 kVp, 400 mAs, 1 s gantry rotation, 0.6 mm collimator, 0.6 mm axial slice thickness, 0.6 mm axial slice interval, 512 × 512 pixel matrix, and B50s (medium sharp) reconstruction algorithm. The estimated voxel size was 0.2 mm by 0.2 mm in the X–Y plane and 0.6 mm in the Z direction. For measurement of abdominal fat, each rat was scanned twice. Five sequential 0.6-mm slices (3 mm slab) located immediately cranial to the tip of the iliac crests were loaded into the Phillips Brilliance three-dimensional software viewer. Total abdominal fat (TAF) was selected using Hounsfield Unit (HU) threshold technique assuming that fat attenuation is between −30 and −190 HU. The subcutaneous abdominal fat (SAF) and visceral abdominal fat (VAF) compartments were separated by a region of interest (ROI) drawn along the abdominal wall musculature as an anatomic landmark separating the two compartments. TAF, VAF, and SAF volumes were measured using the two separate image data sets for each rat, and the mean of each compartment was used for comparison between rats. Data are presented as means ± SE with units: mm3 factored for body weight (g) or femur length (cm).
Measurement of mean arterial blood pressure.
While under gas anesthesia with isofluorane (Mallinckrodt Veterinary, Hazelwood, CA) and with aseptic technique, HAF rats and controls were implanted at 14 wk of age with radiotelemetry transmitters (TA11PA-C40; Data Sciences International, St. Paul, MN) into the abdominal aorta below the renal arteries, as we previously described (20, 26). Rats were placed into individual cages above a receiver (RLA-3000) and allowed 2 wk of recovery. Mean arterial pressure (MAP) measurements were obtained during a 10-s sampling period (500 Hz) and recorded and averaged every 5 min for 24 h per day. Data are presented as means ± SE and averaged for each group of rats.
MAP in HAF rats treated with α1,β1,2-adrenergic receptor antagonists.
HAF rats and controls, aged 14 wk (n = 5/group), were implanted with telemetry transmitters as described above. After 2 wk recovery period, baseline MAP was measured 24 h per day for 5 days. Then rats were given terazosin (10 mg·kg−1·day−1 sc) and propranolol (10 mg·kg−1·day−1 sc) for 6 days, and MAP was measured. Data were averaged for the group of rats and are presented as means ± SE.
MAP in HAF rats subjected to renal denervation.
Renal denervation or sham surgery was performed in HAF and control rats (n = 5–6/group), as we have previously described (15). Telemetry transmitters were also implanted at the same time. After 2 wk recovery, MAP was measured for 5 days and averaged for each group. Data are presented means ± SE.
Treatment of HAF rats with MC4R antagonist SHU-9119.
HAF (n = 6) and control rats (n = 6) were implanted with radiotelemetry transmitters using isoflurane anesthesia, as described below. After transmitter implantation, intracerebroventricular cannulas were implanted into the right lateral cerebral ventricle, as previously described (20). Cannula placement accuracy was tested by measuring the dipsogenic response (immediate drinking of at least 5 ml of water in 10 min) to an intracerebroventricular injection of 100 ng of angiotensin II.
After 2 wk recovery, MAP was measured during a 5-day baseline period, and then an osmotic minipump (Alzet) was implanted subcutaneously in the scapular region and connected to the intracerebroventricular cannula using polyethylene tubing for infusion of the MC3/4R antagonist SHU-9119 (1 nmol/h in saline, 0.5 μl/h icv) for 5 days. The dose of SHU-9119 was based on our previous studies showing that this dose effectively blocks MC3/4R and markedly increases food intake (9, 10, 12, 20). During SHU-9119 infusion, HAF and control rats were food restricted to the level of diet eaten during baseline blood pressure measurements. Body weights were measured throughout baseline and treatment periods and were similar during SHU-9119 infusion and baseline.
MAP in DHT-treated female MC4R−/− and WT rats.
Female MC4R−/− and WT rats were implanted at 5–6 wk with DHT or placebo pellets (“controls”) (n = 5/group), as described in Animal models. At 14 wk of age, rats were implanted with radiotelemeters, as described in Measurement of mean arterial blood pressure, and 2 wk later, MAP was measured for 7 days. Data are presented as means ± SE over the 7 days. At the end of the study, blood samples were drawn for DHT levels.
Measurement of plasma DHT.
Plasma DHT was measured in all HAF and control rats, using a commercially available radioimmunoassay kit (Diagnostic Systems Laboratories, Webster, TX), as we previously described (26). Data are only presented for MC4R null and WT rats since we have previously shown that DHT levels are increased by three- to fourfold in SD rats.
Western blot for MC4R.
Protein levels of MC4R were evaluated in hypothalamic homogenates from HAF and control rats (n = 3/group) by Western blot analysis, using goat anti-MC4R (R-19) antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA) and donkey anti-goat IgG-HRP-sc2020 secondary antibodies (1:2,000; Santa Cruz Biotechnology). Rabbit anti-GAPDH antibody served as a loading control (1:30,000; Sigma-Aldrich) with donkey anti-rabbit IgG as secondary antibody (1:60,000; Sigma-Aldrich).
Statistical analyses.
All data are expressed as means ± SE. Data were analyzed by Student's t-test (for two groups) or two-way analysis of variance (ANOVA) with Dunnett's post hoc test. Differences were considered statistically significant at P < 0.05. Statistical analyses were performed with SigmaStat software package version 3.1 (Systat Software, San Jose, CA).
RESULTS
Measurement of adipose tissue distribution by computed tomography.
As shown in Table 1, body weight was higher in HAF than control rats. Regardless of whether factored for body weight or femur length, SAF was higher in HAF than control rats. TAF was only higher in HAF rats when factored for femur length. VAF was not different between the groups.
Table 1.
Body weights, TAF, SAF, and VAF measured by computed tomography and factored for body weight or femur length in HAF rats and placebo controls, aged 12 wk
| BW, g | TAF, mm3/g | SAF, mm3/g | VAF, mm3/g | |
|---|---|---|---|---|
|
Fat mass factored for body weight | ||||
| Placebo | 230.6 ± 4.0 | 4.33 ± 0.38 | 1.52 ± 0.11 | 2.69 ± 0.31† |
| HAF | 329.0 ± 11.3* | 4.47 ± 0.27 | 2.16 ± 0.16* | 2.21 ± 0.11 |
| Femur length, cm | TAF, mm3/cm | SAF, mm3/cm | VAF, mm3/cm | |
|---|---|---|---|---|
|
Fat mass factored for femur length | ||||
| Placebo | 3.47 ± 0.11 | 288.5 ± 26.8 | 101.1 ± 7.86 | 179.1 ± 21.0† |
| HAF | 3.58 ± 0.09 | 409.4 ± 23.5* | 197.3 ± 9.78* | 203.4 ± 17.1 |
Values are means ± SE; n = 4 rats/group. HAF, hyperandrogenemic female; BW, body weight; TAF, total abdominal fat; SAF, subcutaneous abdominal fat; VAF, visceral abdominal fat.
P < 0.05, HAF vs. placebo.
P < 0.05, VAF vs. SAF in the same group.
Effect of adrenergic blockade on MAP in HAF rats.
As shown in Fig. 1, treatment of HAF rats with α1,β1,2-adrenergic antagonists terazosin and propranolol reduced the MAP by 14–18 mmHg compared with baseline levels. There was no effect of adrenergic blockers in controls (data not shown).
Fig. 1.
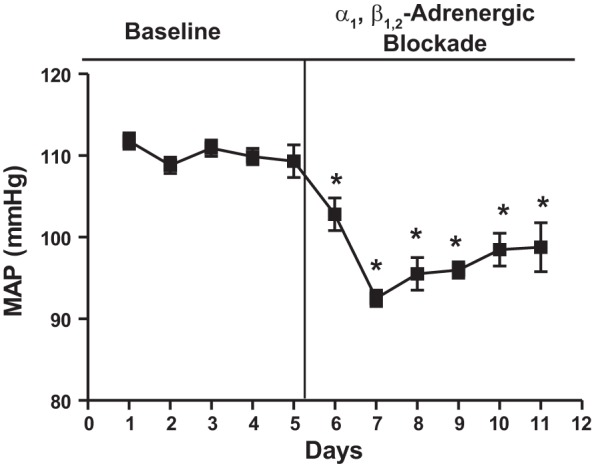
Adrenergic blockade reduced mean arterial pressure (MAP) in hyperandrogenemic female (HAF) rats. HAF rats were treated with α1,β1,2-adrenergic blockers terazosin and propranolol as described in methods, and MAP was measured by telemetry. *P < 0.05 compared with baseline MAP.
Effect of renal denervation on HAF and control rats.
As shown in Fig. 2, MAP was higher in sham HAF rats than control sham rats (101 ± 4 vs. 120 ± 6 mmHg, P < 0.05), and renal denervation reduced MAP in HAF rats to the same level as treated or untreated controls but had no effect in control rats.
Fig. 2.
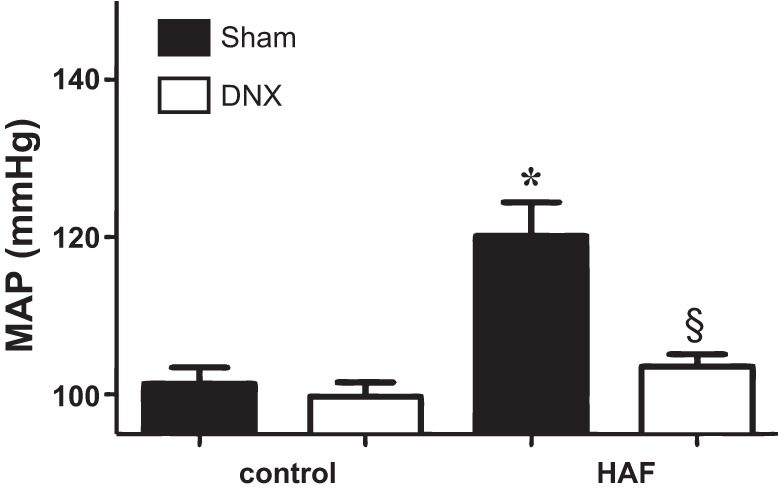
Renal denervation (DNX) decreased MAP in HAF rats. HAF and control rats were renally denervated and implanted with telemetry probes as described in methods. After 2 wk recovery, MAP was measured in both groups. *P < 0.05 HAF compared with control rats; §denervated HAF rats compared with sham HAF rats.
Measurement of MC4R.
As shown in Fig. 3, MC4R expression in the hypothalamus was twofold higher in HAF rats than in controls.
Fig. 3.
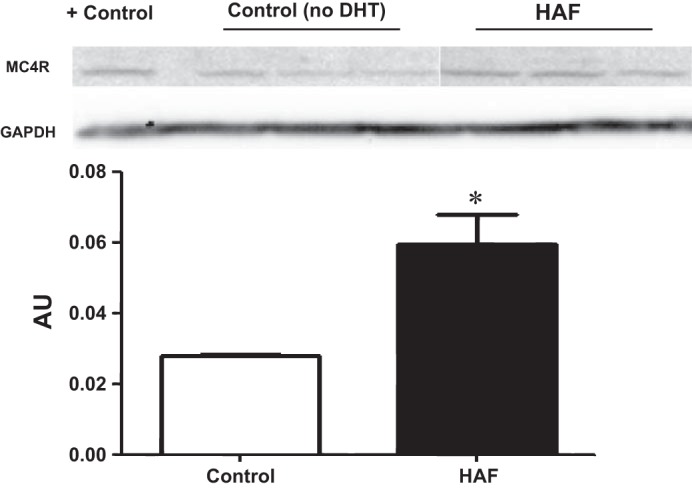
HAF rats exhibited higher protein levels of melanocortin-4 receptor (MC4R) in the hypothalamus than control rats. Western blot analyses were performed as described in methods. *P < 0.05 HAF rats compared with control rats.
Treatment of HAF and placebo controls with MC4R antagonist SHU-9119.
In the baseline period, HAF rats had higher MAP than controls (Fig. 4) (112 ± 1 vs. 105 ± 2, P < 0.05), and SHU-9119 decreased MAP in HAF rats to 94 ± 4 mmHg but had no effect in SHU-9119 treated control rats. Heart rate was higher in control rats than HAF rats during baseline (375 ± 6 vs. 351 ± 6 beats/min, P < 0.05). SHU-9119 reduced heart rate in both groups to similar levels (339 ± 12 vs. 335 ± 9 beats/min, P = NS; P < 0.05 compared with baseline for both groups).
Fig. 4.
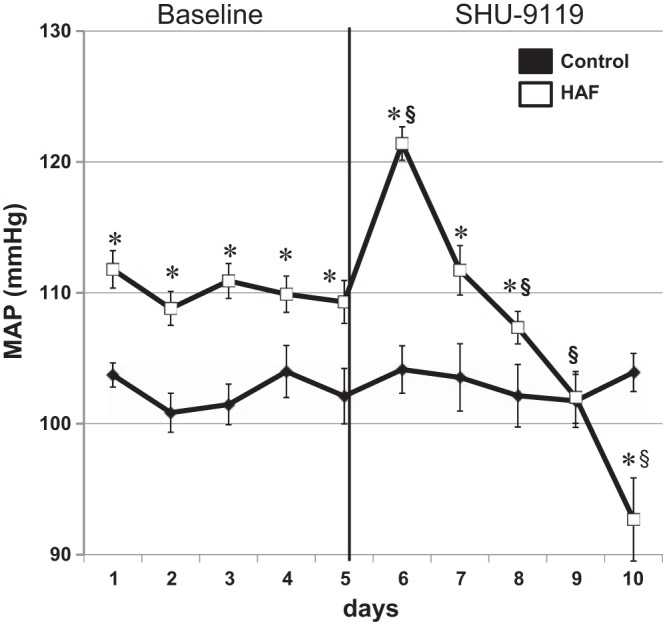
SHU-9119, an MC4R antagonist, reduced MAP in HAF rats. HAF and control rats were implanted with telemetry and intracerebroventricular cannulas. After 2 wk recovery, baseline MAP was measured for 5 days, and then SHU-9119 was given intracerebroventricularly via osmotic minipump in both HAF and control rats for 5 days, and MAP was measured, as described in methods. *P < 0.05 compared with controls; §P < 0.05 compared with baseline period in the same group.
Effect of DHT on MAP in MC4R−/− and WT controls.
DHT treatment increased plasma DHT levels by approximately fivefold in both WT rats and MC4R−/− rats (DHT-treated WT: 20.7 ± 3.2; control WT: 4.4 ± 1.1 ng/dl; DHT-treated MC4R−/−: 26.7 ± 2.2; control MC4R−/−: 5.4 ± 1.6 ng/dl; P < 0.01 for DHT-treated rats vs. control rats). Body weights were significantly higher in untreated MC4R−/− rats compared with WT controls (412.3 ± 14.3 vs. 278.7 ± 12.6 g) and increased with chronic DHT treatment in both MC4R−/− and WT rats. DHT in WT rats increased body weights by ∼42% (394.6 ± 31.3 g), but in MC4R−/− rats, body weights only increased by 13% with DHT (466.5 ± 15.9 g) (Fig. 5A). As shown in Fig. 5B, MAP was higher in control MC4R−/− than control WT rats (106 ± 1 vs. 97 ± 1 mmHg, P < 0.01). DHT significantly increased MAP in female WT rats (105 ± 1 mmHg, P < 0.05), whereas DHT had no effect on MAP in female MC4R−/− rats (105 ± 1 mmHg; P = NS vs. untreated MC4R−/−).
Fig. 5.
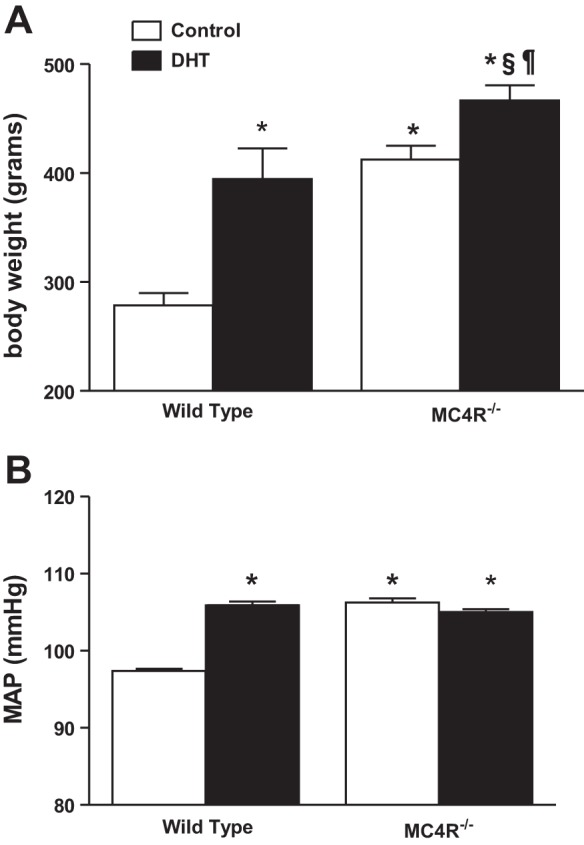
Dihydrotestosterone (DHT) increased MAP in wild-type (WT) but not MC4R−/− rats. WT and MC4R−/− rats were treated with DHT, as described in methods, beginning at 5–6 wk of age. Controls received placebo pellets. At 14 wk of age, rats were implanted with radiotelemeters. After 2 wk, MAP was measured continuously for 8 days, and the data were averaged. A: body weights. B: MAP averaged over 8 days. *P < 0.05 compared with WT control; §P < 0.05 compared with DHT-treated WT; ¶P < 0.05 compared with untreated MC4R−/−.
DISCUSSION
In the present studies we found that: 1) HAF rats have increased subcutaneous abdominal fat compared with control females; 2) adrenergic blockade normalizes MAP in HAF rats; 3) renal denervation normalizes MAP in HAF rats but has no effect on MAP in controls; 4) hypothalamic MC4R expression is increased in HAF rats compared with controls, and the MC4R antagonist SHU-9119 reduces MAP and heart rate in HAF rats; and 5) in the absence of an active MC4R in MC4R null rats, DHT does not increase blood pressure. Taken together, these data suggest that sympathetic activation, renal nerve activation, and MC4R contribute to the elevation in blood pressure that occurs in HAF rats.
We previously showed that DHT in females causes an increase in food intake of ∼3 g per day and leads to an increase in body weight by 14–16 wk of age (26). In the present study we determined the distribution of adipose tissue in HAF rats using computed tomography. Body weight was increased by 40%, but femur length was not different in HAF; only the subcutaneous adipose fat levels were different from controls, not the visceral fat. This is one difference between our rat model and women with PCOS who have an increase in abdominal fat (2, 18). It is also possible that skeletal muscle may be increased in the HAF rats due to the androgen influence but we have not measured this.
The fact that adrenergic blockade and renal denervation normalized MAP in HAF rats suggests that the sympathetic and renal nervous systems contribute to the increase in MAP in HAF rats. As noted in preliminary reports by Schlaich and colleagues (21), renal nerve ablation in women with PCOS also reduced their blood pressure, suggesting that the renal nerves contribute to the elevated blood pressure in women with PCOS as well.
In the present study we found that blockade of MC4R with SHU-9119 in female rats reduced MAP. In addition, we found that DHT treatment in MC4R−/− rats failed to increase blood pressure as found in DHT-treated WT rats. These data suggest that the elevated blood pressure in DHT-treated rats is mediated at least in part by the MC4R. MC4Rs are activated by increases in α-melanocyte stimulating hormone (α-MSH), the neurotransmitter released by activation of pro-opiomelanocortin (POMC) neurons. Hall and colleagues reported that hypertension is mediated, at least in part, by sympathetic activation that is due to MC4R in two distinctive models, male obese rats and male spontaneously hypertensive rats (SHR) that are not obese (9, 10, 12). MC4R antagonism, however, did not decrease blood pressure in lean female SHR, regardless of age, whereas MAP in aged male SHR was significantly decreased with SHU-9119 just as in young males (20). The fact that SHU-9119 attenuated the modest hypertension that occurs with androgens in our HAF model suggests that the MC4R may mediate the sympathetic activation. Perhaps the difference between the SHR and the HAF rats is that the HAF rats exhibit an increase in MC4R expression in the hypothalamus and perhaps the SHR females do not although this hypothesis remains to be tested in female SHR. Elevated androgens have been shown previously to increase gene expression of pro-opiomelanocortin, the precursor for α-MSH (the ligand for MC4R) in monkeys and rats (1, 6), which would in turn activate MC4R.
However, the effect of androgens on POMC-MC4R system is controversial. Adams and colleagues (1) reported that testosterone upregulated POMC gene expression in nonhuman primates, and that castration reduced POMC mRNA levels in the arcuate nucleus. Earlier, these investigators showed that the testosterone-mediated increase in POMC mRNA in rats affected very few of the POMC neurons and only in the more caudal portion of the arcuate nucleus and not the dorsal portion (6). However, in a later study, Fodor and Delemarre-van de Waal (11) found androgen receptors on only 3% of POMC neurons in the arcuate nucleus. Lindblom and colleagues (17) reported that the anabolic androgenic steroid nandrolone decanoate actually reduced expression of POMC mRNA expression in the hypothalamus. However, whether and how POMC precursor actually contributes to changes in α-MSH is not clear. Cakir and colleagues (4) reported that with diet-induced obesity, α-MSH levels are reduced, while POMC levels are unchanged due to endoplasmic reticulum stress that inhibits posttranslational processing of POMC to α-MSH. Another caveat for all of these studies is that they were performed in male animals; thus whether similar effects of androgens would occur in females as in males is not clear. For example, we found previously in obese male Zucker rats that are androgen deficient, androgen supplementation caused weight loss, improvement in insulin resistance, and reductions in plasma cytokines (7). In the HAF rats, androgens cause an increase in food intake, weight increase, insulin resistance, and increase in plasma cytokines (26). The only effect that we have found that is common to both males and females treated with androgens is an increase in blood pressure (7, 26). In addition, whether androgens increase hypothalamic MC4R expression in obese male Zucker rats, as we found in the HAF rats, has not, to our knowledge, been reported. Thus whether MC4R and POMC neurons are similarly affected in males and females in response to androgens needs further study.
Another finding of our studies is that female MC4R−/− rats had higher blood pressure than did WT females. Spradley and colleagues (22) reported that blood pressure as measured by carotid arterial catheter in conscious, restrained rats was not significantly different between female MC4R+/− and WT rats. These investigators did not evaluate blood pressure in female MC4R−/− rats however. However, our present data are different from those published by Stepp and colleagues (23) who found that there was no difference in blood pressure between male MC4R−/− and WT, as measured by radiotelemetry, thus suggesting a sex difference in blood pressure in these genetically modified rats.
The hyperandrogenemic female rat has limitations as a model for PCOS in women and not all the characteristics present in women with PCOS are present in our model, as noted above with the lack of visceral adiposity in our model. However, most of the characteristics of PCOS that are important for the present study, such as insulin resistance, hyperlipidemia, and abnormal estrous cycling, do occur in our model. In addition, while the model is developed using exogenous DHT because DHT cannot be converted to estradiol, the DHT levels are low enough that endogenous steroid production is intact and estradiol levels remain normal (26), as in women with PCOS. The HAF rats do exhibit an abnormal estrous cycle, however, with a 6-day cycle rather than a typical 4-day cycle (21). Increased androgen levels in women with PCOS are directly associated with increased sympathetic activity (21, 24), and likely hypertension, just as in our model. Thus the model is relevant for studies into potential mechanisms for the elevated blood pressure.
Perspectives and Significance
PCOS is a syndrome in which reproductive age women have increased androgens, fertility difficulty, and increased risk factors for cardiovascular disease, including elevated blood pressure. The mechanisms responsible for the elevated blood pressure are unclear, but sympathetic activation has been implicated, although the mechanism(s) for sympathetic activation in women with PCOS is not clear. In the present study we found that MC4R antagonism or genetic MC4R deficiency attenuates the elevated blood pressure in our novel rat model of hyperandrogenemia. In addition to the implications of our study for women with PCOS, the model may also be important in evaluating potential mechanisms of cardiovascular disease in transgender individuals who take high doses of androgens for masculinization and also have a high incidence of PCOS (3). Androgens are elevated in many postmenopausal women (23), and hypertension in postmenopausal women is often associated with increased sympathetic activity (13, 14, 25). The model thus provides a means to study potential therapeutic targets that may be useful in treatment of hypertension in hyperandrogenemic women in general.
GRANTS
These studies were supported by National Heart, Lung, and Blood Institute Grants P01 HL51971 (to J. E. Hall, J. P. Granger, and J. F. Reckelhoff), R01 HL66072 (to J. F. Reckelhoff), and National Research Service Award HL105324 (to F. T. Spradley); National Institute of General Medical Sciences Grant P20GM104357 (to J. E. Hall and J. M. do Carmo); National American Heart Association Scientist Development Grant 11DG5680016 (to J. M. do Carmo); and Southeast Affiliate, American Heart Association Postdoctoral Fellowship Awards [14POST18640015 (to R. Maranon), 11POST6960000 (to R. Lima), 11POST7450006 to (M. Moulana)].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.O.M., R.L., F.T.S., H.Z., A.S., M.M., J.E.H., J.P.G., and J.F.R. conception and design of research; R.O.M., R.L., F.T.S., J.M.d.C., H.Z., A.S., E.B., L.T., M.M., and J.F.R. performed experiments; R.O.M., R.L., F.T.S., J.M.d.C., H.Z., A.S., E.B., L.T., M.M., J.E.H., J.P.G., and J.F.R. analyzed data; R.O.M., R.L., F.T.S., J.M.d.C., H.Z., A.S., E.B., L.T., M.M., J.E.H., J.P.G., and J.F.R. interpreted results of experiments; R.O.M., R.L., and J.F.R. prepared figures; R.O.M., R.L., J.E.H., J.P.G., and J.F.R. drafted manuscript; R.O.M., R.L., F.T.S., J.M.d.C., H.Z., A.S., E.B., L.T., M.M., J.E.H., J.P.G., and J.F.R. edited and revised manuscript; R.O.M., R.L., F.T.S., J.M.d.C., H.Z., A.S., E.B., L.T., M.M., J.E.H., J.P.G., and J.F.R. approved final version of manuscript.
REFERENCES
- 1.Adams LA, Vivian L, Clifton DK, Steiner RA. Testosterone regulates pro-opiomelanocortin gene expression in primate brain. Endocrinology 128: 1881–1886, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Jannsen O, Legro R, Normal R, Taylor A, Witchel S. Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and Society. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91: 456–488, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Baba T, Endo T, Honnma H, Kitajima Y, Hayashi Ikeda H, Masumori N, Kamiya H, Moriwaka O, Saito T. Association between PCOS and female-male transsexuality. Hum Reprod 22: 1011–1016, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Cakir I, Cyr NE, Perello M, Litvinov BP, Romero A, Stuart RC, Nillni EA. Obesity induces hypothalamic endoplasmic reticulum stress and impairs proopiomelanocortin (POMC) post-translational processing. J Biol Chem 288: 17675–17688, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension 49: 1442–1447, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Chowen-Breed JA, Clifton DK, Steiner RA. Regional specificity of testosterone regulation of proopiomelanocortin gene expression in arcuate nucleus of the male rat. Endocrinology 124: 2875–2881, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Davis DD, Lopez Ruiz A, Yanes LL, Iliescu R, Yuan K, Moulana M, Racusen LC, Reckelhoff JF. Testosterone supplementation in male obese Zucker rats reduces body weight and improves insulin sensitivity but increases blood pressure. Hypertension 59: 726–731, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Silva AA, do Carmo JM, Kanyicska B, Dubinion J, Brandon E, Hall JE. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension 51: 884–890, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.do Carmo JM, Da Silva AA, Rushing JS, Pace B, Hall JE. Differential control of metabolic and cardiovascular functions by melanocortin 4 receptors in proopiomelanocortin neurons. Am J Physiol Regul Integr Comp Physiol 305: R359–R368, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.do Carmo JM, da Silva AA, Dubinion J, Sessums PO, Ebaady SH, Wang Z, Hall JE. Control of metabolic and cardiovascular function by leptin-brain melanocortin pathway. IUBMB Life 65: 692–698, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fodor M, Delemarre-van de Waal HA. Are POMC neurons target for sex steroids in the arcuate nucleus of the rat? Neuroreport 12: 3989–3991, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Hall JE, DaSilva AA, DoCarmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 285: 17271–17276, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart EC, Charkoudian N. Sympathetic neural regulation of blood pressure: influences of sex and aging. Physiology 29: 8–15, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Hogarth AJ, Graham LN, Corrigan JH, Deuchars J, Mary DA, Greenwood JP. Sympathetic nerve hyperactivity and its effect in postmenopausal women. J Hypertens 29: 2167–2175, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Iliescu R, Yanes LL, Bell W, Dwyer T, Baltatu OC, Reckelhoff JF. Role of the renal nerves in blood pressure in male and female SHR. Am J Physiol Regul Integr Comp Physiol 290: R341–R344, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Kargili A, Karakurt F, Kasapoglu B, Derbent A, Koca C, Selcoki Y. Association of polycystic ovary syndrome and a non-dipping blood pressure pattern in young women. CLINICS 65: 475–479, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindblom J, Kindlundh AM, Nyberg F, Berstron L, Wikberg JE. Anabolic androgenic steroid nandrolone decanoate reduces hypothalamic proopiomelanocortin mRNA levels. Brain Res 986: 139–147, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Luque-Ramirez M, Marti D, Fernande-Duran E, Alpanes M, Alvarez-Blosco F, Escobar-Morreale HF. Office blood pressure, ambulatory blood pressure monitoring and echocardiography abnormalities in women with polycystic ovary syndrome: role of obesity and androgen excess. Hypertension 63: 624–629, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Manneras L, Cajander S, Holmang A, Seleskovic Z, Lystig T, Lonn M, Steiner-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of PCOS. Endocrinology 148: 3781–3791, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Maranon RO, Lima R, Mathbout M, do Carmo JM, Hall JE, Roman RJ, Reckelhoff JF. Postmenopausal hypertension: role of the sympathetic nervous system in an animal model. Am J Physiol Regul Integr Comp Physiol 306: R248–R256, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlaich MP, Straznicky N, Grima M, Ika-Sari C, Dawood T, Mahfoud F, Lambert E, Chopra R, Socratous F, Hennebry S, Eikelis N, Böhm M, Krum H, Lambert G, Esler MD, Sobotka PA. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens 29: 991–996, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Spradley FT, Palei AC, Granger JP. Obese melanocortin-4 receptor-deficient rats exhibit augmented angiogenic balance and vasorelaxation during pregnancy. Physiol Rep 1: e00081, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stepp DW, Osakwe CC, de Chantemele EJ, Mintz JD. Vascular effects of deletion of melanocortin-4 receptors in rats. Physiol Rep 1: e00146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sverrisdottir YB, Mogren T, Ktaoka J, Janson PO, Stener-Victorin E. Is polycystic ovary syndrome associated with high sympathetic activity and size at birth? Am J Physiol Endocrinol Metab 294: E576–E581, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Yanes LL, Reckelhoff JF. Postmenopausal hypertension. Am J Hypertens 24: 740–749, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanes LL, Romero DG, Moulana M, Lima R, Davis DD, Zhang H, Lockhart R, Reckelhoff JF. Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gender Med 8: 103–115, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


