Abstract
In the past 10 years the prevalence of type 2 diabetes mellitus (T2DM) has increased hugely worldwide, driven by a rise in the numbers of overweight and obese individuals. A number of diets have been shown to be effective for the management of T2DM: the Mediterranean diet, the vegetarian diet and the low-calorie diet. Results of studies clearly indicate, however, that the efficacy of these diets is not solely related to the biochemical structure of the individual nutrients they contain. This review discusses this point with reference to the potential role of the intestinal microbiota in diabetes. The macrobiotic Ma-Pi 2 diet is rich in carbohydrates, whole grains and vegetables, with no animal fat or protein or added sugar. In short- and medium-term trials conducted in patients with T2DM, the Ma-Pi 2 diet has been found to significantly improve indicators of metabolic control, including fasting blood glucose, glycosylated hemoglobin, the serum lipid profile, body mass index, body weight and blood pressure. The diet may also alter the gut microbiota composition, which could additionally affect glycemic control. As a result, the Ma-Pi 2 diet could be considered a valid additional short- to medium-term treatment for T2DM.
Keywords: Ma-Pi 2 macrobiotic diet, Type 2 diabetes, Low-grade inflammation, Gut microbiota, Metabolic control
Core tip: Imbalances in the intestinal microbiota (dysbiosis) have been linked to diseases, including diabetes. In short- and medium-term trials conducted in patients with type 2 diabetes mellitus (T2DM), the Ma-Pi 2 diet, which is rich in carbohydrates, whole grains and vegetables, with no animal fat or protein or added sugar, has been found to significantly improve indicators of metabolic control, including fasting blood glucose, glycosylated hemoglobin, the serum lipid profile, body mass index, body weight and blood pressure. The diet may also alter the gut microbiota composition. Hence, the Ma-Pi 2 diet could be considered a valid additional short- to medium-term treatment for T2DM.
INTRODUCTION
In the past 10 years, the prevalence of type 2 diabetes mellitus (T2DM) in Italy has increased greatly; currently, approximately 3.3 million people are diagnosed with T2DM (5.5% of the total population) and another million people are thought to be affected but as yet undiagnosed. These numbers are expected to increase in the near future, and it has been estimated that 9.0% of the population will have T2DM by 2030[1]. Similar increases are anticipated in other European countries and, globally, the number of people with diabetes is predicted to reach 592 million by the year 2035 - an increase of 55% from 2013 data[2].
This increase in the prevalence of T2DM, which accounts for approximately 90% of all diabetes cases, is clearly being driven by an increase in the numbers of individuals who are overweight or obese[3]. Indeed, the risk of diabetes appears to literally soar as the weight piles on; a rise in body mass index (BMI) from 21 kg/m2 (healthy) to 35 kg/m2 (obese) can increase the likelihood of developing the disease by a factor of 80[4]. In line with the increase in prevalence of diabetes, the number of obese adults, together with the rates of obesity-related diseases (e.g., coronary heart disease and stroke, hypertension and arthritis) and associated healthcare costs, are all expected to increase dramatically worldwide over the next 20 years[4,5].
T2DM and obesity, and their associated complications and costs, are therefore among the most pressing healthcare problems that society is currently facing. Despite improvements in our understanding of T2DM and the development of new diagnostic and treatment methods, we are still failing to control this epidemic. There is a crucial need to find effective, simple, pragmatic, sustainable and cheap interventions (e.g., those aimed at changing lifestyle, such as exercise and diet).
Recent reviews highlight the benefits of physical exercise in T2DM[6]. In Italy, the Italian Diabetes and Exercise Study[7-10] has contributed to this evidence and has helped provide definitions and standards used by the American College of Sports and Medicine and the American Diabetes Association (ADA). Yet, despite the accepted benefits of physical exercise for patients with T2DM, the implementation of exercise recommendations has proved difficult. Reasons for this include a lack of patient compliance, insufficient knowledge/awareness of benefits among general practitioners, diabetologists or exercise professionals, and a lack of dedicated facilities[11]. In addition, in order to be successful, exercise interventions in individuals with T2DM tend to require intensive counseling and supervision from medical or exercise professionals.
As a result, recent attention has focused less on exercise and more on diet; dietary therapy (as a lifestyle intervention), with or without additional drug treatment, represents an efficacious and safe alternative for T2DM management[12]. Various cohort studies have shown that selected healthy eating patterns, mainly characterized by a higher intake of fruit and vegetables, are associated with a lower risk of diabetes[13]. In this review, we look at some of the various diets that have been shown to be effective for the management of T2DM, in particular the Ma-Pi 2 macrobiotic diet, and explore the potential role of the intestinal microbiota in T2DM.
MEDITERRANEAN DIET
A Mediterranean-type diet is one involving: a high consumption of cereals, grains, fruits, vegetables, legumes, nuts; olive oil as the principal source of fat; low-to-moderate consumption of fish and poultry; relatively low consumption of red meat; and moderate consumption of wine, normally with meals[14].
Based on this definition, numerous epidemiological studies have indicated the favorable effects of this diet[15-17]. Such diets have been reported in two prospective studies[18,19] and one intervention study[20] to be associated with a reduced risk of diabetes. However, the role of the Mediterranean diet in weight control remains controversial[21], which suggests that the protective effect of this diet against diabetes are not based on weight control but through several of its dietary characteristics. Currently it is unclear which component of the Mediterranean diet contributes most to its favorable effects.
LOW CALORIE DIET
Weight loss following a reduction in energy intake (caloric restriction) and/or an increase in energy expenditure (through exercise) improves insulin sensitivity, hyperglycemia and other cardio-metabolic risk factors[22]. Caloric restriction (CR) is defined as a reduction in caloric intake by around 20%-40%, with adequate intakes of protein and micronutrients to avoid malnutrition[23,24]. Caloric intake can be reduced by eliminating the consumption of energy-dense foods (e.g., refined carbohydrates, potatoes, white bread, white rice, sweets and sweetened drinks) and by increasing the intake of a nutrient-dense foods (a wide variety of vegetables, fruits, nuts, low-fat dairy products, egg whites, wheat and soy proteins, fish and meat). The energy intake of such a diet is around 1100-2000 kcal/d, with approximately 26% of calories obtained from protein, 28% from fat and 46% from complex carbohydrates[23]. CR may be administered as a short- (1 mo-1 year) or long-term diet. CR in healthy individuals as well as those who are obese or have T2DM has been reported to lower blood glucose levels, fasting insulin values and BMI[25]. Weight loss induced by caloric restriction mediates a reduction in inflammatory markers and improves insulin resistance and sensitivity[26]. With adequate nutrition, CR can improve cardio-metabolic health, prevent T2DM and may be a powerful tool against obesity and insulin resistance.
VEGETARIAN DIET
Vegetarian diets typically include large amount of fruits, vegetables and legumes, nuts and soya-protein and usually aim to eliminate all animal products. Studies have shown that, generally, vegetarians residing in Western countries have a lower BMI and a higher ratio of polyunsaturated to saturated fat intake than individuals consuming a non-vegetarian diet (people eating meat and/or fish)[27,28]. Vegetarians have also been shown to have lower concentrations of low-density lipoprotein (LDL)-cholesterol[29-31], lower blood pressure[32,33] and a lower risk of diabetes[34] than non-vegetarians. Factors associated with the higher fiber content in vegetarian diets promote increased insulin sensitivity[35]. Vegetarian diets are also associated with other health benefits. According to the largest study ever conducted in the United Kingdom from the University of Oxford, the risk of hospitalization or death from heart disease was 32% lower in vegetarians than in people who ate meat and fish, with most of the difference in risk probably caused by effects on cholesterol and blood pressure[36]. In addition to a lower risk of cardiovascular disease[37,38], vegetarian diets have been reported to reduce the incidence of cancer in a low-risk population[39,40].
MICROBIOTA
The results of some studies indicate that the efficacy of a diet is not solely related to the biochemical structure of the individual nutrients it contains and the current standard definition of macronutrients fails to capture important information[41]. This has become more obvious as knowledge of the gut microbiota[42-46] has been gained and the “food as hormone” hypothesis[47] has emerged.
The human gut microbiota weighs about 1500 g and the number of intestinal microbial cells it contains is tenfold greater than the total number of human body cells[48]. The gut microbiome, which represents the collective genomes of all gut microbiota, is 150 times greater than the human gene complement[46]. The gut microflora plays several metabolic active roles: it produces vitamins, synthesizes amino-acids, transforms bile acid, and is able to ferment non-digestible substrates and endogenous mucus, stimulating bacterial growth and producing short-chain fatty-acids (SCFA)[49], which work in the gut and at distance after absorption[49]. Moreover, the gut microbiota also causes pathogen displacement, by competing for attachment sites and nutrients, and by secreting antimicrobials[50]. The microbiota plays a fundamental role in the development of the immune system[51], as SCFA have a strong immunomodulatory activity, leading to the production and release of cytokines, chemokines and phagocytes[52].
The gut microbiota is also involved in the development of cells and tissues[53], and is responsible for the integrity of the gut barrier[53] through its involvement in glucagon-like peptide (GLP)-2 production by enteroendocrine L-cells, and the circulation of endotoxins[54]. In addition, the microbiota triggers the production of peptide YY, which regulates gut motility and the hungry sensation[55].
Dysbiosis is a bacterial imbalance in the gut resulting in a modification of the normal local distribution of microbial communities that correlates with biochemical and clinical modifications in the host: hyperglycemia and T2DM have been observed in the presence of a low percentage of bacterial Firmicutes and Clostridia species[56]. Diet and increase in bodyweight are linked to gut microbial imbalance, in both the presence and absence of obesity[57].
Recent studies have shown a link between dysbiosis and several diseases, including diabetes (Figure 1).
Figure 1.
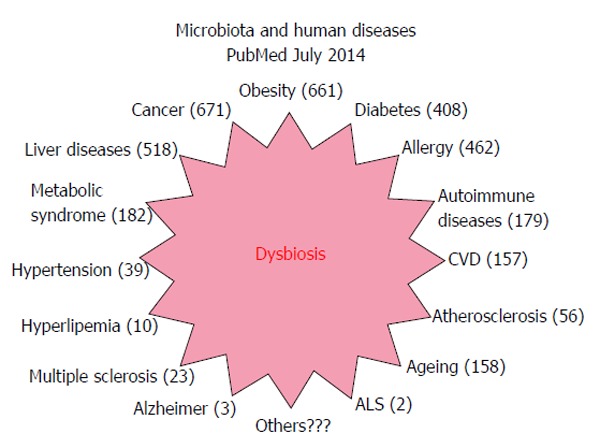
Articles indexed in PubMed concerning the microbiota and human diseases. Results of a research in PubMed looking for articles concerning gut microbiota that associated dysbiosis and human diseases. Number of articles for each kind of disease is indicated in brackets. CVD: Cardiovascular diseases; ALS: Amyotrophic lateral sclerosis.
Obesity and T2DM are associated with chronic low-grade inflammation and endotoxemia. Lipopolysaccharide (LPS) consumption and a diet rich in fat increase adiposity, impaired glucose tolerance, and insulin resistance those raises are reversible by the consumption of a pre-probiotic rich diet[44,58,59], or a meal based on fiber and fruit, as recommended by the American Heart Association[60]. Bacterial LPS derived from Gram-negative bacteria residing in the gastrointestinal microflora could act as a trigger for the development of diabetes and obesity; in a series of experiments in mice, it was observed that high-fat feeding favors an increase in the Gram-negative to Gram-positive colonizing bacteria ratio, which has been associated with an increased intestinal permeability that precedes the development of metabolic endotoxemia, inflammation, and associated disorders (obesity and diabetes)[44]. Different nutrients have different enhancing-effects on endotoxemia (LPS production by microflora or for higher gut permeability) and the consequent inflammatory response[61]. Other factors may also influence our microbiota, such as age[62-64], chemical products and antibiotics[65], and prebiotics and probiotics[58,66-68].
The internationally endorsed definition of probiotics is “live microorganisms which when administered in adequate amounts confer a health benefit on the host”[69], whereas, definition for prebiotics is “a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microflora, that confer benefits upon host well-being and health”[66]. Therefore, in order to be classified as a prebiotic, the ingredient should resist gastric acidity, hydrolysis and should be fermented by the intestinal microflora. The consumption of prebiotic foods enhances a reduction of triglyceride and postprandial glucose levels, gut permeability and inflammatory effect[66,70]. It has been observed that administering prebiotics to obese mice induces a microbiota modulation, lowering both diet-induced LPS endotoxemia, and systemic inflammation and liver inflammation[44]. The main positive effects of pro- and prebiotic administration are increases in GLP-1 and peptide YY secretion[71-73], a reduction in the complications of pregnancy[74], and improvements in metabolic diseases (obesity, T2DM, cardiovascular disease)[75].
New findings in microbiota knowledge are opening new fields of study about human health and disease. However, the research regarding the interaction between bacteria and human health is at the beginning and many aspects are still not properly understood; well-defined clinical studies are needed in order to develop the potential of this new therapeutic area.
Until now, scientists have focused primarily on identifying human genetic markers, but research has shown that other factors, such as the intestinal flora, play a role in the development of T2DM[45,46]. The results of metagenome-wide association studies in T2DM patients and non-diabetic controls[45,46] clearly indicate that T2DM is a significant factor in the observed variation in gut microbial samples, and suggest that the gut microbiota in T2DM patients features dysbiosis. These studies[45,46] indicate that a gut-microbiota-based index could be more effective and accurate in assessing and predicting the risk of T2DM than a human genome variation-based one.
Knowledge of the microbiota and the general concept of “food as hormone”, as proposed by Ryan et al[47], suggest that diet has an enormous impact on our health. In fact, circulating substrates derived from food have both direct and indirect actions and, ultimately, food may be considered a cocktail of hormones that exert their effects on target tissues, activating cell-surface or nuclear receptors. Food components also interact with the gut flora to induce indirect signals. Therefore, if we consider this new role for food, we can make dietary recommendations to promote health or treat specific diseases, taking into account that until now macronutrients have been classified according to their energy-yielding biochemical properties and not by their ability to work in a manner similar to that of hormones. Identifying these food- and food metabolite-receptor interactions will provide new opportunities for studying the relationship between the food we eat and diseases, including obesity.
MA-PI 2 MACROBIOTIC DIET
Macrobiotic diets are originally derived from an ancient Eastern philosophy of life based on two ancient Asian theories (Yin/Yang and the Five Transformations), formulated for Western culture by the Japanese philosopher Georges Ohsawa[76] and further updated by Mario Pianesi who created the 5 Ma-Pi diets[77]. The Ma-Pi 2 diet was specifically designed for patients with metabolic disorders, and consists of 50%-55% whole-grain (rice, millet and barley), 35%-40% vegetables (carrots, savoy cabbage, chicory, red radish, onions, parsley, cabbage) and 8%-10% legumes (adzuki beans, chickpeas, lentils and black beans), plus gomashio (roasted ground sesame seeds with unrefined sea salt), and fermented products [miso, wandadou jiangyou (soy sauce) and yanzimei (pickled ume plums)], which could have probiotic effect; seaweeds [Kunbu (Laminaria japonica, Aresch), Qundaicai (Undaria pinnatifida, Harv.), Haitai (Porphyria tenera, Kjell.) and Hiziki (Sargassum fusiforme, Harv.)] and Beicha tea (caffeine-free green tea). The daily average energy intake is in the range of 1700-2200 kcal (12% from proteins, 18% from fats and 70% from carbohydrates, mainly complex). The Ma-Pi 2 diet contains, on average, 18% saturated, 46% monounsaturated and 36% polyunsaturated fat, with no trans-fatty acids and an n-6:n-3 polyunsaturated fatty acid ratio of 5:1. It provides nutrients and phytocompounds with antioxidant, hypoglycemic and hypolipidemic effects, such as vitamin C, β carotene, magnesium (average 700 mg/d), manganese (average 16 mg/d), zinc (average 15 mg/d), chromium, phytosterols (average 326 mg/d), dietary fiber (average 50-60 g/d), inulin (average 9 g/d), polyphenols, tocotrienols, folates (more than 500 μg/d), quercetin, and prebiotic and probiotic products. The Ma-Pi 2 diet excludes all animal products, (including egg and dairy), and has no added sugars[77].
All of the ingredients used to prepare the Ma-Pi 2 diets are grown, stored and processed without the use of synthetic chemicals. The crops are from old seed varieties, which were produced using natural methods. All of the products used are labeled, providing detailed information on the origin and characteristics of the product and its supply chain (the Pianesian Transparent Label)[78]. The nutritional content of one Ma-Pi 2 diet used in a clinical study in T2DM patients is presented in Table 1[79].
Table 1.
Example of the average daily energy and nutrient intake for the Ma-Pi 2 diet during in one dietary intervention study[79]
| Nutrient | Ma-Pi 2 diet |
| Energy (kcal) | 2174 |
| Protein (g) | 66 |
| Tryptophan1 | 13 |
| Threonine1 | 35 |
| Isoleucine1 | 41 |
| Leucine1 | 73 |
| Lysine1 | 42 |
| Met + cystine1 | 34 |
| Phen + tyrosine1 | 78 |
| Valine1 | 50 |
| Total fat (g) | 38 |
| Cholesterol (mg) | 0 |
| Carbohydrates (g) | 392 |
| Fiber (g) | 54 |
| Vitamin C (mg) | 164 |
| Folic acid (g) | 751 |
| Vitamin B1 (mg) | 3.52 |
| Vitamin B2 (mg) | 1.3 |
| Vitamin B6 (mg) | 5.55 |
| Niacin (mg) | 25 |
| Vitamin B12 (g) | 0.45 |
| Vitamin E (mg) | 10 |
| Vitamin A (g) | 3266 |
| Potassium (mg) | 3646 |
| Manganese (mg) | 16 |
| Iron (mg) | 24 |
| Calcium (mg) | 982 |
| Phosphorus (mg) | 1632 |
| Zinc (mg) | 15.4 |
| Magnesium (mg) | 754 |
| Sodium (mg) | 1724 |
mg of amino acid per gram of protein.
Since 2001, various clinical studies have been carried out to assess the effect of the Ma-Pi 2 diet in T2DM patients.
Single-arm studies of the Ma-Pi 2 diet in patients with T2DM conducted in several countries and continents (e.g., America, Asia, Africa and Europe) have consistently shown that consumption of the diet for 3 or 6 mo resulted in statistically significant improvements from baseline in indicators of metabolic control, including fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), the serum lipid profile [including reductions in total cholesterol, LDL cholesterol and triglyceride values, and the LDL:high-density lipoprotein (HDL) cholesterol ratio], BMI, and insulin resistance, as well as body weight and blood pressure (P < 0.001) (Table 2)[79,80-84]. These results were apparent even in patients with poor glycemic control (HbA1c > 8.5%)[85]. In patients being treated with insulin, use of this agent fell during the intervention[79,85].
Table 2.
Metabolic effects of the Ma-Pi 2 dietary intervention after variable periods of time
| Metabolic parameters | Duration of Ma-Pi 2 intervention | Effect |
| Body mass index | 3 wk-6 mo | ↓ |
| Body weight (kg) | 3 wk-6 mo | ↓ |
| Body fat mass (%) | 3 wk-6 mo | ↓ |
| Body fat free mass (%) | 3 wk-6 mo | ↔ |
| Fasting glucose (mg/dL) | 3 wk-6 mo | ↓ |
| Fasting insulin (mIU/mL) | 3 wk | ↓ |
| HbA1c | 3 wk-6 mo | ↓ |
| HOMA-R index | 3 wk-6 mo | ↓ |
| Triglycerides (mg/dL) | 3 wk-6 mo | ↓ |
| Total cholesterol (mg/dL) | 3 wk-6 mo | ↓ |
| HDL-cholesterol (mg/dL) | 3 wk-6 mo | ↔ |
| LDL-cholesterol (mg/dL) | 3 wk-6 mo | ↓ |
| Systolic blood pressure (mmHg) | 3 wk-6 mo | ↓ |
| Diastolic blood pressure (mmHg) | 3 wk | ↓ |
| Urinary pH | 3 wk | ↑ |
| Serum anion gap (mEq/L) | 3 wk | ↓ |
| Serum bicarbonate (mEq/L) | 3 wk | ↑ |
| Tumor necrosis factor alpha (ng/mL) | 3 wk | ↓ |
| Interleukin 6 (pg/mL) | 3 wk | ↔ |
| Insulin-like growth factor 1 (ng/mL) | ↓ |
Taken from the results of studies investigating the Ma-Pi 2 diet in men and women with type 2 diabetes over variable periods. ↑: Increase; ↓: Decrease; ↔: No effect; HbA1c: Glycosylated hemoglobin; HDL: High-density lipoprotein; LDL: Low-density lipoprotein.
Similar results were obtained later in short-term (21-d) intervention studies[86,87], thus demonstrating the ability of the diet to achieve rapid metabolic control.
The first randomized, controlled trial comparing the Ma-Pi 2 diet (in 25 participants) with Italian dietary recommendation for T2DM (in 26 participants)-the MADIAB trial-was carried out in 2013[88]. After 21 d on the prescribed diet, which was administered under supervised conditions, the average daily energy intake was 1803 kcal (12% protein, 15% fat, and 73% complex carbohydrates, with 29 g/1000 kcal fiber) in the Ma-Pi 2 group and 1798 kcal (18% protein, 32% fat, and 49% complex carbohydrates, with 20.5 g/1000 kcal fiber) in the control group (P = 0.860).
The multivariate analysis (adjusted for age, gender, BMI at baseline, and physical activity) showed that significantly higher percentage reductions in FBG, postprandial blood glucose (PPBG), HbA1c, total cholesterol, LDL-cholesterol, LDL:HDL ratio, BMI, weight, waist and hip circumference, and insulin resistance were seen in the Ma-Pi 2 group than in the control group. Although the triglyceride levels were reduced in all subjects, the reduction detected in the controls was significantly higher than that in intervention subjects. Furthermore, all subjects in the Ma-Pi 2 group achieved FBG and PPBG target levels (< 110 mg/dL for FBG and < 140 mg/dL for PPBG) at the end of the 21-d dietary treatment[88].
Further results from this trial, presented at the 2014 ADA 74th Scientific Sessions, have also demonstrated that the Ma-Pi 2 diet is a safe strategy by which to reduce markers of insulin resistance and inflammation. In addition, a significant reduction in insulin-like growth factor-1 was observed in the Ma-Pi 2 subjects vs the controls (P < 0.001)[89].
The MADIAB trial is also currently assessing the hypothesis that the Ma-Pi 2 diet may affect the composition of the gut microbiota to a greater extent than the control diet. The results of this analysis will be published later this year.
These results strengthen the idea that the Ma-Pi 2 diet could play an important role in the treatment of T2DM, as whole cereals, non-animal fats and protein, and the high fiber content (> 50 g/d) and probiotic presence could improve gut microbiota composition, favoring a reduction in inflammation and insulin resistance[90].
Eliminating all animal products from the diet may increase the risk of certain nutritional deficiencies in some micronutrients such as vitamin D, calcium, iron, zinc and long-chain n-3 (omega-3) fatty acids[40]. However, the Ma-Pi 2 diet content of those micronutrients seems to be adequate[77,79]. Special concern should be given to possible deficiencies of vitamin B-12[77], even though clinical evidences for deficiency in this vitamin are described only after several years of insufficient consumption[91]. The MA-PI 2 diet was especially conceived for the treatment of T2DM and studies in adults with T2DM consistently showed that a 3 or 6-mo consumption of the Ma-Pi 2 diet was nutritionally safe, at least for the evaluated time[79-83,85]. These evidences suggest that Ma-Pi 2 diet can be used as a short- and medium-term treatment, aimed to achieve a good metabolic control and that further research is needed to demonstrate the safety of this diet in the long-term.
However, currently available studies on the Ma-Pi 2 diet in patients with T2DM have a number of limitations, such as the use of relatively small sample sizes. This was due to the particular design of the studies, where subjects were required to dwell in isolated environment 24 h per day, in order to ensure a good compliance to the diets[80-85,87-89]. However the MADIAB randomized trial was adequately powered and corroborative analyses were performed to ensure consistency and robustness of the clinical trial results[88]. Other limitations in available studies on the Ma-Pi 2 diets include their short- or medium-term durations and the fact that, in some studies, participants (in both the intervention and control groups) were studied in a supervised environment[84,85,87-89]. Subject compliance to dietary recommendations for T2DM are required to obtain clinically significant changes in outcomes, and improvements obtained with dietary interventions in clinical trials are not easy to reproduce in daily life. Future studies should aim to address all of these issues.
CONCLUSION
A variety of diets have been shown to be effective for the management of T2DM, including the Mediterranean-style, vegetarian and low-calorie diets. However, the Ma-Pi 2 diet, in both uncontrolled and controlled short- and medium-term trials conducted in patients with T2DM in several countries and continents, has been found to achieve a speed of metabolic control that has not been reported in studies of other diets.
This finding could be attributable to the composition of the Ma-Pi 2 diet, which is high in whole grains, vegetables and legumes, and fermented products, with no animal products and no added sugars. These characteristics allow to improve glycemic control, reduce insulin requirements, improve insulin sensitivity, lower blood cholesterol and triglycerides, reduce body weight control, and lower systemic blood pressure. The dietary habits may also modify the gastrointestinal microflora composition, which could influence glucose control.
Hence, the Ma-Pi 2 diet could be considered a valid additional short- to medium-term treatment for T2DM, particularly when glycemic control needs to be rapidly improved. Further research is needed to demonstrate the efficacy of this diet in the long-term management of T2DM.
Footnotes
P- Reviewer: Bagyánszki M S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
Conflict-of-interest: The authors declare that they have no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 30, 2014
First decision: December 17, 2014
Article in press: February 2, 2015
References
- 1.Nicolucci A, Rossi C, Lucisano G. Facts and figures about diabetes in Italy. Italian Diabetes Monitor: Fondazione Mario Negri Sud; 2014. Available from: http: //www.ibdo.it/pdf/DiabetesMonitor-FactandFigure2014.pdf. [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas, 6th ed. Brussels, Belgium: International Diabetes Federation; 2013. [Google Scholar]
- 3.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 4.Bilous R, Donnelly R. Handbook of Diabetes, 4th ed. Oxford: Wiley Blackwell; 2010. p. 46. [Google Scholar]
- 5.Levi J, Segal LM, St Laurent R, Lang A, Rayburn J. F as in Fat: How Obesity Threatens America’s Future 2012. Project Report. Princeton, NJ: Trust for America’s Health/Robert Wood Johnson Foundation; 2012. [Google Scholar]
- 6.Balducci S, Sacchetti M, Haxhi J, Orlando G, D’Errico V, Fallucca S, Menini S, Pugliese G. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab Res Rev. 2014;30 Suppl 1:13–23. doi: 10.1002/dmrr.2514. [DOI] [PubMed] [Google Scholar]
- 7.Balducci S, Zanuso S, Massarini M, Corigliano G, Nicolucci A, Missori S, Cavallo S, Cardelli P, Alessi E, Pugliese G, et al. The Italian Diabetes and Exercise Study (IDES): design and methods for a prospective Italian multicentre trial of intensive lifestyle intervention in people with type 2 diabetes and the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2008;18:585–595. doi: 10.1016/j.numecd.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Balducci S, Zanuso S, Nicolucci A, De Feo P, Cavallo S, Cardelli P, Fallucca S, Alessi E, Fallucca F, Pugliese G. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES) Arch Intern Med. 2010;170:1794–1803. doi: 10.1001/archinternmed.2010.380. [DOI] [PubMed] [Google Scholar]
- 9.Nicolucci A, Balducci S, Cardelli P, Zanuso S, Pugliese G. Improvement of quality of life with supervised exercise training in subjects with type 2 diabetes mellitus. Arch Intern Med. 2011;171:1951–1953. doi: 10.1001/archinternmed.2011.561. [DOI] [PubMed] [Google Scholar]
- 10.Balducci S, Zanuso S, Cardelli P, Salvi L, Bazuro A, Pugliese L, Maccora C, Iacobini C, Conti FG, Nicolucci A, et al. Effect of high- versus low-intensity supervised aerobic and resistance training on modifiable cardiovascular risk factors in type 2 diabetes; the Italian Diabetes and Exercise Study (IDES) PLoS One. 2012;7:e49297. doi: 10.1371/journal.pone.0049297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes Care. 2010;33 Suppl 1:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montonen J, Knekt P, Härkänen T, Järvinen R, Heliövaara M, Aromaa A, Reunanen A. Dietary patterns and the incidence of type 2 diabetes. Am J Epidemiol. 2005;161:219–227. doi: 10.1093/aje/kwi039. [DOI] [PubMed] [Google Scholar]
- 14.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61:1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 15.Esposito K, Giugliano D. Mediterranean diet and type 2 diabetes. Diabetes Metab Res Rev. 2014;30 Suppl 1:34–40. doi: 10.1002/dmrr.2516. [DOI] [PubMed] [Google Scholar]
- 16.Esposito K, Kastorini CM, Panagiotakos DB, Giugliano D. Prevention of type 2 diabetes by dietary patterns: a systematic review of prospective studies and meta-analysis. Metab Syndr Relat Disord. 2010;8:471–476. doi: 10.1089/met.2010.0009. [DOI] [PubMed] [Google Scholar]
- 17.Esposito K, Maiorino MI, Ceriello A, Giugliano D. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract. 2010;89:97–102. doi: 10.1016/j.diabres.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-González MA, de la Fuente-Arrillaga C, Nunez-Cordoba JM, Basterra-Gortari FJ, Beunza JJ, Vazquez Z, Benito S, Tortosa A, Bes-Rastrollo M. Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ. 2008;336:1348–1351. doi: 10.1136/bmj.39561.501007.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romaguera D, Guevara M, Norat T, Langenberg C, Forouhi NG, Sharp S, Slimani N, Schulze MB, Buijsse B, Buckland G, et al. Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: the InterAct project. Diabetes Care. 2011;34:1913–1918. doi: 10.2337/dc11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salas-Salvadó J, Bulló M, Babio N, Martínez-González MÁ, Ibarrola-Jurado N, Basora J, Estruch R, Covas MI, Corella D, Arós F, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34:14–19. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi M, Negri E, Bosetti C, Dal Maso L, Talamini R, Giacosa A, Montella M, Franceschi S, La Vecchia C. Mediterranean diet in relation to body mass index and waist-to-hip ratio. Public Health Nutr. 2008;11:214–217. doi: 10.1017/S1368980007000833. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415. [PubMed] [Google Scholar]
- 23.Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age (Dordr) 2010;32:97–108. doi: 10.1007/s11357-009-9118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- 25.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontana L. Neuroendocrine factors in the regulation of inflammation: excessive adiposity and calorie restriction. Exp Gerontol. 2009;44:41–45. doi: 10.1016/j.exger.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davey GK, Spencer EA, Appleby PN, Allen NE, Knox KH, Key TJ. EPIC-Oxford: lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003;6:259–269. doi: 10.1079/PHN2002430. [DOI] [PubMed] [Google Scholar]
- 28.Tonstad S, Butler T, Yan R, Fraser GE. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care. 2009;32:791–796. doi: 10.2337/dc08-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burslem J, Schonfeld G, Howald MA, Weidman SW, Miller JP. Plasma apoprotein and lipoprotein lipid levels in vegetarians. Metabolism. 1978;27:711–719. doi: 10.1016/0026-0495(78)90009-4. [DOI] [PubMed] [Google Scholar]
- 30.Lock DR, Varhol A, Grimes S, Patsch W, Schonfeld G. ApoA-I/ApoA-II ratios in plasmas of vegetarians. Metabolism. 1983;32:1142–1145. doi: 10.1016/0026-0495(83)90061-6. [DOI] [PubMed] [Google Scholar]
- 31.Thorogood M, Carter R, Benfield L, McPherson K, Mann JI. Plasma lipids and lipoprotein cholesterol concentrations in people with different diets in Britain. Br Med J (Clin Res Ed) 1987;295:351–353. doi: 10.1136/bmj.295.6594.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong B, van Merwyk AJ, Coates H. Blood pressure in Seventh-day Adventist vegetarians. Am J Epidemiol. 1977;105:444–449. doi: 10.1093/oxfordjournals.aje.a112403. [DOI] [PubMed] [Google Scholar]
- 33.Appleby PN, Davey GK, Key TJ. Hypertension and blood pressure among meat eaters, fish eaters, vegetarians and vegans in EPIC-Oxford. Public Health Nutr. 2002;5:645–654. doi: 10.1079/PHN2002332. [DOI] [PubMed] [Google Scholar]
- 34.Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis. 2013;23:292–299. doi: 10.1016/j.numecd.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukagawa NK, Anderson JW, Hageman G, Young VR, Minaker KL. High-carbohydrate, high-fiber diets increase peripheral insulin sensitivity in healthy young and old adults. Am J Clin Nutr. 1990;52:524–528. doi: 10.1093/ajcn/52.3.524. [DOI] [PubMed] [Google Scholar]
- 36.Crowe FL, Appleby PN, Travis RC, Key TJ. Risk of hospitalization or death from ischemic heart disease among British vegetarians and nonvegetarians: results from the EPIC-Oxford cohort study. Am J Clin Nutr. 2013;97:597–603. doi: 10.3945/ajcn.112.044073. [DOI] [PubMed] [Google Scholar]
- 37.Key TJ, Fraser GE, Thorogood M, Appleby PN, Beral V, Reeves G, Burr ML, Chang-Claude J, Frentzel-Beyme R, Kuzma JW, et al. Mortality in vegetarians and nonvegetarians: detailed findings from a collaborative analysis of 5 prospective studies. Am J Clin Nutr. 1999;70:516S–524S. doi: 10.1093/ajcn/70.3.516s. [DOI] [PubMed] [Google Scholar]
- 38.Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford) Am J Clin Nutr. 2009;89:1613S–1619S. doi: 10.3945/ajcn.2009.26736L. [DOI] [PubMed] [Google Scholar]
- 39.Tantamango-Bartley Y, Jaceldo-Siegl K, Fan J, Fraser G. Vegetarian diets and the incidence of cancer in a low-risk population. Cancer Epidemiol Biomarkers Prev. 2013;22:286–294. doi: 10.1158/1055-9965.EPI-12-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 41.Colditz GA, Manson JE, Stampfer MJ, Rosner B, Willett WC, Speizer FE. Diet and risk of clinical diabetes in women. Am J Clin Nutr. 1992;55:1018–1023. doi: 10.1093/ajcn/55.5.1018. [DOI] [PubMed] [Google Scholar]
- 42.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 43.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 44.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 45.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 46.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan KK, Seeley RJ. Physiology. Food as a hormone. Science. 2013;339:918–919. doi: 10.1126/science.1234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 49.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 50.Tlaskalová-Hogenová H, Stepánková R, Hudcovic T, Tucková L, Cukrowska B, Lodinová-Zádníková R, Kozáková H, Rossmann P, Bártová J, Sokol D, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salminen S, Bouley C, Boutron-Ruault MC, Cummings JH, Franck A, Gibson GR, Isolauri E, Moreau MC, Roberfroid M, Rowland I. Functional food science and gastrointestinal physiology and function. Br J Nutr. 1998;80 Suppl 1:S147–S171. doi: 10.1079/bjn19980108. [DOI] [PubMed] [Google Scholar]
- 54.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–24.e1-2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes. 2014;5:3–17. doi: 10.3920/BM2012.0065. [DOI] [PubMed] [Google Scholar]
- 59.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 60.Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32:2281–2287. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zoetendal EG, Vaughan EE, de Vos WM. A microbial world within us. Mol Microbiol. 2006;59:1639–1650. doi: 10.1111/j.1365-2958.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- 64.Biagi E, Candela M, Fairweather-Tait S, Franceschi C, Brigidi P. Aging of the human metaorganism: the microbial counterpart. Age (Dordr) 2012;34:247–267. doi: 10.1007/s11357-011-9217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 67.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104 Suppl 2:S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 68.Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90:1236–1243. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- 69.Food and Agriculture Organization/World Health Organization. Health and Nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Switzerland, Basel: World Health Organization; 2001. [Google Scholar]
- 70.Strowski MZ, Wiedenmann B. Probiotic carbohydrates reduce intestinal permeability and inflammation in metabolic diseases. Gut. 2009;58:1044–1045. doi: 10.1136/gut.2009.179325. [DOI] [PubMed] [Google Scholar]
- 71.Nilsson AC, Ostman EM, Holst JJ, Björck IM. Including indigestible carbohydrates in the evening meal of healthy subjects improves glucose tolerance, lowers inflammatory markers, and increases satiety after a subsequent standardized breakfast. J Nutr. 2008;138:732–739. doi: 10.1093/jn/138.4.732. [DOI] [PubMed] [Google Scholar]
- 72.Solga SF, Buckley G, Clark JM, Horska A, Diehl AM. The effect of a probiotic on hepatic steatosis. J Clin Gastroenterol. 2008;42:1117–1119. doi: 10.1097/MCG.0b013e31816d920c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 2009;89:1751–1759. doi: 10.3945/ajcn.2009.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luoto R, Kalliomäki M, Laitinen K, Isolauri E. The impact of perinatal probiotic intervention on the development of overweight and obesity: follow-up study from birth to 10 years. Int J Obes (Lond) 2010;34:1531–1537. doi: 10.1038/ijo.2010.50. [DOI] [PubMed] [Google Scholar]
- 75.Delzenne NM, Neyrinck AM, Cani PD. Modulation of the gut microbiota by nutrients with prebiotic properties: consequences for host health in the context of obesity and metabolic syndrome. Microb Cell Fact. 2011;10 Suppl 1:S10. doi: 10.1186/1475-2859-10-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohsawa G. Le zen macrobiotique ou l’art du rajeunissement et de la longevitè. Paris: Librairie Philosophique J Vrin; 2004. [Google Scholar]
- 77.Porrata C, Hernández M, Abuín A, Campa C, Pianesi M. Caracterización y evaluación nutricional de las dietas macrobióticas Ma-Pi. Rev Cubana Investig Bioméd. 2008;27:1–36. [Google Scholar]
- 78.Mancini L. Conventional, organic and polycultural farming practices: material intensity of italian crops and Foodstuffs. Resources. 2013;2:628–650. [Google Scholar]
- 79.Porrata-Maury C, Hernández-Triana M, Rodríguez-Sotero E, Vilá-Dacosta-Calheiros R, Hernández-Hernández H, Mirabal-Sosa M, Campa-Huergo C, Pianesi M. Medium- and short-term interventions with ma-pi 2 macrobiotic diet in type 2 diabetic adults of bauta, havana. J Nutr Metab. 2012;2012:856342. doi: 10.1155/2012/856342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhumisawasdi J, Vanna O, Surinpang N. The self-reliant system for alternative care of diabetes mellitus patients--experience macrobiotic management in Trad Province. J Med Assoc Thai. 2006;89:2104–2115. [PubMed] [Google Scholar]
- 81.Porrata C, Abuin Landin A, Zayas AM, Vilà Dacosta-Calheiros R, Hernandez Triana M, Menendez Triana J, Diaz Sanchez ME, Sosa MM, Huergo CC, Pianesi M. Efecto terapeutico de la dieta macrobiotica MAPI-2 en 25 adultos con diabetes mellitus tipo 2. Rev Cubana Invest Biomed. 2007;26:71–87. [Google Scholar]
- 82.Monsan Yapo M, Katchè Adueni V, Ehouman G, Porrata C, Pianesi M. MAPI-2 macrobiotic diet intervention during six momths in adults with type 2 diabetes mellitus, Cote D’Ivoire, 2010. Revue Soc Sci Nat de Tunisie. 2010;37:62–71. [Google Scholar]
- 83.Kablan BJ, Kouassi D, N’guetta KF, Adoueni KV, Konè M, Diafouka F, Boa YF, Lokrou A, Gbanè A, Ehouman G, et al. Curative effects of MAPI-2 macrobiotic diet in Ivoirian type 2 diabetic. Cahier de Santè Publique. 2011;10:96–125. [Google Scholar]
- 84.Fallucca F, Fallucca S, Pianesi M. The effects of the MA-PI 2 macrobiotic diet in the treatment of type 2 diabetes and diet-induced metabolic acidosis. Diabetes Metab Res Rev. 2014;30:659–660. doi: 10.1002/dmrr.2602. [DOI] [PubMed] [Google Scholar]
- 85.Porrata C, Sánchez J, Correa V, Abuín A, Hernández-Triana M, Dacosta-Calheiros RV, Díaz ME, Mirabal M, Cabrera E, Campa C, et al. Ma-pi 2 macrobiotic diet intervention in adults with type 2 diabetes mellitus. MEDICC Rev. 2009;11:29–35. doi: 10.37757/MR2009V11.N4.8. [DOI] [PubMed] [Google Scholar]
- 86.Porrata-Maury C, Hernández-Triana M, Ruiz-Álvarez V, Díaz-Sánchez ME, Fallucca F, Bin W, Baba-Abubakari B, Pianesi M. Ma-Pi 2 macrobiotic diet and type 2 diabetes mellitus: pooled analysis of short-term intervention studies. Diabetes Metab Res Rev. 2014;30 Suppl 1:55–66. doi: 10.1002/dmrr.2519. [DOI] [PubMed] [Google Scholar]
- 87.Fallucca F, Porrata C, Monaco G, Bufacchi A, Pianesi M. MAPI macrobiotic diet intervention during 21 days in adults with type 2 diabetes mellitus, Rome. Minerva Endocrinol. 2012;37 Suppl 1:116. [Google Scholar]
- 88.Soare A, Khazrai YM, Del Toro R, Roncella E, Fontana L, Fallucca S, Angeletti S, Formisano V, Capata F, Ruiz V, et al. The effect of the macrobiotic Ma-Pi 2 diet vs. the recommended diet in the management of type 2 diabetes: the randomized controlled MADIAB trial. Nutr Metab (Lond) 2014;11:39. doi: 10.1186/1743-7075-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Behavioral medicine, clinical nutrition, education , and exercise. Diabetes. 2014;63 Suppl 1:A170–A212. [Google Scholar]
- 90.Fallucca F, Porrata C, Fallucca S, Pianesi M. Influence of diet on gut microbiota, inflammation and type 2 diabetes mellitus. First experience with macrobiotic Ma-Pi 2 diet. Diabetes Metab Res Rev. 2014;30 Suppl 1:48–54. doi: 10.1002/dmrr.2518. [DOI] [PubMed] [Google Scholar]
- 91.Dong A, Scott SC. Serum vitamin B12 and blood cell values in vegetarians. Ann Nutr Metab. 1982;26:209–216. doi: 10.1159/000176565. [DOI] [PubMed] [Google Scholar]


