Abstract
In many neural networks, mechanisms of compensatory plasticity respond to prolonged reductions in neural activity by increasing cellular excitability or synaptic strength. In the respiratory control system, a prolonged reduction in synaptic inputs to the phrenic motor pool elicits a TNF-α- and atypical PKC-dependent form of spinal plasticity known as inactivity-induced phrenic motor facilitation (iPMF). Although iPMF may be elicited by a prolonged reduction in respiratory neural activity, iPMF is more efficiently induced when reduced respiratory neural activity (neural apnea) occurs intermittently. Mechanisms giving rise to iPMF following intermittent neural apnea are unknown. The purpose of this study was to test the hypothesis that iPMF following intermittent reductions in respiratory neural activity requires spinal TNF-α and aPKC. Phrenic motor output was recorded in anesthetized and ventilated rats exposed to brief intermittent (5, ∼1.25 min), brief sustained (∼6.25 min), or prolonged sustained (30 min) neural apnea. iPMF was elicited following brief intermittent and prolonged sustained neural apnea, but not following brief sustained neural apnea. Unlike iPMF following prolonged neural apnea, spinal TNF-α was not required to initiate iPMF during intermittent neural apnea; however, aPKC was still required for its stabilization. These results suggest that different patterns of respiratory neural activity induce iPMF through distinct cellular mechanisms but ultimately converge on a similar downstream pathway. Understanding the diverse cellular mechanisms that give rise to inactivity-induced respiratory plasticity may lead to development of novel therapeutic strategies to treat devastating respiratory control disorders when endogenous compensatory mechanisms fail.
Keywords: inactivity, phrenic, plasticity, TNF-α, atypical PKC
an emerging principle of neuroscience is that prolonged perturbations in neural activity evoke compensatory mechanisms of plasticity that act to correct that perturbation (7, 26, 27, 40, 43); such “homeostatic plasticity” is thought to maintain neuronal activity within physiologically appropriate levels. Although mechanisms that stabilize neuronal activity levels in response to prolonged changes in neuronal activity have been relatively well studied in the cortex (41, 44) and hippocampus (9, 19), little is known regarding the role of ongoing activity in shaping the output of neurons in the respiratory control system—a system in which preservation of neural activity is paramount. We recently demonstrated that a prolonged reduction in respiratory neural activity (i.e., a neural apnea), even in the absence of hypoxia or hypercapnia, elicits a rebound increase in phrenic motor output, a form of plasticity known as inactivity-induced phrenic motor facilitation (iPMF) (2, 4, 6, 21, 35, 36, 38). Mechanisms local to the phrenic motor pool sense and respond to reduced respiratory neural activity to give rise to iPMF since a unilateral withdrawal of spinal synaptic inputs to the phrenic motor pool (while maintaining brain stem and contralateral phrenic respiratory neural activity) elicits ipsilateral but not contralateral iPMF (35). Evidence indicates that iPMF consists of an early, transient phase that stabilizes into a long-lasting increase in phrenic motor output by a TNF-α-induced increase in activation of an atypical protein kinase C (aPKC) isoform known as PKCζ (6, 38). However, mechanisms regulating the iPMF phenotype following prolonged neural apnea vary with genetic/epigenetic background, since in some rat substrains, iPMF is long-lasting (2, 6, 38), while in others, phrenic amplitude is only transiently increased due to a N-methyl d-aspartate (NMDA) receptor-dependent constraint on the stabilization of long-lasting iPMF (36). While a physiological role for iPMF has not yet been directly shown, mechanisms allowing phrenic motor neurons to compensate for abnormal changes in activity may function to preserve an optimal level of activity, preventing catastrophic decreases in respiratory motor output (37).
Although it is rare to experience a single prolonged period of reduced respiratory neural activity, brief intermittent neural apneas are comparatively common during a variety of physiological and pathophysiological conditions that can occur throughout development and aging (37), such as apnea of prematurity (11), central sleep apnea (12, 15), and chronic heart failure (31). We recently demonstrated that brief intermittent disruptions in respiratory neural activity more efficiently induce iPMF than sustained patterns (2); however, cellular mechanisms giving rise to iPMF have only been investigated following a prolonged neural apnea. The purpose of this study was to test the hypothesis that intermittent neural apnea elicits long-lasting iPMF via spinal TNF-α and aPKC-dependent mechanisms. Our results reveal that distinct mechanisms initiate and regulate expression of inactivity-induced phrenic plasticity in response to different patterns of reduced respiratory activity, but that similar downstream mechanisms function to stabilize it into its long-lasting form. An understanding of the diverse cellular mechanisms giving rise to inactivity-induced respiratory plasticity under varied stimulus patterns may lead to the development of novel treatment and/or prevention strategies for devastating respiratory control disorders associated with reduced respiratory neural activity, such as spinal cord injury (sustained) and central sleep apnea (intermittent) (37).
METHODS
Animals.
Experiments were performed on 2.5–3.5-mo-old male Sprague-Dawley rats from Charles River Laboratories (colony P09; n = 62). Rats were housed two per cage with a 12:12-h light-dark cycle and food and water provided ad libitum. All experimental protocols were approved by the Animal Care and Use Committee at the University of Wisconsin, Madison.
Experimental preparation.
Isoflurane anesthesia was induced in a closed container and then continued on a nose cone with 2.5–3.5% isoflurane (50% O2-N2 balance) flowing from a vaporizer. Body temperature was monitored with a rectal thermometer (Physitemp, model 700 1H) and maintained near 37.0°C with a custom heated surgery table. A tail vein catheter was placed for delivery of fluids (lactated Ringer solution; ∼20% sodium bicarbonate, as necessary) to maintain blood pressure and pH homeostasis throughout surgery and experimental protocols. The trachea was cannulated, and mechanical ventilation was begun (model no. 683; Harvard Apparatus, Holliston MA; ∼70 breaths/min, 2.5–3 ml; 50% O2-N2 balance). End-tidal CO2 (ETCO2) was measured continuously from the expired line of the ventilator circuit as an index of arterial Pco2 with a flow-through capnograph (Capnogard, Respironics). In preliminary studies, we found that under our anesthesia protocol, spontaneously breathing rats typically have an ETCO2 of ∼45 mmHg. Thus, CO2 was added to the inspired gas mixture to maintain ETCO2 near ∼45 mmHg to avoid unintended neural apnea during mechanical ventilation. Tracheal pressure was monitored, and inspired CO2 was adjusted to ensure rats continued respiratory efforts throughout the surgery. The vagus nerve was isolated and cut bilaterally at the cervical level to prevent entrainment of respiratory frequency to the ventilator. The right femoral artery was isolated and catheterized to monitor arterial blood pressure and draw blood samples (∼0.3 ml) for pH and blood-gas analysis (ABL800; Radiometer). Rats were then transferred to urethane anesthesia (1 ml/100 g of 0.175 g/ml urethane infused at 6 ml/h iv), and isoflurane was gradually withdrawn. Pressor responses to paw pad-pinch were tested to confirm adequate depth of anesthesia. The left phrenic nerve was dissected via a dorsal approach, cut distally, desheathed, placed on bipolar silver electrodes, and submerged in mineral oil. In some rats, a lamenectomy was performed at cervical vertebrae 2 (C2), a small hole was cut in the dura, and a silicone catheter (2 French; Access Technologies) was connected to a 50-μl syringe (Hamilton) was inserted into the intrathecal space and advanced caudally (∼5 mm) to rest above spinal segment C4. Pancuronium bromide was infused (1 mg/kg iv) to induce neuromuscular paralysis.
Experimental protocols.
At least 1 h following termination of isoflurane anesthesia, “baseline” was established when the phrenic nerve burst amplitude and frequency remained stable under isocapnic conditions for at least 15–20 min. An arterial blood sample was drawn to obtain baseline Pco2, Po2, and pH measurements (temperature-corrected).
Rats were then exposed to one of three patterns of reduced respiratory neural activity: five brief intermittent neural apneas (∼1.25 min each, separated by ∼5 min; n = 9), a single brief “massed” neural apnea of a similar cumulative duration (∼6.25 min; n = 9), or a single prolonged neural apnea (∼30 min; n = 9). A fourth group received a similar surgical preparation, but no neural apnea to account for any time-dependent changes in neural activity under isocapnic conditions (time controls; n = 5). Neural apnea was induced by reducing inspired CO2 until all rhythmic phrenic activity ceased (CO2 apneic threshold). In rats receiving brief intermittent neural apnea, inspired CO2 was then immediately increased until respiratory neural activity resumed (CO2 recruitment threshold) and baseline ETCO2 was restored. In rats receiving brief massed or prolonged neural apnea, ETCO2 was maintained ∼2 mmHg below the CO2 apneic threshold for 6 or 30 min, respectively, at which point, neural apnea was reversed, as described above. Importantly, in all cases, arterial Po2 was maintained with the ventilator during neural apnea. Arterial blood samples were drawn 5, 15, 30, and 60 min following neural apnea to confirm Pco2 was within 1.5 mmHg of baseline and to allow maintenance of Po2 and pH. At the end of each protocol, rats were exposed to a hypercapnic challenge (90 mmHg < ETCO2 < 100 mmHg) to ensure observed results were not due to deterioration of the preparation or a lack of dynamic range in phrenic output.
Pharmacological treatments.
Separate groups of rats were exposed to brief intermittent neural apnea and intrathecal injection of the following pharmacological compounds: recombinant human soluble TNF receptor 1 (sTNFR1; R&D Systems), myristoylated ζ-pseudosubstrate inhibitory peptide (PKCζ-PS; Tocris Bioscience), and myristoylated scrambled ζ-pseudosubstrate peptide (scrPKCζ-PS; Tocris Bioscience). Solutions were prepared in artificial CSF (aCSF) (in mM: 120 NaCl, 3 KCl, 2 CaCl, 2 MgCl, 23 NaHCO3, 10 glucose bubbled with 95% O2-5% CO2) and stored at −20°C. All solutions were delivered to the intrathecal space in 10 μl volumes at concentrations known to be sufficient to block iPMF induced by prolonged neural apnea [sTNFR1: 0.13 μg/μl (6, 35, 36) and PKCζ-PS: 2 μg/μl (35, 36, 38)]. Vehicle-treated rats received 10 μl of aCSF. Rats were treated with sTNFR1 (n = 6) or aCSF (n = 6) 20–30 min prior to brief intermittent apnea, PKCζ-PS (n = 6), or scrPKCζ-PS (n = 5) 5 min following brief intermittent apnea. Separate groups of time control rats were also treated with intrathecal sTNFR1 (n = 3) or PKCζ-PS (n = 4) at corresponding time points to ensure there were no drug-dependent effects on baseline phrenic motor output.
Data analysis.
Phrenic burst activity was amplified (×10K), band-pass filtered (0.3–10 kHz; AM Systems), integrated (time constant 50 ms), and rectified. The resulting signal was digitized and analyzed with PowerLab (AD Instruments; LabChart 7.0 software). Sixty-breath bins were taken immediately prior to blood samples taken at baseline and 5, 15, 30, and 60 min after neural apnea and analyzed for peak phrenic burst amplitude. Nerve burst amplitude was expressed as a percent change from baseline (% baseline). Statistical differences between groups and individual time points were determined using a two-way repeated-measures ANOVA design and Bonferroni's post hoc tests, respectively. Group differences in maximum phrenic and hypoglossal amplitude elicited during hypercapnic challenge were determined using a one-way ANOVA and Tukey's post hoc test. Linear regression analysis was used to determine the relationship of iPMF magnitude with the magnitude of the phrenic response to a hypercapnic challenge (Prism 6, Graph Pad Software). A significance level of P < 0.05 was used for all comparisons. All data are shown as means ± SE.
RESULTS
Rat groups were organized into three experimental series (Table 1). In series 1, reduced respiratory neural activity was presented in three patterns: five brief neural apneas (∼1.25 min each) separated by ∼5 min of resumed respiratory neural activity (brief intermittent neural apnea), a single brief massed neural apnea of similar cumulative duration (∼6.25 min), or a prolonged neural apnea (∼30 min) that approximated the duration of the stimulus period (neural apneas plus intervening rest periods). The cumulative duration of brief intermittent neural apnea (6:14 ± 0:25 min:s) was not significantly different than the duration of brief massed neural apnea (6:23 ± 0:20 min:s; P > 0.05), nor was the total stimulus period of brief intermittent neural apnea (28:01 ± 1:04 min:s) different than the duration of prolonged neural apnea (28:46 ± 0:31 min:s; P > 0.05). In series 2, the cumulative duration and total stimulus period of brief intermittent neural apnea were not significantly different between groups receiving sTNFR1 (7:02 ± 0:33 and 31:11 ± 2:06 min:s, respectively) or aCSF (5:25 ± 24 and 30:59 ± 2:46 min:s, respectively; P > 0.05). Similarly, in series 3, the cumulative duration and total stimulus period of brief intermittent neural apnea was not significantly different between groups receiving PKCζ-PS (5:45 ± 0:30 and 29:16 ± 0:58 min:s, respectively) or scrPKCζ-PS (6:36 ± 0:58 and 28:45 ± 1:13 min:s, respectively; P > 0.05).
Table 1.
Arterial PaO2, Pco2, pH, and mean arterial pressure at baseline and 60 min following treatment in all rat groups
| Treatment Group | Time | PaCO2, mmHg | PaO2, mmHg | pH | MAP |
|---|---|---|---|---|---|
| Series 1: Intermittent Neural Apnea Is Sufficient for iPMF | |||||
| Brief Intermittent (n = 9) | baseline | 49 ± 1 | 282 ± 5 | 7.34 ± 0.01 | 127 ± 6 |
| 60 min | 49 ± 1 | 267 ± 7* | 7.33 ± 0.01 | 98 ± 7* | |
| Brief Massed (n = 9) | baseline | 48 ± 1 | 291 ± 5 | 7.35 ± 0.01 | 114 ± 7 |
| 60 min | 48 ± 1 | 286 ± 6 | 7.35 ± 0.01 | 108 ± 3 | |
| Prolonged (n = 9) | baseline | 47 ± 2 | 281 ± 3 | 7.36 ± 0.01 | 111 ± 5 |
| 60 min | 47 ± 1 | 269 ± 7 | 7.35 ± 0.01 | 98 ± 2 | |
| Time Control (n = 5) | baseline | 47 ± 1 | 292 ± 10 | 7.36 ± 0.01 | 111 ± 9 |
| 60 min | 47 ± 1 | 264 ± 11* | 7.36 ± 0.01 | 93 ± 11* | |
| Series 2: Spinal TNF-α Is not Necessary | |||||
| sTNFR1 + Brief Intermittent (n = 6) | baseline | 46 ± 1 | 292 ± 4 | 7.37 ± 0.01 | 108 ± 4 |
| 60 min | 46 ± 1 | 285 ± 7 | 7.35 ± 0.01 | 92 ± 8* | |
| aCSF + Brief Intermittent (n = 6) | baseline | 46 ± 1 | 274 ± 5 | 7.37 ± 0.01 | 93 ± 3 |
| 60 min | 46 ± 1 | 262 ± 7 | 7.36 ± 0.02 | 85 ± 6 | |
| sTNFR1 + Time Control (n = 3) | baseline | 45 ± 2 | 286 ± 3 | 7.36 ± 0.02 | 91 ± 6 |
| 60 min | 45 ± 2 | 271 ± 5 | 7.38 ± 0.00 | 99 ± 4 | |
| Series 3: Spinal aPKC Is Necessary | |||||
| Brief Intermittent + PKCζ-PS (n = 6) | baseline | 45 ± 1 | 267 ± 3 | 7.35 ± 0.01 | 110 ± 8 |
| 60 min | 45 ± 1 | 251 ± 4* | 7.37 ± 0.01 | 86 ± 11* | |
| Brief Intermittent + scrPKCζ-PS (n = 5) | baseline | 47 ± 1 | 273 ± 6 | 7.35 ± 0.02 | 121 ± 13 |
| 60 min | 47 ± 1 | 250 ± 4* | 7.32 ± 0.02 | 104 ± 11 | |
| Time Control + PKCζ-PS (n = 4) | baseline | 48 ± 0 | 285 ± 8 | 7.35 ± 0.01 | 124 ± 4 |
| 60 min | 48 ± 1 | 267 ± 18 | 7.36 ± 0.00 | 97 ± 8* | |
Data are presented as means ± SE. MAP, mean arterial pressure.
Significantly different than baseline, P < 0.05. No significant differences in any variable were detected at any time point between groups.
Table 1 shows PaCO2, PaO2, pH, and mean arterial pressure (MAP) during baseline and 60 min after neural apnea. Average PaCO2, PaO2, pH, and MAP were not significantly different at any time point when compared across treatment groups (P > 0.05). There were no time-dependent changes in PaCO2 or pH from baseline to 60 min after neural apnea within any treatment group (P > 0.05). In some groups, we observed a small but significant decrease in PaO2 and/or MAP from baseline to 60 min. However, these changes were not considered to have contributed to our results since 1) changes were small and inconsistent, 2) PaO2 was maintained well above levels that stimulate breathing (>200 mmHg), 3) small changes in MAP (∼20 mmHg) do not significantly alter phrenic motor output in rats (42), and 4) other rat groups and previous studies have demonstrated similar iPMF without changes in PaO2 (2, 6, 38) or MAP (21).
Series 1: intermittent neural apnea elicits long-lasting iPMF.
We used a rat substrain in which spinal NMDA receptor activation during transient iPMF restrains stabilization of long-lasting iPMF following prolonged neural apnea (36); thus, we sought to determine whether a similar NMDA receptor-mediated constraint was operative following intermittent neural apnea. Representative compressed phrenic neurograms from prolonged, brief intermittent, and brief massed neural apnea and time control groups are shown in Fig. 1A. Average changes in phrenic burst amplitude are shown in Fig. 1B. In time control rats, there were no changes in phrenic burst amplitude from baseline at any time point (5 min: −1 ± 5, 15 min: 4 ± 4, 30 min: 6 ± 3, 60 min: 9 ± 3, % baseline; P > 0.05), suggesting that our preparation was stable during the recording period. A brief sustained neural apnea was not sufficient to elicit iPMF since phrenic amplitude was not different from time controls at any time point following neural apnea (5 min: −3 ± 5, 15 min: 2 ± 4, 30 min: −1 ± 4, 60 min: 6 ± 4, % baseline; P > 0.05). However, when the sustained neural apnea was prolonged, phrenic burst amplitude was initially increased relative to time controls and rats exposed to brief massed neural apnea (5 min: 52 ± 10, 15 min: 43 ± 9, % baseline; P < 0.05), but progressively decayed by 30 (31 ± 7, % baseline) and 60 min (19 ± 5, % baseline), such that phrenic amplitude was no longer significantly different from either group (P > 0.05), confirming that iPMF is transient in this substrain when NMDA receptors are not blocked during early iPMF (36). By contrast, rats exposed to intermittent neural apnea expressed a long-lasting increase in phrenic burst amplitude upon resumption of respiratory neural activity (5 min: 60 ± 9, 15 min: 73 ± 11, 30 min: 67 ± 12, 60 min: 71 ± 11, % baseline) that was significantly different from time controls (P < 0.0001) and brief sustained neural apnea (P < 0.0001) at all time points, and prolonged neural apnea at 30 and 60 min (P < 0.05). Collectively, these data indicate that brief repeated episodes of neural apnea elicit long-lasting iPMF without the necessity for spinal NMDA receptor inhibition.
Fig. 1.
Long-lasting inactivity-induced phrenic motor facilitation (iPMF) is pattern dependent in Charles River Sprague-Dawley rats. A. representative compressed phrenic (Ph) neurograms depicting integrated nerve burst amplitude before (baseline), during, and for 60 min following either brief intermittent, brief massed, or prolonged neural apnea, or an equivalent duration in time controls (top to bottom, respectively). B: mean changes in phrenic burst amplitude (% change from baseline) 5, 15, 30, and 60 min following brief intermittent (●), brief massed (■), prolonged (▲), or no neural apnea (time controls; ▼). C: Average phrenic burst amplitude responses to hypercapnic challenge 60 min after neural apnea. Shaded bar indicates a significant long-lasting iPMF was expressed. D: linear regression comparing the magnitude of iPMF 60 min post neural apnea to the maximum phrenic amplitude elicited during a subsequent hypercapnic challenge, indicating iPMF is associated with a proportional increase in respiration-related phrenic dynamic range. *Significantly different from time control. #Significantly different from brief massed neural apnea. ϕSignificantly different from prolonged neural apnea; P < 0.05.
At the end of each protocol (60 min), rats were exposed to a severe hypercapnic challenge to determine whether expression of inactivity-induced plasticity resulted in an increase in phrenic dynamic range (i.e., greater maximum amplitude). The average maximum phrenic response to hypercapnia is shown in Fig. 1C. As expected, all rats (including time controls) expressed a significant increase in phrenic burst amplitude from baseline in response to hypercapnia (P < 0.05). The maximum phrenic burst amplitude elicited 60 min following prolonged (102 ± 15% baseline) and brief massed (70 ± 5% baseline) neural apnea (treatments that did not induce long-lasting iPMF) were not significantly different from the response in time controls (74 ± 9% baseline; P > 0.05). However, 60 min following brief intermittent neural apnea, when iPMF was expressed, the maximum phrenic amplitude during hypercapnia was significantly increased compared with all other groups (173 ± 30% baseline; P < 0.05). Fig. 1D illustrates the relationship between iPMF and phrenic burst amplitude response to hypercapnia. Sixty minutes following brief intermittent neural apnea, there was a significant linear relationship between the magnitude of iPMF and phrenic burst amplitude during hypercapnia (P = 0.005; R2 = 0.699). Although prolonged neural apnea elicited only a transient iPMF (due to NMDA receptor-mediated constraint of long-lasting iPMF; 36) and did not increase the phrenic hypercapnic response on average, a few individual rats continued to express a small amount of iPMF at 60 min, which was associated with an elevated phrenic burst amplitude response to hypercapnia (P = 0.002; R2 = 0.756). However, changes in phrenic amplitude 60 min following brief massed neural apnea did not have a significant relationship with the phrenic amplitude during hypercapnia (P = 0.940; R2 = 0.001, respectively). Similarly, there was not a significant relationship among time controls (P = 0.478; R2 = 0.071). Thus, expression of iPMF following intermittent neural apnea is associated with a proportional increase in respiration-related phrenic dynamic range.
Series 2: iPMF following intermittent neural apnea does not require spinal TNF-α.
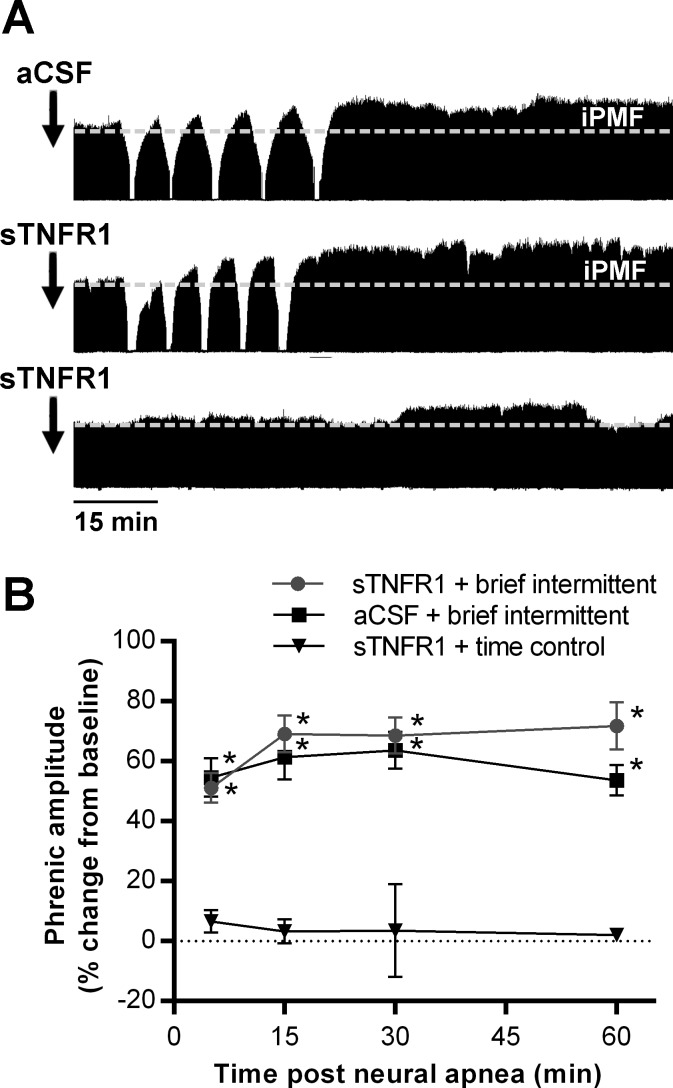
Since spinal TNF-α is required for long-lasting iPMF following a prolonged neural apnea, we sought to determine whether TNF-α is similarly required for iPMF following brief intermittent neural apnea. Representative compressed phrenic neurograms from rats receiving aCSF or a TNF-α scavenger (sTNFR1) 15–20 min prior to brief intermittent neural apnea or at the corresponding time point in time controls is shown in Fig. 2A. Average changes in phrenic burst amplitude are shown in Fig. 2B. In time controls, intrathecal sTNFR1 did not change phrenic amplitude at any time point (5 min: 7 ± 4, 15 min: 3 ± 4, 30 min: 4 ± 15, 60 min: 2 ± 2, % baseline) compared with baseline (P > 0.05). In rats receiving intrathecal vehicle (aCSF) prior to intermittent neural apnea, phrenic burst amplitude was significantly increased from time controls at all time points after neural apnea (5 min: 55 ± 6, 15 min: 61 ± 7, 30 min: 64 ± 6, 60 min: 54 ± 5, % baseline; P < 0.001), indicating long-lasting iPMF. Treatment with intrathecal sTNFR1 prior to brief intermittent neural apnea did not impair iPMF since, similar to rats receiving aCSF, phrenic burst amplitude was significantly increased from time controls at all time points after neural apnea (5 min: 51 ± 5, 15 min: 69 ± 6, 30 min: 69 ± 6, 60 min: 71 ± 8; P < 0.001). Indeed, there were no significant differences in phrenic burst amplitude between rats receiving intrathecal aCSF or sTNFR1 prior to brief intermittent neural apnea (P > 0.05), indicating that spinal TNF-α signaling is not required for iPMF following brief intermittent neural apnea.
Fig. 2.
iPMF following brief intermittent neural apnea does not require spinal TNFα. A: representative compressed phrenic neurograms from rats receiving intrathecal vehicle (aCSF; top) or sTNFR1 (middle) ∼15 min prior to brief intermittent neural apnea, and intrathecal sTNFR1 in a rat receiving no neural apnea (time control; bottom). B: mean changes in phrenic burst amplitude (%baseline) 5, 15, 30, and 60 min following brief intermittent neural apnea in rats receiving intrathecal sTNFR1 (●) or aCSF (■), and at equivalent time points in time control rats receiving intrathecal sTNFR1 (▼). *Significantly different from sTNFR1 time controls, P < 0.05.
Series 3: iPMF following intermittent neural apnea requires spinal aPKC.
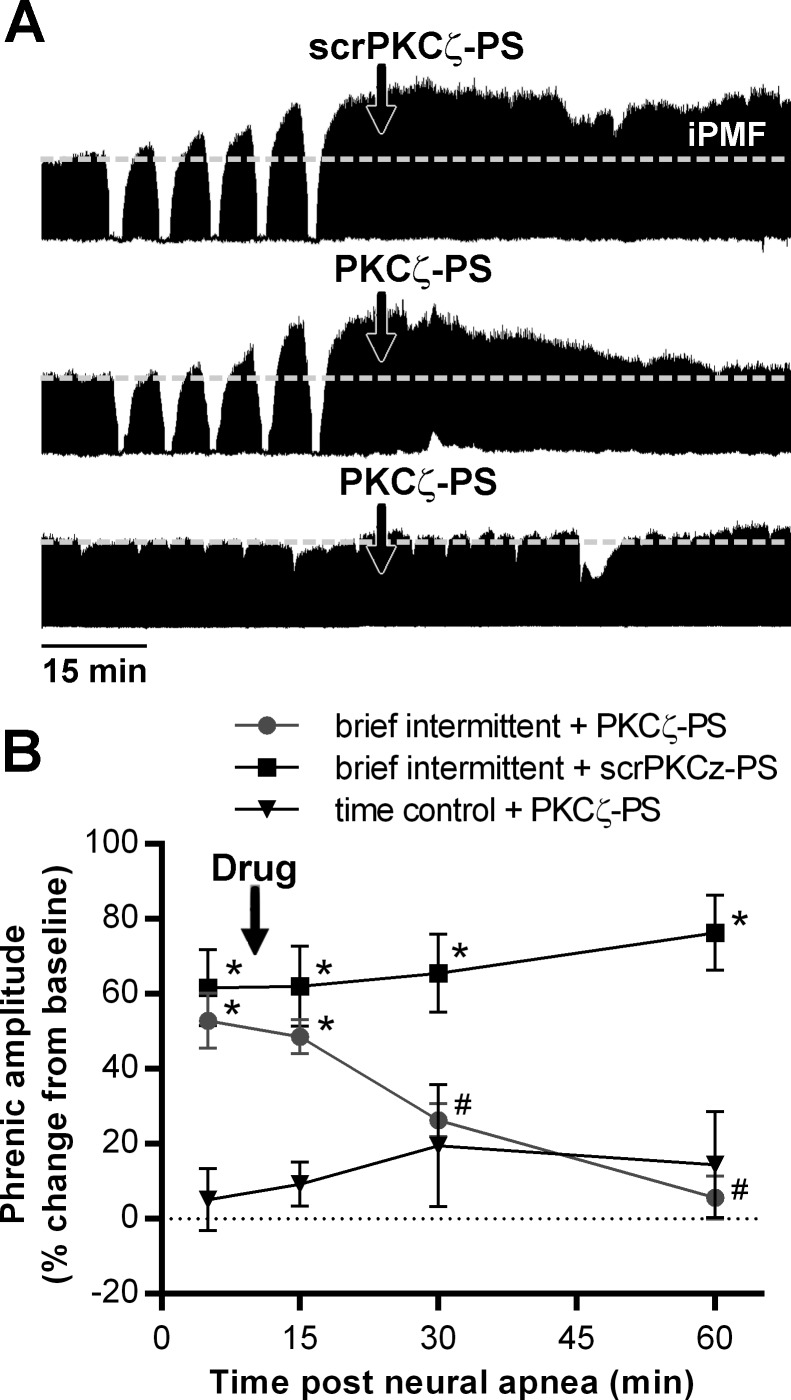
To test the hypothesis that stabilization of iPMF following brief intermittent neural apnea requires spinal aPKC activity, rats were treated with intrathecal scrPKCζ-PS or PKCζ-PS ∼10 min following brief intermittent neural apnea or at the corresponding time point in time controls. Representative compressed phrenic neurograms are shown in Fig. 3A. Average change in phrenic nerve burst amplitude are shown in Fig. 3B. In time controls, intrathecal PKCζ-PS had no effect on phrenic burst amplitude (15 min: 8 ± 4, 30 min: 17 ± 12, 60 min: 11 ± 10), compared with predrug levels (5 min: 2 ± 7; P > 0.05) and baseline (P > 0.05). In rats receiving intrathecal scrPKCζ-PS following brief intermittent neural apnea, phrenic burst amplitude was significantly increased from time controls at all time points after neural apnea (5 min: 62 ± 10, 15 min: 62 ± 11, 30 min: 66 ± 10, 60 min: 76 ± 10, % baseline; P < 0.001), indicating scrPKCζ-PS did not impair iPMF. Rats receiving intrathecal PKCζ-PS expressed significant increases in phrenic burst amplitude prior to (5 min: 53 ± 7% baseline) and immediately following (15 min: 49 ± 5% baseline) delivery of PKCζ-PS, compared with time controls (P < 0.001), indicating iPMF. However, shortly after PKCζ-PS delivery, phrenic burst amplitude began to gradually decline, such that by 30 (26 ± 4% baseline) and 60 (6 ± 6% baseline) min, phrenic burst amplitude was significantly decreased compared with rats receiving scrPKCζ-PS (P < 0.01) and no longer different from time controls (P > 0.05), indicating iPMF had been abolished. These data indicate that spinal aPKC is necessary for iPMF following brief intermittent neural apnea.
Fig. 3.
Spinal aPKC is necessary for iPMF elicited by brief intermittent neural apnea. A: representative compressed phrenic neurograms from rats receiving intrathecal injection of a scrambled control peptide (scrPKCζ-PS; top) or PKCζ-PS (middle) ∼10 min following brief intermittent neural apnea, and intrathecal PKCζ-PS in a rat receiving no neural apnea (time control, bottom). B: mean changes in phrenic burst amplitude (% change from baseline) 5, 15, 30, and 60 min following brief intermittent neural apnea in rats receiving intrathecal PKCζ-PS (●) or scrPKCζ-PS (■), and at equivalent time-points in time control rats receiving intrathecal PKCζ-PS (▼). *Significantly different from PKCζ-PS time controls. #Significantly different from brief intermittent neural apnea and scrPKCζ-PS, P < 0.05.
DISCUSSION
Although inactivity-induced phrenic motor facilitation was only recently described (21), we are beginning to grasp its underlying mechanisms. Our working model suggests that iPMF following prolonged neural apnea requires a spinal TNF-α-induced increase in aPKC activity to stabilize a transient increase in phrenic burst amplitude into long-lasting iPMF (6, 38); stabilization of long-lasting iPMF may be disrupted by NMDA receptor activation during transient iPMF expression, at least in some rat substrains (36). Here, we tested the hypothesis that similar TNF-α- and aPKC-dependent mechanisms give rise to iPMF following brief intermittent neural apnea. Contrary to our hypothesis, we found that iPMF following intermittent neural apnea does not require TNF-α, but retains its requirement for aPKC. Further, we show that unlike iPMF following prolonged neural apnea (36), stabilization of iPMF following brief intermittent reductions in respiratory neural activity does not require NMDA receptor inhibition. We conclude that distinct cellular processes initiate and regulate iPMF expression following different patterns of reduced respiratory neural activity that then converge on a similar downstream mechanism. Heterogeneous mechanisms giving rise to iPMF may increase system flexibility and ensure that the phrenic motor system responds appropriately during conditions that result in different patterns of reduced respiratory neural activity.
Distinct mechanisms give rise to iPMF following intermittent vs. prolonged reductions in respiratory neural activity.
Although typically thought of as a proinflammatory cytokine, TNF-α is also important in the healthy CNS and has been shown to play a key role in neuronal homeostatic responses to prolonged activity deprivation (5, 6, 26, 34). For example, in cultured hippocampal neurons, a prolonged reduction in neural activity induces a TNF-α-dependent increase in AMPA receptor membrane expression and synaptic transmission in vitro (5, 34); further, TNF-α is necessary for scaling up of visual cortical neurons responses following monocular deprivation in vivo (18). Glia are likely the source of diffusible TNF-α, which is released, presumably in response to low extracellular glutamate levels caused by reduced network activity, to initiate a compensatory increase in synaptic strength (26, 34). Similarly, multiple studies from our laboratory have demonstrated a necessary role for TNF-α in iPMF following a prolonged neural apnea (6, 35, 36). Although greater than 24 h of activity deprivation is typically required to elicit TNF-α-dependent plasticity in the hippocampus (34) and visual cortex (18), a sustained reduction in the activity of the phrenic motor pool induces TNF-α-dependent iPMF relatively rapidly (∼30 min) (6, 35, 36). Indeed, a rapid response to reduced activity of the phrenic neural network may be appropriate given the life-threatening consequences of a loss of phrenic motor output. Thus, we hypothesize that a prolonged duration of reduced respiratory neural activity within the phrenic motor nucleus is sensed by nearby glia and responded to through the release of diffusible TNF-α and initiation of an aPKC-dependent signaling cascade within phrenic motor neurons, giving rise to iPMF.
In this study, we show that spinal TNF-α is not required for iPMF elicited by brief intermittent episodes of reduced respiratory neural activity. These pattern-specific differences in the initiation of iPMF may reflect the existence of two different types of plasticity utilizing different “sensors” of reduced neural activity. For example, reducing the activity of single cortical neurons with focal application of TTX in vitro induces a relatively rapid (1–2 h) scaling up of synaptic strength that does not likely involve TNF-α release, but requires reduced neuronal spiking and intracellular Ca2+ levels (14). Thus, in addition to TNF-α-dependent homeostatic regulation of network activity, mechanisms intrinsic to neurons may allow relatively rapid adjustments in the activity of individual cells (26). Moreover, the expression of TNF-α-dependent homeostatic plasticity in the visual cortex transitions to a TNF-α-independent and CamKII-dependent mechanism during adulthood in some mouse substrains (29), demonstrating that multiple mechanisms can elicit a phenotypically similar response to reduced neural activity. Cellular cascades initiating iPMF following brief intermittent neural apnea are currently unknown.
Mechanisms of iPMF elicited by different patterns of inactivity converge on aPKC.
Despite utilizing different mechanisms of induction, spinal aPKC activity is necessary for iPMF elicited by both brief intermittent and prolonged neural apnea. Specifically, spinal aPKC activity is necessary during the early phase of iPMF since intrathecal delivery of the aPKC inhibitor PKCζ-PS abolishes iPMF when delivered shortly (∼10 min) following the resumption of respiratory neural activity (see results and Ref. 38). Therefore, we speculate that this shared requirement for aPKC during the formation and stabilization of iPMF represents a “convergence” on similar downstream cellular machinery that ultimately gives rise to the iPMF phenotype. This type of convergence between distinct mechanisms of plasticity is not uncommon since different forms of homoeostatic plasticity can involve conserved cellular cascades. For example, β-3 integrin is thought to be a point of convergence between TNF-α-dependent synaptic scaling and other forms of homeostatic synaptic scaling with shorter time-courses in hippocampal neurons (1). Further, other well-studied effectors of synaptic plasticity (e.g., BDNF, MSK1, and MAPK) have been implicated in both homeostatic (i.e., activity-dependent) and adaptive (i.e., experience-dependent) plasticity elicited in response to different types of stimuli (8). Moreover, a similar convergence on aPKC among mechanisms of plasticity with different modes of induction has been observed in other highly active oscillatory systems. For example, in the pacemaker nucleus of weakly electric fish, aPKC is required for both the regulation of a basal “set point” to maintain stable neural activity, as well as adaptive, experience-dependent, plasticity in response to social interaction (13). Interestingly, protein phosphatases regulated by Ca2+ influx (calcineurin) determine the duration of this plasticity (13), perhaps similar to the NMDA receptor-mediated constraint on iPMF following prolonged neural apnea. Thus, we suspect that iPMF elicited by sustained and brief intermittent reductions in respiratory neural activity utilize conserved downstream mechanisms that are subject to different regulatory constraints.
Our observation that long-lasting, but not transient, iPMF is associated with an enhanced phrenic response during a hypercapnic challenge is consistent with previous reports (2, 35, 36). Indeed, the phrenic hypercapnic response is also enhanced following prolonged neural apnea under conditions of NMDA receptor inhibition in this rat substrain (36). Similar shifts in the phrenic hypercapnic response may suggest that mechanisms downstream from aPKC that ultimately give rise to long-lasting iPMF are similar, independent of how iPMF is induced. However, we cannot rule out that the signaling pathways underlying iPMF elicited by different patterns of neural apnea diverge and become distinct once again downstream of aPKC. For example, TNF-receptor activation can lead to the interaction of aPKC with its scaffolding protein SQSTM1/p62, which can then initiate the NF-κB cascade to increase transcription of inflammatory and cell survival genes (24, 32, 33). Also, TNF receptor-associated factor 6, TRAF6, can induce SQSTM1/p62-dependent membrane receptor internalization and degradation (24), which likely involves interactions with aPKC. In a hippocampal LTP paradigm, aPKC interactions with SQSTM1/p62 are required for AMPAR trafficking to the membrane and expression of LTP (16). Therefore, different inducing stimuli can activate cellular mechanisms involving aPKC, which then diverge through directed and selective protein-protein interactions. Studies investigating the cellular mechanisms downstream of aPKC will be necessary to determine whether similar processes underlie the maintenance of iPMF elicited by different patterns of reduced respiratory neural activity.
Regulation of iPMF is pattern-dependent.
Similar to other forms of respiratory neuroplasticity (3, 10), iPMF is pattern-sensitive, and the degree of sensitivity appears to be subject to genetic/epigenetic differences (2, 36). While iPMF is not expressed following a brief massed neural apnea, iPMF may be revealed if the duration of the massed neural apnea is broken up into several shorter episodes (2). Interestingly, in rat substrains that lack the NMDA receptor-dependent regulatory constraint on iPMF, this sensitivity to pattern may be overcome if the duration of neural apnea is prolonged, resulting in robust long-lasting iPMF (2, 38). However, prolonged neural apnea only revealed a transient increase in phrenic motor output in the rat substrain examined in this study due to a NMDA receptor-dependent regulatory constraint on the stabilization of long-lasting iPMF (36). In contrast to the transient iPMF observed following prolonged neural apnea, intermittent neural apnea elicited a robust and long-lasting iPMF, suggesting that similar regulatory mechanisms do not constrain iPMF when respiratory neural activity is reduced intermittently. Thus, an important question is why does spinal NMDA receptor activation restrain iPMF following a prolonged episode of reduced neural activity but not following intermittent episodes? One possibility is that periods of restored activity between intermittent neural apneas desensitize NMDA receptors, diminishing their regulatory effect during the stabilization of iPMF. Indeed, NMDA receptors can be desensitized rapidly by stimuli such as excessive NMDA receptor activation, hyperactivity, and increased intracellular Ca2+, altered activity of intracellular kinases and/or phosphatases, and G protein-coupled receptor activation (17, 20, 22, 23, 28, 30, 39), and a greater desensitization is often observed following repeated stimulation (25). Alternatively, mechanisms downstream of NMDA receptor activation may lead to the differential regulation of iPMF following distinct patterns of reduced respiratory neural activity. Further investigation of the mechanisms regulating the expression of iPMF will be necessary to understand how they are affected by the pattern of reduced respiratory neural activity.
Perspectives and Significance
We are beginning to appreciate that mechanisms local to the phrenic motor pool respond vigorously and rapidly to reductions in neural activity, without changes in blood gases (4, 35, 37). Importantly, complete absence of phrenic neural activity is not necessary for iPMF to be induced (35). As such, iPMF may represent a mechanism of “homeostatic” or compensatory plasticity that enables phrenic motor neurons to adapt to changes in neuronal excitability and/or synaptic inputs that may occur throughout life, thereby preventing catastrophic decreases in phrenic motor output. Data presented here suggest that the phrenic motor pool can initiate and regulate compensatory responses to different patterns of reduced respiratory neural activity through distinct mechanisms, a characteristic that may lead to increased system flexibility and allow adaptation to physiological and pathological conditions associated with heterogeneous manifestations of reduced respiratory neural activity (37). Although we are beginning to grasp the underlying mechanisms of iPMF elicited by different patterns of reduced respiratory neural activity and under varied genetic/epigenetic contexts, the robustness and physiological significance of this plasticity during pathological conditions associated with reduced activity of respiratory motor pools in a highly variable human population remain unknown. Future studies investigating inactivity-induced respiratory plasticity under such conditions may lead to the development of novel therapeutic strategies for when these endogenous mechanisms of compensatory plasticity fail.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: N.A.B. and T.L.B.-H. conception and design of research; N.A.B. performed experiments; N.A.B. analyzed data; N.A.B. and T.L.B.-H. interpreted results of experiments; N.A.B. prepared figures; N.A.B. and T.L.B.-H. drafted manuscript; N.A.B. and T.L.B.-H. edited and revised manuscript; N.A.B. and T.L.B.-H. approved final version of manuscript.
ACKNOWLEDGMENTS
Research support was provided by the National Heart, Lung and Blood Institute Grant HL-105511.
REFERENCES
- 1.Aizenman CD, Pratt KG. There's more than one way to scale a synapse. Neuron 58: 651–653, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Baertsch NA, Baker-Herman TL. Inactivity-induced phrenic and hypoglossal motor facilitation are differentially expressed following intermittent versus sustained neural apnea. J Appl Physiol 114: 1388–1395, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529: 215–219, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker-Herman TL, Strey KA. Similarities and differences in mechanisms of phrenic and hypoglossal motor facilitation. Respir Physiol Neurobiol 179: 48–56, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNF-α. Science 295: 2282–2285, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Broytman O, Baertsch NA, Baker-Herman TL. Spinal TNF is necessary for inactivity phrenic motor facilitation. J Physiol 591: 5585–5598, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Lau AG, Sarti F. Synaptic retinoic acid signaling and homeostatic synaptic plasticity. Neuropharmacology 78: 3–12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrêa SA, Hunter CJ, Palygin O, Wauters SC, Martin KJ, McKenzie C, McKelvey K, Morris RG, Pankratov Y, Arthur JS, Frenguelli BG. MSK1 regulates homeostatic and experience-dependent synaptic plasticity. J Neurosci 32: 13,039–13,051, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeg KE. Synapse-specific homeostatic mechanisms in the hippocampus. J Neurophysiol 101: 503–506, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann NY Acad Sci 1279: 143–153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Fiore JM, Martin RJ, Gauda EB. Apnea of prematurity—perfect storm. Respir Physiol Neurobiol 189: 213–222, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: Pathophysiology and treatment. Chest 131: 595–607, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George AA, Macleod GT, Zakon HH. Calcium-dependent phosphorylation regulates neuronal stability and plasticity in a highly precise pacemaker nucleus. J Neurophysiol 106: 319–331, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron 57: 819–826, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Javaheri S, Dempsey JA. Central sleep apnea. Compr Physiol 3: 141–163, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J, Parameshwaran K, Seibenhener ML, Kang MG, Suppiramaniam V, Huganir RL, Diaz-Meco MT, Wooten MW. AMPA receptor trafficking and synaptic plasticity require SQSTM1/p62. Hippocampus 19: 392–406, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci 19: 96–101, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron 58: 673–680, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leininger E, Belousov AB. Homeostatic plasticity: comparing and contrasting cortical and hippocampal studies. A review. Crit Rev Neurobiol 18: 125–134, 2006. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald JF, Jackson MF, Beazely MA. G protein-coupled receptors control NMDARs and metaplasticity in the hippocampus. Biochim Biophys Acta 1768: 941–951, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Mahamed S, Strey KA, Mitchell GS, Baker-Herman TL. Reduced respiratory neural activity elicits phrenic motor facilitation. Respir Physiol Neurobiol 175: 303–309, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer ML, Vyklicky L, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature 338: 425–427, 1989. [DOI] [PubMed] [Google Scholar]
- 23.McBain CJ, Mayer ML. N-methyl-d-aspartic acid receptor structure and function. Physiol Rev 74: 723–760, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Moscat J, Diaz-Meco MT, Wooten MW. Signal integration and diversification through the p62 scaffold protein. Trends Biochem Sci 32: 95–100, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Nakamichi N, Yoneda Y. Functional proteins involved in regulation of intracellular Ca2+ for drug development: desensitization of N-methyl-d-aspartate receptor channels. J Pharmacol Sci 97: 348–350, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 66: 337–351, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pribiag H, Stellwagen D. Neuroimmune regulation of homeostatic synaptic plasticity. Neuropharmacology 78: 13–22, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Raman IM, Tong G, Jahr CE. β-adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron 16: 415–421, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Ranson A, Cheetham CE, Fox K, Sengpiel F. Homeostatic plasticity mechanisms are required for juvenile, but not adult, ocular dominance plasticity. Proc Natl Acad Sci USA 109: 1311–1316, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rojas A, Dingledine R. Ionotropic glutamate receptors: regulation by G-protein-coupled receptors. Mol Pharmacol 83: 746–752, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosen D, Roux FJ, Shah N. Sleep and breathing in congestive heart failure. Clin Chest Med 35: 521–534, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Sanz L, Diaz-Meco MT, Nakano H, Moscat J. The atypical PKC-interacting protein p62 channels NF-κB activation by the IL-1-TRAF6 pathway. EMBO J 19: 1576–1586, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanz L, Sanchez P, Lallena MJ, Diaz-Meco MT, Moscat J. The interaction of p62 with RIP links the atypical PKCs to NF-κB activation. EMBO J 18: 3044–3053, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature 440: 1054–1059, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Streeter KA, Baker-Herman TL. Decreased spinal synaptic inputs to phrenic motor neurons elicit localized inactivity-induced phrenic motor facilitation. Exp Neurol 256: 46–56, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Streeter KA, Baker-Herman TL. Spinal NMDA receptor activation constrains inactivity-induced phrenic motor facilitation in Charles River Sprague-Dawley rats. J Appl Physiol (1985). 117: 682–693, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strey KA, Baertsch NA, Baker-Herman TL. Inactivity-induced respiratory plasticity: protecting the drive to breathe in disorders that reduce respiratory neural activity. Respir Physiol Neurobiol 189: 384–394, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strey KA, Nichols NL, Baertsch NA, Broytman O, Baker-Herman TL. Spinal atypical protein kinase C activity is necessary to stabilize inactivity-induced phrenic motor facilitation. J Neurosci 32: 16,510–16,520, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science 267: 1510–1512, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol 4: a005736, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Ann Rev Neurosci 34: 89–103, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Walker JK, Jennings DB. Respiratory effects of pressor and depressor agents in conscious rats. Can J Physiol Pharmacol 76: 707–714, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Wenner P. Homeostatic synaptic plasticity in developing spinal networks driven by excitatory GABAergic currents. Neuropharmacology 78: 55–62, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitt JL, Petrus E, Lee HK. Experience-dependent homeostatic synaptic plasticity in neocortex. Neuropharmacology 78: 45–54, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]