Abstract
Norovirus gastroenteritis is a major public health burden worldwide. Although fecal shedding is important for transmission of enteric viruses, little is known about the immune factors that restrict persistent enteric infection. We report here that while the cytokines interferon-α (IFN-α) and IFN-β prevented the systemic spread of murine norovirus (MNoV), only IFN-λ controlled persistent enteric infection. Infection-dependent induction of IFN-λ was governed by the MNoV capsid protein and correlated with diminished enteric persistence. Treatment of established infection with IFN-λ cured mice in a manner requiring non-hematopoietic cell expression of the IFN-λ receptor, Ifnlr1, and independent of adaptive immunity. These results suggest the therapeutic potential of IFN-λ for curing virus infections in the gastrointestinal tract.
Human noroviruses (HNoVs) are a leading cause of gastroenteritis worldwide (1, 2). Asymptomatic fecal shedding of HNoVs may be important epidemiologically by providing a reservoir between outbreaks (1, 3–9). Some strains of murine norovirus (MNoV) also establish persistent enteric infection, providing a model for analyzing mechanisms of enteric NoV persistence and immunity in a natural host (1, 10, 11). Interferons (IFNs) are critical for control of both murine and human NoV replication (12–18). IFN-α and IFN-β (also called Type I IFNs and hereafter IFN-αβ), IFN-γ (also called Type II IFN) and IFN-λ (also called Type III IFN or interleukin 28/9) signal through the distinct heterodimeric receptors Ifnar1/Ifnar2, Ifngr1/Ifngr2 and Ifnlr1/Il10rb, to regulate gene expression through phosphorylation of Stat proteins (19, 20). Although the roles of IFNs in control of persistent enteric infection have not been elucidated, it is of interest that IFN-λ, but not IFN-αβ, is important for control of acute rotavirus infection in the intestine of mice (21).
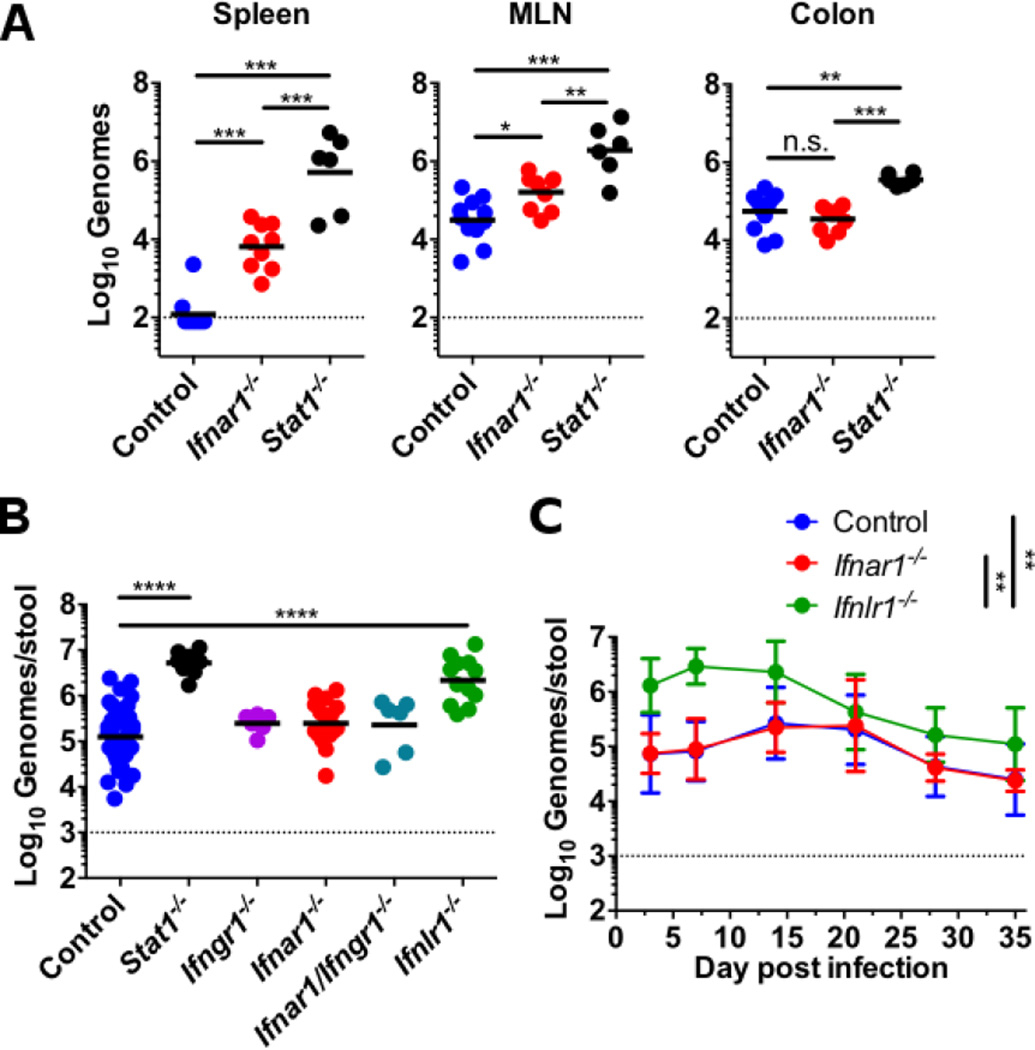
To define the role of IFNs in MNoV enteric persistence, we measured levels of the persistent MNoV strain CR6 in different tissues and in feces after oral inoculation of control mice and mice deficient in Ifnar1, Ifnlr1, Ifngr1, or Stat1 (Fig. 1) (also see supplementary materials and methods). As expected, Ifnar1 and Stat1 were important for limiting replication in the spleen and mesenteric lymph node (MLN) (12, 13, 16, 17), whereas Stat1 rather than Ifnar1 controlled levels of replication in the colon (Fig. 1A) suggesting that IFN-αβ responses did not explain Stat1-dependent control of replication in the intestine. Consistent with this, comparison of the requirement for each IFN receptor in control of fecal shedding revealed that only Stat1 and Ifnlr1 limited levels of fecal shedding of MNoV (Fig. 1B). Furthermore, we observed increased fecal shedding compared to controls in Ifnlr1−/− but not Ifnar1−/−mice over 35 days of infection (Fig. 1C).
Figure 1. Systemic and intestinal persistence of MNoV are controlled by IFN-αβ and IFN-λ respectively.
Mice were orally inoculated with 106 plaque-forming units (PFU) of MNoV strain CR6 and genome copies in the indicated tissue (A) or feces (B and C) were quantitated by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Genome copies were compared between control, Stat1−/− and Ifnar1−/− mice at day 21 in tissues (A); between control, Stat1−/−, Ifngr1−/−, Ifnar1−/−, Ifnlr1−/−, and Ifnar1−/− × Ifngr1−/− mice at day 14 in feces (B); and between control, Ifnar1−/−, and Ifnlr1−/− over time in feces (C). Data shown are pooled from at least two independent experiments with each point representing an individual animal in (A) and (B). Points in (C) represent at least four animals pooled from two to four independent experiments. Dashed line represents limit of detection. Statistical significance determined by one-way (A and B) or two-way (C) analysis of variance (ANOVA). n.s., not significant (P > 0.05); *P ≤ 0.05, **P ≤ 0.01, *** P ≤ 0.001, ****P ≤ 0.0001. Error bars in (C) denote SD.
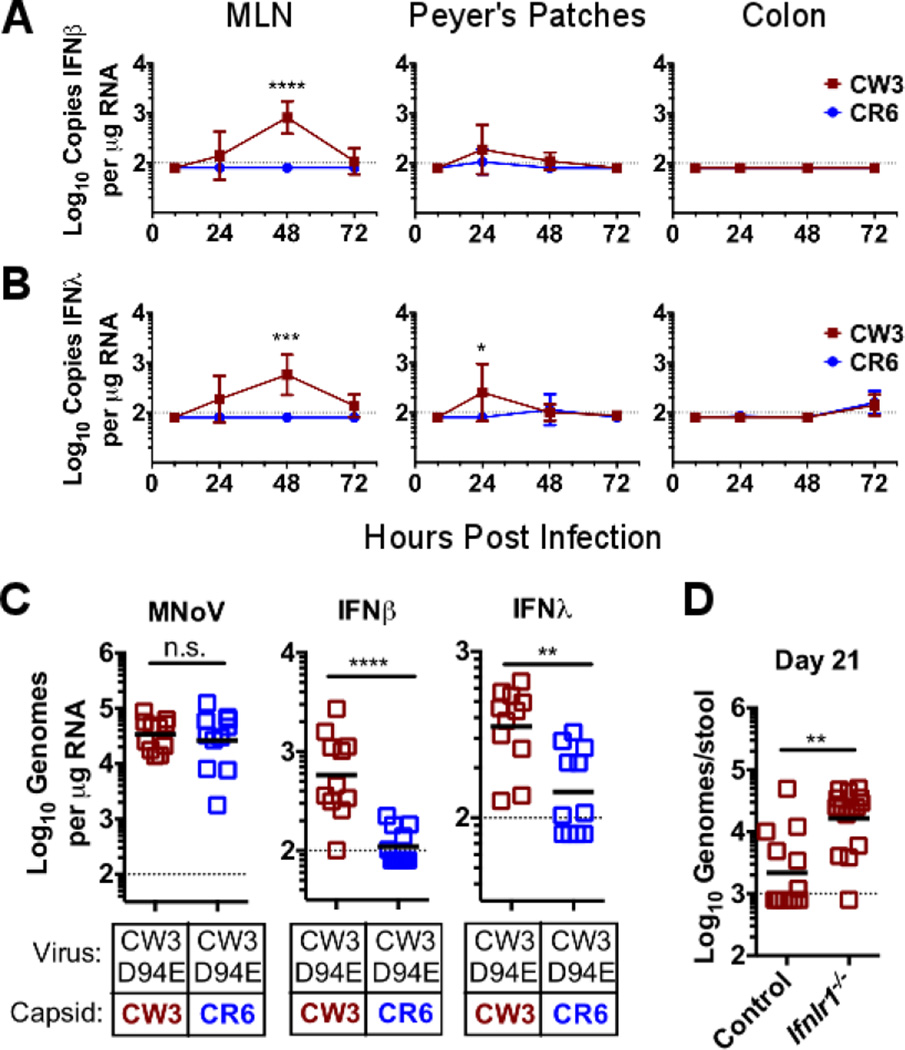
To define the basis for the intestine-specific role of IFN-λ in control of enteric persistence, we inoculated mice with the persistent MNoV strain CR6 or the non-persistent MNoV strain CW3 and compared viral replication and induction of IFN-λ and IFN-β in Peyer’s patches, mesenteric lymph nodes (MLNs), and the colon. As expected, CW3 replicated preferentially in MLN, CR6 and CW3 replicated equivalently in Peyer's patches, and CR6 replicated preferentially in colon (fig. S1) (11, 22). CW3 induced both IFN-β and IFN-λ (Fig. 2, A and B) in MLN and Peyer's patches. In contrast, CR6 did not induce detectable IFN-β or IFN-λ mRNA in any organ despite the high level of replication in the intestine (Fig. 2, A and B). Both strains induced equivalent amounts of IFN-β from bone marrow-derived dendritic cells (BMDCs) in vitro (fig. S2). The capacity of strain CW3 to infect systemic organs maps to the protruding domain of the viral capsid protein (11, 22), whereas a single coding change (Asp94→Glu94, hereafter D94E) in the NS1–2 protein confers the capacity for enteric persistence upon CW3 (11, 23). In chimeric viruses, the presence of the entire CW3 capsid gene or the protruding domain of the CW3 capsid gene correlated with IFN-β and IFN-λ induction (fig. S3, A to F). Furthermore, in CW3-derived viruses carrying the NS1–2 D94E mutation that confers persistence (CW3D94E), the presence of the CR6 capsid lessened IFN-β and IFN-λ induction in MLNs despite similar levels of viral replication (Fig. 2C). This phenotype allowed us to use a chimeric virus to test the hypothesis that IFN-λ responses are required for prevention of persistence. The CW3D94E strain is capable of efficiently establishing enteric persistence only at low doses (fig. S4). When control mice were inoculated with a high dose of CW3D94E, many mice failed to establish persistence (fig. S4 and Fig. 2D). This failure of CW3D94E to persist was rescued by either the CR6 capsid protein, which is associated with diminished IFN-β and IFN-λ responses (fig. S4), or by infection of Ifnlr1−/− mice (Fig. 2D). Persistence of parental CW3, lacking intestinal tropism conferred by the D94E mutation, was not rescued in Ifnlr1−/− mice (fig. S5). These data indicate that induction of IFN-λ interferes with the establishment of enteric MNoV persistence.
Figure 2. Induction of IFN-λ prevents enteric NoV persistence.
(A and B) Control mice were orally inoculated with 106 PFU of either MNoV CW3 or CR6, and total RNA was isolated from MLN, Peyer’s patches, or colon at the indicated times. Relative copy numbers for IFN-β transcripts (A) and IFN-λ transcripts (B) were quantified by qRT-PCR. (C) MNoV genomes, IFN-β transcripts and IFN-λ transcripts in MLNs 48 hours after inoculation were compared between mice infected with CW3D94E containing either the CW3 or CR6 capsid gene. (D) MNoV genomes in feces from control or Ifnlr1−/− mice were measured at day 21 after inoculation with 106 PFU of CW3D94E. Data in (A) to (C) are pooled from three independent experiments for a total of three to four mice per time point. Data points in (D) are individual mice pooled from three independent experiments. Dashed lines represent limit of detection. Statistical significance determined by two-way ANOVA (A and B) or Mann-Whitney test (C and D). n.s., not significant (P > 0.05); *P ≤ 0.05, **P ≤ 0.01, *** P ≤ 0.001, ****P ≤ 0.0001. Error bars in (A) and (B) denote SD.
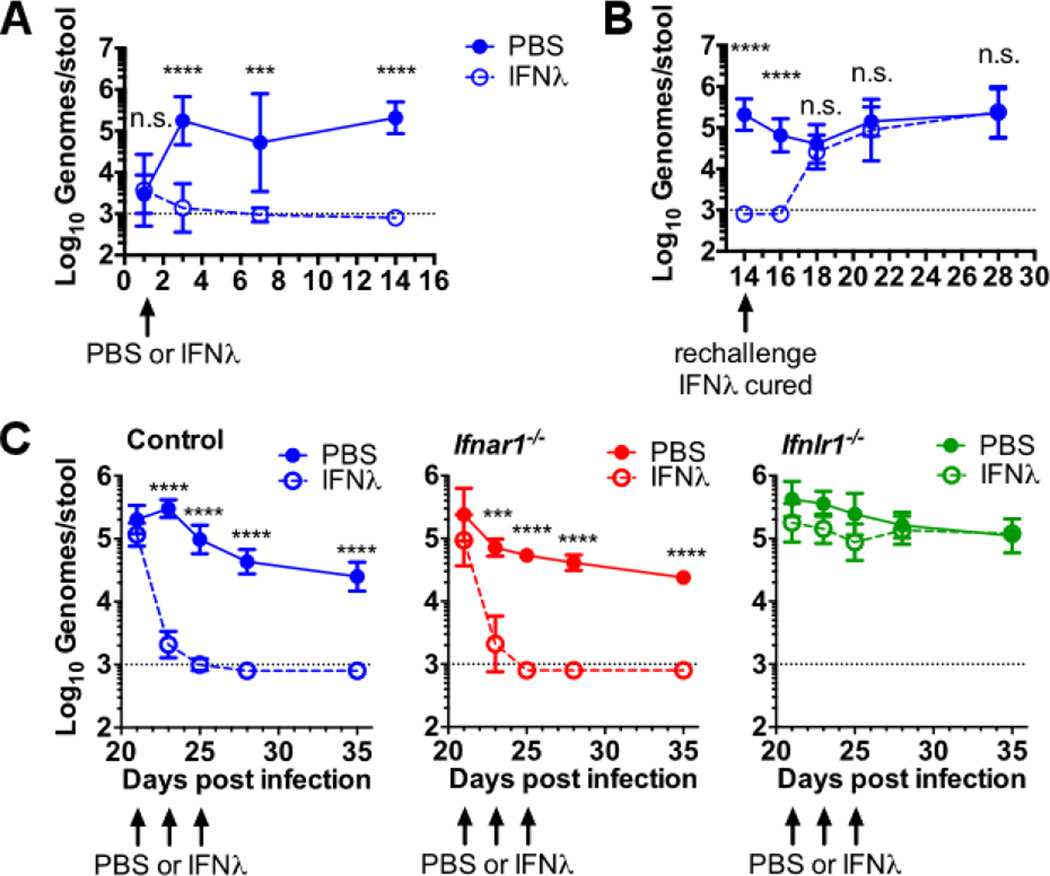
These findings suggested a primary role for IFN-λ in control of enteric MNoV persistence. Consistent with this, intraperitoneal treatment of mice with IFN-λ 1 day after oral inoculation with CR6 prevented persistent enteric MNoV infection (Fig. 3A). When cured mice were re-challenged with CR6 2 weeks later, persistent infection was established (Fig. 3B), indicating that IFN-λ acted by stimulating innate immunity rather than promoting adaptive immunity. These data also indicate that therapeutic levels of IFN-λ wane in animals within 2 weeks of administration.
Figure 3. IFN-λ treatment prevents and cures persistent enteric MNoV infection.
(A to C) Feces were collected at the indicated day after oral inoculation and MNoV genomes were quantified by qRT-PCR. (A) Mice were injected with 25 µg of IFN-λ or phosphate-buffered saline (PBS) intraperitoneally 1 day after oral inoculation with 106 PFUs of CR6. (B) The IFN-λ-treated mice from (A) were rechallenged with 106 PFUs of CR6 at day 14 after initial infection. (C) Persistent infection with CR6 was established in control, Ifnar1−/−, or Ifnlr1−/−mice followed by intraperitoneal injection of 25 µg of IFN-λ on days 21, 23, and 25. Data shown are pooled from two (A) or three (B and C) independent experiments for a total of four to eight mice per time point. Dashed lines represent limit of detection. Statistical significance was determined by two-way ANOVA. n.s., not significant (P > 0.05); *P ≤ 0.05, **P ≤ 0.01, *** P ≤ 0.001, ****P ≤ 0.0001. Error bars in (A) and (B) denote SD and in (C) denote SEM.
To determine if therapeutic IFN-λ was effective against established persistent infection, we treated mice with IFN-λ at 21, 23 and 25 days after oral inoculation with CR6, a time at which stable enteric persistence is established (11). Shedding of virus into the feces was reduced ~100-fold within 2 days, and was undetectable within 1 week (Fig. 3C). Analysis of viral genomes in tissues 2 weeks after IFN-λ treatment revealed reduced or undetectable levels of virus in the MLN and undetectable virus levels in the colon (fig. S6). As little as a single 1-µg dose of IFN-λ was sufficient to clear persistent MNoV (fig. S7A). Ifnlr1 was required for IFN-λ activity whereas Ifnar1 was dispensable (Fig. 3C and fig. S6) indicating that IFN-λ does not require IFN-αβ signaling to eliminate enteric persistence.
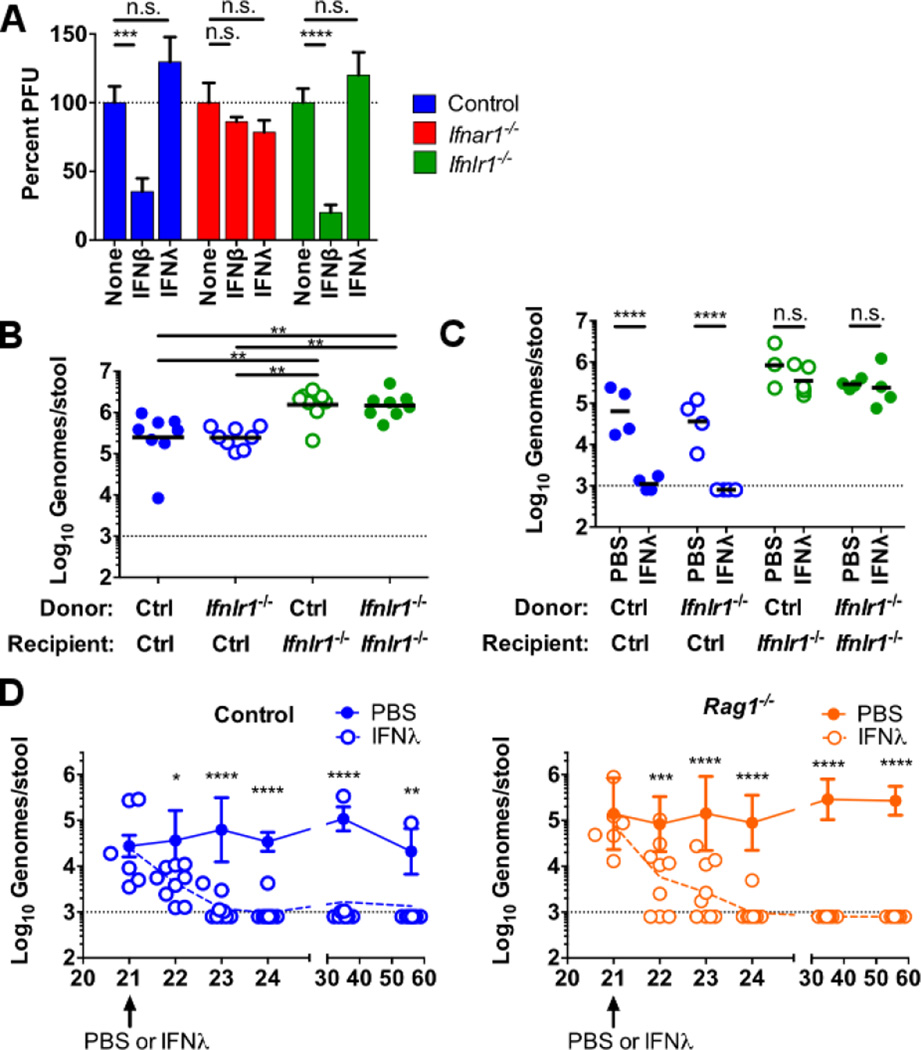
Whereas MNoV is known to replicate in immune cells, attempts to cultivate MNoV or HNoV in epithelial cells have not been successful (1, 24). Treatment of cultured BMDCs with IFN-β, but not IFN-λ, inhibited MNoV replication (Fig. 4A). Taken together with previously published data (21, 25–27), this result raised the possibility that IFN-λ acts on nonhematopoietic cells to control enteric persistence. Experiments in reciprocal bone marrow chimeras revealed that the control of fecal MNoV levels (Fig. 4B) and the capacity for IFN-λ to cure enteric persistence (Fig. 4C) mapped to the nonhematopoietic rather than radiation-sensitive hematopoietic cells. These data were consistent with an Ifnar1-independent effect of IFN-λ on enteric persistence through stimulation of immunity via signaling in radiation-insensitive cells.
Figure 4. IFN-λ durably clears enteric MNoV persistence through effects on radiation-insensitive cells in the absence of adaptive immunity.
(A) BMDCs were treated with media, 100 IU/mL IFN-β, or 100 ng/mL IFN-λ for 24 hours, then inoculated with CR6 at a multiplicity of infection of five, and viral titers were determined by plaque assay 12 hours later. Data are pooled from three independent experiments performed in triplicate and normalized to untreated. (B and C) Bone marrow chimeras were generated using control and Ifnlr1−/− donor and recipient mice as indicated and 8 to 10 weeks later were orally inoculated with 106 PFUs of CR6. (B) MNoV shedding in feces was quantified on day 14. (C) A single 25-µg dose of IFN-λ or PBS was administered intraperitoneally 21 days after inoculation and MNoV shedding was quantified on day 23. Data in (B) and (C) are pooled from two independent experiments and shown as individual mice. (D) Control or Rag1−/− mice orally inoculated with CR6 were treated 21 days later with a single 25-µg dose of IFN-λ. Shedding was monitored for 35 days postinjection. Dashed lines in (B) to (D) represent limit of detection. Statistical significance was determined by one-way (B) or two-way (A, C, D) ANOVA. n.s., not significant (P > 0.05); *P ≤ 0.05, **P ≤ 0.01, *** P ≤ 0.001, ****P ≤ 0.0001. Error bars in (A) denote SEM and in (D) denote SD.
Despite the capacity of mice to mount protective adaptive immune responses to MNoV infection (28, 29), their susceptibility of mice to reinfection after treatment with IFN-λ (Fig. 3B) suggested that IFN-λ might act preferentially through the innate immune system. However, it is generally thought that in mammals, viral clearance requires adaptive B and T cell immunity. To test whether IFN-λ can clear established infection in the absence of an adaptive immune response, we inoculated Rag1−/− mice with CR6 and 21 days later treated with a single dose of IFN-λ. Enteric persistence of CR6 was cured by IFN-λ treatment of both control and Rag1−/− mice (Fig. 4D). All Rag1−/− mice and the majority of control mice remained MNoV-free for 35 days following treatment, well after therapeutic levels of IFN-λ waned (Fig. 3B). The absence of infectious MNoV was confirmed by fecal transplantation from IFN-λ-cleared Rag1−/− mice to naïve Rag1−/−or Stat1−/− mice (fig. S7, B and C). Together, these data demonstrate that IFN-λ treatment both prevents and cures established enteric persistence of MNoV in the absence of an adaptive immune response.
Our study establishes the importance of IFN-λ in innate immunity to persistent enteric viral infection. Establishment of persistent enteric infection by certain strains of MNoV was related to failure to induce IFN-λ responses. Whereas MNoV replicates in hematopoietic cells, IFN-λ was found to act on nonhematopoietic cells, suggesting that the mechanism of action is indirect. The efficacy of IFN-λ in prevention and cure of enteric persistence did not require adaptive immunity. These findings provide a different view of the immune system because it is generally believed that the development of an adaptive immune response is required for clearance of viral infection by antigen-specific targeting. One implication of our findings is that immune therapies may be able to control persistent virus infection regardless of their effects on adaptive immune responses. We propose this is an example of sterilizing innate immunity wherein viral clearance can be determined by immune responses apart from adaptive immunity. A similar observation of viral clearance by an independent mechanism of a second enteric virus, rotavirus, supports the existence of sterilizing innate immunity (30). Analogously, metazoan organisms lacking adaptive immune systems likely are capable of clearing some pathogens and preventing persistent infection. We speculate that evolutionarily conserved innate immune mechanisms with sterilizing potential exist, perhaps with particular importance in protection against persistent infection at mucosal surfaces such as the intestine.
Supplementary Material
Acknowledgments
We thank D. Kreamalmeyer for maintaining our mouse colony and S. Doyle and Bristol-Myers Squibb for providing Ifnlr1−/− mice and recombinant IFN-λ protein. The mouse norovirus strains used in this paper are available from Washington University under a material transfer agreement (MTA). Ifnlr1−/− mice were made available from ZymoGenetics (Bristol-Myers Squibb) under a MTA with Washington University School of Medicine. H.W.V. is a co-inventor on a patent filed by Washington University School of Medicine related to the use of murine norovirus. The data presented in this manuscript are tabulated in the main paper and in the supplementary materials. H.W.V. was supported by NIH grants R01 AI084887 and U19 AI109725, the Crohn's and Colitis Foundation Genetics Initiative grant 274415, and Broad Foundation grant IBD-0357. M.S.D. and H.M.L. were supported by NIH grants U19 AI083019 and U19 AI106772. T.J.N. was supported by NIH training grant 5T32A100716334 and postdoctoral fellowships from the Cancer Research Institute and American Cancer Society. M.T.B. was supported by NIH training grant 5T32CA009547 and the W.M. Keck Fellowship from Washington University. B.T.M. was supported by NIH award F31CA177194-01. J.M.N. was supported by NIH training grant 5T32AI007163.
Footnotes
Supplementary Materials:
www.sciencemag.org/content/347/6219/269/suppl/DC1
Materials and Methods
Supplementary Text
Figs. S1 to S7
References (31–33)
References
- 1.Karst SM, Wobus CE, Goodfellow IG, Green KY, Virgin HW. Advances in Norovirus Biology. Cell Host Microbe. 2014 Jun 11;15:668. doi: 10.1016/j.chom.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. [10/29/2009];N Engl.J Med. 2009 361:1776. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips G, Tam CC, Rodrigues LC, Lopman B. Prevalence and characteristics of asymptomatic norovirus infection in the community in England. Epidemiol Infect. 2010 Oct;138:1454. doi: 10.1017/S0950268810000439. [DOI] [PubMed] [Google Scholar]
- 4.Saito M, et al. Multiple norovirus infections in a birth cohort in a Peruvian peri-urban community. Clin Infect Dis. 2013 Dec 2; doi: 10.1093/cid/cit763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayukekbong J, et al. Enteric viruses in healthy children in Cameroon: viral load and genotyping of norovirus strains. J Med Virol. 2011 Dec;83:2135. doi: 10.1002/jmv.22243. [DOI] [PubMed] [Google Scholar]
- 6.Cheon DS, et al. Seasonal prevalence of asymptomatic norovirus infection in Korean children. Foodborne Pathog Dis. 2010 Nov;7:1427. doi: 10.1089/fpd.2010.0547. [DOI] [PubMed] [Google Scholar]
- 7.Sukhrie FH, Siebenga JJ, Beersma MF, Koopmans M. Chronic shedders as reservoir for nosocomial transmission of norovirus. J Clin Microbiol. 2010 Nov;48:4303. doi: 10.1128/JCM.01308-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukhrie FH, et al. Nosocomial transmission of norovirus is mainly caused by symptomatic cases. Clin Infect Dis. 2012 Apr;54:931. doi: 10.1093/cid/cir971. [DOI] [PubMed] [Google Scholar]
- 9.Lopman B, Simmons K, Gambhir M, Vinje J, Parashar U. Epidemiologic implications of asymptomatic reinfection: a mathematical modeling study of norovirus. Am J Epidemiol. 2014 Feb 15;179:507. doi: 10.1093/aje/kwt287. [DOI] [PubMed] [Google Scholar]
- 10.Tomov VT, et al. Persistent enteric murine norovirus infection is associated with functionally suboptimal virus-specific CD8 T cell responses. J Virol. 2013 Jun;87:7015. doi: 10.1128/JVI.03389-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nice TJ, Strong DW, McCune BT, Pohl CS, Virgin HW. A single-amino-acid change in murine norovirus NS1/2 is sufficient for colonic tropism and persistence. J Virol. 2013 Jan;87:327. doi: 10.1128/JVI.01864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW. STAT1-dependent innate immunity to a Norwalk-like virus. Science. 2003 Mar 7;299:1575. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 13.Wobus CE, et al. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004 Dec;2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang KO, George DW. Interferons and ribavirin effectively inhibit Norwalk virus replication in replicon-bearing cells. [11/2007];J Virol. 2007 81:12111. doi: 10.1128/JVI.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang KO, Sosnovtsev SV, Belliot G, King AD, Green KY. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. [9/30/2006];Virology. 2006 353:463. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Thackray LB, et al. Critical role for interferon regulatory factor 3 (IRF-3) and IRF-7 in type I interferon-mediated control of murine norovirus replication. J Virol. 2012 Dec;86:13515. doi: 10.1128/JVI.01824-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCartney SA, et al. MDA-5 recognition of a murine norovirus. PLoS Pathog. 2008 Jul;4:e1000108. doi: 10.1371/journal.ppat.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang S, et al. Nondegradative role of Atg5-Atg12/ Atg16L1 autophagy protein complex in antiviral activity of interferon gamma. Cell Host Microbe. 2012 Apr 19;11:397. doi: 10.1016/j.chom.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taniguchi T, Takaoka A. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. [2/2002];Curr. Opin. Immunol. 2002 14:111. doi: 10.1016/s0952-7915(01)00305-3. [DOI] [PubMed] [Google Scholar]
- 20.Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. [7/2008];Nat. Rev. Immunol. 2008 8:559. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pott J, et al. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci U S A. 2011 May 10;108:7944. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strong DW, Thackray LB, Smith TJ, Virgin HW. Protruding domain of capsid protein is necessary and sufficient to determine murine norovirus replication and pathogenesis in vivo. J Virol. 2012 Mar;86:2950. doi: 10.1128/JVI.07038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borin BN, et al. Murine norovirus protein NS1/2 aspartate to glutamate mutation, sufficient for persistence, reorients side chain of surface exposed tryptophan within a novel structured domain. Proteins. 2013 Nov 25; doi: 10.1002/prot.24484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones MK, et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014 Nov 7;346:755. doi: 10.1126/science.1257147. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ank N, et al. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008 Feb 15;180:2474. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- 26.Mordstein M, et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010 Jun;84:5670. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008 Mar;4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu G, Kahan SM, Jia Y, Karst SM. Primary high-dose murine norovirus 1 infection fails to protect from secondary challenge with homologous virus. J Virol. 2009 Jul;83:6963. doi: 10.1128/JVI.00284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chachu KA, LoBue AD, Strong DW, Baric RS, Virgin HW. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog. 2008 Dec;4:e1000236. doi: 10.1371/journal.ppat.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B, et al. Prevention and cure of rotavirus infection via TLR5/NLRC4–mediated production of IL-22 and IL-18. Science. 2014 Nov 14;346:861. doi: 10.1126/science.1256999. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.