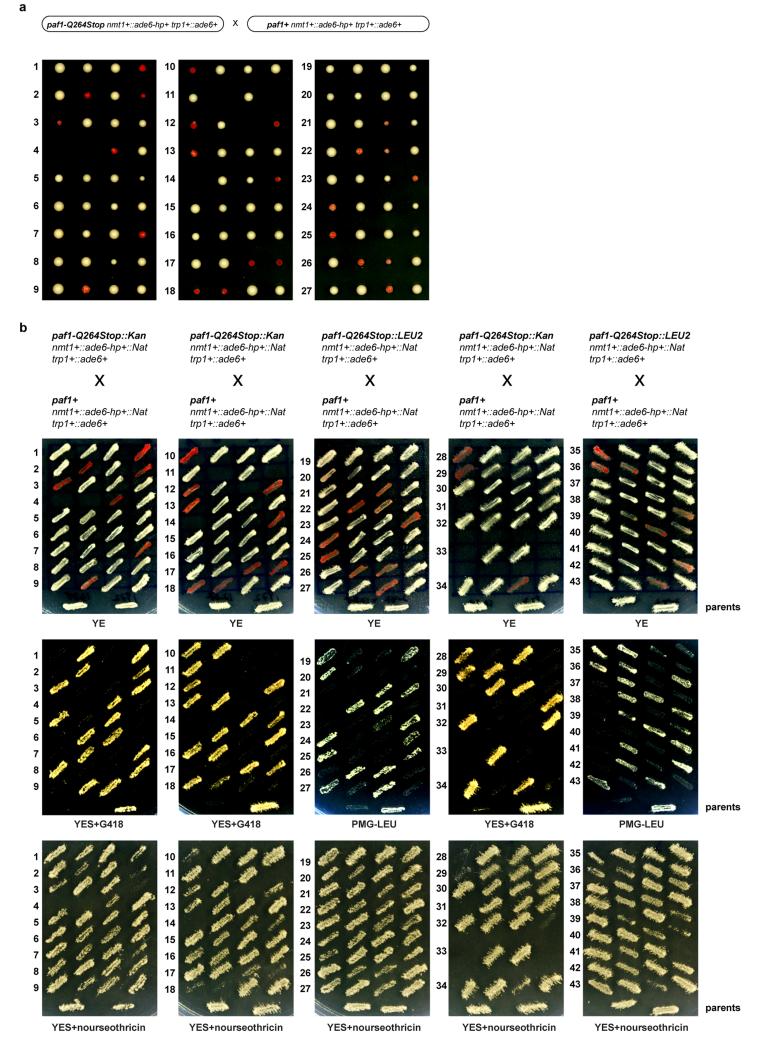
Extended Data Figure 7. Pronounced siRNA-directed heterochromatin formation in trans during meiosis.
a, White (naïve) cells that had not yet established heterochromatin at the trp1+::ade6+ locus were isolated from populations of freshly generated paf1-Q264Stop strains and crossed with paf1+ cells. Both mating partners expressed ade6-hp siRNAs and contained the same trp1+::ade6+ reporter. Spores were dissected on YE plates and incubated for 3-4 days at 30°C. Note the non-Mendelian inheritance pattern of the parental white phenotype and the high incidence of heterochromatin formation (red phenotype) in paf1-Q264Stop cells after meiosis. b, Spores from 43 tetrads were dissected in total. Colonies formed by the individual spores (a) were then struck on YE plates and incubated for 3-4 days at 30°C, followed by replica-plating onto YES-G418 and YES+nourseothricin (Nat) plates for genotyping. Thus, the cells visible on the YE plates have gone through roughly 50-80 mitotic divisions after mating and sporulation. This analysis shows that de novo formation of heterochromatin by trans-acting siRNAs during meiosis occurs more frequently than in mitosis. However, once established, heterochromatin is remarkably stable in mitotic cells (see also figure 2). Intriguingly, growth of some paf1-Q264Stop descendants was reduced on YES+nourseothricin plates, demonstrating spreading of heterochromatin into the neighboring Nat-resistance cassette that marks the nmt1+::ade6-hp+ locus (see Extended Data Fig. 1). Note that genes repressed by heterochromatin can be derepressed under strong negative selection. Thus, this observation indicates extraordinary repressive activity of the heterochromatin that forms in cis at the ade6-hp siRNA-producing locus. Finally, note that paf1+ cells (no growth on YES-G418 or PMG-LEU) never turned red, demonstrating the high repressive activity of Paf1. This explains unsatisfactory results of previous attempts to induce the formation of stable heterochromatin in trans by expressing synthetic siRNAs.