Abstract
A complex social life serves as one of the main driving forces behind the evolution of higher cognitive abilities in vertebrates. In birds, however, data are primarily derived from captive animals, which strongly contrast with free-flying birds in terms of the number of interaction partners as well as available space. In captivity, Common Raven Corvus corax, nonbreeder groups show strong social bonds and complex tactical manoeuvring, whereas wild non-breeders are thought to resemble anonymous aggregations. Over 2 years, we observed a free-flying population of Ravens that visits a game park in the northern Alps. We here focus on the daily fission–fusion dynamics, individual spacing, and the influence of spacing on the birds’ agonistic and affiliative behaviour. The composition of marked Ravens in the local population changed slowly but constantly, although often remaining stable for several weeks. Birds only flocked for feeding, mobbing and roosting, and spent the rest of the day in loose aggregations, characterised by temporary small subgroups of 2–5 individuals. Aggression was high during crowd foraging but low outside of a feeding context. Affiliative behaviours, such as sitting within reaching distance, allo-preening and social play, were observed particularly in the small subgroups. These findings suggest that Raven aggregations are not as unstructured as previously thought. Birds may spend time and/or interact affilliatively with multiple individuals during the day. This, along with temporary stability in group composition, provides the opportunity for social relationships to develop, and enables the existence of socialised subgroups within free-flying Raven aggregations.
Keywords: Common Raven, Fission–fusion dynamics, Group life, Complex societies, Corvids
Introduction
Higher cognitive abilities can be found in many animal species and are especially renowned in some families of birds and mammals. The underlying driving forces behind such abilities are thought to be the same in the different vertebrate taxa. Indeed, the most supported hypothesis for cognitive evolution in mammals, the social brain hypothesis (Byrne and Whiten 1988; Dunbar 1998), has also been successfully applied to a bird family, the corvids (Bond et al. 2003, 2007). This hypothesis states that life in complex societies provides special kinds of intellectual problems leading to increased brain size. As such, corvids may use their bigger brains to tactically manoeuvre the social domain: they form alliances and differentiated relationships (Emery et al. 2007; Fraser and Bugnyar 2010a, 2012), reconcile conflicts (Fraser and Bugnyar 2011) and console distressed partners (Fraser and Bugnyar 2010b; Seed et al. 2007); moreover, they infer social dominance from observing interactions among others (Paz-y-Mino et al. 2004), employ tactical deception in competition for hidden food (Bugnyar and Kotrschal 2002; Emery et al. 2004) and seemingly attribute knowledge about others’ perception and knowledge state when protecting their own food caches (Emery and Clayton 2001; Dally et al. 2006) and when pilfering the caches of others (Bugnyar 2011; Bugnyar and Heinrich 2005, 2006).
However, all these results were obtained from captive birds, where group size and interaction patterns are limited by space. In contrast, free-living corvid flocks are characterised by a strong fluidity, changing their flock size often within a single day. Flocks of Common Ravens Corvus corax, Western Jackdaws Corvus monedula and Rooks Corvus frugilegus vary from large groups of several hundred birds at the night roost to small groups of a handful of individuals during the day. Alternatively, Eurasian Nutcrackers Nucifraga nucifraga form large congregations at fruiting trees, but small groups at roosts in the core areas of their home ranges (Haffer 1993; Vander Wall and Balda 1977). Such fluidity is resplendent of some mammalian systems, such as those found in elephants Loxodonta africana (McComb et al. 2001), hyenas Crocuta crocuta (Holekamp et al. 1997), bottlenose dolphins Tursiops truncatus (Connor et al. 1999), spider monkeys Ateles geoffroyi (Symington 1990) and chimpanzees Pan troglodytes (Smuts et al. 1987). On the one hand, a high degree of fission–fusion dynamics may increase the socio-cognitive challenges faced by a species (Aureli et al. 2008; Barrett et al. 2003), and may correlate with the ability to perform certain cognitively demanding skills, such as inhibition (Amici et al. 2008). On the other hand, the potential for social bonding may be limited under high fission–fusion dynamics and such a society may drift towards anonymity (Byrne and Bates 2007a). To characterise a species as socially complex, we would thus expect (at least) the formation of longitudinally stable (sub)groups that enable group members to build up differentiated relationships based on a history of give-and-take interactions (Byrne and Bates 2007a). Furthermore, strong learning effects on social behaviour and survival strategies would necessitate a strong degree of dependence on other group members (de Waal and Tyack 2003). Thus, if the social brain hypothesis does indeed apply to flocks of wild corvids, it needs to be shown that they are not just temporary aggregations resembling an anonymous crowd, but that they represent semi-permanent groups in which such social manoeuvring could be applied (Byrne and Bates 2007b).
Ravens are ideal subjects to illustrate the problem. They typically join non-breeder aggregations in their first 3 years when they are sexually immature. Birds may also remain in these aggregations as adults, if they are not able to occupy a territory. As territories are large (minimum of 10 km2; Rösner and Selva 2005), defended for life (up to 30 years; Haffer 1993) year round by socially monogamous breeding pairs (Heinrich 1989), and thus are limited in supply, birds may spend several years in non-breeder aggregations, which they mainly use to gather information about, and access to, food locations (Marzluff et al. 1996; Wright et al. 2003). Indeed, Ravens can gain a lot by learning from and cooperating with others: they are specialised scavengers on carcasses of large mammals, a type of food which typically is unpredictable in supply and thus difficult to find. Moreover, carcasses are often defended either by dominant conspecifics or predators such as Wolves Canis lupus (Stahler et al. 2002). What is yet unclear is to what extent Raven aggregations are anonymous or represent groups in which (some) individuals meet regularly over time. Some studies suggest that aggregations of wild Ravens lack any form of stability. In one such study, Ravens caught from one feeding place, and thus assumed to belong to the same group, dispersed randomly after being marked and released (Heinrich et al. 1994). Additionally, marked non-breeding Ravens, observed at various feeding places within an area of 10,000 km2 over 2 years, did not travel in groups or stay together (Huber 1991). Conversely, other Raven populations have shown sub-roost formation (Wright et al. 2003) and environment-dependant stable ‘gang’ foraging (Dall and Wright 2009), where birds that hung out together over the day also slept in closer proximity in the night roosts. Furthermore, non-breeding Ravens are often reported to engage in frequent social play (Drack and Kotrschal 1995; Eklow 1988; Gwinner 1966; Heinrich and Smolker 1998), a behaviour which we would expect from individuals that know each other and maintain close bonds (Diamond and Bond 2003). Are the sophisticated patterns of social manoeuvring that characterise studies of captive Ravens therefore the result of unnaturally stable and densely populated conditions in captivity or might studies on free-flying Ravens have missed crucial aspects of the social life of Raven non-breeder aggregations?
To our knowledge, all previous studies on free-flying Ravens were conducted during feeding, either on different garbage dumps (Boarman 2003; Huber 1991) or on deliberately placed carcasses in the wild (Dall and Wright 2009; Heinrich et al. 1994; Parker et al. 1994; Rösner et al. 2005). Aggression rates during crowd foraging are typically very high (Heinrich 1994a), most likely reducing the chances of observing affiliative interactions; under captive situations, affiliative relationships between individuals are best detected in a relaxed context, outside feeding (Drack and Kotrschal 1995), when birds are more likely to approach one another for allo-preening and/or engage in playful interactions. Here, we studied free-flying wild Ravens within a local zoo in the Austrian Alps, where the birds spend much of the day. Specifically, they use the park for snatching food from the zoo animals and, notably, also for resting, socialising and playing (Drack and Kotrschal 1995). By concentrating our study on this location, we were able to habituate the Ravens to close-distance observations (down to 25 m) not only at feeding sites but also at areas used for resting and social interactions throughout the day. Our hypothesis was that Raven aggregations do not represent an anonymous crowd but contain socialised sub-groups. This should become apparent through the formation of (small) groups during the day, aside from crowd foraging situations, characterised by high levels of affiliative behaviours and low levels of aggression. We therefore investigated the daily fission–fusion dynamics of free-flying non-breeder Ravens, the spacing of the individuals during the day, and the dependence of behaviours on individual spacing. We also recorded the change in “flock” composition across the year, whereby the term “flock” infolds all Ravens present in the valley on the same day, not a cohesive crowd which moves together. We expected flock composition not to change randomly but to exhibit patterns of stability, so that individual Ravens would have the opportunity to build up relationships based on repeated interactions.
Methods
Field site and study animals
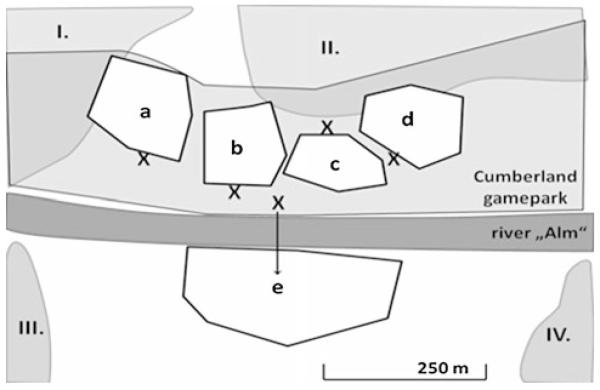
The study subjects were derived from a population of free-flying wild Common Ravens in the inner Almtal, a small valley in the Northern Austrian Alps. These Ravens regularly use parts of the area of the Cumberland Wildpark, a local zoo housing typical Eurasian wild animals. Most of the Ravens were usually found in and/or around four large enclosures (Przewalsky horse, wolf/bear, wild boar and red/fallow deer; Fig. 1) or on an adjacent cliff throughout the day, including the winter months and the breeding season. Hence, they were considered to be non-breeders. The rest of the valley, including the southern and northern part of the game park, is rarely used by non-breeders but is aggressively defended by territorial Raven pairs (Drack and Kotrschal 1995). The communal night roost is situated approximately 500 m from the park, on the other side of the cliff; its exact location has changed over the years but it has been consistently used by Ravens since the early 1990s (own unpublished data; compare Wright et al. 2003). Non-breeders typically arrive at the game park at dawn and leave towards the night roost around sunset. Territorial breeders (all adults and identified as such by defending a territory and/or feeding young) visit the park only occasionally, notably during the morning feeding of the zoo animals (between 0700 and 1000 hours). They typically show up in pairs, engage in joint displays and generally dominate the non-breeders, including adult birds (own unpublished data). During the breeding season, only one of the pair partners shows up at the feeding places, carrying off consecutive loads of food towards their territory and nest.
Fig. 1.
Distribution of the wild Common Ravens Corvus corax in the inner Almtal with the areas a–e (a Przewalsky horse Equus ferus przewalski, b wolf/bear Canis lupus/Ursus arctos, c wild boar Sus scrofa and d red/fallow deer Cervus elaphus/Dama dama; e cliff along the park’s border) and the surrounding four territories of wild Ravens (I.–IV.); X represent the spots from which the areas were observed
We caught Ravens with a drop-in trap (Stiehl 1978) situated in the game park with a mean marking rate of 6 Ravens per month (see Table 1 for an overview over the number and sex of the birds trapped), starting in spring 2008. All birds were weighed and marked with individual wing tags (Caffrey 2000) and coloured ring combinations on both legs. Age class was assigned as juvenile (within first year), subadult (second and third year) or adult (older than 3 years) using mouth and feather colour (Heinrich 1994b); sex was determined from blood samples. To ensure the least possible stress for the birds, they were taken out of the trap, handled and released within the shortest possible time after trapping.
Table 1.
List of the number and sex of birds marked over the course of the study, mean number of Common Ravens Corvus corax present in feeding crowds, % of marked birds in feeding crowds and number of days taken records of the individuals present in the valley
| Month | Year | Marked females | Marked males | Total | No. of birds present | % Ravens marked at feeds | No. of days recorded flock composition |
|---|---|---|---|---|---|---|---|
| Jan–Mar | 2008 | 7 | 2 | 9 | 19 ± 11 | 13.10 ± 13.18 | |
| Apr–Jun | 2008 | 4 | 7 | 11 | 18 ± 11 | 23.07 ± 17.01 | |
| Jul–Sept | 2008 | 18 | 9 | 27 | 16 ± 11 | 30.21 ± 14.98 | |
| Oct–Dec | 2008 | 7 | 11 | 18 | 24 ± 19 | 33.96 ± 16.90 | |
| Jan–Mar | 2009 | 13 | 10 | 23 | 25 ± 16 | 43.16 ± 20.43 | |
| Apr–Jun | 2009 | 2 | 1 | 3 | 28 ± 16 | 53.54 ± 19.06 | |
| Jul–Sept | 2009 | 6 | 6 | 12 | 27 ± 16 | 51.04 ± 20.75 | |
| Oct–Dec | 2009 | 5 | 1 | 6 | 38 ± 22 | 44.59 ± 13.83 | 27 |
| Jan–Mar | 2010 | - | - | - | 42 ± 20 | 45.26 ± 12.57 | 25 |
| Apr–Jun | 2010 | 11 | 13 | 24 | 36 ± 17 | 59.54 ± 18.43 | 62 |
| Jul–Sept | 2010 | - | - | - | 29 ± 11 | 58.58 ± 13.35 | 11 |
Data collection
Flock size and composition
After a habituation phase of 3 months in autumn 2007, we started data collection at the five different observation areas in and around the gamepark (Fig. 1). Data for the present study were not taken before summer 2008, so the Ravens were already fully habituated and did not change their behaviour due to the observer’s presence. Overall flock size estimates and identities of marked Ravens present were taken each morning while the bears/wolves and wild boars were being fed (0730–0900 hours), as all marked Ravens recorded during the day participated in the morning feeds. Only one marked Raven was not present at any of the feeds despite being observed twice in the gamepark during the day. All data were collected with binoculars and a tape recorder.
To check for variation in flock composition, we analysed data on the presence of marked Ravens over 1 year. For these analyses, we used data from October 2009 until the end of September 2010, because during that time we had 100 marked Ravens and sufficient recordings of the flock composition during the morning feeds to run our analyses. We recorded the flock composition on 125 days distributed over 8 months within that year. Within these 8 months, we investigated the variation in the presence of the 100 birds. Depending on how often they were in the valley, we assigned all birds to one of four ‘presence categories’ for each of the months. If a marked subject was present on more than two-thirds of the days within a month, they were labelled ‘stable’. Birds which were present between one-third and two-thirds of the sampling days were categorised as ‘changing’, because 86% of them either arrived within that month for a longer stay, or left within that month after a longer period of presence, and were thus changing to or from residency. If birds were present for less than one-third of observations per month, they were labelled ‘visitors’, and finally, those that were not present at all were counted as ‘absent’.
Individual spacing and behaviour
In addition to data collected during the scheduled feeding times of the gamepark animals, we conducted 33 observation rounds between June 2008 and March 2009 either between 0900 and 1130 hours (‘morning’ observation), between 1200 and 1430 hours (‘midday’ observation), or between 1500 and 1730 hours (‘evening’ observation). To control for the influence of the time of day on the patterns of spacing and behaviour, we distributed the number of rounds evenly over the three times of day. Each observation round was started at the horse enclosure, followed by the wolf/bear enclosure, the cliff, wild boars and finally the fallow deer enclosure. At each location, scan samples were conducted every 90 s to systematically record all Ravens present. Typically, 10 such scans were done before switching to the next location; however, time constraints resulted in a maximum of only 6 scans per location for some rounds. In total, 23 full rounds were sampled (with 10 scans per location), 8 in the morning and at midday, and 7 in the evening. Ten shorter rounds (with 6 scans per location) were conducted, 3 in the morning, 3 at mid-day, and 4 in the evening. During each scan, the identities (if possible) of all Ravens within the enclosure/on the cliff were recorded along with the distances to their two nearest neighbours and their behaviour (see Table 2 for behavioural definitions). Within 1 m of another Raven, individual spacing was estimated in 25-cm steps, from 1 m onwards we proceeded in 50-cm steps until 3 m, from 3 to 50 m we estimated distances in 5-m steps, starting with 5 m, and from 50 m on we used 10-m steps. To facilitate estimates of close distances between the birds (within 3 m), the body size of the Ravens (approx. 50 cm body length from head to tail, and 10 cm body width) was taken as a reference. For longer distances, we took enclosure structures with 3- and 10-m lengths as references. If a Raven was alone in the enclosure, we used 300 m as a standard nearest neighbour distance as this was double the maximal distance that we observed between two Ravens during all observational rounds. Ravens were recorded as being in a group if the intergroup distance (the spacing from the edge of the group to the next Raven) was at least 10 times as long as the intragroup distance (mean distance from each group member to its two nearest neighbours within the group). All other Ravens were labelled “solitary”. As Ravens have typical calls and displays that result in conspecifics either approaching (food calls for unspecific receivers, courtship displays for a specific receiver) or staying at distance (territory calls for unspecific receivers, vocal show-off for a specific receiver) from the signaller (Gwinner 1964; Heinrich 1989), Ravens may signal their social intentions even when solitary (Table 2).
Table 2.
Behavioural categories for solitary individuals and groups
| Category | Behaviours |
|
|---|---|---|
| Solitary individuals | Groups | |
| Agonistic | Territory calls | Approach retreat |
| Vocal show off | Forced retreat | |
| Fight | ||
| Chase flight | ||
| Neutral Affiliative | Locomotion and sitting | Locomotion and sitting |
| Food calls | Social play | |
| Courtship displays | Sitting close | |
| Allopreening | ||
| Invite preening | ||
| Courtship displays | ||
| Comfort behaviour + foraging | Preening/shaking | Preening/shaking |
| Resting/sleeping | Resting/sleeping | |
| Singing | Singing | |
| Individual play | Individual play | |
| Foraging | Foraging | |
| Others | Departing | Departing |
| Undefined vocalisations | Undefined vocalisations | |
Aggression
To have a closer look at the effect of number of birds present and food context on aggression rates, over 46 days from February to May 2010, T.W. recorded data on aggressive conflicts at either one or two (n = 17) morning feedings (bear/wolf and wild boars) for 30 min each, and took additional 30-min samples outside feeding times, once (n = 5), twice (n = 28) or thrice (n = 13) a day. Data on aggression were only collected at the wolf/bear, wild boar and deer enclosures and comprised 43 h of observation time during morning feeds and 50 h during the rest of the day (non-feeding context).
Data analyses
Statistical analyses were conducted using SPSS 19.0 (SPSS, Chicago, IL, USA). We used multinomial generalised linear mixed models to determine the effects of age and sex on the presence patterns of the birds. The presence category served as the dependent variable, age and sex as fixed factors and subject identity and month as random factors. To account for possible seasonal effects on sociality we also checked whether the distribution of presence categories changed over the months. We therefore ran a separate model with month as a fixed factor and subject identity as random factor. Wilcoxon signed-ranks tests were used for comparisons between the morning feedings and observation rounds on the same day, either for the number of Ravens present (n = 31 days), or the aggression rates during morning feeds and non-feeding situations (n = 46 days). A Mann–Whitney U test assessed differences in the number of birds present in groups or remaining solitary. We ran separate linear mixed models on each behavioural category to see whether being in a group or solitary had an effect on the extent to which a particular behavioural category was expressed. We therefore took the mean number of birds per observation round that were solitary or in groups, and calculated the proportion of grouped and solitary birds engaged in the different behavioural categories. The five behavioural categories served as dependent variables and whether the subject was solitary or in a group was taken as a fixed factor. The mean number of grouped and solitary individuals and the recording date were entered as random factors. All tests performed were two-tailed and α was set to 0.05.
Results
Flock size and composition
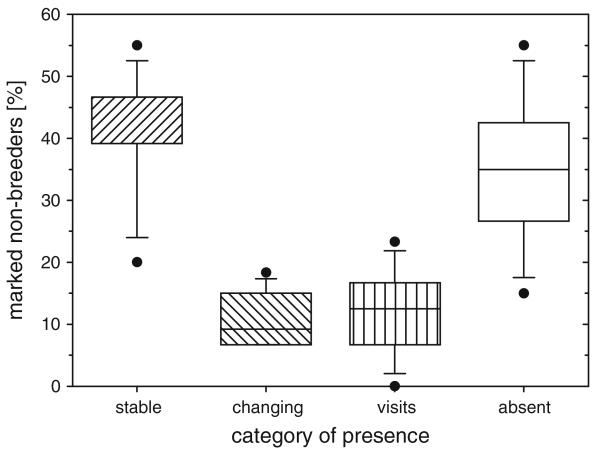
We recorded a mean number of 38 ± 17 birds (mean ± SD) present at the morning feeds (n = 442 days of data collection). Over the particular year in which the threshold of 100 marked birds was reached, only 60 of the 100 birds were seen (29 female: 10 adult, 13 subadult, 6 juvenile; 31 male: 11 adult, 15 subadult, 5 juvenile). When comparing the distribution of presence of the marked Ravens over the year, more birds were either ‘stable’ or ‘absent’ than ‘changing’ or ‘visiting’ (Fig. 2). Neither sex (F3.468 = 0.630, P = 0.596), nor age (F6.468 = 1.781, P = 0.101) had an effect on the presence patterns. There was a change in the distribution of presence categories over the year (F21.456 = 2.663, P < 0.001), which was mainly due to a constant increase of absent birds and a constant decrease of stable present birds. When dropping those two from the data, the percentage of visiting birds and changing birds did not differ within the year (F7.101 = 2.663, P = 0.286). All birds switched their presence category in at least 1 month with the exception of six birds whose presence was stable over the whole period of observation.
Fig. 2.
Median number of marked non-breeders: whose presence was stable, who were changing it, just paying visits to the Almtal, or were absent
Individual spacing and behaviour
The maximum number of birds seen during the morning feeds did not differ from the total number of birds seen the same day during the later observation round (Z31 = −0.284, P = 0.776). This indicates that we were able to find most of the Ravens during the rounds, although we cannot confirm this for unmarked birds.
During non-feeding conditions, there was no difference in the number of birds which were solitary and those in small groups (U33 = −0.475, P = 0.636) with a mean of 15 ± 7 solitary birds and 16 ± 11 Ravens in groups per observation round. Solitary birds kept a mean distance of approximately 70 ± 69 m from their neighbours, whereas mean nearest neighbour distances in groups (intragroup distance) was 2.54 m ± 2.20 m, and intergroup distance (between groups and surrounding individuals) was 65 m ± 80 m (the large standard deviations result from the adopted 300 m distance if the group, or the individual was alone in the observation area). Mean group size was 3.5 ± 1.2 Ravens (n = 192 groups).
Birds differed in the behavioural categories expressed if in groups or solitary. Some behaviours were never observed in groups such as singing (total n = 5), whereas others were confined to groups, like social interactions. Nevertheless, solitary Ravens were also able to express their social intentions through their vocalisations (see “Methods”). Linear mixed models revealed that grouping Ravens engaged in only slightly more agonistic behaviours than solitary ones [grouping birds 6.10 ± 1.59% (mean ± SE) and solitary birds 1.72 ± 1.73%, F1.70 = 3.474, P = 0.067]; however, they differed strongly with regards to affiliative interactions (grouping birds 14.19 ± 2.05% and solitary birds 2.19 ± 2.23%, F1.33 = 16.753, P < 0.001). Solitary Ravens in contrast performed more neutral behaviours like sitting and locomotion than grouping birds (grouping birds 50.37 ± 3.77% and solitary birds 63.13 ± 4.09%, F1.70 = 5.265, P = 0.025). No effect of individual spacing was apparent on the frequency of comfort behaviours expressed (grouping birds 15.36 ± 2.62% and solitary birds 14.84 ± 2.73%, F1.33 = 0.059, P = 0.810) or on the final category, the undefined vocalisations and departures (grouping birds 13.98 ± 3.47% and solitary birds 18.61 ± 3.78%, F1.29 = 0.864, P = 0.360).
Aggression
Aggression rates were significantly higher during feeds than in non-feeding situations with a mean conflict rate of 0.60 ± 0.31 conflicts per individual during 1 h of feeding compared to 0.07 ± 0.15 conflicts per individual during 1 h of the rest of the day (Z46 = −5.650, P < 0.001). No significant difference was found between the aggression rates per individual between the wolf/bear feeds and wild boar feeds (Z17 = −1.633, P = 0.102), nor between the three different non-feeding time slots per day (, P = 0.568), or if only two enclosures were visited (Z28 = −1.495, P = 0.135).
Discussion
Our study reveals that Raven groups are not as unstructured in their aggregation patterns as previously thought. The dichotomy between anonymous aggregations in the wild and socialised sub-groups with differentiated relationships in captivity cannot be fully resolved with this study; however, we can show that the foundation for socialised sub-groups is present in a free-flying Raven population.
According to the assumption that Ravens form anonymous aggregations, groupings should only be found in response to external factors like increasing foraging efficiency, antipredator defence or reducing thermoregulatory demands (Beauchamp 1999; Shoemaker and Phillips 2011). During our observation periods, Ravens gathered in one area for foraging. In addition, they assembled at their communal roost every evening and flocked for mobbing (personal observation). The formation of large aggregations therefore has a strong external component in those Ravens, which conforms to the findings in most literature (Marzluff et al. 1996; Wright et al. 2003) and our expectations for temporary and anonymous aggregations.
Aside from these gatherings, however, Ravens could be found either alone or in small sub-groups over the day. As predation pressure is relatively weak in these large birds (Haffer 1993), the necessity to remain in groups could, in an anonymous set-up, be explained by increased foraging efficiency (Beauchamp 2010; Dall and Wright 2009). During our observations, no pronounced differences were found in foraging rates between Ravens in groups and solitary birds. Rather, Ravens seemed to selectively join other individuals to engage in affiliative behaviours. In an anonymous setting, affilliative behaviours should be limited to pair partners or those that are in the process of pair formation. In our dataset, Ravens were found in sub-groups of a mean of 3.5 individuals, exchanging affiliative behaviours with more than one partner. This makes it unlikely that pair formation would be the only explanation for grouping in Ravens.
Small sub-groups characterised by affiliative interactions might consist of close kin, due to direct or inclusive fitness benefits (Carlisle and Zahavi 1986; Canestrari et al. 2005). The mean group size of 3.5 individuals is well within the range of typical clutch sizes of Ravens (Haffer 1993), so this scenario might be possible. However, so far, hardly any effects of kinship have been reported in wild Ravens (Parker et al. 1994; but see Webb et al. 2009). Future research is needed to disentangle whether or not small groups of Ravens may form around siblings in wild populations.
Irrespective of kinship, it is worth to point out that, aside from crowd-foraging, group formation in Ravens does correlate with affiliative behaviours. This supports the idea that Ravens form and maintain bonds within the aggregation of non-breeders (Hinde 1976); something that has so far only been shown in captive Ravens (Fraser and Bugnyar 2010b). The stability in flock composition over several months would also provide the opportunity for repeated interactions within dyads to occur, a fundamental requirement for differentiated relationships to form (Byrne and Bates 2007b).
Such valuable relationships could be used by non-breeder Ravens to cope with the scramble competition that characterises crowd-foraging (Heinrich 1988). Individuals may support each other during and/or after conflicts (Fraser and Bugnyar 2010a, b, 2012). Social embedding may also enable Ravens to better access food (Huber 1991) and minimise distress during food competition (Fraser and Bugnyar 2010a). Indeed, aggression rates in small groups were virtually absent in comparison to the situation in the foraging crowd. We have to bear in mind that our study site is characterised by a relatively stable environment with a predictable food supply, possibly affecting aggression rates as well as the time needed for foraging; notably, it might allow individuals to allocate extra time for socialising. Still, Ravens have a long history with humans, from joining hunter-gatherers to feeding on the dead bodies at battle fields and, more recently, at garbage dumps. Hence, it remains speculative how socially ‘artificial’ the situation at our study site actually is, and underlines the importance of taking into account the huge flexibility of the species.
Taken together, our study confirms that Raven life as a non-breeder is characterised by competition and yet also by the need to cooperate (Heinrich 2011). This may drive the need for increased social complexity and differentiated relationships of the type previously described for captive Ravens. Our data provide the first suggestive evidence that such socialised sub-groups exist in free-flying Raven flocks.
Acknowledgments
This work was financially supported from the FWF—Fonds zur Förderung der wissenschaftlichen Forschung (START: Y366-B17) and the DAAD—Deutscher Akademischer Austausch Dienst (D/07/44470), and we gratefully acknowledge the support provided by the Cumberland Gamepark and the Verein der Förderer der Konrad Lorenz Forschungsstelle. We thank Bernd Heinrich for interesting comments on an earlier draft of the manuscript. All experiments and bird manipulations comply with the current laws of Austria, and were authorized by the Central Administration of Upper Austria.
Contributor Information
Anna Braun, University Vienna, Vienna, Austria; Konrad Lorenz Research Station, Gruenau, Austria.
Thomas Walsdorff, University Vienna, Vienna, Austria; Université catholique de Louvain, Louvain-la-Neuve, Belgium.
Orlaith N. Fraser, University Vienna, Vienna, Austria
Thomas Bugnyar, University Vienna, Vienna, Austria; Konrad Lorenz Research Station, Gruenau, Austria.
References
- Amici F, Aureli F, Call J. Fission-fusion dynamics, behavioural flexibility, and inhibitory control in primates. Curr Biol. 2008;18:1415–1419. doi: 10.1016/j.cub.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Aureli F, Schaffner CM, Boesch C, Bearder SK, Call J, Chapman CA, Connor R, Di Fiore A, Dunbar RIM, Henzi SP, Holekamp K, Korstjens AH, Layton R, Lee P, Lehmann J, Manson JH, Ramos-Fernandez G, Strier KB, van Schaik CP. Fission-fusion dynamics, new research frameworks. Curr Anthropol. 2008;49:627–654. [Google Scholar]
- Barrett L, Henzi P, Dunbar R. Primate cognition: from ‘what now?’ to ‘what if?’. Trends Cogn Sci. 2003;7:494–497. doi: 10.1016/j.tics.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Beauchamp G. The evolution of communal roosting in birds: origin and secondary losses. Behav Ecol. 1999;10:675–687. [Google Scholar]
- Beauchamp G. Relaxed predation risk reduces but does not eliminate sociality in birds. Biol Lett. 2010;6:472–474. doi: 10.1098/rsbl.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boarman WI. Managing a subsidized predator population: reducing common raven predation on desert tortoises. Environ Manag. 2003;32:205–217. doi: 10.1007/s00267-003-2982-x. [DOI] [PubMed] [Google Scholar]
- Bond AB, Kamil AC, Balda RP. Social complexity and transitive inference in corvids. Anim Behav. 2003;65:479–487. [Google Scholar]
- Bond AB, Kamil AC, Balda RP. Serial reversal learning and the evolution of behavioral flexibility in three species of North American corvids (Gymnorhinus cyanocephalus, Nucifraga columbiana, Aphelocoma californica) J Comp Psychol. 2007;121:372–379. doi: 10.1037/0735-7036.121.4.372. [DOI] [PubMed] [Google Scholar]
- Bugnyar T. Knower-guesser differentiation in ravens: others’ viewpoints matter. Proc R Soc Lond B. 2011;278:634–640. doi: 10.1098/rspb.2010.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnyar T, Heinrich B. Ravens, Corvus corax, differentiate between knowledgeable and ignorant competitors. Proc R Soc Lond B. 2005;272:1641–1646. doi: 10.1098/rspb.2005.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnyar T, Heinrich B. Pilfering ravens, Corvus corax, adjust their behaviour to social context and identity of competitors. Anim Cogn. 2006;9:369–376. doi: 10.1007/s10071-006-0035-6. [DOI] [PubMed] [Google Scholar]
- Bugnyar T, Kotrschal K. Observational learning and the raiding of food caches in ravens, Corvus corax: is it ‘tactical’ deception? Anim Behav. 2002;64:185–195. [Google Scholar]
- Byrne RW, Bates LA. Brain evolution: when is a group not a group? Curr Biol. 2007a;17:R884. doi: 10.1016/j.cub.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Byrne RW, Bates LA. Sociality, evolution and cognition. Curr Biol. 2007b;17:R714–R723. doi: 10.1016/j.cub.2007.05.069. [DOI] [PubMed] [Google Scholar]
- Byrne RW, Whiten A. Machiavellian intelligence. Social expertise and the evolution of intellect in monkeys, apes and humans. Oxford University Press; New York: 1988. [Google Scholar]
- Caffrey C. Marking crows. N Am Bird Bander. 2000;26:146–148. [Google Scholar]
- Canestrari D, Marcos JM, Baglione V. Effect of parentage and relatedness on the individual contribution to cooperative chick care in carrion crows Corvus corone corone. Behav Ecol Sociobiol. 2005;57:422–428. [Google Scholar]
- Carlisle TR, Zahavi A. Helping at the nest, allofeeding and social status in immature arabian babblers. Behav Ecol Sociobiol. 1986;18:339–351. [Google Scholar]
- Connor RC, Heithaus MR, Barre LM. Superalliance of bottlenose dolphins. Nature. 1999;397:571–572. [Google Scholar]
- Dall SRX, Wright J. Rich pickings near large communal roosts favor ‘gang’ foraging by juvenile common ravens, Corvus corax. PLoS ONE. 2009;4:e4530. doi: 10.1371/journal.pone.0004530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dally JM, Clayton NS, Emery NJ. The behaviour and evolution of cache protection and pilferage. Anim Behav. 2006;72:13–23. [Google Scholar]
- de Waal FBM, Tyack PL. Preface. In: de Waal FBM, Tyack PL, editors. Animal social complexity: intelligence, culture, and individualized societies. Harvard University Press; Cambridge: 2003. pp. ix–xiv. [Google Scholar]
- Diamond J, Bond AB. A comparative analysis of social play in birds. Behaviour. 2003;140:1091–1115. [Google Scholar]
- Drack G, Kotrschal K. Aktivitätsmuster und Spiel von freilebenden Kolkraben Corvus corax im inneren Almtal/Oberösterreich. Monticula. 1995;7:159–174. [Google Scholar]
- Dunbar RIM. The social brain hypothesis. Evol Anthropol. 1998;6:178–190. [Google Scholar]
- Eklow A. The behaviour of ravens Corvus corax, in fresh snow. Var Fagelvarld. 1988;47:89–90. [Google Scholar]
- Emery NJ, Clayton NS. Effects of experience and social context on prospective caching strategies by scrub jays. Nature. 2001;414:443–446. doi: 10.1038/35106560. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Dally JM, Clayton NS. Western scrub-jays (Aphelocoma californica) use cognitive strategies to protect their caches from thieving conspecifics. Anim Cogn. 2004;7:37–43. doi: 10.1007/s10071-003-0178-7. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Seed AM, von Bayern AM, Clayton NS. Cognitive adaptations of social bonding in birds. Philos Trans R Soc Lond B. 2007;362:489–505. doi: 10.1098/rstb.2006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser ON, Bugnyar T. The quality of social relationships in ravens. Anim Behav. 2010a;79:927–933. doi: 10.1016/j.anbehav.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser ON, Bugnyar T. Do ravens show consolation? Responses to distressed others. PLoS ONE. 2010b;5:e10605. doi: 10.1371/journal.pone.0010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser ON, Bugnyar T. Ravens reconcile after aggressive conflicts with valuabe partners. PLoS ONE. 2011;6:e18118. doi: 10.1371/journal.pone.0018118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser ON, Bugnyar T. Reciprocity of agonistic support in ravens. Anim Behav. 2012;83:171–177. doi: 10.1016/j.anbehav.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinner E. Untersuchungen über das Ausdrucks- und Sozial-verhalten des Kolkraben (Corvus corax corax L.) Z Tierpsychol. 1964;21:657–748. [Google Scholar]
- Gwinner E. Über einige Bewegungsspiele des Kolkraben (Corvus corax L.) Z Tierpsychol. 1966;23:28–36. [PubMed] [Google Scholar]
- Haffer J. Corvidae—Rabenvögel. In: Glutz von Blotzheim UN, editor. Handbuch der Vögel Mitteleuropas. Aula; Wiesbaden: 1993. pp. 1947–2022. [Google Scholar]
- Heinrich B. Winter foraging at carcasses by three sympatric corvids, with emphasis on recruitment by the raven, Corvus corax. Behav Ecol Sociobiol. 1988;23:141–156. [Google Scholar]
- Heinrich B. Ravens in winter. Simon & Schuster; New York: 1989. [Google Scholar]
- Heinrich B. Dominance and weight changes in the common raven, Corvus corax. Anim Behav. 1994a;48:1463–1465. doi: 10.1006/anbe.1998.0906. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Age and mouth color in common ravens. Condor. 1994b;94:549–550. [Google Scholar]
- Heinrich B. Conflict, cooperation and cognition in the common raven. Adv Stud behav. 2011;43:189–237. [Google Scholar]
- Heinrich B, Smolker R. Play in common ravens (Corvus corax) In: Bekoff M, Byers JA, editors. Animal play: evolutionary, comparative and ecological perspectives. Cambridge University Press; Cambridge: 1998. pp. 27–44. [Google Scholar]
- Heinrich B, Kaye D, Knight T, Schaumburg K. Dispersal and association among common ravens. Condor. 1994;96:545–551. [Google Scholar]
- Hinde RA. Interactions, relationships and social structure. Man. 1976;11:11–17. [Google Scholar]
- Holekamp KE, Cooper SM, Katona CI, Berry NA, Frank LG, Smale L. Patterns of association among female spotted hyenas (Crocuta crocuta) J Mammal. 1997;78:55–64. [Google Scholar]
- Huber B. Bildung, Alterszusammensetzung und Sozialstruktur von Gruppen nichtbrütender Kolkraben (Corvus corax L.) Metelen Schr Natursch. 1991;2:45–59. [Google Scholar]
- Marzluff JM, Heinrich B, Marzluff CS. Raven roosts are mobile information centres. Anim Behav. 1996;51:89–103. [Google Scholar]
- McComb K, Moss C, Durant SM, Baker L, Sayialel S. Matriarchs as repositories of social knowledge in African elephants. Science. 2001;292:491–494. doi: 10.1126/science.1057895. [DOI] [PubMed] [Google Scholar]
- Parker PG, Waite TA, Heinrich B, Marzluff JM. Do common ravens share ephemeral food resources with kin? DNA finger-printing evidence. Anim Behav. 1994;48:1085–1093. [Google Scholar]
- Paz-y-Mino CG, Bond AB, Kamil AC, Balda RP. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430:778–781. doi: 10.1038/nature02723. [DOI] [PubMed] [Google Scholar]
- Rösner S, Selva N. Use of the bait-marking method to estimate the territory size of scavenging birds: a case study on ravens Corvus corax. Wildl Biol. 2005;11:183–191. [Google Scholar]
- Rösner S, Selva N, Müller T, Pugacewicz E, Laudet F. Raven Corvus corax ecology in a primeval temperate forest. In: Jerzak L, Kavanagh BP, Tryjanowski P, editors. Corvids of Poland. Bogucki Wyd, Nuak; Poznan: 2005. pp. 385–405. [Google Scholar]
- Seed AM, Clayton NS, Emery NJ. Postconflict third-party affiliation in rooks, Corvus frugilegus. Curr Biol. 2007;17:152–158. doi: 10.1016/j.cub.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Shoemaker CM, Phillips RS. Observation of ground roosting by American crows. Wilson J Ornithol. 2011;123:185–187. [Google Scholar]
- Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT. Primate societies. University of Chicago Press; Chicago: 1987. [Google Scholar]
- Stahler D, Heinrich B, Douglas S. Common ravens, Corvus corax, preferentially associate with grey wolves, Canis lupus, as a foraging strategy in winter. Anim Behav. 2002;64:283–290. [Google Scholar]
- Stiehl RB. Aspects of the ecology of the common raven in Harney Basin. Portland State University; Oregon: 1978. PhD thesis. [Google Scholar]
- Symington MM. Fission-fusion social organization in Ateles and Pan. Int J Primatol. 1990;11:47–61. [Google Scholar]
- Vander Wall SB, Balda RP. Coadaptations of the clark’s nutcracker and the pinon pine for efficient seed harvest and dispersal. Ecol Monogr. 1977;47:89–111. [Google Scholar]
- Webb WC, Boarman WI, Rotenberry JT. Movements of juvenile Common ravens in an arid landscape. J Wildlife Manag. 2009;73:72–81. [Google Scholar]
- Wright J, Stone RE, Brown N. Communal roosts as structured information centres in the raven, Corvus corax. J Anim Ecol. 2003;72:1003–1014. [Google Scholar]