Abstract
Pulmonary hypertension (PH) eventually leads to right ventricular (RV) fibrosis and dysfunction that is associated with increased morbidity and mortality. Although angiotensin II plays an important role in RV remodeling associated with hypoxic PH, the molecular mechanisms underlying RV fibrosis in PH largely remain unresolved. We hypothesized that PKC-p38 signaling is involved in RV collagen accumulation in PH and in response to angiotensin II stimulation. Adult male Sprague-Dawley rats were exposed to 3 wk of normoxia or hypoxia (10% FiO2) as a model of PH. Hypoxic rats developed RV hypertrophy and fibrosis associated with an increase in PKC βII and δ protein expression and p38 dephosphorylation in freshly isolated RV cardiac fibroblasts. Further mechanistic studies were performed in cultured primary cardiac fibroblasts stimulated with angiotensin II, a key activator of ventricular fibrosis in PH. Angiotensin II induced a reduction in p38 phosphorylation that was attenuated following chemical inhibition of PKC βII and δ. Molecular and chemical inhibition of PKC βII and δ abrogated angiotensin II-induced cardiac fibroblast proliferation and collagen deposition in vitro. The effects of PKC inhibition on proliferation and fibrosis were reversed by chemical inhibition of p38. Conversely, constitutive activation of p38 attenuated angiotensin II-induced increase of cardiac fibroblast proliferation and collagen accumulation. PKC βII- and δ-dependent inactivation of p38 regulates cardiac fibroblast proliferation and collagen deposition in response to angiotensin II, which suggests that the PKC-p38 signaling in cardiac fibroblasts may be involved and important in the pathophysiology of RV fibrosis in PH.
Keywords: right ventricle, cardiac fibroblast, pulmonary hypertension, PKC, p38, angiotensin II
right ventricular (RV) failure is the main cause of death in patients with pulmonary hypertension (PH) (7). PH causes severe and prolonged RV pressure overload resulting in compensatory RV hypertrophy as an adaptive response. However, a persistent increase in RV afterload results in RV fibrosis and dysfunction that is maladaptive and observed in heart failure (2). Cardiac fibroblasts play a key role in cardiac remodeling and fibrosis through proliferation and matrix generation and degradation in addition to other functions (37). Although many studies have evaluated the signaling mechanisms important in RV hypertrophy with a focus on cardiac myocytes, the molecular mechanisms underpinning RV fibrosis in settings of PH remain unclear.
Although several factors affect the phenotype and function of cardiac fibroblasts including TGF-β (29) and insulin-like growth factor (3), angiotensin II, together with many components of the renin-angiotensin-aldosterone system produced locally in the heart, appears to be one of the most important factors regulating cardiac fibrosis and remodeling (42). In left ventricular hypertrophy and dysfunction, inhibitors of angiotensin signaling are associated with significantly improved outcomes in humans and thus are established therapeutic agents (36). Recent studies indicate that angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers influence RV mass and volume (40). Moreover, in the hypoxia-associated PH model in rats, activity of angiotensin-converting enzyme is significantly increased in the RV, whereas it was decreased in the left ventricle (LV) and septum (32). These data suggest that angiotensin II may also play a key role in RV remodeling associated with hypoxic PH.
Protein kinase C (PKC) are serine-threonine protein kinases that have been shown to play an important role in cardiac diseases including heart failure and cardiac fibrosis (reviewed in Ref. 9). Several PKC isoforms have been shown to be involved in signaling underlying the profibrotic effects of angiotensin II (9). We and others have shown that in the presence of increased RV afterload and fibrosis, PKC isoform expression is altered in tissue lysates from RV, but not the LV (4, 8, 39). However, the identity of specific PKC isoforms mediating RV fibrosis, and the underlying regulatory mechanisms, in cardiac fibroblasts remains unclear.
p38 mitogen-activated protein kinases (MAPK) are one of the downstream targets of PKC isozymes and believed to play an role in cardiac ventricular fibrosis depending on the experimental model and underlying cause. For example, activation of p38 was shown to be associated with increased fibrosis related to inflammation in hypertension and myocardial infarction (24, 26, 31), whereas in LV pressure overload attenuation of p38 activity was shown to increase hypertrophy and fibrosis (5). Hence, a role for p38, and its upstream effectors, in cardiac fibrosis in settings of PH has yet to be elucidated.
In this study, we sought to identify the signaling mechanism related with angiotensin II involved in RV fibrosis using hypoxic PH as our model of increased RV afterload. We hypothesized that PKC-p38 signaling is involved in RV collagen accumulation in PH and in response to angiotensin II stimulation.
MATERIALS AND METHODS
Materials.
All materials were obtained from Sigma (St. Louis, MO), unless otherwise noted. Collagenase II was obtained from Worthington (Lakewood, NJ). DMEM was obtained from Invitrogen (Carlsbad, CA). The vectors encoding dominant negative cDNA for PKC βII (pHACE-PKCβIIK371R) and δ (pHACE-PKCδK376R), wild-type MKK6 (pCDNA3-FLAG-MKK6wt) and dominant negative p38, mutated at the T180/Y182 phosphorylation site (pCMV5-p38agf), were purchased from Addgene (Cambridge, MA). cDNA for phosphorylated green fluorescent protein (pGFP-C1) was obtained from Clontech (Mountain View, CA) and wild-type p38 (pCMV-FLAG-p38wt) cDNA was a kind gift from Dr. Roger Davis (Howard Hughes Medical School/University of Massachusetts Medical School) (33). Sircol collagen assay was purchased from Accurate (Westbury, NY). Antibodies against phosphorylated p38 (T180/Y182) and total p38 were purchased from Cell Signaling (Beverly, MA). Actin, procollagen, angiotensin II receptor (AT1R), PKC βII and PKC δ antibodies, and PKC βII chemical inhibitor, LY333531, were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Lipofectamine 2000 reagent was purchased from Life Technologies (Grand Island, NY).
Echocardiographic measurements.
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were placed in either a normoxic or a normobaric hypoxic (10% FiO2) environment. Normobaric hypoxia was achieved by placing the rats in Biospherix A-chamber with Pro-Ox oxygen controller (Biospherix, Lacona, NY). Following 3 wk of exposure to normoxia or hypoxia, transthoracic echocardiography was performed on animals anesthetized with continuous isoflurane inhalation (2–5%), with a single-element transducer (Vevo 2100; VisualSonics, Toronto, ON, Canada). Adequacy of anesthesia was assessed by pedal reflex. Two-dimensional, Doppler, and M-mode recordings were obtained to measure LV fractional shortening, LV, and RV dimensions. The pulsed-wave Doppler recording at the right ventricular overflow tract was used to measure pulmonary acceleration time (PAT). Tricuspid annular plane systolic excursion (TAPSE) was measured by use of M-mode across the tricuspid valve annulus at the RV free wall. TAPSE was determined by measuring the excursion of the tricuspid annulus from its highest position to the peak descent during ventricular systole. Tissue Doppler was used to measure the early diastolic velocity of the septum (at mitral annulus) and RV lateral wall (at tricuspid annulus). After echocardiographic measurements, rats were euthanized under isoflurane and the heart and lungs were collected. The RV, LV, and interventricular septum (IVS) were dissected, frozen, and homogenized for Western blotting or cardiac fibroblasts from RV, LV, and IVS were isolated (outlined in Cardiac Fibroblast Isolation).
All animal experimental protocols were approved by the Institutional Animal Care and Use Committees of the Providence Veterans Affairs Medical Center and comply with the Health Research Extension Act, US Public Health Service, and US National Institutes of Health policy (protocol no. 2011-001).
Cardiac fibroblast isolation.
Cardiac fibroblasts were isolated as previously described (45) from normoxic or hypoxic rats, or from healthy adult male Sprague-Dawley rats that were anesthetized with continuous isoflurane inhalation (2–5%, assessed by pedal reflex). In brief, hearts were rapidly excised, retrogradely perfused for 2 min in Krebs-Henseleit bicarbonate (KHB) buffer at 37°C, and then switched to enzyme buffer 1 (KHB buffer containing 0.3 mg/ml collagenase II, 0.3 mg/ml hyaluronidase, and 50 μM CaCl2). After perfusion, ventricular tissue was cut and further digested at 37°C in enzyme buffer 1 supplemented with increased CaCl2 (500 μM), trypsin IX (0.6 mg/ml), and deoxyribonuclease (0.6 mg/ml). Cell suspensions were filtered into DMEM supplemented with 10% FBS, F12, and penicillin and streptomycin (complete medium) and centrifuged at 20 g for 2 min followed by removal of the pelleted myocytes and centrifugation of the supernatant at 800 g for 5 min. The resulting fibroblast pellet was resuspended in complete medium and plated into four 10-cm dishes. In the studies evaluating expression profile of PKC and activation status of p38 in rats with or without PH, fibroblasts isolated from the right ventricles were collected for Western blot analysis after they adhered to 10-cm culture dishes (in 2 h). For the in vitro proliferation studies, cells were isolated from healthy rats and cultured in six-well dishes for 2–3 days before they were trypsinized and used for the experiments. All experiments were performed on P1 (Passage 1) cells.
Cardiac fibroblast inhibitor studies.
Subconfluent P1 cells were exposed to serum-free DMEM, supplemented with 10 μg/ml insulin, 5.5 μg/ml transferrin and 5 ng/ml sodium selenite (ITS) and penicillin and streptomycin. After 24 h, cells were pretreated with SB203580 (p38 inhibitor) (100 nM) followed by treatment with LY333531 (PKC βII inhibitor, 50 nM) or rottlerin (PKC δ inhibitor, 3 μM) for an additional 30 min. Cardiac fibroblasts were then exposed to angiotensin II (1 μM) for 15 min to 48 h, followed by proliferation assay, collagen assay, and Western blotting.
Cardiac fibroblast transfections.
Cardiac fibroblasts were transiently transfected with cDNA (4 μg/well from six-well plate) encoding dominant negative PKC βII (PKC βIIK371R) or PKC δ (PKC δK376R), wild-type p38 (p38wt), dominant negative p38 (p38agf), or MKK6 (MKK6wt) or GFP, with use of Lipofectamine 2000 reagent according to the manufacturer's instructions. At 24 h posttransfection, cardiac fibroblasts were quiesced for 24 h, and then the cells were used for experiments as described.
Proliferation assay.
Cardiac fibroblast proliferation was assessed by both cell counting and incorporation of [3H]thymidine into cells. Following pretreatment with inhibitors, cardiac fibroblasts were exposed to angiotensin II (1 μM) and [3H]thymidine (0.025 μCi) for 48 h, then rinsed with ice-cold PBS three times and incubated with 5% trichloroacetic acid (TCA) for 20 min on ice. After two washings, cells were solubilized in 0.5 N NaOH and an aliquot of TCA-insoluble material was neutralized in 0.5 N HCl. Radioactivity was measured by liquid scintillation counter (LSM 6500, Beckman Instruments). Cell proliferation was assessed as counts per minute and normalized to vehicle conditions.
Western blot analysis.
RV, LV, and IVS tissues were homogenized at 4°C in homogenization buffer (20 mM HEPES, 250 mM sucrose, 100 mM NaCl, 0.2 mM EDTA, 200 μM PMSF, 0.5 mM DTT, 1 μM leupeptin, 1 μM aprotinin, and phosphatase inhibitor cocktail III). Homogenates were then centrifuged at 10,000 g for 10 min at 4°C. Supernatants were used for total protein analysis.
Cardiac fibroblasts from the in vitro studies were collected in radioimmunoprecipitation assay buffer and incubated on ice for 10 min prior to centrifugation for 10 min at 15,000 g. The supernatant was subjected to protein determination (Bradford).
Proteins (50 μg/lane) were resolved on 7.5% (procollagen) and 10% (p38 and PKCs) separating gels by SDS-PAGE. Resolved proteins were transferred to polyvinylidene fluoride membranes and immunoblot analysis was performed by appropriate antibody dilution of 1:1,000 for all antibodies with the exception of procollagen (1:200). Equal loading was confirmed by probing for vinculin (1:3,000) or actin (1:1,000) with similar results.
Quantitative densitometry was performed by use of the public domain ImageJ program. Phosphorylated proteins and total proteins were calculated as a ratio of vinculin or actin expression. Data are presented as a ratio of phosphorylated protein to total protein expression. Expression data were then normalized to experimental conditions as indicated (i.e., vs. vehicle or normoxia).
Collagen content measurement.
Collagen content measurements were made by use of Sircol collagen dye binding assay, following the manufacturer's instructions. Cardiac fibroblast lysates were sonicated three times for 5 s and centrifuged at 3,000 g for 5 min. The resulting supernatant was analyzed for collagen content. Sircol dye reagent was mixed to equal amounts of protein from crude homogenate and cells for 30 min with agitation. The collagen-dye complex was centrifuged at 12,000 g for 10 min and the resulting pellet was washed in ice-cold acid-salt wash reagent (acetic acid, sodium chloride, and surfactants). Samples were then centrifuged at 12,000 g for 10 min, and the pellet was dissolved by adding alkali agent (0.5 M sodium hydroxide) and incubating at room temperature for 5 min. Collagen content was measured by spectrophotometer at 555 nm. Data were normalized to vehicle or normoxic conditions.
Statistics.
All data are presented as means ± SE for the indicated number of rats studied (n). Statistical differences were assessed by unpaired, two-tailed Student's t-test or ANOVA for comparison of individual means. P ≤ 0.05 is considered as significant.
RESULTS
RV dysfunction and fibrosis in hypoxic PH.
Rats exposed to chronic hypoxia displayed significantly reduced RV systolic function and elevated pulmonary arterial pressure compared with normoxic animals, indicated by TAPSE and PAT, respectively (Table 1). Furthermore, animals with PH had a lower RV lateral wall e′ (early diastolic tissue Doppler velocity) consistent with presence of RV diastolic dysfunction (Table 1). Hypoxic PH significantly increased RV hypertrophy, assessed by elevated RV free wall thickness (Table 1) and RV mass (RV/BW and RV/LVS) (Fig. 1A). Also, we noted a significant increase in procollagen I expression in the RV, but not LV, from hypoxic PH rats compared with normoxic controls (Fig. 1B). Hence, the model recapitulated the maladaptive changes associated with PH, namely RV fibrosis and dysfunction. We further observed an increase in AT1R expression in the RV from rats with hypoxic PH compared with normoxic controls (Fig. 1C), suggesting increased angiotensin II signaling in settings of PH.
Table 1.
Pulmonary hypertension causes right ventricular dysfunction associated with the disease
| Normoxia | Hypoxia | |
|---|---|---|
| TAPSE, mm | 2.41 ± 0.24 | 1.68 ± 0.09* |
| Ejection fraction, % | 81.73 ± 3.41 | 72.60 ± 3.81 |
| TV LW e′, mm/s | −72.09 ± 14.48 | −34.67 ± 4.31* |
| LV septal wall e′, mm/s | −55.47 ± 12.76 | −25.09 ± 1.23* |
| PAT, ms | 32.84 ± 2.43 | 25.11 ± 1.93* |
| RV diastolic dimension, mm | 1.90 ± 0.2 | 2.45 ± 0.22 |
| RV free wall thickness, mm | 0.62 ± 0.04 | 1.18 ± 0.15* |
Echocardiogram data were obtained at experimental end point and are presented as means ± SE; n = 6,
P < 0.05 vs. normoxia. TAPSE, tricuspid annular plane systolic excursion; TV LW e′, early diastolic tissue Doppler velocity of right ventricular (RV) lateral wall at tricuspid valve annulus; LV, left ventricle; PAT, pulmonary acceleration time.
Fig. 1.
Pulmonary hypertension causes hypertrophy, collagen accumulation, and increased angiotensin II receptor expression in the right ventricle. A: weights of right and left ventricles (RV, LV) and interventricular septum (IVS) [normalized to body weight (BW)] in rats exposed to normoxic and hypoxic (10% FiO2) conditions (n = 6) for 3 wk. RV weight was also expressed as a ratio to LV plus IVS weight. B and C: Western blot analysis of equal protein (50 μg/lane) from tissue homogenates of RV, LV, and IVS from rats (n = 6) exposed to 3 wk of normoxia (N) or hypoxia (H) (10% FiO2) that were probed with an antibody specific to procollagen or angiotensin II receptor type 1 (AT1R). Data are expressed as ratio to vinculin and normalized to respective normoxic controls. *P < 0.05 vs. normoxia.
Effect of PH on the expression profile of PKC and activation status of p38 in ventricular cardiac fibroblasts.
To study the expression profile of PKC in response to PH, cardiac fibroblasts from the RV of rats exposed to chronic hypoxia were isolated and subjected to Western blot analysis for PKC isoforms α, βII, δ, and ϵ. Hypoxic PH resulted in elevated protein expression of PKC βII and δ in RV cardiac fibroblasts (Fig. 2A). Since p38 activation is an important signaling pathway downstream of PKC, we next studied the effect of hypoxic PH on p38 activation in isolated RV cardiac fibroblasts. We observed a significant decrease in p38 activity, assessed by phosphorylation at residues T180/Y182, in the RV fibroblasts (Fig. 2B), whereas no change in p38 phosphorylation status was noted in fibroblasts isolated from the LV or septum (Fig. 2, C and D). In contrast, hypoxic PH had no effect on total ERK expression (data not shown) or ERK phosphorylation status, assessed as ratio of phosphorylated ERK1/2 to total ERK1/2, in isolated RV cardiac fibroblasts (Fig. 2E).
Fig. 2.
Differential PKC isoform expression and MAPK phosphorylation status in cardiac fibroblasts from rats with pulmonary hypertension. Western blot analysis of equal protein (50 μg/lane) from isolated RV (A, B, and E), LV (C), or IVS (D) cardiac fibroblast lysates of rats (n = 4) exposed to 3 wk of normoxia or hypoxia (10% FiO2) that were probed with antibodies specific to either PKC α, βII, δ, ϵ (A) or phosphorylated p38 or ERK1/2 and total p38 or ERK1/2 (B–E). Data are calculated as a ratio of phosphorylated protein to total protein expressed and normalized to respective normoxic controls. *P < 0.05 vs. normoxia.
Effect of angiotensin II on p38 phosphorylation and PKC expression in isolated cardiac fibroblasts.
We performed further mechanistic studies in cultured primary cardiac fibroblasts stimulated with angiotensin II, a key activator of ventricular fibrosis. We did not observe significant changes in the level of p38 phosphorylation following acute exposure to angiotensin II (15 min to 2 h), (Fig. 3A). However, upon longer term exposure to angiotensin II (48 h), we observed a significant decrease in p38 phosphorylation (Fig. 3A) and a significant increase in expression of PKC βII and δ (Fig. 3B) similar to our in vivo observations. Next, we characterized the effect of angiotensin II on proliferation and collagen synthesis in isolated cardiac fibroblasts. Cell proliferation, as measured by both cell count and thymidine incorporation, was significantly elevated from 6 h posttreatment to 48 h (Fig. 3C). Accumulation of collagen was also significantly increased at both 24 and 48 h following exposure to angiotensin II (Fig. 3D). Thus the angiotensin II-induced collagen accumulation, increase in PKC βII and δ expression, and p38 dephosphorylation in isolated cardiac fibroblasts mimic the changes seen in the RV in hypoxic PH.
Fig. 3.
Angiotensin II-induced increase in cardiac fibroblast proliferation and collagen accumulation concomitant with enhanced PKC βII and δ expression and p38 dephosphorylation. Cardiac fibroblasts from healthy control rats that were treated with angiotensin II (1 μM) or vehicle for indicated times (0 to 2, 24, or 48 h), were assessed for p38 phosphorylation (n = 3) (A), PKCβII and δ expression (n = 4) (B), cell proliferation assessed by cell count (n = 6) and thymidine incorporation (n = 4) (C), and collagen content (n = 3) (D). Data are normalized to vehicle and presented as means ± SE; *P < 0.05 vs. vehicle, #P < 0.05 vs. 0-h time point.
Effect of PKC βII and δ inhibition on angiotensin II-induced cell proliferation and collagen deposition.
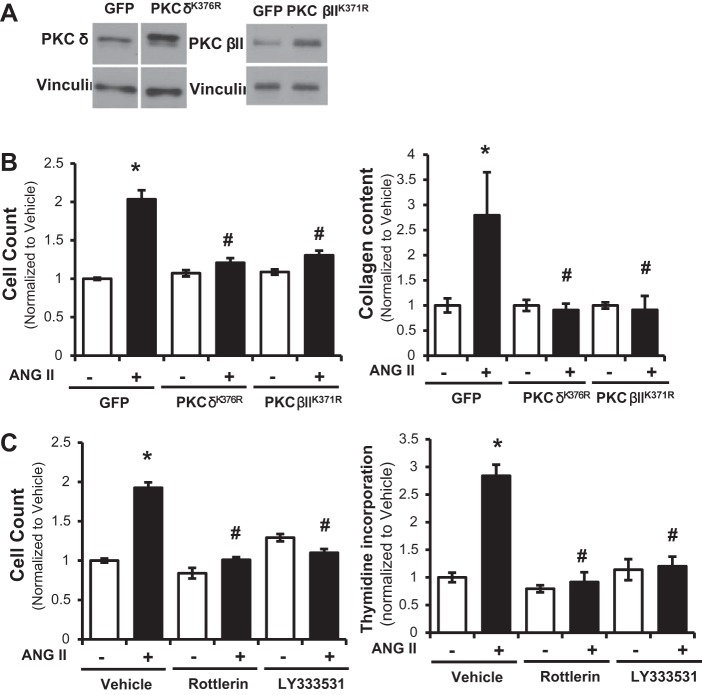
Since PH in rats caused an increase in expression of PKC βII and δ (Fig. 2A) in cardiac fibroblasts in vivo, we next sought to study whether inhibition of these PKC isoforms would play a role in the elevated proliferation and collagen accumulation observed following exposure to angiotensin II (Fig. 3). Molecular inhibition of PKC βII and δ was induced by overexpression of the dominant negative plasmid cDNAs encoding PKC βIIK371R or PKC δK376R (Fig. 4, A and B) or by chemical inhibition of PKC βII and δ using LY333531 and rottlerin (Fig. 4C). Transfection efficiency, as assessed by percentage of GFP-positive cells compared with total number of cardiac fibroblasts, was 42.5 ± 9.9%. As noted for untransfected cardiac fibroblasts (Fig. 3C), GFP-transfected control cells exposed to angiotensin II for 48 h displayed a significant increase in cell number (Fig. 4B) and collagen accumulation (Fig. 4C). Cardiac fibroblasts transiently transfected with either PKC βIIK371R or PKC δK376R displayed no difference in proliferation or collagen deposition (Fig. 4, B and C), in the presence or absence of angiotensin II. Likewise, preincubation of cardiac fibroblasts with either LY333531 or rottlerin, the PKC βII and PKC δ inhibitors, blocked angiotensin II-induced increase in cell proliferation (Fig. 4C). Thus inhibition of PKC βII or δ in cardiac fibroblasts blocks the elevated proliferation and collagen accumulation observed following exposure to angiotensin II.
Fig. 4.
Molecular and chemical inhibition of PKC βII and δ abolishes angiotensin II-induced increases in cardiac fibroblast proliferation and collagen accumulation. Cardiac fibroblasts from healthy control rats that were transfected with PKC βIIK371R and PKC δK376R, or green fluorescent protein (GFP) as control. Transfections were confirmed by Western blot analysis for PKC βII and δ (A). Transfected cardiac fibroblasts treated with angiotensin II (1 μM), or vehicle, for 48 h were measured for cell count (n = 7) (B) or collagen content (n = 5) (B). Cardiac fibroblasts preincubated with LY333531 (50 nM, 30 min) and rottlerin (3 μM, 30 min) followed by treatment with angiotensin II (1 μM), or vehicle, for 48 h were measured by cell count (n = 6) and thymidine incorporation (n = 4) (C). Data are normalized to vehicle and presented as means ± SE. *P < 0.05 vs. vehicle, #P < 0.05 vs. GFP, angiotensin II treatment.
Effect of p38 activation on angiotensin II-induced cell proliferation and collagen deposition.
We next sought to understand the mechanism through which inhibition of PKC βII or δ blocked cell proliferation and collagen deposits in cardiac fibroblasts exposed to angiotensin II. We first studied whether inhibition of PKC βII or δ affected angiotensin II-induced dephosphorylation of p38, as observed in Fig. 3B. p38 dephosphorylation, following angiotensin II treatment, was attenuated by chemical inhibition of PKC βII and δ using LY333531 and rottlerin, respectively, whereas no significant change in total p38 expression was observed in these experiments (Fig. 5). Because p38 activity was downregulated in RV cardiac fibroblasts isolated from rats with PH (Fig. 2B) as well as angiotensin II-treated cardiac fibroblasts in vitro (Fig. 3B), our next studies assessed the effect of altering p38 activity on isolated cardiac fibroblasts in vitro.
Fig. 5.

Chemical inhibition of PKC βII and δ inhibits angiotensin II-induced dephosphorylation of p38. Cardiac fibroblasts from healthy control rats that were preincubated with LY333531 (50 nM, 30 min) and rottlerin (3 μM, 30 min), followed by treatment with angiotensin II (1 μM) or vehicle for 48 h, were assessed for p38 phosphorylation status and total p38 expression. Data are calculated as a ratio of phosphorylated protein to total protein expressed, or total protein to vinculin, and normalized to respective normoxic controls. Grouped data from 4 separate experiments are averaged and presented as means ± SE. *P < 0.05 vs. vehicle/vehicle, #P < 0.05 vs. vehicle/angiotensin II treatment.
Upregulation of p38 activity in cardiac fibroblasts was achieved via overexpression of the p38 protein or by overexpressing the upstream activator MKK6 with wild-type plasmid cDNA (Fig. 6). Activation of p38 was determined with Western blot analysis of transfected cardiac fibroblasts. As expected, transient p38wt cDNA transfection increased p38 phosphorylation and total protein expression, whereas MKK6wt cDNA transfection led to an increase in phosphorylation of endogenously expressed p38 but did not increase total p38 protein expression (Fig. 6A). In both cases, angiotensin II-induced cardiac fibroblasts proliferation (Fig. 6B) and collagen accumulation (Fig. 6C) were prevented by upregulation of p38 expression (p38wt) and/or phosphorylation (MKK6wt). Therefore, activation of p38 attenuates angiotensin II-induced proliferation and collagen accumulation.
Fig. 6.

Molecular activation of p38, via overexpression of p38 or the upstream activator MKK6, abolishes angiotensin II-induced increases in cardiac fibroblast proliferation and collagen accumulation. Cardiac fibroblasts from healthy control rats were transfected with wild-type p38 and MKK6 (p38wt and MKK6wt) or with GFP as control. Transfections were confirmed by Western blot analysis for p38 phosphorylation status and total p38 expression (A). Transfected cardiac fibroblasts treated with angiotensin II (1 μM), or vehicle, for 48 h were measured for cell count (n = 7) (B) and collagen content (n = 4) (C). Data are calculated as a ratio of phosphorylated protein to total protein expressed, or total protein to vinculin, and normalized to respective normoxic controls. Grouped data from 4 separate experiments are averaged and presented as means ± SE. *P < 0.05 vs. vehicle, #P < 0.05 vs. GFP, angiotensin II treatment.
Since p38 has been previously observed to function downstream of PKC (1, 23), we next sought to understand whether the effect of PKC βII or δ inhibition, on collagen accumulation, was dependent on p38 activity. p38 was inhibited in cardiac fibroblasts via preincubation with the chemical inhibitor SB203580, prior to treatment with PKC βII or δ inhibition (Fig. 7). Under baseline conditions, exposure to SB203580 exhibited no effect on vehicle or angiotensin II-induced cell count and collagen accumulation (Fig. 7). Similarly, inhibition of p38, following transient transfection of dominant negative p38 (p38agf), exerted no significant effect on proliferation of cardiac fibroblasts in absence of angiotensin II. Cell count (normalized to GFP) was 1 ± 0.05 arbitrary units (a.u.) for GFP and 1.11 ± 0.04 a.u. for p38agf, despite a significant fourfold increase in p38 expression in p38agf-overexpressing cardiac fibroblasts. As previously observed (Fig. 4), inhibition of PKC βII or δ attenuated angiotensin II-induced increases in proliferation and collagen accumulation (Fig. 7). Following p38 inhibition, these effects of LY333531 and rottlerin on proliferation and collagen levels were abrogated (Fig. 7).
Fig. 7.

Inhibition of p38 prevents the effect of PKC βII and δ inhibition on angiotensin II-induced proliferation and collagen content. Cardiac fibroblasts from healthy control rats that were preincubated with SB203580 (100 nM, 30 min), followed by treatment with LY333531 (50 nM, 30 min) and rottlerin (3 μM, 30 min), and angiotensin II (1 μM), or vehicle, for 48 h were measured for cell count (n = 9) (A) and collagen content (n = 5) (B). Data are normalized to vehicle and presented as means ± SE. *P < 0.05 vs. vehicle/vehicle, #P < 0.05 vs. vehicle/angiotensin II treatment.
In summary, our data suggest that PKC βII and δ mediate cardiac fibroblast proliferation and collagen accumulation through the inhibition of a p38 pathway that may result in RV fibrosis and dysfunction in settings of PH.
DISCUSSION
PH is associated with significant morbidity and mortality related to RV failure. Maladaptive changes in the RV, in response to PH, are associated with RV fibrosis and dysfunction (2). We show that, in a well-established PH model exhibiting RV fibrosis and dysfunction, increases in PKC βII and δ occur concomitant with RV-specific inhibition of p38 in cardiac fibroblasts. Using isolated cardiac fibroblasts, we demonstrate that angiotensin II-induced collagen accumulation is dependent on PKC βII and δ activity. To the best of our knowledge, this is the first study to show that PKC βII and δ-mediated cardiac fibroblast proliferation and collagen content is blocked following p38 inhibition, whereas overexpression or activation of p38 prevents angiotensin II-induced collagen production and proliferation in the cardiac fibroblast. We propose that downregulation of p38 activity, in cardiac fibroblasts, is responsible for the characteristic RV fibrosis seen in PH. Thus regulating the activity of p38 may represent a therapeutic tool for RV failure in PH.
We focused our mechanistic investigation on angiotensin II, an important profibrotic mediator in the heart (30). It has been shown that increase in membrane bound angiotensin-converting enzyme (ACE), and higher ACE activity is present in the RV of hypoxic rats (32). In contrast, no changes are seen in the LV and septum. Furthermore, higher ACE activity was noted in areas with myocardial fibrosis in the RV (32). Similarly, we demonstrate increased expression of the angiotensin II receptor AT1R, a key component of the angiotensin II signaling mechanism. Thus higher local levels of angiotensin II may be present in the RV in settings of hypoxic PH resulting in increased fibroblast proliferation and fibrosis. Angiotensin II results in an increase in cytosolic calcium and diacylglycerol (13) that can increase the activity of PKC isoforms (38). Thus the PH-induced increases in PKC βII and δ expression in the RV observed in the present study are consistent with known downstream signaling related to angiotensin II. We further demonstrate that abrogation of angiotensin II-mediated cardiac fibroblast proliferation and collagen synthesis occurs via inhibition of PKC δ and PKC βII activity. Consistent with our studies, inhibition of PKC δ attenuates collagen I and III synthesis in vitro and in vivo, attributed to a rottlerin-sensitive gene promoter segment in collagen (19, 20). Likewise, increased activation of PKC βII, via IL-7 exposure or inhibition of 14-3-3 activity, correlates with enhanced collagen I and II production and cardiac fibrosis (15, 27). Thus, in settings of PH, PKC βII and PKC δ in RV cardiac fibroblasts are likely to be key regulators promoting ventricular fibrosis. Although we focused on angiotensin II, other profibrotic mediators such as endothelin-1 and TGF-β have been shown to signal via PKCs as well. Further investigation is needed in how these mediators interact in signaling through these pathways resulting in RV fibrosis. Also, although the evidence suggests increased angiotensin II signaling in the RV in hypoxic PH, further experiments are needed to definitively link the response of RV cardiac fibroblasts to increased angiotensin II in vivo in settings of PH, such as using mice with targeted deletion of AT1R in cardiac fibroblasts.
Studies investigating the role of p38 in cardiac fibroblasts and ventricular fibrosis have shown conflicting results. We demonstrate that a reduction in p38 activity results in cardiac fibroblast proliferation and collagen deposition and is associated with RV fibrosis and dysfunction. Our findings are consistent with those of Braz et al. (5), who demonstrated an antifibrotic effect of p38 activation in unstressed and angiotensin II-exposed hearts using transgenic mouse models expressing dominant negative p38, MKK3, or MKK6 kinases. In contrast, short-term angiotensin II (5 min–24 h) exposure has been shown to activate the Ras/p38/CREB pathway in isolated rat cardiac fibroblasts (25, 41). TGF-β-mediated extracellular matrix deposition is associated with increased p38 activity in dermal and lung fibroblasts, and chemical inhibition of p38 decreases stretch-induced collagen I transcripts in lung fibroblasts (22, 34). Whether these discrepancies may be related to the nature and duration of stimulus provided, or heterogeneity of fibroblasts from different tissue, remains to be resolved.
There are several possible mechanisms that may explain the relationship between reduced p38 activity and increased fibrosis that merit further investigation in settings of cardiac fibrosis. In cardiac fibroblasts, calcineurin/NFAT signaling, which is inhibited by p38 in other cell types, suppresses expression of the fibrosis markers collagen and fibronectin (5, 17, 44). In addition, p38 activation results in G1-phase arrest of the cell cycle, and thus decreased proliferation, due to increased p21 levels and phosphorylation of GADD153 (11, 43).
We demonstrate that p38 inhibition abrogates the reduced collagen accumulation noted following PKC βII and δ inhibition, whereas p38 activation correlates with inhibition of PKC βII and δ in cardiac fibroblasts. We further show that angiotensin II-induced dephosphorylation of p38 is attenuated by PKC βII and δ inhibition. Other studies have observed a similar relationship between PKC and p38 (6, 12). In contrast, some studies suggest that increased p38 activity is associated with PKC activation (14, 18, 21). A potential explanation for these discrepancies may be related to differential p38-mediated signaling dependent on the cellular stress or mitogenic stimuli applied (11). Although reduced p38 phosphorylation was necessary to increase cardiac fibroblast proliferation in response to angiotensin II, we did not find that the p38 inhibition was sufficient to increase cardiac fibroblast proliferation in absence of angiotensin II. These data suggest that other parallel signaling by angiotensin II that may interact with p38 signaling plays a role in cardiac fibroblast proliferation and collagen production as well. Also, although we show that inhibition of PKC βII and δ prevents a decrease in p38 phosphorylation, the underlying mechanism remains to be elucidated. One possible mechanistic pathway may include MAPK phosphatases that can dephosphorylate p38; however, there are currently more than 15 members of the MAPK phosphatase family that need to be systematically evaluated for candidate phosphatase(s) in future studies (10, 16).
p38 inhibitors are under evaluation as therapeutic tools to attenuate cardiac remodeling and heart failure (28, 35). However, our study and another report (5) suggest that a decrease in p38 activity may be associated with increased cardiac fibrosis in settings of increased afterload. Furthermore, p38 inhibition exhibited no effect on angiotensin II-induced cardiac fibroblast proliferation or collagen deposition. Hence, these data suggest that more investigation is needed prior to the use of p38 inhibitors as therapeutic agents in heart failure.
In conclusion, the data presented show an association between increased RV collagen and p38 inactivation. In PH, p38 inactivation was specific to RV cardiac fibroblasts. Furthermore, inhibition of PKC βII or δ attenuated angiotensin II-induced cardiac fibroblast proliferation and collagen production in a p38-dependent manner. We therefore propose that PKC βII and δ-dependent inactivation of p38 is involved in increasing in RV collagen content, a mechanism that may be important in the pathophysiology of RV fibrosis in PH.
GRANTS
This material is based on work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development: Biomedical Laboratory Research and Development Service (MERIT Review Award to G. Choudhary, IBX000711A) and support from the Department of Medicine, Alpert Medical School. H. Chichger was supported by American Heart Association Grant 13POST16860031. E. O. Harrington was supported by the National Heart, Lung, and Blood Institute Grant R01 HL-67795 and American Heart Association Grant 10GRNT4160055. K. A. O'Connell was supported by National Heart, Lung, and Blood Institute Grant T32 HL094300-05. U. Mende was supported by the National Heart, Lung, and Blood Institute Grant R01 HL-114784. P. Zhang was supported by the American Heart Association National Scientist Development Grant 13SDG14370008 and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM103652. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.C. and G.C. conception and design of research; H.C., A.V., K.A.O., and P.Z. performed experiments; H.C., A.V., and G.C. analyzed data; H.C., K.A.O., P.Z., U.M., E.O.H., and G.C. interpreted results of experiments; H.C. and A.V. prepared figures; H.C. and G.C. drafted manuscript; H.C., A.V., K.A.O., P.Z., U.M., E.O.H., and G.C. approved final version of manuscript; A.V., K.A.O., P.Z., U.M., E.O.H., and G.C. edited and revised manuscript.
REFERENCES
- 1.Bartha E, Solti I, Kereskai L, Lantos J, Plozer E, Magyar K, Szabados E, Kalai T, Hideg K, Halmosi R, Sumegi B, Toth K. PARP inhibition delays transition of hypertensive cardiopathy to heart failure in spontaneously hypertensive rats. Cardiovasc Res 83: 501–510, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 135: 794–804, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Booz GW, Baker KM. Protein kinase C in angiotensin II signalling in neonatal rat cardiac fibroblasts. Role in the mitogenic response. Ann NY Acad Sci 752: 158–167, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Braun MU, LaRosee P, Simonis G, Borst MM, Strasser RH. Regulation of protein kinase C isozymes in volume overload cardiac hypertrophy. Mol Cell Biochem 262: 135–143, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest 111: 1475–1486, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Du J, Feng W, Song Y, Lu Z, Xu M, Li Z, Zhang Y. beta-Adrenergic receptors stimulate interleukin-6 production through Epac-dependent activation of PKCdelta/p38 MAPK signalling in neonatal mouse cardiac fibroblasts. Br J Pharmacol 166: 676–688, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension. Coron Artery Dis 16: 13–18, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Choudhary G, Troncales F, Martin D, Harrington EO, Klinger JR. Bosentan attenuates right ventricular hypertrophy and fibrosis in normobaric hypoxia model of pulmonary hypertension. J Heart Lung Transplant 30: 827–833, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Churchill E, Budas G, Vallentin A, Koyanagi T, Mochly-Rosen D. PKC isozymes in chronic cardiac disease: possible therapeutic targets? Annu Rev Pharmacol Toxicol 48: 569–599, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal 16: 769–779, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Faust D, Schmitt C, Oesch F, Oesch-Bartlomowicz B, Schreck I, Weiss C, Dietrich C. Differential p38-dependent signalling in response to cellular stress and mitogenic stimulation in fibroblasts. Cell Commun Signal 10: 6, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng W, Song Y, Chen C, Lu ZZ, Zhang Y. Stimulation of adenosine A(2B) receptors induces interleukin-6 secretion in cardiac fibroblasts via the PKC-delta-P38 signalling pathway. Br J Pharmacol 159: 1598–1607, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Force T, Kuida K, Namchuk M, Parang K, Kyriakis JM. Inhibitors of protein kinase signaling pathways: emerging therapies for cardiovascular disease. Circulation 109: 1196–1205, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Grden M, Podgorska M, Kocbuch K, Rzepko R, Szutowicz A, Pawelczyk T. High glucose suppresses expression of equilibrative nucleoside transporter 1 (ENT1) in rat cardiac fibroblasts through a mechanism dependent on PKC-zeta and MAP kinases. J Cell Physiol 215: 151–160, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Gurusamy N, Watanabe K, Ma M, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Inactivation of 14-3-3 protein exacerbates cardiac hypertrophy and fibrosis through enhanced expression of protein kinase C beta 2 in experimental diabetes. Biol Pharm Bull 28: 957–962, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Haneda M, Sugimoto T, Kikkawa R. Mitogen-activated protein kinase phosphatase: a negative regulator of the mitogen-activated protein kinase cascade. Eur J Pharmacol 365: 1–7, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Herum KM, Lunde IG, Skrbic B, Florholmen G, Behmen D, Sjaastad I, Carlson CR, Gomez MF, Christensen G. Syndecan-4 signaling via NFAT regulates extracellular matrix production and cardiac myofibroblast differentiation in response to mechanical stress. J Mol Cell Cardiol 54: 73–81, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Husse B, Isenberg G. Cyclic mechanical strain causes cAMP-response element binding protein activation by different pathways in cardiac fibroblasts. Heart Int 5: e3, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jimenez SA, Gaidarova S, Saitta B, Sandorfi N, Herrich DJ, Rosenbloom JC, Kucich U, Abrams WR, Rosenbloom J. Role of protein kinase C-delta in the regulation of collagen gene expression in scleroderma fibroblasts. J Clin Invest 108: 1395–1403, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jinnin M, Ihn H, Yamane K, Mimura Y, Asano Y, Tamaki K. Alpha2(I) collagen gene regulation by protein kinase C signaling in human dermal fibroblasts. Nucleic Acids Res 33: 1337–1351, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein G, Schaefer A, Hilfiker-Kleiner D, Oppermann D, Shukla P, Quint A, Podewski E, Hilfiker A, Schroder F, Leitges M, Drexler H. Increased collagen deposition and diastolic dysfunction but preserved myocardial hypertrophy after pressure overload in mice lacking PKCepsilon. Circ Res 96: 748–755, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Kucich U, Rosenbloom JC, Abrams WR, Rosenbloom J. Transforming growth factor-beta stabilizes elastin mRNA by a pathway requiring active Smads, protein kinase C-delta, and p38. Am J Respir Cell Mol Biol 26: 183–188, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Lee SE, Yang H, Jeong SI, Jin YH, Park CS, Park YS. Induction of heme oxygenase-1 inhibits cell death in crotonaldehyde-stimulated HepG2 cells via the PKC-delta-p38-Nrf2 pathway. PLoS One 7: e41676, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei J, Xue S, Wu W, Zhou S, Zhang Y, Yuan G, Wang J. Sdc1 overexpression inhibits the p38 MAPK pathway and lessens fibrotic ventricular remodeling in MI rats. Inflammation 36: 603–615, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, Zhou Y, Wu LL. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-beta1 pathways in cardiac fibroblasts. Cardiovasc Res 91: 80–89, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, Saffitz J, Chien K, Xiao RP, Kass DA, Wang Y. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci USA 98: 12283–12288, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Zhu H, Su Z, Sun C, Yin J, Yuan H, Sandoghchian S, Jiao Z, Wang S, Xu H. IL-17 contributes to cardiac fibrosis following experimental autoimmune myocarditis by a PKCbeta/Erk1/2/NF-kappaB-dependent signaling pathway. Int Immunol 24: 605–612, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, Wang C, Lee JC, Feuerstein GZ, Yue TL. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation 99: 1685–1691, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res 91: 1103–1113, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Melendez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension 56: 225–231, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrell NW, Danilov SM, Satyan KB, Morris KG, Stenmark KR. Right ventricular angiotensin converting enzyme activity and expression is increased during hypoxic pulmonary hypertension. Cardiovasc Res 34: 393–403, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 16: 1247–1255, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato M, Shegogue D, Gore EA, Smith EA, McDermott PJ, Trojanowska M. Role of p38 MAPK in transforming growth factor beta stimulation of collagen production by scleroderma and healthy dermal fibroblasts. J Invest Dermatol 118: 704–711, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Shao Z, Bhattacharya K, Hsich E, Park L, Walters B, Germann U, Wang YM, Kyriakis J, Mohanlal R, Kuida K, Namchuk M, Salituro F, Yao YM, Hou WM, Chen X, Aronovitz M, Tsichlis PN, Bhattacharya S, Force T, Kilter H. c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circ Res 98: 111–118, 2006. [DOI] [PubMed] [Google Scholar]
- 36.SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325: 293–302, 1991. [DOI] [PubMed] [Google Scholar]
- 37.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res 105: 1164–1176, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev 88: 1341–1378, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uenoyama M, Ogata S, Nakanishi K, Kanazawa F, Hiroi S, Tominaga S, Seo A, Matsui T, Kawai T, Suzuki S. Protein kinase C mRNA and protein expressions in hypobaric hypoxia-induced cardiac hypertrophy in rats. Acta Physiol (Oxf) 198: 431–440, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Ventetuolo CE, Lima JA, Barr RG, Bristow MR, Bagiella E, Chahal H, Kizer JR, Lederer DJ, Bluemke DA, Kawut SM. The renin-angiotensin system and right ventricular structure and function: the MESA-Right Ventricle Study. Pulm Circ 2: 379–386, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang LP, Wang Y, Zhao LM, Li GR, Deng XL. Angiotensin II upregulates K(Ca)3.1 channels and stimulates cell proliferation in rat cardiac fibroblasts. Biochem Pharmacol 85: 1486–1494, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Weber KT, Sun Y. Recruitable ACE and tissue repair in the infarcted heart. J Renin Angiotensin Aldosterone Syst 1: 295–303, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Wong ES, Le Guezennec X, Demidov ON, Marshall NT, Wang ST, Krishnamurthy J, Sharpless NE, Dunn NR, Bulavin DV. p38MAPK controls expression of multiple cell cycle inhibitors and islet proliferation with advancing age. Dev Cell 17: 142–149, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Yang TT, Xiong Q, Enslen H, Davis RJ, Chow CW. Phosphorylation of NFATc4 by p38 mitogen-activated protein kinases. Mol Cell Biol 22: 3892–3904, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang P, Su J, King ME, Maldonado AE, Park C, Mende U. Regulator of G protein signaling 2 is a functionally important negative regulator of angiotensin II-induced cardiac fibroblast responses. Am J Physiol Heart Circ Physiol 301: H147–H156, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]