Abstract
Excessive inflammation is a major cause of organ damage during sepsis. The elderly are highly susceptible to sepsis-induced organ injury. Sirt1 expression is reduced during aging. In the present study, we investigated the role of Sirt1, a histone deacetylase, in controlling inflammatory responses in a murine sepsis model induced by cecal ligation and puncture (CLP). We examined lung inflammatory signaling in inducible Sirt1 knockout (Sirt1−/−) mice and wild-type littermates (Sirt1+/+) after CLP. Our results demonstrated that Sirt1 deficiency led to severe lung inflammatory injury. To further investigate molecular mechanisms of Sirt1 regulation of lung inflammatory responses in sepsis, we conducted a series of experiments to assess lung inflammasome activation after CLP. We detected increased lung inflammatory signaling including NF-κB, signal transducer and activator of transcription 3, and ERK1/2 activation in Sirt1−/− mice after CLP. Furthermore, inflammasome activity was increased in Sirt1−/− mice after CLP, as demonstrated by increased IL-1β and caspase-7 cleavage and activation. Aggravated inflammasome activation in Sirt1−/− mice was associated with the increased production of lung proinflammatory mediators, including ICAM-1 and high-mobility group box 1, and further disruption of tight junctions and adherens junctions, as demonstrated by dramatic reduction of lung claudin-1 and vascular endothelial-cadherin expression, which was associated with the upregulation of matrix metallopeptidase 9 expression. In summary, our results suggest that Sirt1 suppresses acute lung inflammation during sepsis by controlling inflammasome activation pathway.
Keywords: caspase, interleukin-1, inflammation, lung, transcription factor
sepsis is a devastating disease with high mortality. The epidemiological studies from North America suggest a sepsis incidence of 3 cases per 1,000 persons annually (1). Because of its complexity and heterogeneity, sepsis remains a great challenge for scientists and clinicians. The lung and respiratory tract constitute a large surface of the body in direct contact with the outside environment, including inhaled microbes and particles (6). Pulmonary infection is the most common cause of sepsis, and meanwhile lung as the blood gas exchange site is the first line of sepsis-induced inflammation and tissue damage (48, 49). Molecular mechanisms of sepsis-induced lung inflammatory injury are yet to be determined. Tight regulation of the immune system appears to be critical to better maintain the balance between protective and tissue-damaging innate immune responses (9, 27).
Innate immune system is the first line of defense against invading bacterial pathogens (5, 6, 39, 42). The innate immune receptors such as pattern recognition receptors recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) (5, 6, 42). PAMP-induced Toll-like receptor signaling often results in the activation of MAPK cascades, including ERK1/2, c-JNK, and p38, and nuclear translocation of NF-κB, which consists of the first signal pathway of inflammasome activation (17, 19, 27, 35, 42, 44, 59). The nuclear translocation of NF-κB leads to the production of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, and chemokines, such as IL-8 (17, 19, 26, 27, 35, 42, 50). The inflammasome complex, a central player of the second signal pathway of inflammasome activation, comprises the nucleotide oligomerization domain-like receptor (NLR) protein such as NLRP3, the adapter apoptosis-associated speck-like protein containing caspase-recruitment domain (ASC), and pro-caspase-1 (30). The assembly and activation of the NLRP inflammasome complex cause caspase-1 and IL-1β cleavage and activation, IL-1β secretion (2, 46), and subsequent inflammatory responses (2, 30, 46).
Sirt1, a NAD+-dependent class III histone deacetylase, has been reported to exert its anti-inflammatory function by regulating the production of proinflammatory cytokines (4, 14, 16, 24, 40). However, the role of Sirt1 in sepsis-induced acute inflammatory injury and its function in modulating inflammasome activation pathway have yet to be determined. In the present study, we used a cecal ligation and puncture (CLP) sepsis model to investigate the role of Sirt1 in sepsis-induced acute lung inflammation by inducible deletion of Sirt1 in mice. Our studies demonstrated that Sirt1 knockout mice are highly susceptible to sepsis-induced inflammatory lung injury.
MATERIALS AND METHODS
Reagents.
Tamoxifen was purchased from Sigma-Aldrich (St. Louis, MO). TNF-α and IL-6 ELISA kits were obtained from Biolegend (San Diego, CA). o-Dianisidine dihydrochloride and H2O2 were obtained from Sigma-Aldrich. ICAM-1, claudin-1, and vascular endothelial (VE)-cadherin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-signal transducer and activator of transcription 3 (STAT3) (Thr705), STAT3 (124H6), phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204), p44/42MAPK (ERK1/2), phosphorylated Iκ-Bα, Iκ-Bα, IL-1β, cleaved caspase-3, cleaved caspase-7, high-mobility group box 1 (HMGB1), and β-actin antibodies were obtained from Cell Signaling Technology (Beverly, MA). Matrix metallopeptidase 9 (MMP-9) antibody was obtained from R&D Systems (Minneapolis, MN).
Animal studies.
Mice were housed in cages with free access to food and water in a temperature-controlled room with a 12-h:12-h dark/light cycle. All experiments and animal care procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler. The generation of inducible Sirt1 knockout mice was described previously (32, 60). Six to seven weeks after the birth, mice were given tamoxifen (100 mg/kg body wt in corn oil) by intraperitoneal (ip) injection daily for 5 days to induce nuclear translocation of Cre recombinase, as described previously (60). Twenty to twenty-eight weeks after Sirt1 deletion, age-matched female Sirt1−/− and Sirt1+/+ littermates were used in the studies. CLP was performed as described by us previously (61). Sham animals underwent the same surgical procedure except for ligation and puncture of the cecum.
Lung MPO assay, ELISA, and immunoblotting assays.
Neutrophil accumulation in the lung tissue was assessed by myeloperoxidase (MPO) activity as previously described (58). Briefly, 24 h after CLP surgery, lung tissue samples were frozen and homogenized in Hexadecyltrimethylammonium bromide lysis buffer. MPO activity was examined. Six hour after CLP, mouse lung tissues were collected. Lung tissue IL-6 and TNF-α levels were determined by ELISA kits following the instruction of the commercial kits. For immunoblotting assay, 6 or 24 h after CLP surgery, lung tissues were obtained and homogenized in tissue lysis buffer. Immunoblotting assay was carried out as previously described (60).
Statistical analysis.
Data were analyzed by two-way ANOVA with Bonferroni's multiple-comparisons test using the GraphPad Prism version 6 (GraphPad Software, La Jolla, CA) and expressed as means ± SE. Statistical significance was assigned to P values <0.05.
RESULTS
Sirt1 deletion increases lung proinflammatory cytokine production and aggravates lung inflammation after sepsis induction.
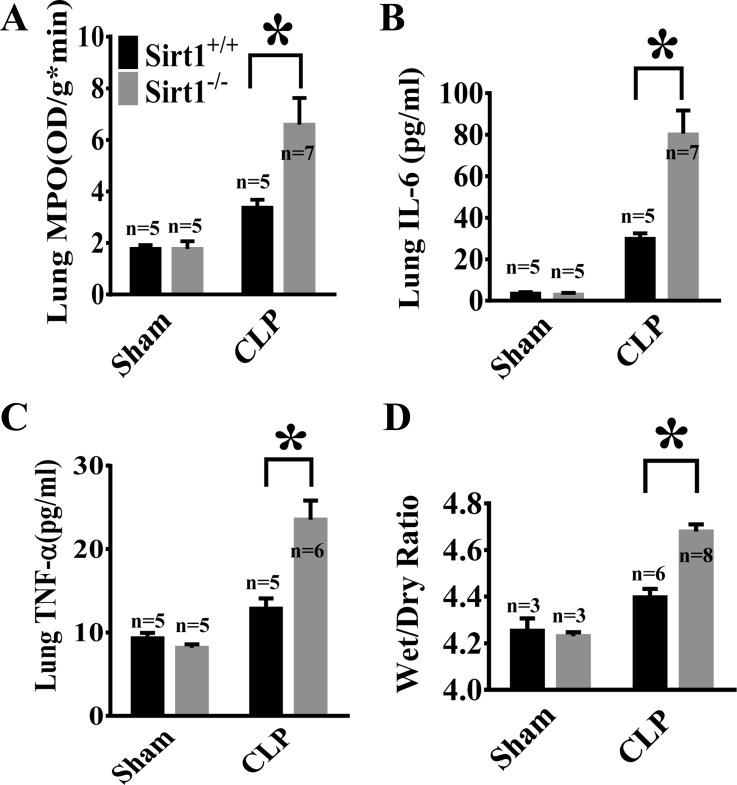
To assess lung inflammation in sepsis after Sirt1 deletion, lung MPO activity, an indicator of neutrophil infiltration was examined in Sirt1−/− mice and Sirt1+/+ littermates (Fig. 1A). Furthermore, lung IL-6 and TNF-α (Fig. 1, B and C) levels were evaluated by ELISA. Proinflammatory cytokines IL-6 and TNF-α and MPO levels in the lung tissues were increased after CLP and much higher in Sirt1−/− mice, suggesting that Sirt1 plays a protective role against sepsis-induced lung inflammation. To assess the role of Sirt1 in sepsis-induced lung injury, we examined edema formation in the lungs of Sirt1−/− mice and Sirt1+/+ littermates after the induction of sepsis. Lung wet/dry weight ratio was significantly increased in Sirt1−/− mice after CLP when compared with that of wild-type littermates (Fig. 1D).
Fig. 1.
Aggravated lung inflammation in Sirt1 knockout mice after cecal ligation and puncture (CLP). Sirt1−/− mice and Sirt1+/+ littermates were divided into 4 groups (Sirt1+/+/Sham, Sirt1−/−/Sham, Sirt1+/+/CLP, Sirt1−/−/CLP). A: lung myeloperoxidase (MPO) was measured 24 h after CLP. 6 h after CLP, proinflammatory factor IL-6 (B) and TNF-α (C) levels were measured by ELISA. D: 24 h after CLP, lungs of Sirt1−/− and Sirt1+/+ littermate mice were collected. Lung wet/dry weight ratio was examined. *P < 0.05 vs. CLP/Sirt1+/+ group. OD, optical density.
Sirt1 deletion leads to increased NF-κB/STAT3/ERK1/2 activation in the lung after sepsis induction.
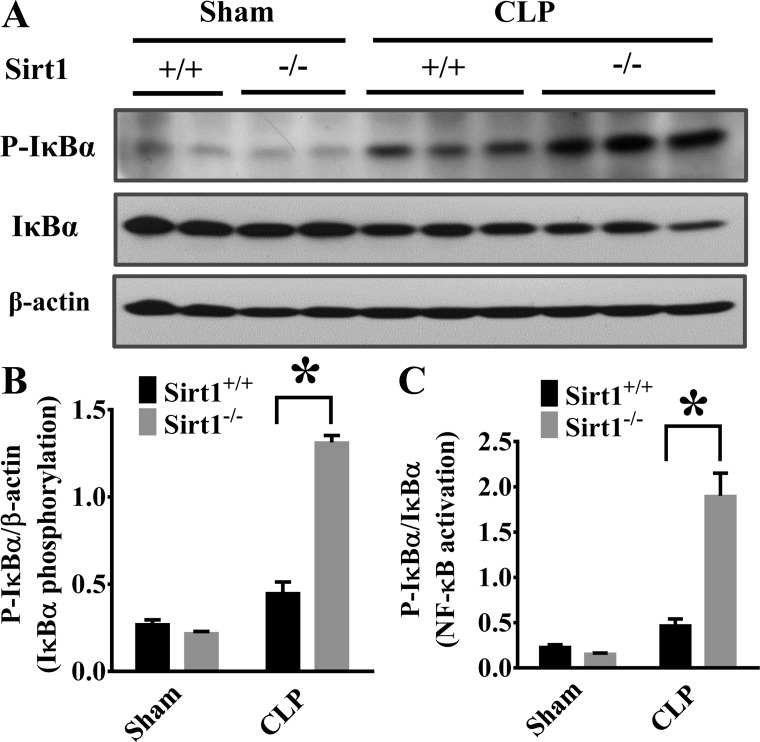
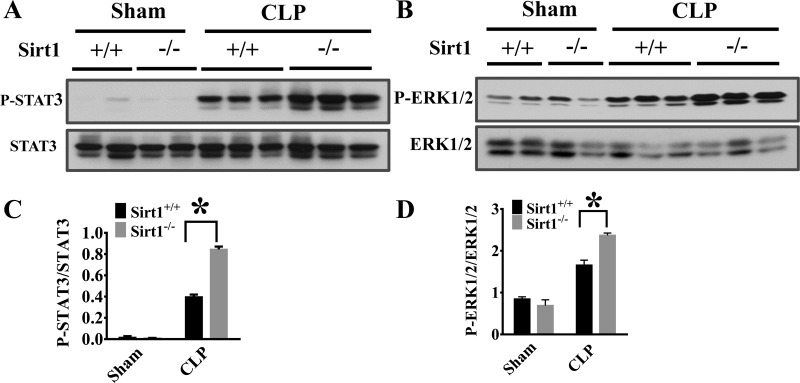
NF-κB is a master transcription factor of proinflammatory mediators (35). To explore molecular mechanisms of Sirt1 protection against sepsis-induced lung inflammation, we examined NF-κB activation. NF-κB activation is dependent on the phosphorylation and degradation of its inhibitory regulator Iκ-Bα (43). We examined Iκ-Bα phosphorylation and degradation. The increased Iκ-Bα phosphorylation and degradation indicate that NF-κB was activated in the lung tissues of Sirt1−/− mice after CLP (Fig. 2, A–C). We also conducted experiments to assess the effects of Sirt1 deletion on other lung inflammatory signaling including STAT3 and ERK1/2 activation. Both STAT3 and ERK1/2 phosphorylation were increased in Sirt1−/− mice (Fig. 3, A–D).
Fig. 2.
Sirt1 deletion enhances CLP-induced NF-κB activation. Lung tissues were harvested 6 h after CLP (Sirt1+/+/Sham, n = 5; Sirt1−/−/Sham, n = 5; Sirt1+/+/CLP, n = 5; Sirt1−/−/CLP, n = 8). Lung samples were then subjected to immunoblotting assay. Iκ-Bα phosphorylation and total Iκ-Bα levels were examined. A: representative blots of Iκ-Bα phosphorylation and total Iκ-Bα. B and C: densitometry analysis. *P < 0.05 vs. Sirt1+/+/CLP group.
Fig. 3.
Sirt1 deletion increases CLP-induced signal transducer and activator of transcription 3 (STAT3) and ERK1/2 activation in the lung. Lung tissues were harvested 6 h after CLP challenge (Sirt1+/+/Sham, n = 5; Sirt1−/−/Sham, n = 5; Sirt1+/+/CLP, n = 5; Sirt1−/−/CLP, n = 8). Lung samples were then subjected to immunoblotting assay. STAT3 (A and C) and ERK1/2 (B and D) phosphorylation were examined. *P < 0.05 vs. Sirt1+/+/CLP group.
Sirt1 deletion leads to increased IL-1β precursor and mature form production in the lung after sepsis induction.
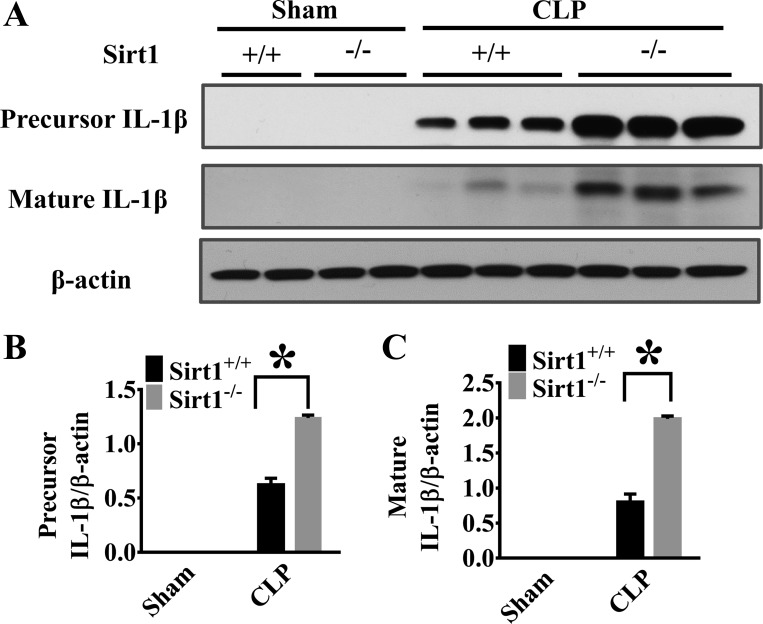
Inflammasome activation pathway contains two essential phases (2, 35, 37, 43). The first is the activation of proinflammatory transcription factors, such as NF-κB (2, 35, 43, 62), which induces proinflammatory cytokine expression e.g., pro-IL-1β and IL-18 production. The second compulsory phase is to generate the mature forms of the cytokines by inflammasome-mediated cleavage of the precursors (2, 12, 30, 42, 46). To assess the role of Sirt1 in this process, we examined both the precursor and mature forms of IL-1β production in the lung tissues after CLP. Our results showed that Sirt1 deletion led to increased pro-IL-1β and the mature form generation (Fig. 4, A–C), suggesting that downregulation of inflammasome activation pathway could be one of the mechanisms contributing to Sirt1 protection against lung inflammation in sepsis.
Fig. 4.
Sirt1 deletion enhances CLP-induced precursor and mature IL-1β production in the lung. Lung tissues were harvested 6 h after CLP (Sirt1+/+/Sham, n = 5; Sirt1−/−/Sham, n = 5; Sirt1+/+/CLP, n = 5; Sirt1−/−/CLP, n = 8). Lung samples were then subjected to immunoblotting assay. The precursor and matured IL-1β levels were examined. A: representative blots of the precursor and mature IL-1β. B and C: densitometry analysis. *P < 0.05 vs. Sirt1+/+/CLP group.
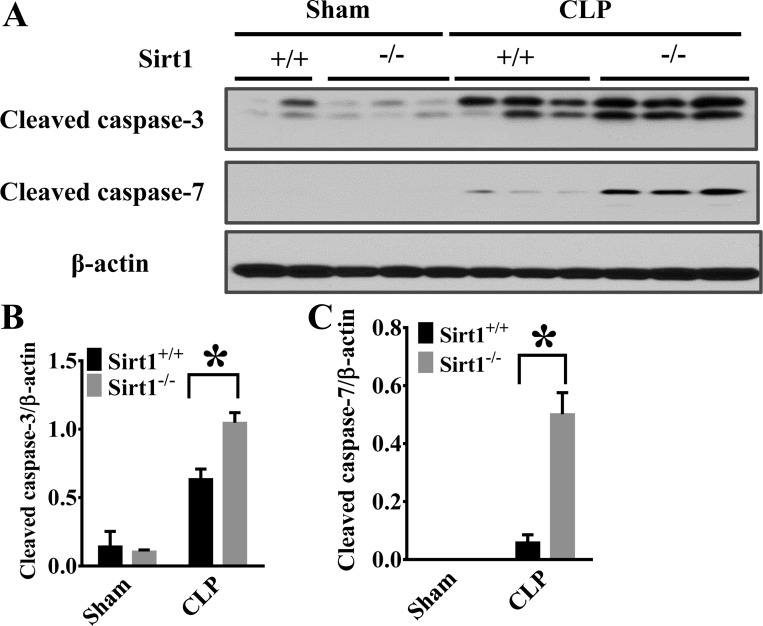
Sirt1 deletion leads to increased caspase-3 and caspase-7 activation in the lung after sepsis induction.
Caspases are highly conserved cysteine-dependent aspartate-specific proteases, which can be grouped into inflammatory and apoptotic caspases (8, 37). Caspase-1, -3, and -7 can also mediate inflammatory responses, even though caspase-3 and caspase-7 are still considered major players in apoptosis or necrosis (8, 12, 37). Caspase-1 is a critical component of the inflammasome complex (11, 12, 37, 46). Caspase-7 is a substrate of caspase-1 (12, 37). To confirm the function of Sirt1 in inflammasome activation, we examined the production of cleaved caspase-3 and -7 in the lung tissues 6 h after CLP surgery. We observed that the activation of both caspase-3 and -7 was increased in Sirt1−/− mice after CLP with the level of caspase-7 cleavage much higher in the lungs of Sirt1−/− mice (Fig. 5, A–C). Our results indicate that Sirt1 regulation of caspase-1-mediated caspase-7 activation may also contribute to its protection against inflammatory signaling in sepsis.
Fig. 5.
Sirt1 deletion increases CLP-induced caspase-3 and caspase-7 activation in the lung. 6 h after CLP, lung tissues were collected and subjected to immunoblotting assay (Sirt1+/+/Sham, n = 5; Sirt1−/−/Sham, n = 5; Sirt1+/+/CLP, n = 5; Sirt1−/−/CLP, n = 8). The cleavage of caspase-3 and caspase-7 was examined. A: representative blots of cleaved caspase-3 and caspase-7. C and D: densitometry analysis. *P < 0.05 vs. Sirt1+/+/CLP group.
Sirt1 deletion leads to increased lung ICAM-1 and HMGB1 expression after sepsis induction.
Proinflammatory cytokines can regulate the expression of cell adhesion molecules such as ICAM-1 (45, 56). We examined the effects of Sirt1 deletion on ICAM-1 expression in lung tissues after CLP. Immunoblotting assays showed that Sirt1−/− mice exhibited significantly higher ICAM-1 expression after CLP (Fig. 6, A and C). Additionally, PAMPs and DAMPs activate the double-stranded RNA-activated protein kinase R to trigger the inflammasome-dependent HMGB1 release (33). HMGB1 plays a role in mediating late inflammatory responses in sepsis (15, 25, 55, 63). Because it is released later than other proinflammatory cytokines, it became known as a “late mediator of sepsis” (41). We examined lung HMGB1 expression in Sirt1−/− mice. Our results showed that Sirt1 deletion led to increased lung HMGB1 production (Fig. 6, B and D).
Fig. 6.
Sirt1 deletion enhances CLP-induced ICAM-1 and high-mobility group box 1 (HMGB1) expression in the lung. ICAM-1 and HMGB1 levels (6 h) were examined after CLP (Sirt1+/+/Sham, n = 5; Sirt1−/−/Sham, n = 5; Sirt1+/+/CLP, n = 5; Sirt1−/−/CLP, n = 8). A and B: representative blots showing ICAM-1 and HMGB1 expression in the lung. C and D: densitometry analysis. *P < 0.05 vs. Sirt1+/+/CLP group.
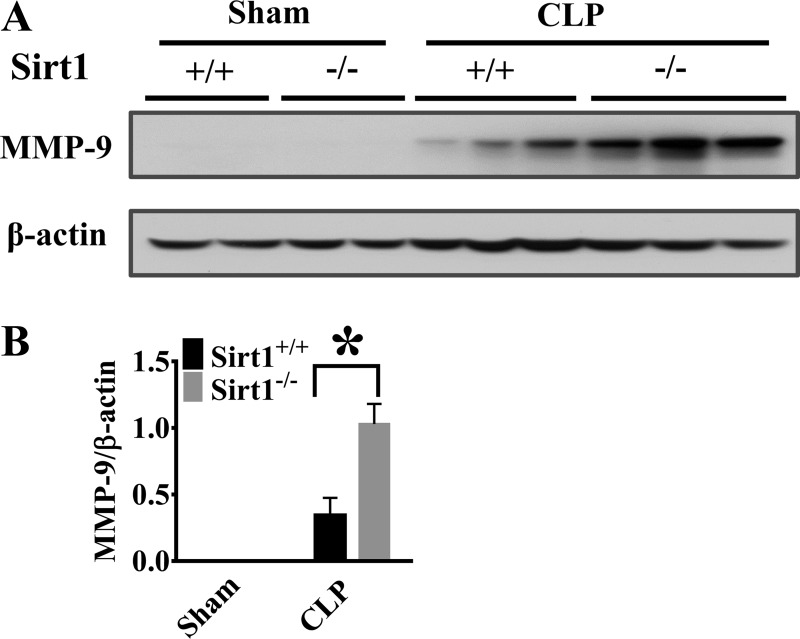
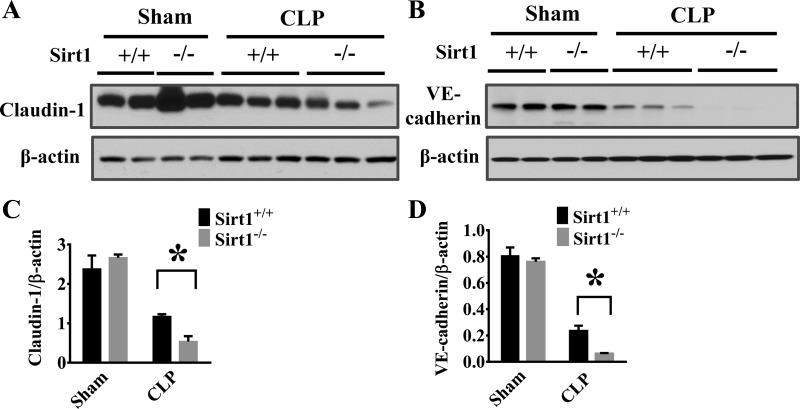
Sirt1 deletion increases lung MMP-9 production and decreases lung claudin-1/VE-cadherin levels after sepsis induction.
Extracellular proteases, such as MMPs, have been reported to mediate inflammatory responses (51, 57) and induce endothelial/epithelial barrier disruption through cleavage and shedding of extracellular fragment of junction proteins (13, 34). We detected a significant increase of MMP-9 expression in the lung tissues of Sirt1−/− mice after CLP (Fig. 7, A and B). The increased extracellular protease activity may lead to degradation of cell-cell junction proteins in the lung. VE-cadherin and claudin are key components of cell-cell junctions (10, 18). Here, we examined the effects of Sirt1 deletion on VE-cadherin and claudin-1 protein levels in the lung tissues after CLP. Consistent with increased MMP-9 production, Sirt1-deficient mice exhibited markedly reduced VE-cadherin and claudin-1 levels after CLP (Fig. 8, A–D).
Fig. 7.
Sirt1 deletion increases CLP-induced matrix metallopeptidase 9 (MMP-9) expression in the lung. Lung tissues were harvested 6 h after CLP (Sirt1+/+/Sham, n = 5; Sirt1−/−/Sham, n = 5; Sirt1+/+/CLP, n = 5; Sirt1−/−/CLP, n = 8). MMP-9 expression (A and B) was examined in the lung tissues by immunoblotting assay. *P < 0.05 vs. Sirt1+/+/CLP group.
Fig. 8.
Sirt1 deletion aggravates CLP-induced lung endothelial barrier disruption. Lung tissues were harvested 6 h after CLP challenge (Sirt1+/+/Sham, n = 5; Sirt1−/−/Sham, n = 5; Sirt1+/+/CLP, n = 5; Sirt1−/−/CLP, n = 8). Samples were subjected to immunoblotting assay. Expression of claudin-1 (A) and vascular endothelial (VE)-cadherin (B) was examined. C and D: Densitometry analysis. *P < 0.05 vs. Sirt1+/+/CLP group.
DISCUSSION
Sepsis is a life-threatening disease with high morbidity and mortality (22, 23). The balance between pro- and anti-inflammatory pathways plays an important role in the fate of the patients (22). Up to now, no efficient therapeutics is available to treat this devastating disease (22, 23). A better understanding of the pathophysiological basis of the disorder is needed to develop novel strategies against sepsis.
It is now well established that early organ damage in sepsis is caused by overwhelming inflammation. However, both inflammatory organ injury and late-stage immune suppression, which leads to secondary infection, likely contribute to the high mortality in sepsis (20–22). Acute lung injury is usually associated with excessive lung inflammation (31, 35, 36). Immunosuppression, attributable to early exhaustion of immune response, is extremely common in the middle and late phases of sepsis and is associated with increased nosocomial infection and mortality (20–22). Therefore, early control of inflammatory responses is critical in the prognosis of sepsis. Sirt1, a member of histone deacetylase, has been reported to play a critical role in regulating metabolism, senescence, and aging-related diseases (3, 47). Our data demonstrate that sepsis-induced lung inflammatory injury in Sirt1-deficient mice is aggravated, suggesting that Sirt1 plays a protective role against lung inflammatory injury in sepsis.
Sirt1 has been reported to control inflammatory cytokine production and activation through NF-κB acetylation (40, 64). Our studies demonstrate that Sirt1 regulates NF-κB activation in sepsis. Upregulation of proinflammatory cytokines by NF-κB activation induces the expression of cell adhesion molecules such as ICAM-1 to promote early leukocyte infiltration and lung inflammatory injury. HMGB1, a late mediator of inflammation in sepsis, is also increased subsequently (7, 52, 54). The increased lung ICAM-1 and HMGB1 expression in Sirt1-deficient mice is consistent with the aggravated lung injury after CLP. The increases of both the early and late proinflammatory mediators in Sirt1-deficient mice likely lead to the excessive inflammatory responses.
Inflammasome pathway has two main signal phases: transcription factor such as NF-κB-regulated gene expression of proinflammatory mediators, followed by mature cytokine production and release phase regulated by inflammsome complex activation (17, 19, 26, 27, 30, 35, 42, 46, 50). The first phase is triggered by the PAMPs or DAMPs, leading to the degradation and activation of the NF-κB inhibitor Iκ-Bα (35). NF-κB activation leads to increased expression of proinflammatory mediators such as ICAM-1 and the precursor of proinflammatory cytokines such as IL-1β and IL-18, which are further processed by the inflammsome complex to generate the active forms (2, 30, 46). The inflammasome complex, which consists of pro-caspase-1, NLRP, and ASC, is required for IL-1β and IL-18 cleavage and release (2, 30, 46). Our data suggest that Sirt1 regulates both the first signal phase (NF-κB) and the second signal phase (inflammasome complex activation and precursor processing) in sepsis.
Members of the inflammatory caspases, also known as group I caspases, include caspase-1, -4, and -5 in human and caspase-1, -11, and -12 in mouse (29, 37). Caspase-1, an aspartate-specific cysteine protease, is a component of the inflammasome complex assembled upon pathogen recognition, which cleaves IL-1β and IL-18 to generate the mature and active forms (29, 37). Caspase-7 is also cleaved and activated by caspase-1 (12). Poly(ADP-ribose) polymerase-1 (PARP1), a target of caspase-7, has been reported to bind to the promoters of NF-κB target genes and to negatively regulate their gene expression (12). Caspase-7 activation by caspase-1 cleavage has been reported to modulate the expression of NF-κB target genes through PARP-1 cleavage (12). In our studies, caspase-7 activation was markedly increased in the Sirt1 knockout mice during sepsis, which may further induce NF-κB-regulated proinflammatory gene expression by PARP-1 cleavage.
The disruption of endothelial cell junctions leads to increased vascular permeability and edema formation during inflammatory lung injury (28, 38, 53). The VE-cadherin and claudins are key members of adherens junctions and tight junctions, respectively. VE-cadherin and claudins are targets of extracellular protease-mediated degradation (13, 18, 34). In our studies, we demonstrated that VE-cadherin and claudin-1 protein levels in the lung tissues of Sirt1-deficient mice were dramatically reduced during sepsis, which was associated with higher MMP-9 expression. Our results suggest that upregulated extracellular proteases such as MMP-9 activity in Sirt1-deficient mice may lead to the greater breakdown of the endothelial barrier function.
In summary, Sirt1 plays a protective role against inflammatory lung injury in CLP-induced sepsis. Sirt1 suppresses inflammasome activation pathway, which prevents the overproduction of proinflammatory mediators and associated tissue damage. Our studies indicate that Sirt1 is a potential therapeutic target to treat sepsis-induced lung inflammatory injury.
GRANTS
The study was supported by the National Institutes of Health grant (1R01GM099744) to J. Fu.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.G., Z.M., and J.F. conception and design of research; R.G., Y.H., and J.C. performed experiments; R.G. analyzed data; R.G., Z.M., S.S., and J.F. interpreted results of experiments; R.G. prepared figures; R.G. drafted manuscript; R.G., Z.M., S.S., and J.F. edited and revised manuscript; R.G., Z.M., Y.H., J.C., S.S., and J.F. approved final version of manuscript.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Barker BR, Taxman DJ, Ting JP. Cross-regulation between the IL-1beta/IL-18 processing inflammasome and other inflammatory cytokines. Curr Opin Immunol 23: 591–597, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer 9: 123–128, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao L, Liu C, Wang F, Wang H. SIRT1 negatively regulates amyloid-beta-induced inflammation via the NF-kappaB pathway. Braz J Med Biol Res 46: 659–669, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carneiro LA, Travassos LH, Girardin SE. NOD-like receptors in innate immunity and inflammatory diseases. Ann Med 39: 581–593, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Chaput C, Sander LE, Suttorp N, Opitz B. NOD-like receptors in lung diseases. Front Immunol 4: 393, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M, Frankfurt M, Volpe BT, Tracey KJ, Diamond B. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med 18: 930–937, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhury I, Tharakan B, Bhat GK. Caspases—an update. Comp Biochem Physiol B Biochem Mol Biol 151: 10–27, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 19: 198–208, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Dejana E, Giampietro C. Vascular endothelial-cadherin and vascular stability. Curr Opin Hematol 19: 218–223, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, Haspel JA, Landazury R, Eppanapally S, Christie JD, Meyer NJ, Ware LB, Christiani DC, Ryter SW, Baron RM, Choi AM. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 185: 1225–1234, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erener S, Petrilli V, Kassner I, Minotti R, Castillo R, Santoro R, Hassa PO, Tschopp J, Hottiger MO. Inflammasome-activated caspase 7 cleaves PARP1 to enhance the expression of a subset of NF-kappaB target genes. Mol Cell 46: 200–211, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Feng S, Cen J, Huang Y, Shen H, Yao L, Wang Y, Chen Z. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS One 6: e20599, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Wang Y, Du L, Xu C, Cao J, Fan T, Liu J, Su X, Fan S, Liu Q, Fan F. Resveratrol inhibits ionising irradiation-induced inflammation in MSCs by activating SIRT1 and limiting NLRP-3 inflammasome activation. Int J Mol Sci 14: 14105–14118, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao XP, Liu Q, Broman M, Predescu D, Frey RS, Malik AB. Inactivation of CD11b in a mouse transgenic model protects against sepsis-induced lung PMN infiltration and vascular injury. Physiol Genom 21: 230–242, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Gillum MP, Kotas ME, Erion DM, Kursawe R, Chatterjee P, Nead KT, Muise ES, Hsiao JJ, Frederick DW, Yonemitsu S, Banks AS, Qiang L, Bhanot S, Olefsky JM, Sears DD, Caprio S, Shulman GI. Sirt1 regulates adipose tissue inflammation. Diabetes 60: 3235–3245, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 25: 6680–6684, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Goncalves A, Ambrosio AF, Fernandes R. Regulation of claudins in blood-tissue barriers under physiological and pathological states. Tissue Barriers 1: e24782, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol 5: 352, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13: 260–268, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13: 862–874, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol 6: 813–822, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Hwang JW, Yao H, Caito S, Sundar IK, Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med 61: 95–110, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiao H, Zhang Y, Yan Z, Wang ZG, Liu G, Minshall RD, Malik AB, Hu G. Caveolin-1 Tyr14 phosphorylation induces interaction with TLR4 in endothelial cells and mediates MyD88-dependent signaling and sepsis-induced lung inflammation. J Immunol 191: 6191–6199, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jitprasertwong P, Jaedicke KM, Nile CJ, Preshaw PM, Taylor JJ. Leptin enhances the secretion of interleukin (IL)-18, but not IL-1beta, from human monocytes via activation of caspase-1. Cytokine 65: 222–230, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Keyel PA. How is inflammation initiated? Individual influences of IL-1, IL-18 and HMGB1. Cytokine 69: 136–145, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci STKE 2007: re8, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Diff 9: 358–361, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol 13: 397–411, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol 76: 467–492, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Rajendran GK, Liu N, Ware C, Rubin BP, Gu Y. SirT1 modulates the estrogen-insulin-like growth factor-1 signaling for postnatal development of mammary gland in mice. Breast Cancer Res 9: R1, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W, Li J, Sama AE, Wang H. Carbenoxolone blocks endotoxin-induced protein kinase R (PKR) activation and high mobility group box 1 (HMGB1) release. Mol Med 19: 203–211, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci 32: 3044–3057, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 290: L622–L645, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Lomas-Neira J, Chung CS, Ayala A. RNA interference as a potential therapeutic treatment for inflammation associated lung injury. Int J Clin Exp Med 1: 154–160, 2008. [PMC free article] [PubMed] [Google Scholar]
- 37.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117: 561–574, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell JA, Paul-Clark MJ, Clarke GW, McMaster SK, Cartwright N. Critical role of toll-like receptors and nucleotide oligomerisation domain in the regulation of health and disease. J Endocrinol 193: 323–330, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Moon MH, Jeong JK, Lee YJ, Seol JW, Jackson CJ, Park SY. SIRT1, a class III histone deacetylase, regulates TNF-alpha-induced inflammation in human chondrocytes. Osteoarthritis Cartilage 21: 470–480, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Naglova H, Bucova M. HMGB1 and its physiological and pathological roles. Bratisl Lek Listy 113: 163–171, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 4: a006049, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1: a000034, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paciello I, Silipo A, Lembo-Fazio L, Curcuru L, Zumsteg A, Noel G, Ciancarella V, Sturiale L, Molinaro A, Bernardini ML. Intracellular Shigella remodels its LPS to dampen the innate immune recognition and evade inflammasome activation. Proc Natl Acad Sci USA 110: E4345–E4354, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palenski TL, Sorenson CM, Sheibani N. Inflammatory cytokine-specific alterations in retinal endothelial cell function. Microvasc Res 89: 57–69, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol 13: 333–342, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rehan L, Laszki-Szczachor K, Sobieszczanska M, Polak-Jonkisz D. SIRT1 and NAD as regulators of ageing. Life Sci 105: 1–6, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Russell JA, Walley KR. Update in sepsis 2012. Am J Respir Crit Care Med 187: 1303–1307, 2013. [DOI] [PubMed] [Google Scholar]
- 49.Seeley EJ. Updates in the management of acute lung injury: a focus on the overlap between AKI and ARDS. Adv Chronic Kidney Dis 20: 14–20, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Simard JC, Cesaro A, Chapeton-Montes J, Tardif M, Antoine F, Girard D, Tessier PA. S100A8 and S100A9 induce cytokine expression and regulate the NLRP3 inflammasome via ROS-dependent activation of NF-kappaB(1.). PLoS One 8: e72138, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorsa T, Mantyla P, Tervahartiala T, Pussinen PJ, Gamonal J, Hernandez M. MMP activation in diagnostics of periodontitis and systemic inflammation. J Clin Periodontol 38: 817–819, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med 33: 564–573, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann NY Acad Sci 1123: 134–145, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 10: 1216–1221, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med 255: 320–331, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JP, Mackman N. Monocytic microparticles activate endothelial cells in an IL-1beta-dependent manner. Blood 118: 2366–2374, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warner RL, Bhagavathula N, Nerusu KC, Lateef H, Younkin E, Johnson KJ, Varani J. Matrix metalloproteinases in acute inflammation: induction of MMP-3 and MMP-9 in fibroblasts and epithelial cells following exposure to pro-inflammatory mediators in vitro. Exp Mol Pathol 76: 189–195, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Wei D, Huang Z. Anti-inflammatory effects of triptolide in LPS-induced acute lung injury in mice. Inflammation 37: 1307–1316, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Wilson SP, Cassel SL. Inflammasome-mediated autoinflammatory disorders. Postgrad Med 122: 125–133, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Z, Liu MC, Liang M, Fu J. Sirt1 protects against thrombomodulin down-regulation and lung coagulation after particulate matter exposure. Blood 119: 2422–2429, 2012. [DOI] [PubMed] [Google Scholar]
- 61.Wu Z, Sawamura T, Kurdowska AK, Ji HL, Idell S, Fu J. LOX-1 deletion improves neutrophil responses, enhances bacterial clearance, and reduces lung injury in a murine polymicrobial sepsis model. Infect Immun 79: 2865–2870, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao J, Zhang H, Huang Y, Wang H, Wang S, Zhao C, Liang Y, Yang N. Bay11-7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-kappaB activation. Int Immunopharmacol 17: 116–122, 2013. [DOI] [PubMed] [Google Scholar]
- 63.Zhu S, Li W, Li J, Sama AE, Wang H. Caging a beast in the inflammation arena: use of Chinese medicinal herbs to inhibit a late mediator of lethal sepsis, HMGB1. Int J Clin Exp Med 1: 64–75, 2008. [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu X, Liu Q, Wang M, Liang M, Yang X, Xu X, Zou H, Qiu J. Activation of Sirt1 by resveratrol inhibits TNF-alpha induced inflammation in fibroblasts. PLoS One 6: e27081, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]