Abstract
Cysteine-rich protein-61 (CYR61), also known as connective tissue growth factor, CYR61, and nephroblastoma overexpressed gene 1 (CCN1), is a heparin-binding protein member of the CCN family of matricellular proteins. Gene expression profiles showed that Cyr61 is upregulated in human acute lung injury (ALI), but its functional role is unclear. We hypothesized that CYR61 contributes to ALI in mice. First, we demonstrated that CYR61 expression increases after bleomycin-induced lung injury. We then used adenovirus-mediated gene transfer to determine whether CYR61 overexpression in the lungs was sufficient to cause ALI. Mice instilled with CYR61 adenovirus showed greater weight loss, increased bronchoalveolar lavage total neutrophil counts, increased protein concentrations, and increased mortality compared with mice instilled with empty-vector adenovirus. Immunohistochemical studies in lungs from humans with idiopathic pulmonary fibrosis revealed CYR61 expression on the luminal membrane of alveolar epithelial cells in areas of injury. We conclude that CYR61 is upregulated in ALI and that CYR61 overexpression exacerbates ALI in mice.
Keywords: Cysteine-rich protein-61, CCN1, acute lung injury
acute respiratory distress syndrome (ARDS) remains an important cause of morbidity and mortality in adults and children worldwide (29, 34). ARDS is characterized by an excessive inflammatory response and damage to the alveolar epithelial-capillary barrier. The standard treatment for ARDS is supportive and includes mechanical ventilation. In the absence of specific treatments for ARDS, the study of new modulators of the inflammatory process represents an important step for the development of new therapeutic agents.
The cysteine-rich protein 61 (CYR61) (also known as connective tissue growth factor, CYR61, and nephroblastoma overexpressed gene 1, CCN1) is a 40-kDa heparin-binding protein rich in cysteine residues (25). CYR61 belongs to a family of secreted proteins referred to as CCN. CCN proteins bind to both the extracellular matrix and to cell-surface proteins and are therefore called matricellular. As a heparin-binding protein, CYR61 binds matrix proteins such as heparan sulfate and similar glycoaminoglycans, which provides a protected environment and may allow CYR61 to exert its functions for a prolonged period of time. CYR61 is involved in a wide range of cellular processes, such as cell adhesion, proliferation, migration, differentiation, and survival (2, 10–12, 14, 17). CYR61 assumes these diverse and sometimes opposing functions by its ability to bind different combinations of coreceptors (e.g., integrins) on the cell surface (17). For example, CYR61 promotes endothelial cell proliferation and survival through binding to integrin-αvβ3 (9, 18) but promotes fibroblast adhesion, apoptosis, and senescence through binding to integrin-α6β1 and to heparan sulfate proteoglycans (HSPGs) such as syndecan-4 (6, 11). Furthermore, CYR61 is able to reprogram macrophages toward a proinflammatory M1 polarization phenotype through binding to integrin-αMβ2 on the macrophage cell surface (1). By its ability to regulate all these different cellular functions, CYR61 is involved in different biological activities, including angiogenesis (9), tumorigenesis (19, 30, 32), and wound injury repair (11).
A growing body of evidence indicates that CYR61 plays a role in lung disease (21, 24, 27, 31). We have shown that Cyr61 gene expression is upregulated in mice after activation of the Fas/Fas ligand system (22). These results were corroborated by additional lung-expression profiles from the literature showing that Cyr61 is upregulated in humans with chronic obstructive pulmonary disease (COPD) as well as in animal models of hyperoxia (27), lung fibrosis (13), asthma (4), and ventilation-induced lung injury (VILI) (7, 31). However, it is unclear whether CYR61 plays a protective role in acute lung injury or instead contributes to its pathogenesis. We hypothesized that CYR61 plays an important role in the development of acute lung injury by enhancing lung inflammation and contributing to tissue injury in the lungs. We show that CYR61 expression increases in the lungs after bleomycin instillation. Moreover, CYR61 overexpression in murine lungs after adenovirus-mediated gene transfer was associated with weight loss, lung injury, and increased mortality.
MATERIALS AND METHODS
Reagents
Polyclonal rabbit anti-human CYR61 antibody was from Abcam (Cambridge, MA), monoclonal rabbit anti human β-actin antibody was from Cell Signaling (Danvers, MA), and bleomycin was from Hospira (Lake Forest, IL). Other reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Construction of Adenoviral Vectors
We created adenoviral vectors to overexpress CYR61. To create the adenoviral vectors, the murine Cyr61 open reading frame was used as a template for Cyr61 amplification, using PCR and the following primers: forward 5′-GAT CGG CCA AAT CGG CCG CCG CCA CCA TGA GCT-3′; reverse 5′-GAT CGG CCA TAA GGG CCT TAG TCC CTG AAC TTG-3′. The Cyr61 DNA fragment was fused into a pENTR-1G entry vector (3) and subsequently transferred into the destination vector pAd/CMV/V5-DEST (Gateway; Invitrogen, Carlsbad, CA) by homologous recombination. The CYR61 adenovirus (AdCyr61) was produced in 293A cells and purified by cesium chloride gradient ultracentrifugation (3). A control adenovirus (AdCtr) was made lacking Cyr61.
Animal Protocols
All of the animal experiments were performed according to protocols approved by the institutional animal research committee of the University of Washington. Mice were housed in a pathogen-free environment according to University of Washington animal use guidelines. Male C57BL/6 mice aged 8 wk were anesthetized with 5% inhaled isofluorane and then intubated endotracheally with a 20-gauge angiocatheter. Placement of the catheter in the trachea was verified by visualizing the movement of a 100-μl bubble of water in response to respiratory efforts. After intubation was confirmed, the trachea was instilled with bleomycin 1(.2 U/kg) or PBS in a volume ranging from 48 to 56 μl (n = 5/group). After the installations, the mice were extubated, returned to their cages, and allowed free access to food and water. At 3 days after PBS instillation and 10 days after bleomycin instillations, the mice were euthanized with 0.30 ml/kg ip of Beuthanasia-D (Schering-Plough Animal Health, Union, NJ). The thorax was rapidly opened, and the mouse was exsanguinated by direct cardiac puncture. The left lung was removed and flash frozen in liquid nitrogen. The right lung was lavaged with 0.6 mM EDTA in PBS; an aliquot of the bronchoalveolar lavage fluid (BALF) was removed for cell counts and differentials, the remaining fluid was spun at 1,200 g, and the supernatants were stored at −80°C in individual aliquots.
For the experiments using adenoviral vectors, C57BL/6 mice aged 6–9 mo were anesthetized as described above. The mice then received intratracheal instillations of AdCyr61 or AdCtr, 1 × 1010 virus particles. The mice were allowed to recover from anesthesia, given free access to food and water for 8 days, and then euthanized and studied as described above.
BALF Measurements
Total cell counts in the BALF were performed with an automated cell counter (Countess, Invitrogen). Differential counts were performed on cytospin preparations using the Diff-quick method (Fisher Scientific, Kalamazoo, MI). BALF total protein was measured with the bicinchoninic acid method (Pierce, Rockford, IL).
Measurements of CYR61
PCR.
Lung tissues were homogenized, and the cells were lysed in TRIzol (Life Technologies, Carlsbad, CA). RNA was extracted in chloroform/isopropanol, washed in 75% ethanol, and resuspended in water. The samples were treated with DNAse for 30 min (DNA-free kit; Ambien, Austin, TX), and then cDNA was made with the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Quantitative PCR was performed using the SYBR-green method with the following primers for mouse CYR61: forward 5′-CTGCCA CCG CTC TGA AAG GGA T-3′; reverse 5′-CCC CCT TTTGGT AGA TTC TGG-3′. Hypoxanthine phosphoribosyltransferase was used as housekeeping gene (forward 5′-CAG GCC AGA CTT TGT TGG AT-3′; reverse 5′-CTT GCG CTC ATC TTA GGC TT-3′). Data were interpreted using the ΔΔCT method.
Immunoblots.
Briefly, samples containing equal amounts of protein were separated by SDS-PAGE under reducing conditions using NuPAGE Novex 4–12% Bis Tris polyacrylamide gradient gel with MES-SDS running buffer (Invitrogen). After electrophoresis, the gel contents were transferred onto a nitrocellulose membrane (Hybond-ECL; Amersham Biosciences, Piscataway, NJ) using NuPAGE transfer buffer (Invitrogen). Posttransfer, the membranes were incubated for 1 h with 5% nonfat milk, rinsed three times for 5 min in PBS with 0.1% Tween-20 (PBS-T) (Sigma-Aldrich), and then incubated overnight at 4°C with primary antibody. After primary antibody labeling, the membranes were washed three times with PBS-T, and bound antibody was detected by incubation for 1 h with goat anti-rabbit IgG-horseradish peroxidase (HRP)-conjugated secondary antibody (Invitrogen). The membranes were then washed five times with PBS-T and developed with ECL Prime Western Blotting detection reagent (Amersham). Visualization was accomplished using an Omega Ultra-Lum digital camera (Claremont, CA). After visualization of the signal for each respective antibody, the membranes were stripped of antibody by incubation for 15 min at room temperature in Restore Plus Western blot stripping buffer (Pierce). After being stripped, the membranes were washed three times in PBS-T and incubated for 30 min at room temperature in 5% nonfat milk. The blots were washed three times in PBS-T, then incubated overnight at 4°C with rabbit anti-human β-actin monoclonal antibody, labeled with goat anti-rabbit HRP-conjugated secondary antibody (Invitrogen) for 1 h at room temperature, developed with the chemiluminescent substrate as described above, and visualized as described. Densitometry analysis of the immunoblots was performed using ImageJ software (National Institutes of Health, Bethesda, MD).
ELISA.
ELISA was developed in house to measure CYR61. To generate murine CYR61 standard, the cDNA of mouse Cyr61 (accession number NM 010516.2), was obtained from Sino Biological (Beijing, China) and cloned into a mammalian expression vector. The construct contains the whole Cyr61-coding cDNA, including the native start codon, and a 10-histidine tag on the 3′ end of the gene. HEK 293 cells were transfected with the plasmid using Lipofectamine from Life Technologies. Cell lysates were collected 5 days after transfection. The expressed mouse CYR61-HIS fusion protein was purified with a Ni+-loaded affinity column. The specificity of purified mouse CYR61 was determined by immunoblotting and silver staining of SDS-PAGE. For the antigen capture mouse CYR61 ELISA, goat anti-mouse CYR61 IgG (Santa Cruz Biotechnology, Dallas, TX) was used as a capture antibody, and biotinylated sheep anti-mouse CYR61 IgG (R&D Systems, Minneapolis, MN) was used as a detection antibody. The lower limit of detection of the assay was 31.25 ng/ml.
Detection of CYR61 in Human Lung Tissue
All the human studies were performed according to protocols submitted to and approved by the Institutional Review Committee of the University of Washington. Briefly, human samples were obtained from deidentified biopsies from patients with idiopathic pulmonary fibrosis (IPF). Deparaffinized lung sections were quenched with Peroxo-Block (Zymed Laboratories, San Francisco, CA). Antigenic retrieval was performed with heated 10 mM citric acid. Slides were blocked with protein block (Dako, Carpenteria, CA) and then incubated with goat anti-mouse Cyr61 (Santa Cruz Biotechnology). The slides were developed with diaminobenzidine (Zymed Laboratories). All samples underwent hematoxylin and eosin staining.
Data Analysis
Data are expressed either as individual data points or as means ± SE. Comparisons between two groups were done with two-tailed t-test; comparisons between multiple groups were done by ANOVA with Tukey's post hoc analysis. A P value <0.05 was considered significant.
RESULTS
Lung CYR61 Expression Increases Following Bleomycin Instillation
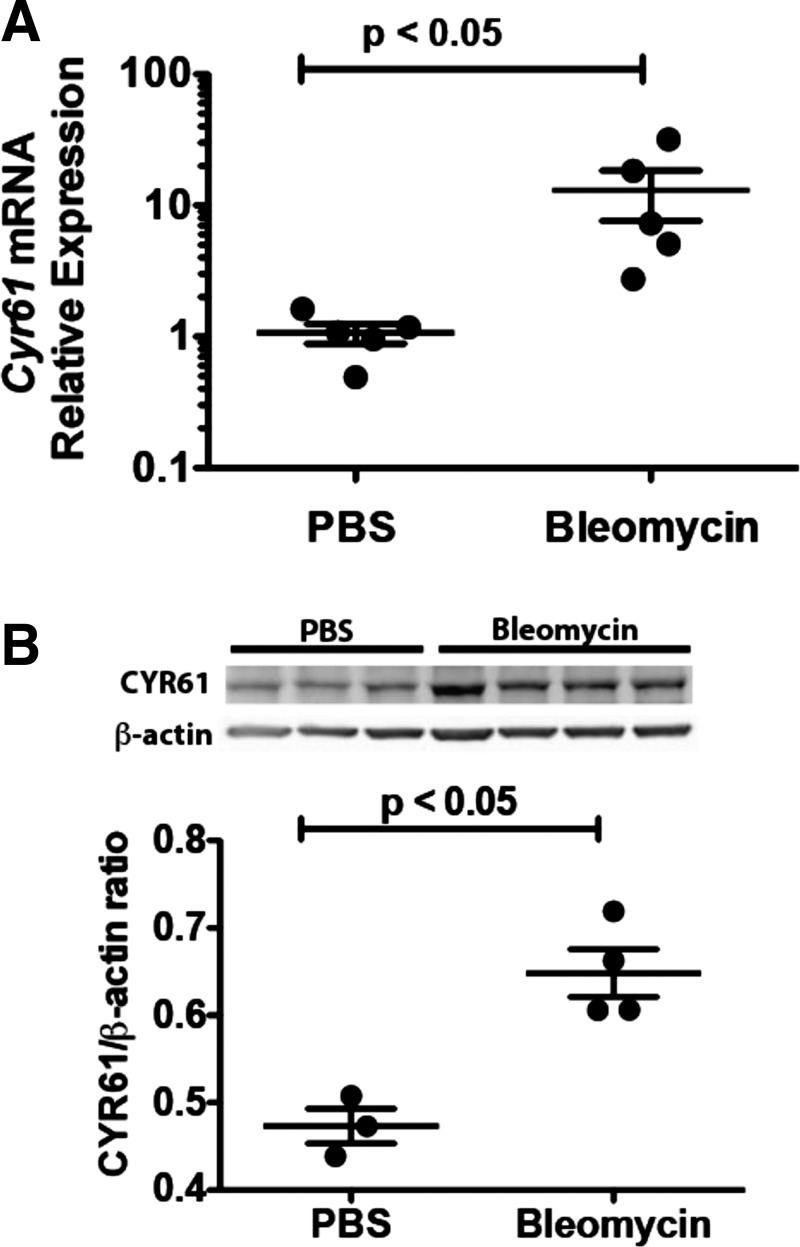
First, we investigated whether CYR61 expression increases in the lungs after bleomycin-induced lung injury. BALF from bleomycin-treated mice showed a significant increase in total neutrophil counts (0.35 ± 0.22 × 104 cells in the PBS-treated mice vs. 4.14 ± 0.99 × 104 in the bleomycin-treated mice, P < 0.05) (Fig. 1A) and in total protein concentrations (131.20 ± 23.06 μg/ml vs. 1,357.00 ± 283.60 μg/ml, P < 0.05) (Fig. 1B). We found that, compared with mice treated with PBS, Cyr61 mRNA expression increased in whole-lung lysates on day 10 after bleomycin instillation (Fig. 2A) (P < 0.05). CYR61 protein expression was confirmed by immunoblotting on lung homogenates from mice 10 days after bleomycin (Fig. 2B). These data demonstrate that CYR61 expression increases in the setting of acute injury induced by bleomycin instillation.
Fig. 1.
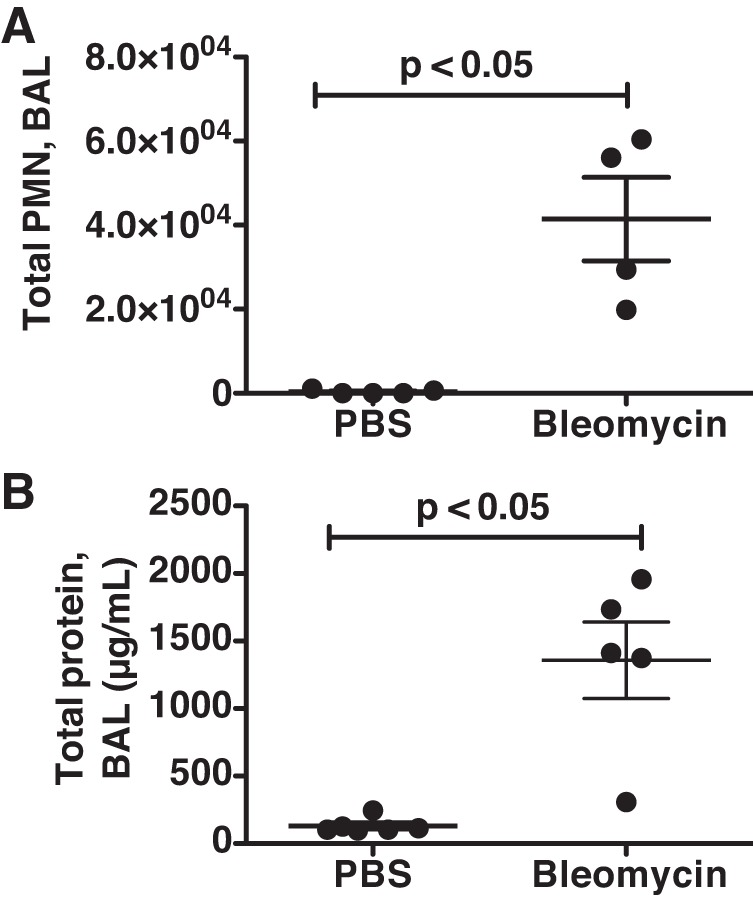
Mice developed lung injury 10 days after bleomycin administration. Bronchoalveolar lavage fluid (BALF) was collected in mice 10 days after intratracheal instillation of either bleomycin (1.2 IU/kg) or PBS for determination of total polymorphonuclear neutrophils (PMN) cell count (A) and protein concentrations (B). Both the total BALF PMN cell count and the BALF protein concentrations were significantly increased in the mice treated with bleomycin compared with PBS-treated mice (P < 0.05). Dots represent individual mice; lines represent means ± SE (PBS control group, n = 5–6; bleomycin group, n = 4–5).
Fig. 2.
Cysteine-rich protein-61 (CYR61) expression increases in murine lungs 10 days after bleomycin instillation. C57BL/6 mice received intratracheal instillations of either bleomycin (1.2 U/kg) or PBS (day 3). 10 days after bleomycin instillation, the lungs were homogenized for quantitative real-time PCR and immunoblots. The lung Cyr61 mRNA expression increased on day 10 after bleomycin administration (P < 0.05) (A). Similarly, the lung CYR61 protein expression was increased on day 10 after bleomycin administration, compared with mice studied 3 days after PBS administration (B). Dots represent individual data points; lines represent means ± SE.
Adenoviral Expression of CYR61 in Healthy Lungs Exacerbates Lung Injury and Is Associated with Weight Loss and Increased Mortality
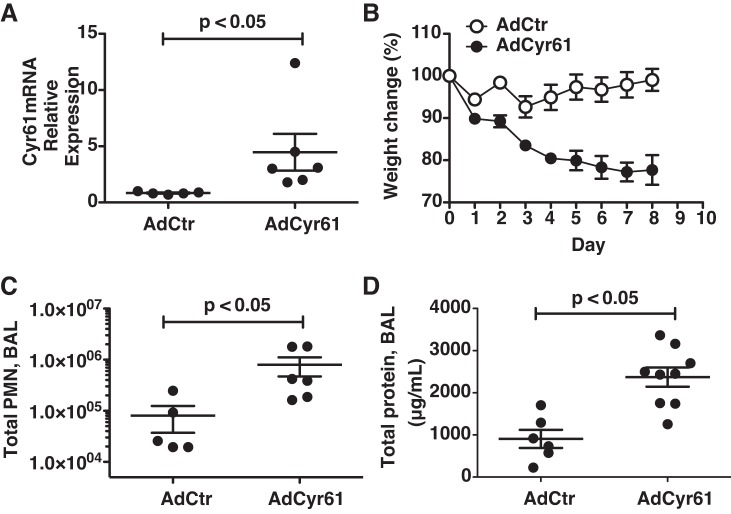
To investigate the effect of CYR61 in lungs directly, we generated adenovirus particles capable of expressing Cyr61 in infected cells (AdCyr61). The effect of CYR61 overexpression in vivo was determined by delivering AdCyr61 intratracheally to C57BL/6 mice. Control mice received an equal dose of virus (AdCtr). Cyr61 mRNA increased in the lungs of mice instilled with AdCyr61 compared with mice instilled with AdCtr and remained elevated on day 8 postinstillation (Fig. 3A). Instillation of AdCyr61 was associated with an increase in mortality: 10 out of 12 mice instilled with AdCyr61 met criteria for euthanasia, compared with 0 mice instilled with AdCtr. The increased mortality was associated with increased evidence of illness, measured as progressive weight loss in the AdCyr61 group, compared with mild weight gain in the AdCtr group (Fig. 3B). This was associated with the development of a neutrophilic alveolitis in the lungs of the mice receiving AdCyr61, measured as increased total BALF neutrophil counts compared with AdCtr mice (0.81 ± 0.44 × 105 cells for AdCtr vs. 7.94 ± 0.32 × 105 cells for AdCyr61, P < 0.05) (Fig. 3C). In addition, mice in the AdCyr61 group had increased lung permeability, measured as elevated total BALF protein compared with the AdCtr group (907.8 ± 215.3 μg/ml for the AdCtr group vs. 2,373.0 ± 228.9 μg/ml for the AdCyr61 group, P < 0.05) (Fig. 3D). Thus CYR61 overexpression was associated with increased lung injury, weight loss, and mortality.
Fig. 3.
Intratracheal delivery of CYR61 adenovirus (AdCyr61) increases CYR61 expression in the lungs and results in increased weight loss, mortality, and lung injury. C57BL/6 mice received intratracheal instillation of 1 × 1010 virus particles of AdCyr61 or vehicle adenovirus (AdCtr). 8 days following the instillations, BALF was collected, and murine lung tissues were homogenized for RNA extraction. AdCyr61 instillation increased Cyr61 mRNA expression in the lungs (A) and was associated with progressive weight loss (B) and signs of alveolitis with increased total BALF PMN count (C) and protein concentration (D) compared with mice instilled with AdCtr. Dots represent individual mice; lines represent means ± SE.
CYR61 is Expressed in the Lungs of Humans with IPF
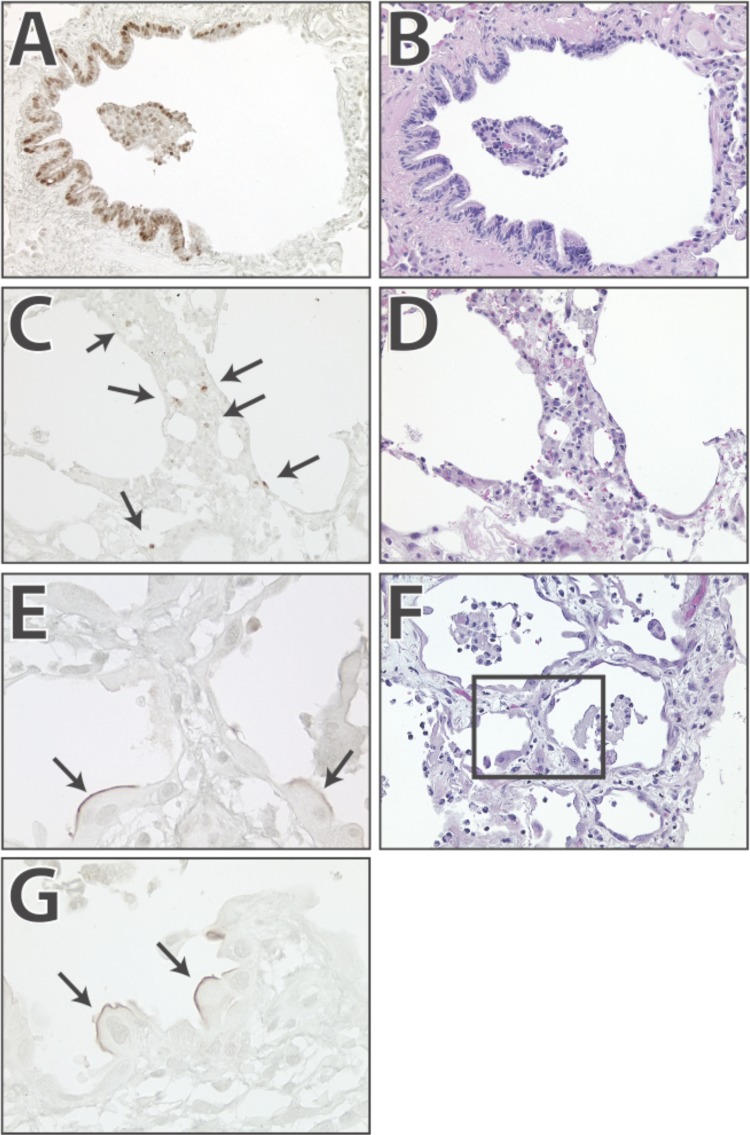
Finally, we investigated the human relevance of the murine data by performing CYR61 immunohistochemistry in tissue sections from one patient who died from ARDS and three patients with IPF. CYR61 expression was seen in three different patterns. Pattern 1 consisted of strong staining in airway epithelial cells (Fig. 4). Pattern 2 was seen in large cuboidal cells in alveoli with evidence of active bronchiolarization. These cuboidal cells showed expression of CYR61 on the luminal surface but not on the cytoplasm or nuclei. Pattern 3 consisted of nuclear and cytoplasmic staining in cells located within thickened alveolar septa and on the alveolar surface. Of these three patterns, Pattern 1 (airway expression) was seen in affected and nonaffected areas of the lung, whereas Patterns 2 and 3 were seen exclusively in areas of ongoing fibrosis.
Fig. 4.
CYR61 is expressed in alveolar epithelial cells in humans with idiopathic pulmonary fibrosis (IPF). To determine whether CYR61 is expressed in the lungs of humans with lung disease, we performed immunohistochemistry in the lungs of 3 patients that died from IPF and 1 patient that died from acute respiratory distress syndrome. The results were similar. The most common pattern is staining of airway epithelial cells (A and B show hematoxylin and eosin, H and E). In addition, there is staining in cells located in thickened airway walls (C and D show H and E). A third pattern consists of staining on the luminal membrane of cuboidal alveolar cells (closed arrows, E and G; F shows H and E staining). Inset: area shown in E.
DISCUSSION
In this study, we showed that CYR61 expression increases in the lungs after bleomycin-induced lung injury. Furthermore, we showed that adenovirus-mediated Cyr61 gene transfer in murine lungs was associated with weight loss, lung injury, and increased mortality. These results are important because they demonstrate that CYR61 expression is activated in the context of lung injury and may contribute to tissue injury.
CYR61 is expressed constitutively in a large number of cells in different organs, and its expression is highly sensitive to environment perturbations. Animal studies have shown that CYR61 expression increases following tissue injury in various tissues, including skin, kidney, and cardiovascular injuries (5). In healthy lungs, CYR61 is primarily expressed in airway epithelial cells (2). A number of studies using gene expression analysis found that Cyr61 was present among different genes upregulated in humans with COPD (22), as well as in an animal model of lung fibrosis (15, 21), hyperoxia (23), and VILI (7, 27). Thus the data available from the literature show that CYR61 is expressed in healthy lungs, and its expression increases in a number of lung disease processes.
We first investigated whether CYR61 expression increases in the bleomycin model of lung injury and specifically focused on the early stages after instillation. The response to bleomycin is characterized by an initial inflammatory phase followed by a remodeling and repair phase that results in lung fibrosis. In our study, Cyr61 expression increased significantly during the inflammatory phase at day 10 postinstillation. The mechanism that triggers CYR61 upregulation during lung inflammation is not clear. CYR61 is coded by an immediate-early gene, which is regulated by various stimuli, including environmental perturbations, growth factors, hormones, and cytokines (5). Transforming growth factor-β (TGF-β), which plays a major role in bleomycin-induced lung fibrosis, is also a potent inductor of CYR61 expression (26). Studies in kidneys have shown that TGF-β can upregulate CYR61 expression in renal tubular epithelial cells in animal models of obstructive nephropathy (15). Because both TGF-β and CYR61 demonstrate a peak expression during the inflammatory phase postbleomycin instillation, it is possible that TGF-β is involved in CYR61 upregulation after bleomycin, but this needs to be confirmed by further studies.
Given the robust expression of CYR61 after bleomycin-induced lung injury, we next determined the function played by CYR61 in the pathophysiology of lung injury using adenovirus-mediated Cyr61 gene transfer to murine lungs. Adenovirus-mediated transfer of Cyr61 to the lungs of healthy mice resulted in lung injury, weight loss, and increased mortality. This finding is inconsistent with previous studies on CYR61, which attributed to CYR61 an important role in tissue remodeling and repair after injury. In the skin, CYR61 promotes wound healing by inducing skin fibroblast senescence and antifibrotic gene activation (11). In an animal model of autoimmune myocarditis, systemic Cyr61 gene transfer resulted in a reduced autoimmune myocarditis score and immune cell infiltration by reducing in vivo and in vitro immune cell migration (28). In the lungs, CYR61 has been shown to protect alveolar epithelial cells from apoptosis in the context of hyperoxia (27) and to accelerate alveolar epithelial repair in vitro (33). Thus the proinflammatory effect of CYR61 in the lungs seen in our study represents a novel in vivo function of CYR61 that differs from the protective effect shown in the literature so far (11).
Several lines of evidence suggest that CYR61 promotes a proinflammatory response by different mechanisms. First, CYR61 has a biphasic effect on immune cell migration. Initially, CYR61 attracts immune cells to sites of injury, but later it renders them refractory to further chemotactic stimulation, resulting in the immobilization of inflammatory cells at the injury site (20). Second, in addition to its effect on cell migration, Bai et al. (1) demonstrated that CYR61 induces a proinflammatory genetic program in macrophages with M1 characteristics and promotes adhesion of activated macrophages through binding to integrin-αMβ2 and HSPGs (1). Third, CYR61 can modulate the effect of proinflammatory cytokines and contributes to tissue injury by increasing the proapoptotic effect of cytokines, such as TNF-α, FasL, and TRAIL (6, 8). Fourth, CYR61 expression is upregulated in lung epithelial cells after exposure to cigarette smoking and induces IL-8 secretion by lung epithelial cells, resulting in the recruitment of more inflammatory cells, in particular neutrophils (23). Finally, CYR61 blockade with a neutralizing antibody significantly attenuated the severity of renal inflammation and decreased renal fibrosis after ischemic acute kidney injury (16). Thus we postulate that CYR61 is proinflammatory in the lungs.
There are a number of limitations in the present study. First, it is possible that adenovirally mediated gene transfer to the lungs induces inflammation and may account for part of the lung injury found in the study. However, our control empty vector did not induce significant lung injury or weight loss. Second, CYR61 expression in our adenoviral model was only measured at the RNA level. Therefore CYR61 protein concentration after adenovirus instillation and the type of cells overexpressing CYR61 remain unclear. However, the observed differences in inflammatory response suggest that CYR61 was bioactive.
In summary, CYR61 expression increases in mice after bleomycin instillation. Overexpression of CYR61 in murine lungs after adenovirus instillation increases lung injury and is associated with weight loss and increased mortality. In lungs of humans with IPF, CYR61 is constitutively expressed in the airway epithelium and shows a distinct pattern of expression in the alveolar epithelial cells. We conclude that CYR61 is upregulated in the context of lung injury and that CYR61 overexpression induces acute lung injury.
GRANTS
This work was supported by Swiss National Science Foundation Grant PBGEP3-142293 (S. Grazioli), NIH:NHLBI grant HL081764 (G. Matute-Bello), NHLBI grant HL086883 (W. Altemeier), HL103868 (P. Chen), and HL120947 (P. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S. Grazioli, S. Gil, D.A., O.K., A.W.F., J.F.H., and T.B. performed experiments; S. Grazioli, J.S.D., L.M.S., W.A.A., and G.M.-B. analyzed data; S. Grazioli, P.C., J.S.D., W.A.A., and G.M.-B. interpreted results of experiments; S. Grazioli, A.W.F., and G.M.-B. prepared figures; S. Grazioli and G.M.-B. drafted manuscript; S. Grazioli, S. Gil, D.A., O.K., A.W.F., J.F.H., T.B., P.C., J.S.D., L.M.S., W.A.A., and G.M.-B. edited and revised manuscript; S. Grazioli, S. Gil, D.A., O.K., A.W.F., J.F.H., T.B., P.C., J.S.D., L.M.S., W.A.A., and G.M.-B. approved final version of manuscript; G.M.-B. conception and design of research.
ACKNOWLEDGMENTS
The authors acknowledge Kristen Mittelsteadt, Chi F. Hung, and Yu-Hua Chow for laboratory and clinical assistance and Bryce Johnson and Dr. Shuyu Ren for assistance with adenoviral work.
Current address for L. Schnapp: Division of Pulmonary, Critical Care, Allergy, and Sleep Medicine at the Medical University of South Carolina, Charleston, South Carolina; current address for P. Chen: Division of Pulmonary and Critical Care, Women's Guild Lung Institute, Cedars-Sinai Medical Center, Los Angeles, California.
REFERENCES
- 1.Bai T, Chen CC, Lau LF. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J Immunol 184: 3223–3232, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brigstock DR. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61). Angiogenesis 5: 153–165, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Campanholle G, Mittelsteadt K, Nakagawa S, Kobayashi A, Lin SL, Gharib SA, Heinecke JW, Hamerman JA, Altemeier WA, Duffield JS. TLR-2/TLR-4 TREM-1 signaling pathway is dispensable in inflammatory myeloid cells during sterile kidney injury. PLoS One 8: e68640, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y, Chen HL, Cheng S, Xie JG, Xiong WN, Xu YJ, Fang HJ. Inhibitory effect of dexamethasone on expression of cysteine-rich 61 protein in airway epithelial cells of allergic mouse models. J Huazhong Univ Sci Technolog Med Sci 33: 628–631, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol 41: 771–783, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CC, Young JL, Monzon RI, Chen N, Todorovic V, Lau LF. Cytotoxicity of TNFalpha is regulated by integrin-mediated matrix signaling. EMBO J 26: 1257–1267, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolinay T, Kaminski N, Felgendreher M, Kim HP, Reynolds P, Watkins SC, Karp D, Uhlig S, Choi AM. Gene expression profiling of target genes in ventilator-induced lung injury. Physiol Genomics 26: 68–75, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Franzen CA, Chen CC, Todorovic V, Juric V, Monzon RI, Lau LF. Matrix protein CCN1 is critical for prostate carcinoma cell proliferation and TRAIL-induced apoptosis. Mol Cancer Res 7: 1045–1055, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grote K, Salguero G, Ballmaier M, Dangers M, Drexler H, Schieffer B. The angiogenic factor CCN1 promotes adhesion and migration of circulating CD34+ progenitor cells: potential role in angiogenesis and endothelial regeneration. Blood 110: 877–885, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Jedsadayanmata A, Chen CC, Kireeva ML, Lau LF, Lam SC. Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/mouse connective tissue growth factor is mediated through integrin alpha(IIb)beta(3). J Biol Chem 274: 24321–24327, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12: 676–685, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kireeva ML, Mo FE, Yang GP, Lau LF. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol 16: 1326–1334, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, Gunther A, Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 119: 772–787, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyriakides TR, Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb Haemost 90: 986–992, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Lai CF, Chen YM, Chiang WC, Lin SL, Kuo ML, Tsai TJ. Cysteine-rich protein 61 plays a proinflammatory role in obstructive kidney fibrosis. PLoS One 8: e56481, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai CF, Lin SL, Chiang WC, Chen YM, Wu VC, Young GH, Ko WJ, Kuo ML, Tsai TJ, Wu KD. Blockade of cysteine-rich protein 61 attenuates renal inflammation and fibrosis after ischemic kidney injury. Am J Physiol Renal Physiol 307: F581–F592, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Lau LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci 68: 3149–3163, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leu SJ, Lam SC, Lau LF. Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbilical vein endothelial cells. J Biol Chem 277: 46248–46255, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Lin MT, Chang CC, Chen ST, Chang HL, Su JL, Chau YP, Kuo ML. Cyr61 expression confers resistance to apoptosis in breast cancer MCF-7 cells by a mechanism of NF-kappaB-dependent XIAP up-regulation. J Biol Chem 279: 24015–24023, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Lobel M, Bauer S, Meisel C, Eisenreich A, Kudernatsch R, Tank J, Rauch U, Kuhl U, Schultheiss HP, Volk HD, Poller W, Scheibenbogen C. CCN1: a novel inflammation-regulated biphasic immune cell migration modulator. Cell Mol Life Sci 69: 3101–3113, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma SF, Grigoryev DN, Taylor AD, Nonas S, Sammani S, Ye SQ, Garcia JG. Bioinformatic identification of novel early stress response genes in rodent models of lung injury. Am J Physiol Lung Cell Mol Physiol 289: L468–L477, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Matute-Bello G, Wurfel MM, Lee JS, Park DR, Frevert CW, Madtes DK, Shapiro SD, Martin TR. Essential role of MMP-12 in Fas-induced lung fibrosis. Am J Respir Cell Mol Biol 37: 210–221, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon HG, Kim SH, Gao J, Quan T, Qin Z, Osorio JC, Rosas IO, Wu M, Tesfaigzi Y, Jin Y. CCN1 secretion and cleavage regulate the lung epithelial cell functions after cigarette smoke. Am J Physiol Lung Cell Mol Physiol 307: L326–L337, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, Otterbein SL, Song R, Hayashi S, Zhou Z, Pinsky DJ, Watkins SC, Pilewski JM, Sciurba FC, Peters DG, Hogg JC, Choi AM. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci USA 101: 14895–14900, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien TP, Yang GP, Sanders L, Lau LF. Expression of Cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol 10: 3569–3577, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parisi MS, Gazzerro E, Rydziel S, Canalis E. Expression and regulation of CCN genes in murine osteoblasts. Bone 38: 671–677, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Perkowski S, Sun J, Singhal S, Santiago J, Leikauf GD, Albelda SM. Gene expression profiling of the early pulmonary response to hyperoxia in mice. Am J Respir Cell Mol Biol 28: 682–696, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Rother M, Krohn S, Kania G, Vanhoutte D, Eisenreich A, Wang X, Westermann D, Savvatis K, Dannemann N, Skurk C, Hilfiker-Kleiner D, Cathomen T, Fechner H, Rauch U, Schultheiss HP, Heymans S, Eriksson U, Scheibenbogen C, Poller W. Matricellular signaling molecule CCN1 attenuates experimental autoimmune myocarditis by acting as a novel immune cell migration modulator. Circulation 122: 2688–2698, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto S, Yokoyama M, Aoki M, Suzuki K, Kakehi Y, Saito Y. Induction and function of CYR61 (CCN1) in prostatic stromal and epithelial cells: CYR61 is required for prostatic cell proliferation. Prostate 61: 305–317, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Wallace MJ, Probyn ME, Zahra VA, Crossley K, Cole TJ, Davis PG, Morley CJ, Hooper SB. Early biomarkers and potential mediators of ventilation-induced lung injury in very preterm lambs. Respir Res 10: 19, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie D, Yin D, Tong X, O'Kelly J, Mori A, Miller C, Black K, Gui D, Said JW, Koeffler HP. Cyr61 is overexpressed in gliomas and involved in integrin-linked kinase-mediated Akt and beta-catenin-TCF/Lef signaling pathways. Cancer Res 64: 1987–1996, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Zemans RL, McClendon J, Aschner Y, Briones N, Young SK, Lau LF, Kahn M, Downey GP. Role of β-catenin-regulated CCN matricellular proteins in epithelial repair after inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol 304: L415–L427, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics 124: 87–95, 2009. [DOI] [PubMed] [Google Scholar]