Abstract
Diabetic polyneuropathy (DPN) can cause muscle atrophy, weakness, contractile slowing, and neuromuscular transmission instability. Our objective was to assess the response of the impaired neuromuscular system of DPN in humans when stressed with a sustained maximal voluntary contraction (MVC). Baseline MVC and evoked dorsiflexor contractile properties were assessed in DPN patients (n = 10) and controls (n = 10). Surface electromyography was used to record tibialis anterior evoked maximal compound muscle action potentials (CMAPs) and neuromuscular activity during MVCs. Participants performed a sustained isometric dorsiflexion MVC for which task termination was determined by the inability to sustain ≥60% MVC torque. The fatigue protocol was immediately followed by a maximal twitch, with additional maximal twitches and MVCs assessed at 30 s and 2 min postfatigue. DPN patients fatigued ∼21% more quickly than controls (P < 0.05) and featured less relative electromyographic activity during the first one-third of the fatigue protocol compared with controls (P < 0.05). Immediately following fatigue, maximal twitch torque was reduced similarly (∼20%) in both groups, and concurrently CMAPs were reduced (∼12%) in DPN patients, whereas they were unaffected in controls (P > 0.05). Twitch torque and CMAP amplitude recovered to baseline 30 s postfatigue. Additionally, at 30 s postfatigue, both groups had similar (∼10%) reductions in MVC torque relative to baseline, and MVC strength recovered by 2 min postfatigue. We conclude DPN patients possess less endurance than controls, and neuromuscular transmission failure may contribute to this greater fatigability.
Keywords: diabetes, fatigue, electromyography, compound muscle action potential, weakness
diabetic polyneuropathy (DPN), a common complication of diabetes mellitus (DM), can be associated with dysfunction of the neuromuscular system. Some DPN-related neuromuscular changes include the following: muscle atrophy (7), weakness (9), muscle contractile slowing (3), reduced motor unit firing rates (2, 6, 55), and motor unit loss (1, 2, 23). However, at present, little is known regarding how these alterations may affect the performance of the neuromuscular system when its capacity is stressed during a fatiguing task and subsequent recovery. Challenging the compromised system will permit the identification and functional relevance of key underlying factors contributing to DPN.
Neuromuscular fatigue can be defined as the loss of voluntary force-producing capacity during physical activity. In the context of this investigation, this is differentiated from “experienced fatigue,” which refers to a general sense of tiredness, lack of energy, or chronic exhaustion often reported clinically (21, 30). Neuromuscular fatigue is well known to be task dependent and can be affected by a wide array of factors, including age (5), physical training status (43), and neurological disease (15).
One previous study that explored muscle fatigue in insulin-dependent (type 1) DM, concluded that DM patients have greater relative muscle endurance than controls during repeated, maximal isokinetic contractions of the ankle plantar and dorsiflexors (8). However, in that report, the majority of DM patients either did not have symptoms of DPN or had a neuropathy with minimal motor involvement (8). Additionally, no measure of voluntary activation (VA) was made; therefore, it is not known whether participants were producing maximal efforts during their isokinetic contractions (8). It has been shown previously that individuals with metabolic disease without neuropathy (e.g., type 1 DM; Ref. 6), vascular disease (e.g., chronic heart failure; Ref. 13), or nerve disease (e.g., amyotrophic lateral sclerosis; Ref. 50) fatigue more quickly than controls.
Greater fatigability in various other clinical populations compared with controls has been attributed to a range of factors, including the following: insufficient blood flow (13), impaired neuromuscular transmission (61), and central activation failure (49). Indeed, even during minimally fatiguing contractions [20% maximal voluntary contraction (MVC) for 30 s], neuromuscular conduction failure has been reported in DPN patients (4). In contrast, healthy adult aging has been associated with greater resistance to fatigue in certain isometric tasks (5, 16), but not all isometric tasks and muscle groups (26). It is not entirely clear whether slowed contractile and metabolic functions often reported with aging necessarily provide greater endurance (29), although slowed contractile properties also have been reported in DPN patients (3). However, because DPN can involve concurrent pathophysiological changes to metabolism, vasculature, and nerve and muscle, the impact of altered contractile properties may be mitigated. Therefore, DPN provides an interesting model to study various factors contributing to fatigue, as well as to improved understanding about the functional impact of DPN.
Investigating the recovery of neuromuscular properties following fatigue also can provide insight into underlying physiological processes, although it is typically less well studied than fatigue per se. Metabolic disturbances (48), blood flow impairments (18), and dysfunction in myocellular processes related to clearing metabolites and restoring homeostasis (28, 31) could conceivably impair or delay recovery in DPN patients.
The present study used a sustained, isometric dorsiflexion MVC to induce fatigue to a predetermined task termination point (i.e., 60% MVC). Dorsiflexion was selected because the dorsiflexors [i.e., tibialis anterior (TA)] are known to be affected in DPN (1, 2, 3), and participants are readily able to maximally activate the dorsiflexors, with minimal familiarization or training (11). A sustained, isometric MVC was selected to provide a relatively simple model that would substantially stress the neuromuscular system (30, 39). Finally, a predetermined task termination point (60% MVC) was utilized to ensure all participants fatigued to the same degree relative to their maximal strength, thus providing an opportunity to assess recovery (39).
Thus the main objective of this study was to investigate neuromuscular fatigue in patients with DPN compared with age-matched controls using a sustained, dorsiflexion MVC. We also aimed to determine whether impairments in neuromuscular transmission or VA were associated with fatigability in the DPN group. An additional objective was to assess the short-term (i.e., 2 min) neuromuscular recovery of DPN patients compared with controls. We hypothesized that both groups would be able to achieve near maximal VA (∼95%) while fatiguing to 60% of MVC torque, but, due to DPN-related neuromuscular dysfunctions and compromised blood flow, that individuals with DPN would fatigue more quickly and recover more slowly than controls.
METHODS
Participants.
Ten patients (6 men) with DPN (aged 64 ± 11 yr) and 10 age- and sex-matched controls (aged 62 ± 12 yr) were recruited for participation in this study. All participants provided informed, written consent before participation. The study was approved by the local university's Research Ethics Board and conformed to the Declaration of Helsinki. The patients with DPN met accepted clinical criteria for diagnosis of type 2 (insulin independent) DM and DPN (17). The presence of DPN was confirmed via clinical and electrophysiological examination performed by a trained neurologist, who also confirmed the absence of other causes of nerve injury or neuropathy. DPN patients were not taking any medications for neuropathic pain, which could have had an effect on VA. Data outlining some of the neuromuscular impacts of DPN (e.g., loss of motor units, muscle slowing, and neuromuscular transmission instability) in these patients have been previously reported elsewhere (2–4). Patients with metabolic, neurological, or vascular conditions unrelated to DM or DPN were excluded from participation. Data regarding glycemic control were obtained through clinical chart review. Control participants were living independently in the community, free from medications, and were screened by physicians (neurologists) to ensure they met inclusion criteria. Participants in both groups were not engaged in systematic physical exercise regimens.
Evoked contractile properties and maximal strength of dorsiflexors.
The experimental setup has been described in detail in a previous report (3). Participants were seated in a custom isometric dorsiflexion dynamometer to assess voluntary and electrically evoked properties of the dorsiflexor muscles (35). In all participants, the right leg was tested. The ankle was positioned and maintained at 30° of plantar flexion; both knee and hip angles were positioned at 90°. Hip joint movement was minimized by securing a padded, adjustable brace over the distal aspect of the right thigh. Inelastic straps were wrapped over the dorsum of the foot to secure it to the dynamometer (38). The great toe was left uncovered by the straps to minimize torque contributions from extensor hallucis longus.
Maximal evoked single-twitch and compound muscle action potential (CMAP) responses from the dorsiflexors (TA muscle) were obtained using supramaximal electrical stimulation of the fibular nerve just distal to the fibular head. Percutaneous nerve stimulation was performed using a bar electrode, which transmitted the single, 100-μs square-wave pulses triggered from a constant-voltage electrical stimulator (Digitimer stimulator, model DS7AH; Digitimer, Welwyn Garden City, UK). Twitches were analyzed off-line for the following: peak twitch tension (N·m), time to peak tension (ms), and half-relaxation time (HRT; ms). From these data, twitch contraction duration (ms; time to peak tension + HRT) and twitch peak rate of torque development (RTD; peak twitch tension/time to peak twitch tension) were calculated. Accompanying CMAPs were analyzed for maximal negative peak voltage (mV), negative peak duration (ms), and negative peak area (μVms).
At least three dorsiflexion MVCs, and no more than five, were performed by each participant, and each MVC attempt was separated by a minimum of 3 min to minimize the development of any fatigue before the actual fatigue test. Participants were verbally instructed to perform their MVC as hard and as fast as possible to ensure maximal torque and RTD were produced. MVCs were held for ∼3–4 s during each attempt, during which strong verbal encouragement was given. Real-time visual feedback of the torque signal was provided to the participant on a computer monitor throughout the experimental protocol. The interpolated twitch technique (ITT) was used to assess VA during the second and third MVC attempts to ensure adequate muscle activation was achieved (52). The ITT involves supramaximal electrical stimulation of the fibular nerve before, during, and immediately after a voluntary MVC. The amplitude of the interpolated torque electrically evoked during the plateau of the MVC was compared with the twitch elicited immediately following the MVC. %VA was calculated as follows: [1 − (interpolated twitch/resting twitch)] × 100. The peak voluntary torque measured during the three MVC attempts was recorded as the maximal torque for that participant. All torque signals were collected and sampled online at 500 Hz using Spike2 software (version 7.11; Cambridge Electronic Design, Cambridge, UK). Torque was later analyzed off-line to determine voluntary isometric torques (strength) and maximal voluntary RTD (N·m·s−1). Maximal RTD was obtained by transforming the torque signal into its first derivative and recording the peak value for a given contraction.
Throughout the experimental protocol, surface electromyography (EMG) was recorded from the TA and soleus (SOL) muscles using self-adhering Ag-AgCl electrodes (1 × 3 cm; GE Healthcare, Helsinki, Finland). For the TA, the active electrode was placed over the motor point, ∼7 cm distal to the tibial tuberosity and 2 cm lateral to the anterior border of the TA; the reference electrode was placed distally over the tendon of the TA. For the SOL, the active electrode was placed 2 cm below the gastrocnemii border, along the longitudinal axis over the SOL; the reference electrode was placed distally over the tendocalcaneus. Minor adjustments to active electrode placement over the TA were made until the optimal CMAPs (greatest amplitude and fastest rate of rise) were obtained from percutaneous, supramaximal nerve stimulation. Ground electrodes were placed over the patella. Maximal SOL CMAPs were obtained by percutaneous electrical stimulation of the tibial nerve between the origins of the heads of the gastrocnemii muscles. Voluntary root mean square (RMS) EMG for MVC contractions was calculated using a 0.5-s window, which was centered about the peak voluntary dorsiflexion torque. Surface EMG signals were preamplified (×100), amplified (×2), band-pass filtered (10 Hz to 1 kHz), converted by a 12-bit analog-to-digital converter (Power 1401, Cambridge Electronic Design, Cambridge, UK), and sampled online at 2,000 Hz.
Fatigue protocol.
Following the assessment of maximal dorsiflexion strength, participants rested for 10 min to ensure any fatigue or postactivation potentiation had dissipated. The fatigue protocol in the present study involved a single, sustained, isometric dorsiflexion MVC. The MVC was maintained until the participants could no longer sustain 60% of their prefatigue MVC torque. For the fatigue protocol, participants were instructed to contract as hard and as fast as possible, and as long as possible. Additionally participants were instructed to minimize any extraneous movements or contractions aside from dorsiflexion; also they were asked to continue breathing throughout the fatigue protocol. During the fatigue task, participants were provided with continuous and strong verbal encouragement. Throughout the protocol, visual feedback of their torque was provided on a computer monitor. In addition to torque output, the computer monitor displayed horizontal lines demarcating 100% of MVC and 60% MVC. Task termination occurred when the torque dropped and remained below the 60% MVC level despite further verbal encouragement. Participants were not informed that the line on the computer monitor demarcating 60% of MVC torque represented their task termination point. Before instructing the participant to cease the fatiguing MVC, an interpolated twitch was applied to assess %VA at task termination.
Similar to baseline measures, fatigue protocol torque and EMG data were analyzed offline. Time to task termination was determined as the time from which the participant reached their maximal torque until they could no longer produce torque greater than 60% of their baseline MVC. Torque and TA EMG data from the fatigue protocol were subsequently binned into increments of 10% of their time to task termination (i.e., a fatigue protocol lasting 60 s would consist of 10 × 6 s bins). Average torque and TA RMS EMG data for these 10% bins were calculated for each participant. Binned torque and RMS EMG data were subsequently normalized to MVC torque and maximum RMS EMG data, respectively. This served to provide profiles of torque output and neural activation of the TA over the course of the fatigue protocol. Antagonist (SOL) coactivation was quantified during the dorsiflexion MVC fatigue protocol in similar manner to TA activation, but was normalized to SOL RMS EMG during baseline MVCs. The area under the curve of the torque tracing was measured to assess the angular impulse (N·m·s−1) for each participant. This served as an analog for the parameter of work, which is often calculated for dynamic muscle contractions. %VA at the cessation of the fatigue protocol was calculated similarly to baseline MVCs, described above.
Assessment of recovery.
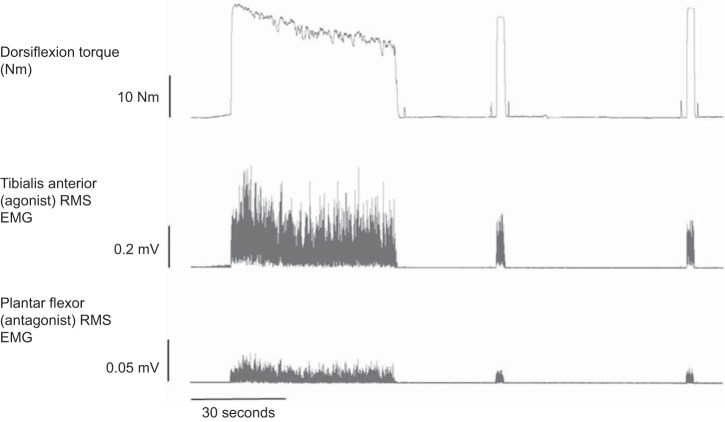
Immediately (∼1 s) following cessation of the fatigue protocol, a supramaximal electrical stimulus was applied to the fibular nerve to assess the degree of fatigue induced by the fatigue protocol. Subsequently, participants performed MVCs with ITT at 30 s and 2 min post-fatigue protocol. Before these MVCs, additional maximal dorsiflexion twitches were elicited to provide an assessment of recovery of the twitch and CMAP properties (CMAP negative peak amplitude, duration, and area) following fatigue. MVCs were assessed for %VA using the ITT technique. Recovery MVC torques and TA EMG data were analyzed similarly to baseline MVCs. Raw torque and EMG tracings outlining the fatigue and recovery protocol are depicted in Fig. 1.
Fig. 1.
Sample torque, tibialis anterior root-mean-squared electromyography (RMS EMG), and plantar flexor RMS EMG during a sustained, isometric dorsiflexion maximal voluntary contraction (MVC) fatigue protocol and subsequent recovery at 30 s and 2 min post-task termination.
Statistical analysis.
Mean values ± SDs are presented in the text, Tables 1–3, and Figs. 2–4, except where noted. The level of significance was set at P ≤ 0.05 for all statistical tests. All baseline data and time to task termination of the fatigue protocol were analyzed using a one-way ANOVA (group). Binned torque and EMG values for fatigue data, as well as recovery data, were analyzed using separate mixed design (split-plot) ANOVAs. For these mixed-design ANOVAs, the between-subjects factor was group (control or DPN patient), with repeated measures over time. Separate repeated-measures ANOVAs were used to analyze fatigue and recovery data. Additionally, fatigue and recovery data were analyzed both as absolute values, as well as relative to baseline. Mauchly's sphericity test and Levene's test were used to ensure that there were no violations of sphericity or homogeneity of variance assumptions, respectively. Main and interaction effects were determined by Pillai's trace (P ≤ 0.05). Following each ANOVA in which a significant main effect or interaction was found, post hoc analysis was performed using the modified Bonferroni correction method. Relationships among variables of interest were tested using Pearson's product moment correlation. Data analyses were performed using IBM SPSS Statistics software (version 20.0; IBM SPSS, Arnock, NY).
Table 1.
Participant characteristics
| Participant Characteristic | Controls | DPN Patients |
|---|---|---|
| n | 10 | 10 |
| Men/women | 6/4 | 6/4 |
| Age, yr | 62.2 ± 12.1 | 64.7 ± 11.3 |
| Height, m | 1.7 ± 0.1 | 1.7 ± 0.1 |
| Weight, kg | 71.4 ± 5.6 | 73.9 ± 8.5 |
| HbA1c, % | 7.5 ± 1.2 | |
| Sural nerve SNAP, μV | 2.1 ± 0* | |
| Fibular nerve CV, m/s | 38.4 ± 7.2 |
Values are means ± SD; n, no. of subjects. DPN, diabetic polyneuropathy; SNAP, sensory nerve action potential; CV, conduction velocity.
SNAP responses were absent in 9 out of 10 DPN patients.
Table 3.
Recovery of maximal twitch, MVC, and CMAP relative to baseline (%)
| Immediate Postfatigue |
30 s Postfatigue |
2 min Postfatigue |
||||
|---|---|---|---|---|---|---|
| Controls | DPN Patients | Controls | DPN Patients | Controls | DPN Patients | |
| Twitch torque, N·m | 80.3 ± 14.3§ | 87.5 ± 12.7§ | 90.2 ± 11.3 | 103.1 ± 10.9 | 98.1 ± 12.6 | 103.1 ± 7.7 |
| Twitch torque/TPT, N·m·s−1 | 88.8 ± 17.6 | 83.6 ± 22.1 | 96.2 ± 8.7 | 94.5 ± 13.8 | 98.1 ± 13.2 | 98.6 ± 17.3 |
| Twitch torque/HRT, N·m·s−1 | 72.7 ± 15.3§ | 67.0 ± 14.5§ | 90.1 ± 15.3 | 85.6 ± 17.8 | 98.2 ± 18.9 | 94.6 ± 12.2 |
| MVC, N·m | 92.7 ± 9.4§ | 88.0 ± 7.9§ | 99.7 ± 10.1 | 98.0 ± 9.0 | ||
| MVC RTD, N·m·s−1 | 78.4 ± 13.5§ | 73.9 ± 11.4§ | 97.7 ± 14.9 | 88.9 ± 13.8 | ||
| MVC RMS EMG | 96.1 ± 16.9 | 92.8 ± 18.3 | 95.4 ± 12.1 | 94.8 ± 15.3 | ||
| CMAP NP amplitude, μV | 93.9 ± 7.8 | 86.9 ± 13.2§* | 93.9 ± 8.7 | 95.6 ± 10.6 | 96.9 ± 7.2 | 95.6 ± 8.7 |
| CMAP NP duration, ms | 108.3 ± 6.4 | 110.6 ± 7.9 | 105.4 ± 7.1 | 104.6 ± 6.8 | 102.9 ± 5.1 | 103.8 ± 6.2 |
| CMAP NP area, μVms | 97.3 ± 7.1 | 93.3 ± 8.4 | 97.5 ± 7.8 | 96.6 ± 6.5 | 99.4 ± 5.8 | 96.5 ± 6.9 |
Values are means ± SD.
Significant difference between groups;
significant difference from baseline (P < 0.05).
Fig. 2.
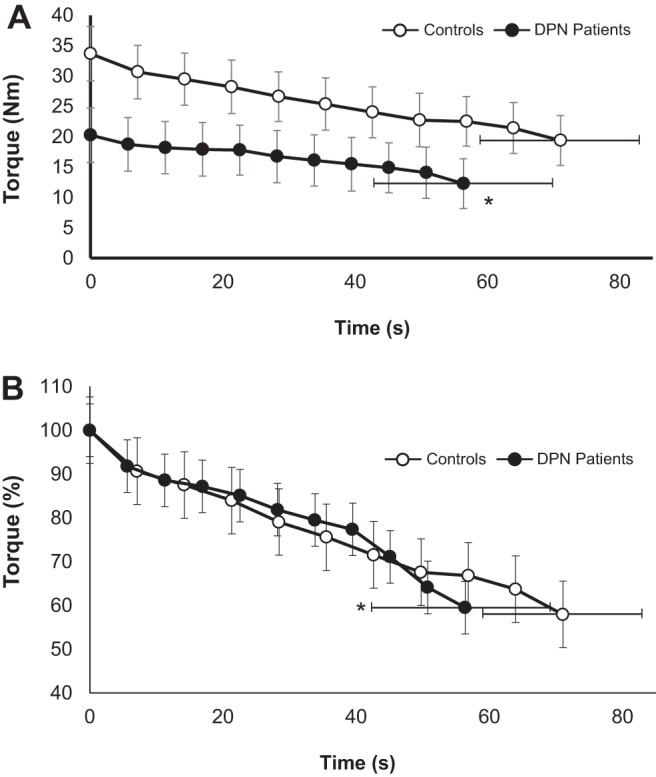
Mean (±SD) absolute dorsiflexion torque [N·m (A); % (B)] produced during a sustained, isometric dorsiflexion MVC fatigue protocol in controls (○; time to task termination = 71 s) and diabetic polyneuropathy (DPN) patients (●; time to task termination = 56 s). *Significant difference between groups in time to task termination (P < 0.05). Controls produced significantly greater torque throughout the protocol (P < 0.05).
Fig. 4.
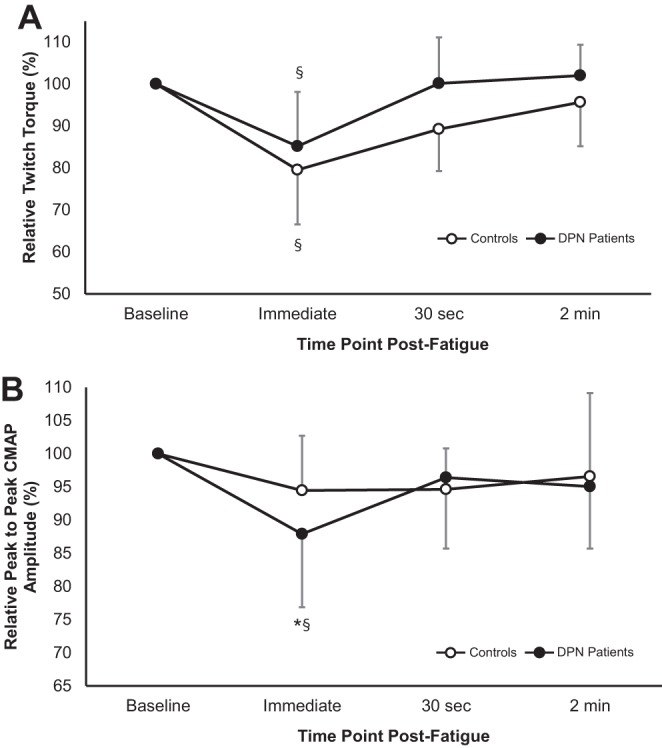
Electrically evoked dorsiflexion relative twitch torques (A) and relative peak-to-peak compound muscle action potential (CMAP) amplitudes (mV; B) at baseline and immediately, 30 s, and 2 min post-task termination in controls (○) and DPN patients (●). Values are means ± SD. §Significant difference from baseline (P < 0.05). *Significant difference between groups (P < 0.05).
RESULTS
Participant characteristics and baseline measures.
Participant characteristics are presented in Table 1. No differences between groups were found for mean age (∼65 yr; P = 0.26), height (∼1.7 m; P = 0.34), or weight (∼72 kg; P = 0.23). Baseline neuromuscular measures are presented in Table 2. DPN patients were found to have 40% weaker (P = 0.005) MVCs with 47% slower maximal RTD (P = 0.0008). Strength differences were not due to differences in VA% (P = 0.02). Compared with controls, the DPN group twitches were ∼36% smaller (P = 0.03), and ∼38% slower (i.e., lower twitch RTD) (P = 0.01) with ∼30% smaller CMAP negative peak amplitudes and areas (P = 0.003). CMAP negative peak durations were not different between groups (P = 0.22). No differences were detected for antagonist muscle coactivation during MVCs between controls (14.5 ± 5.7%) and DPN patients (18.3 ± 8.3%; P = 0.22).
Table 2.
Dorsiflexion neuromuscular parameters at baseline
| Parameter | Controls | DPN Patients | Difference, % |
|---|---|---|---|
| Torque | |||
| MVC torque, N·m | 33.7 ± 8.1 | 20.2 ± 9.2* | −40 |
| MVC RTD, N·m·s−1 | 170.2 ± 41.5 | 89.1 ± 37.9* | −47 |
| Voluntary activation, % | 98.8 ± 2.1 | 97.2 ± 3.7 | |
| Baseline twitch torque, N·m | 5.1 ± 1.7 | 3.2 ± 1.8* | −36 |
| Baseline twitch torque/TPT, N·m·s−1 | 45.0 ± 17.0 | 28.0 ± 14.0* | −38 |
| Baseline twitch torque/HRT, N·m·s−1 | 41.0 ± 12.0 | 19.0 ± 14.0* | −54 |
| Potentiated twitch torque, N·m | 8.3 ± 2.9 | 4.4 ± 1.2* | −47 |
| Potentiated twitch torque/TPT, N·m·s−1 | 98.0 ± 24.0 | 58.0 ± 19.0* | −41 |
| Potentiated twitch torque/HRT, N·m·s−1 | 79.0 ± 15.0 | 32.0 ± 16.0* | −59 |
| EMG | |||
| CMAP NP amplitude, mV | 6.6 ± 0.7 | 4.6 ± 1.1* | −30 |
| CMAP NP duration, ms | 15.0 ± 1.0 | 15.0 ± 2.0 | |
| CMAP NP area, mVms | 43.0 ± 11.0 | 30.0 ± 13.0* | −30 |
Values are means ± SD. MVC, maximal voluntary contraction; RTD, rate of torque development; TPT, time to peak twitch; HRT, half-relaxation time; EMG, electromyography; CMAP, compound muscle action potential; NP, negative peak.
Significant difference between groups (P < 0.05).
Fatigue.
Fatigue profiles of torque over time are presented as absolute values in Fig. 2. The mean time to task termination of the fatigue protocol was ∼21% shorter in DPN patients (56.4 ± 14.2 s) compared with controls (71.1 ± 11.7 s; P = 0.001). Additionally, DPN group angular impulse was ∼50% less than in controls (1,637.4 ± 639.7 vs 852.4 ± 410.5 N·m·s−1; P = 0.001). There was a main effect for time (P < 0.001) and an interaction effect for group and time (P = 0.01) for the changes in relative torque calculated from the binned time intervals. Both groups featured decreases in torque output relative to their baseline MVCs over the course of the fatigue protocol (P < 0.05). Pairwise comparisons between groups over binned time intervals showed the DPN group had lesser decreases in relative torque output from 50–90% of the time to task termination compared with controls (P < 0.05). Just before (1–2 s) task termination, %VA was 97.3 ± 2.9 and 95.8 ± 3.6% in the controls and DPN patients, respectively, with no differences between groups (P = 0.28). No significant relationships were found between glycemic control (i.e., %HbA1c) or severity of neuropathy (i.e., TA CMAP amplitude) and time to fatigue.
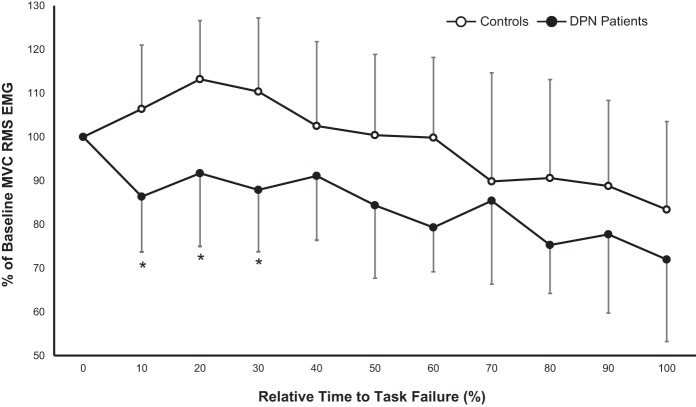
Over the course of the fatigue protocol, normalized TA RMS EMG values are expressed over 10% binned time intervals in Fig. 3. For these variables, a main effect of time (P < 0.001) and an interaction effect between time and group (P < 0.001) were detected. From 0–30% of the time to task termination, the DPN group featured less relative EMG activity from the TA compared with controls. For the remainder of the fatigue protocol, no differences were detected in relative TA RMS EMG between groups (P > 0.05). There were no group differences (P = 0.19) in %antagonist SOL coactivation during the fatigue protocol, however, there was an effect of time (P = 0.01) with both groups featuring decreased %antagonist coactivation over the fatigue protocol (results not shown).
Fig. 3.
Tibialis anterior relative RMS EMG during a sustained, isometric dorsiflexion MVC fatigue protocol in controls (○) and DPN patients (●). Values are means ± SD. *Significant difference between groups (P < 0.05).
Recovery.
Relative data pertaining to neuromuscular recovery following the fatiguing protocol are presented in Table 3. For all voluntary and evoked torque data, main effects for time were detected during recovery, whereas no interaction effects between group and time were found. At 30 s postfatigue, both DPN patients and control groups featured significantly weaker (∼10%) MVCs (P = 0.001; Table 3), but these relative changes were not different between groups. At 2 min postfatigue, the MVC for both groups had recovered to baseline levels (P = 0.35). Similarly, MVC RTD was significantly reduced (∼20%) in both groups 30 s following fatigue (P = 0.001; Table 3); however, both groups were not significantly different from their respective baselines at 2 min postfatigue (P = 0.15). %VA was near maximal (>95%) in both groups during MVCs at 30 s (controls = 96.3 ± 2.2%; DPN patients = 95.3 ± 3.1%; P = 0.34) and 2 min postfatigue (controls = 97.8 ± 1.9%; DPN patients = 96.7 ± 2.6%; P = 0.39).
The twitch evoked immediately following task termination was 15–20% weaker in both groups relative to their respective baseline twitches (P < 0.001; Table 3); however, the relative changes were not different between groups (P = 0.30; Fig. 4A). Peak twitch torque recovered to baseline levels in both groups at 30 s postfatigue (P = 0.08). Twitch HRT was prolonged ∼20% compared with baseline in both groups immediately following the fatigue protocol (P = 0.001; Table 3), and at 30 s postfatigue twitch HRT recovered in both groups (P = 0.21).
Relative to baseline, CMAP negative peak amplitude was significantly reduced by ∼12% in DPN patients immediately following the fatigue protocol (P = 0.003; Fig. 4B), whereas for controls there was no change (P = 0.18; Fig. 4B). In both groups, CMAP durations and areas were not affected by the fatigue protocol (Table 3), although the DPN patients had a nonsignificant 7% reduction in CMAP area immediately following the fatigue protocol (P = 0.12). Additionally, by 30 s postfatigue, the DPN group CMAP negative peak amplitudes had recovered to baseline (P = 0.27).
DISCUSSION
The main findings of this study were are follows: 1) during a sustained isometric dorsiflexion MVC, those with DPN had less endurance compared with controls; 2) the differences in fatigability between groups did not appear to be due to deficiencies in %VA; 3) DPN patients featured evidence of possible neuromuscular transmission failure during and immediately following the fatiguing task, whereas controls did not; 4) at 2 min postfatigue, the maximal evoked and voluntary contractile properties of the dorsiflexors in both groups had recovered similarly to baseline levels.
Decreased neuromuscular endurance in DPN.
In agreement with other studies, baseline neuromuscular properties of the dorsiflexors related to strength (MVC, MVC RTD, twitch torque) and CMAP amplitude were decreased in people with DPN compared with controls (Table 2; Refs. 2, 3, 8, 36). However, novel to the present study, when tasked with maintaining a sustained, isometric dorsiflexion MVC, those with DPN fatigued significantly more quickly (∼21%) compared with controls. Furthermore, the difference in fatigability between groups did not appear to be due to differences in VA, as both groups maintained greater than 95% VA (P > 0.05), as measured via the ITT at task termination. The time to task failure of the control group was similar to that reported previously using a similar task and protocol (71 vs. 85 s; Ref. 30). Additionally, the greater muscle endurance in the control group does not appear to be due to a pacing strategy, as their RMS EMG indicates a maximal or near-maximal effort and follows a not uncommon pattern of enhanced surface EMG for several seconds before a roughly parallel decline with force for a sustained MVC of 60 s in healthy adults (12, 40). It is reasonable to suggest that the brief initial increase in surface EMG in the control group (Fig. 3) reflects underlying processes related to electrogenic potentiation that is often reported to explain facilitation of evoked potentials (CMAPs) during high-intensity muscle activation (24). This EMG potentiation was not evident for the DPN group (Fig. 3), which is perhaps related to reduced concentration of, or dysfunctional, Na+/K+ pumps (31, 33).
DPN patients fatigued significantly more quickly (∼21%; Fig. 2), while producing lower absolute torques, and substantially lesser total angular impulse (∼50%) compared with controls. This is the opposite relationship observed in other muscle endurance comparisons in which greater endurance reported has been attributed partially to less absolute muscle strength (25, 37, 57). Moreover, our results stand in contrast to those previously reported in a study investigating fatigue in a mixed population of insulin-dependent (type 1) DM, with and without DPN, in which DM patients had greater muscular endurance than controls (8). The divergent findings could be accounted for by several key differences in experimental design. Perhaps most importantly, the previous work used repeated isokinetic contractions, which are difficult to assess in terms of VA, and their intermittent nature could attenuate the development of any substantive neuromuscular transmission failure or potential deficits related to impaired muscle blood flow. In contrast, a fatigue study examining muscle endurance in insulin-dependent DM without any clinical signs of DPN found a substantial decrease (approximately −50%) in knee extensor muscle time to fatigue during a sustained isometric task compared with controls (6). Reduced endurance of the DM group in that study was related to lower motor unit firing rates and reduced neuromuscular transmission velocity, despite no clinical evidence of DPN.
There are several possible mechanisms underlying the reduced endurance observed in DPN patients, which are not mutually exclusive. One possible mechanism is the presence of neuromuscular transmission failure (4, 6, 33), which may occur at the motor axon, neuromuscular junction, or muscle fiber membrane. Evidence supporting transmission failure is reflected by the decreased relative surface EMG activity during fatigue in the DPN group compared with controls (Fig. 3) and a reduction in CMAP amplitude immediately following fatigue in DPN but not in controls (Fig. 4B). Furthermore, in DPN patients, there was a nonsignificant trend of a 7% reduction in CMAP area (P = 0.12), whereas no trend was found in controls (P = 0.31; Table 3). In addition to this trend, no significant differences were found postfatigue in CMAP duration (Table 3). This result suggests temporal dispersion of the CMAP was not responsible for the observed decrease in CMAP amplitude in DPN patients (56). A previous investigation presented a strong trend for a reduced CMAP amplitude following a 1-min sustained abductor pollicis brevis contraction in DPN patients, a result that was associated with neuronal hyperexcitability due to presumed Na+/K+ pump dysfunction (33). Moreover, Krishnan and colleagues (33) reported the degree of reduction in maximal CMAP amplitude noted in DPN patients correlated inversely with baseline refractoriness (r = −0.65, P < 0.05), suggesting a relationship between neuronal hyperexcitability and conduction failure in DPN. Indeed, propagation failure of action potentials at any point in the neuromuscular system would impair contractions of the associated muscle fibers, thus limiting the system's force-generating capacity. Demyelination or axonal damage could lead to an increased risk of action potential propagation failure in the motor axon in a manner similar to frequency-dependent conduction block observed in other demyelinating neuropathies (27, 56). Furthermore, given the significant reduction in motor units in DPN patients (1, 2, 23), any neuromuscular transmission failure occurring at the level of the motor axon may have exacerbated decrements in force production compared with other demyelinating conditions, because each remaining motor unit innervates a greater relative proportion of muscle fibers following motor unit loss and subsequent collateral reinnervation. DPN-related pathological alterations at the neuromuscular junction (e.g., reduced numbers of synaptic vesicles) or muscle fiber (e.g., T-tubule disruption) levels could also contribute to failed neuromuscular transmission (20). In addition, dysfunctional axonal Na+/K+ pumps (29, 31) and myocellular Ca2+ channels (41, 42) may contribute to any conduction failure in DPN, as previously postulated (33). Moreover, intermittent neuromuscular transmission failure has been previously reported in DPN during sustained (i.e., 30 s) low-intensity (25% MVC) contractions (4). Thus it is possible the sustained maximal contraction used in the present study could result in equal or greater transmission failure, reducing force generation capacity in DPN patients more quickly than controls during a sustained contraction.
Another potential cause underlying the increased fatigability in DPN observed in the present study is related to abnormal muscle energy metabolism. Previous reports have documented that myocellular PCr loss, pH decrease, and muscle deoxygenation occur significantly faster in type 2 DM patients than controls (48). These factors could lead to an earlier reduction in actomyosin cross-bridge force production during a sustained effort (see Ref. 58 for review). However, given the known task-dependent nature of fatigue (see Ref. 19 for review), it is not completely clear how these previous findings may relate to the results presented here.
An additional consideration regarding DPN and skeletal muscle endurance is related to blood supply. Adequate blood flow is necessary to engage in oxidative metabolism, and if this blood flow is insufficient during muscular contraction, metabolism and the ability to remove metabolic by-products that inhibit force production are limited (58). It has been reported that DM and DPN are associated with dysfunction in the macro- and microvasculature (10, 18, 22, 34, 60), as well as slowed oxygen kinetics during exercise (45, 46). Although muscle blood flow may not present a major limitation to force production during sustained isometric contractions in healthy individuals (51, 59), it should be noted that an impaired ability to deliver oxygen and clear metabolites from contracting muscle could contribute to increased fatigability in DPN patients.
Neuromuscular recovery postfatigue in DPN.
DPN patients performed a fatigue protocol of shorter duration (∼21%; Fig. 1), at lower absolute levels of torque output (∼40%; Fig. 1), and, therefore, markedly less total angular impulse (∼50% less angular impulse) compared with controls. Two minutes following fatigue-evoked twitch and MVC properties had returned to baseline values in both groups. However, immediately following the fatigue protocol, DPN patients and controls both featured evoked twitch responses that were similarly weaker than their respective baseline twitches (Fig. 4A). Additionally, 30-s post-task termination, both groups had similar deficits in MVC torque (∼10%) and MVC maximal RTD (∼20%). The similar recovery profiles between patients and controls are surprising, given the substantial disparity in total angular impulse (i.e., work), and it may be interesting to test these groups with tasks of equal total angular impulse. Notwithstanding, whereas fatigue induced a similar relative change in twitch torque across groups, it appears that the reasons underlying the weaker twitch responses could be different. DPN patients featured smaller CMAP amplitudes compared with baseline, whereas the control CMAP was unchanged immediately following fatigue (Fig. 4B). This finding may indicate, as mentioned earlier, neuromuscular transmission failure may have occurred in DPN patients, contributing to a decrease in strength; whereas twitch torque depression in controls is likely primarily due to accumulation of force-depressing metabolites (e.g., inorganic phosphate, H+ ions). Another consideration is that DPN patients, in a previous study examining the same cohort of patients presented here, have been shown to potentiate to a lesser relative extent (approximately −40%) compared with healthy controls (3), which may be related to changes in relative proportions of fiber types, or calcium sensitivity in relation to muscle atrophy (53). As individuals with DPN appear to benefit less from postactivation potentiation (Ref. 3; Table 2), they may be susceptible to the force-depressing effects of fatigue without gaining as much from the competing force-increasing effects of potentiation during recovery (44). Therefore, to recover from a maximal, sustained fatiguing contraction of short duration, it is possible DPN patients may need to regain both neuromuscular transmission fidelity, as well as adequate clearance of metabolites associated with fatigue.
Summary.
The present study reports decreased neuromuscular endurance in patients with diabetic neuropathy compared with controls, during sustained maximal isometric contractions. Given our results, we propose this DPN-related loss of endurance may be partially attributed to neuromuscular transmission failure and possibly pathological alterations in muscle metabolism or blood flow. Although the DPN group performed ∼50% less angular impulse (an isometric analog of work) than controls, postfatigue, evoked and voluntary muscle strength properties recovered in both groups similarly. These findings may have functional relevance underlying abnormal gait and increased fall risk in patients with DPN (47, 54). Finally, DPN-related fatigability may warrant consideration for the design and implementation of exercise-based rehabilitation strategies for this patient population.
GRANTS
This work was supported by the Natural Sciences and Engineering Research Council of Canada. M. D. Allen was supported by the Ontario Graduate Scholarship program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.D.A., K.K., T.J.D., and C.L.R. conception and design of research; M.D.A. performed experiments; M.D.A. analyzed data; M.D.A., K.K., T.J.D., and C.L.R. interpreted results of experiments; M.D.A. prepared figures; M.D.A. drafted manuscript; M.D.A., K.K., T.J.D., and C.L.R. edited and revised manuscript; M.D.A., K.K., T.J.D., and C.L.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank In Ho (Dennis) Choi for assistance during data collection and all those who participated in the study.
REFERENCES
- 1.Allen MD, Choi I, Kimpinski K, Doherty TJ, Rice CL. Motor unit loss and weakness in association with diabetic neuropathy in humans. Muscle Nerve 48: 298–300, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Allen MD, Kimpinski K, Doherty TJ, Rice CL. Length dependent loss of motor axons and altered motor unit properties in human diabetic polyneuropathy. Clin Neurophysiol 125: 836–843, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Allen MD, Major B, Kimpinski K, Doherty TJ, Rice CL. Skeletal muscle morphology and contractile function in relation to muscle denervation in diabetic neuropathy. J Appl Physiol 116: 545–552, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen MD, Stashuk D, Kimpinski K, Doherty TJ, Hourigan ML, Rice CL. Increased neuromuscular transmission instability and motor unit remodelling with diabetic neuropathy as assessed using novel near fibre motor unit potential parameters. Clin Neurophysiol. In press. [DOI] [PubMed] [Google Scholar]
- 5.Allman BL, Rice CL. Neuromuscular fatigue and aging: central and peripheral factors. Muscle Nerve 25: 785–796, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Almeida S, Riddell MC, Cafarelli E. Slower conduction velocity and motor unit discharge frequency are associated with muscle fatigue during isometric exercise in type 1 diabetes mellitus. Muscle Nerve 37: 231–240, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Andersen H, Gadeberg PC, Brock B, Jakobsen J. Muscular atrophy in diabetic neuropathy: a stereological magnetic resonance imaging study. Diabetologia 40: 1062–1069, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Andersen H. Muscular endurance in long-term IDDM patients. Diabetes Care 21: 604–609, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Andreassen CS, Jakobsen J, Andersen H. Muscle weakness: a progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes 55: 806–812, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Bauer TA, Reusch JE, Levi M, Regenteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care 30: 2880–2885, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Belanger AY, McComas AJ. Extent of motor unit activation during effort. J Appl Physiol 51: 1131–1135, 1981. [DOI] [PubMed] [Google Scholar]
- 12.Bigland-Ritchie B. Human Muscle Fatigue: Physiological Mechanisms (Ciba Foundation Symposium 82). London: Pitman Medical, 1981, p. 130–156. [Google Scholar]
- 13.Buller NP, Jones D, Poole-Wilson PA. Direction measurement of skeletal muscle fatigue in patients with chronic heart failure. Br Heart J 65: 20–24, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callaghan BC, Hur J, Feldman EL. Diabetic neuropathy: one disease or two? Curr Opin Neurol 25: 536–541, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet 363: 978–988, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Christie A, Snook EM, Kent-Braun JA. Systematic review and meta-analysis of skeletal muscle fatigue in old age. Med Sci Sports Exerc 43: 568–577, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyck PJ, Albers JW, Andersen H, Arezzo JC, Biessels GJ, Bril V, Feldman EL, Litchy WJ, O'Brien PC, Russell JW; Toronto Expert Panel on Diabetic Neuropathy. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev 27: 620–628, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Edmonds ME, Roberts VC, Watkins PJ. Blood flow in the diabetic neuropathic foot. Diabetologia 22: 9–15, 1982. [DOI] [PubMed] [Google Scholar]
- 19.Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol 586: 11–23, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fahim MA, Hasan MY, Alshuaib WB. Early morphological remodeling of neuromuscular junction in a murine model of diabetes. J Appl Physiol 89: 2235–2240, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Friedman J, Friedman H. Fatigue in Parkinson's disease. Neurology 43: 2016–2018, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Greenman RL, Khaodhiar L, Lima C, Dinh T, Giurini JM, Veves A. Foot small muscle atrophy is present before the detection of clinical neuropathy. Diabetes Care 28: 1425–1430, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen S, Ballantyne JP. Axonal dysfunction in the neuropathy of diabetes mellitus: a quantitative electrophysiological study. J Neurol Neurosurg Psychiatry 40: 555–564, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hicks A, Fenton J, Garner S, McComas AJ. M wave potentiation during and after muscle activity. J Appl Physiol 66: 2606–2610, 1989. [DOI] [PubMed] [Google Scholar]
- 25.Hunter SK, Enoka RM. Sex differences in the fatigability of arm muscles depends on absolute force during isometric contractions. J Appl Physiol 91: 2686–2694, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Justice JN, Mani D, Pierpoint LA, Enoka RM. Fatigability of the dorsiflexors and associations among multiple domains of motor function in young and old adults. Exp Gerontol 55: 92–101, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaji R. Physiology of conduction block in multifocal motor neuropathy and other demyelinating neuropathies. Muscle Nerve 27: 285–296, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Kent-Braun JA. Skeletal muscle fatigue in old age: whose advantage? Exerc Sport Sci Rev 37: 3–9, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kent-Braun JA. Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur J Appl Physiol Occup Physiol 80: 57–63, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Kjeldsen K, Braendgaard H, Sidenius P, Larsen JS, Norgaard A. Diabetes decreases Na+-K+ pump concentration in skeletal muscles, heart ventricular muscle, and peripheral nerves of rat. Diabetes 36: 842–848, 1987. [DOI] [PubMed] [Google Scholar]
- 32.Kluger BM, Krupp LB, Enoka RM. Fatigue and fatiguability in neurologic illnesses: proposal for a unified taxonomy. Neurology 80: 409–416, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnan AV, Lin CS, Kiernan MC. Activity-dependent excitability changes suggest Na+/K+ pump dysfunction in diabetic neuropathy. Brain 131: 1209–1216, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes 41: 1076–1083, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Marsh E, Sale D, McComas AJ, Quinlan J. Influence of joint position on ankle dorsiflexion in humans. J Appl Physiol 51: 160–167, 1981. [DOI] [PubMed] [Google Scholar]
- 36.Martinelli AR, Mantovani AM, Nozabieli AJ, Ferreira RM, Barela JA, Camargo MR, Fregonesi CE. Muscle strength and ankle mobility for the gait parameters in diabetic neuropathies. Foot (Edinb) 23: 17–21, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Maughan RJ, Harmon M, Leiper JB, Sale D, Delman A. Endurance capacity of untrained males and females in isometric and dynamic muscular contractions. Eur J Appl Physiol 55: 395–400, 1986. [DOI] [PubMed] [Google Scholar]
- 38.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. The effect of contraction intensity on motor unit number estimates of the tibialis anterior. Clin Neurophysiol 116: 1342–1347, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Minotti JR, Pillay P, Chang L, Wells L, Massie BM. Neurophysiological assessment of skeletal muscle fatigue in patients with congestive heart failure. Circulation 86: 903–908, 1992. [DOI] [PubMed] [Google Scholar]
- 40.Moritani T, Muro M, Nagata A. Intramuscular and surface electromyogram changes during muscle fatigue. J Appl Physiol 60: 1179–1185, 1986. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa M, Kobayashi S, Kimura I, Kimura M. Diabetic state-induced modification of Ca, Mg, Fe and Zn content of skeletal, cardiac and smooth muscles. Endocrinol Jpn 36: 795–807, 1989. [DOI] [PubMed] [Google Scholar]
- 42.Nobe S, Aomine M, Arita M, Ito S, Takaki R. Chronic diabetes mellitus prolongs action potential duration of rat ventricular muscles: circumstantial evidence for impaired Ca2+ channel. Cardiovasc Res 24: 381–389, 1990. [DOI] [PubMed] [Google Scholar]
- 43.Pääsuke M, Ereline J, Gapeyeva H. Neuromuscular fatigue during repeated exhaustive submaximal static contractions of knee extensor muscles in endurance-trained, power-trained and untrained men. Acta Physiol Scand 166: 319–326, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Rassier DE, MacIntosh BR. Coexistence of potentiation and fatigue in skeletal muscle. Braz J Med Biol Res 33: 499–508, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Regensteiner JG, Sippel J, McFarling ET, Wolfel EE, Hiatt WR. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med Sci Sports Exerc 27: 875–881, 1995. [PubMed] [Google Scholar]
- 46.Regensteiner JG, Bauer TA, Reusch JE, Brandenburg SL, Sippel JM, Vogelson AM, Smith S, Wolfel EE, Eckel RH, Hiatt WR. Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J Appl Physiol 85: 310–317, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Resnick HE, Stansbery KB, Harris TB, Tirivedi M, Smith K, Morgan P, Vinik AI. Diabetes, peripheral neuropathy, and old age disability. Muscle Nerve 25: 43–50, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 107: 3040–3046, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Schillings ML, Kalkman Janssen JS, HM, van Engelen BG, Bleijenberg G, Zwarts MJ. Experienced and physiological fatigue in neuromuscular disorders. Clin Neurophysiol 118: 292–300, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Sharma KR, Kent-Braun JA, Majumdar S, Huang Y, Mynhier M, Weiner MW, Miller RG. Physiology of fatigue in amyotrophic lateral sclerosis. Neurology 45: 733–740, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Thompson BC, Fadia T, Pincivero DM, Scheuermann BW. Forearm blood flow responses to fatiguing isometric contractions in women and men. Am J Physiol Heart Circ Physiol 293: H805–H812, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Todd G, Gorman RB, Gandevia SC. Measurement and reproducibility of strength and voluntary activation of lower-limb muscles. Muscle Nerve 29: 834–842, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Tubman LA, Rassier DE, MacIntosh BR. Attentuation of myosin light chain phosphorylation and posttetanic potentiation in atrophied skeletal muscle. Pflügers Arch 434: 848–851, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Volpato S, Bianchi L, Lauretani F, Lauretani F, Bandinelli S, Guralnik JM, Zuliani G, Ferrucci L. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care 35: 1672–1679, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe K, Gazzoni M, Holobar A, Miyamoto T, Fukuda K, Merletti R, Moritani T. Motor unit firing pattern of vastus lateralis muscle in type 2 diabetes mellitus patients. Muscle Nerve 48: 806–813, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Watson BV, Brown WF, Doherty TJ. Frequency-dependent conduction block in carpal tunnel syndrome. Muscle Nerve 33: 619–626, 2006. [DOI] [PubMed] [Google Scholar]
- 57.West W, Hicks A, Clements L, Dowling J. The relationship between voluntary electromyogram, endurance time and intensity of effort in isometric handgrip exercise. Eur J Appl Physiol 71: 301–305, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Westerblad H, Allen DG, Bruton JD, Andrade FH, Lannergren J. Mechanisms underlying the reduction of isometric force in skeletal muscle fatigue. Acta Physiol Scand 162: 253–260, 1998. [DOI] [PubMed] [Google Scholar]
- 59.Wigmore DM, Propert K, Kent-Braun JA. Blood flow does not limit skeletal muscle force production during incremental isometric contractions. Eur J Appl Physiol 96: 370–378, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 27: 567–574, 1996. [DOI] [PubMed] [Google Scholar]
- 61.Zwarts MJ, van Weerden TW. Transient paresis in myotonic syndromes. A surface EMG study. Brain 112: 665–680, 1989. [DOI] [PubMed] [Google Scholar]