Abstract
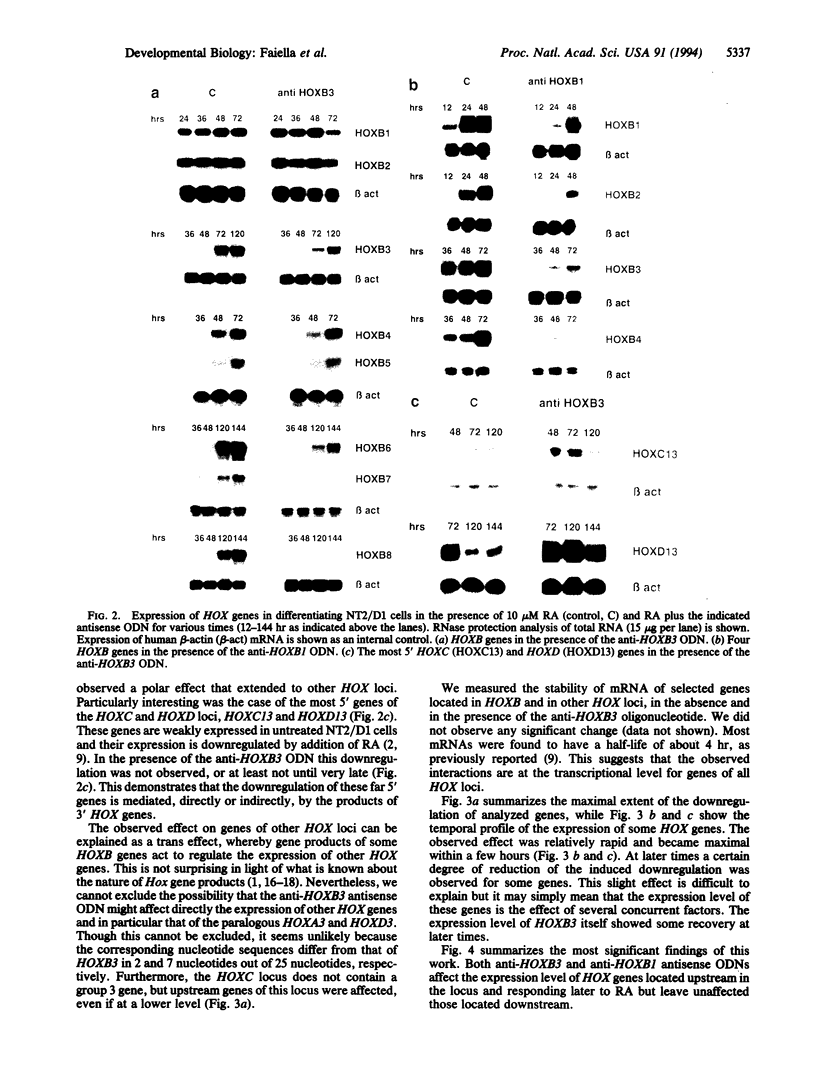
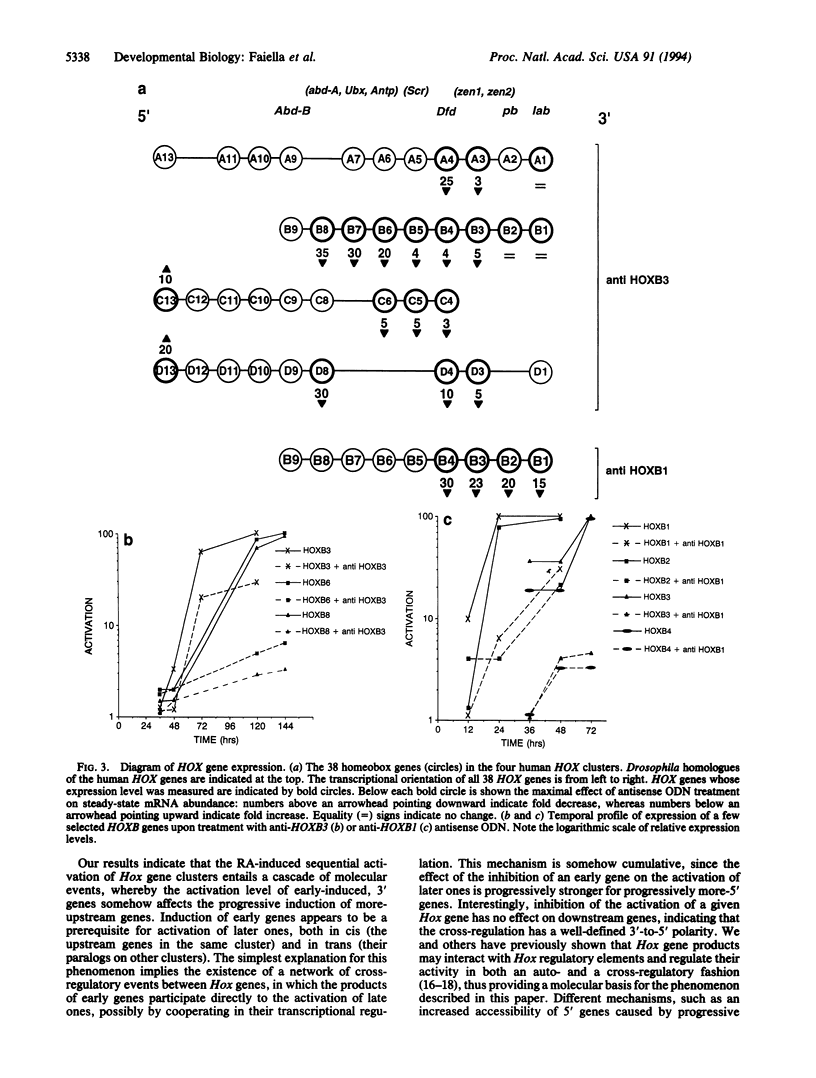
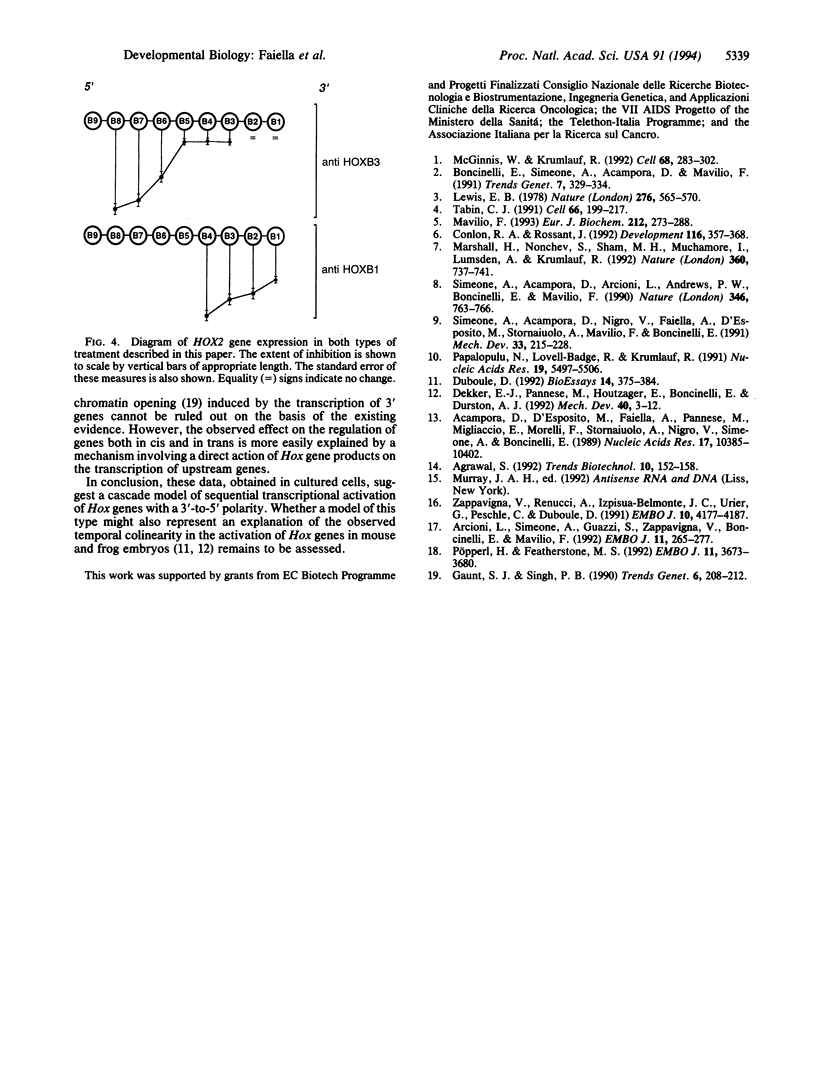
Most homeobox genes belonging to the Hox family are sequentially activated in embryonal carcinoma cells upon treatment with retinoic acid. Genes located at the 3' end of each one of the four Hox clusters are activated first, whereas upstream Hox genes are activated progressively later. This activation has been extensively studied for human HOX genes in the NT2/D1 cell line and shown to take place at the transcriptional level. To understand the molecular mechanisms of sequential HOX gene activation in these cells, we tried to modulate the expression of 3' HOX genes through the use of antisense oligonucleotides added to the culture medium. We chose the HOXB locus. A 5- to 15-fold reduction of the expression of HOXB1 and HOXB3 was sufficient to produce a significant inhibition of the activation of the upstream HOXB genes, as well as of their paralogs in the HOXA, HOXC, and HOXD clusters. Conversely, no effect was detectable on downstream HOX genes. The extent of this inhibition increased for progressively more-5' genes. The stability of the corresponding mRNAs appeared to be unaffected, supporting the idea that the observed effect might be mediated at the transcriptional level. These data suggest a cascade model of progressive activation of Hox genes, with a 3'-to-5' polarity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acampora D., D'Esposito M., Faiella A., Pannese M., Migliaccio E., Morelli F., Stornaiuolo A., Nigro V., Simeone A., Boncinelli E. The human HOX gene family. Nucleic Acids Res. 1989 Dec 25;17(24):10385–10402. doi: 10.1093/nar/17.24.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S. Antisense oligonucleotides as antiviral agents. Trends Biotechnol. 1992 May;10(5):152–158. doi: 10.1016/0167-7799(92)90203-8. [DOI] [PubMed] [Google Scholar]
- Arcioni L., Simeone A., Guazzi S., Zappavigna V., Boncinelli E., Mavilio F. The upstream region of the human homeobox gene HOX3D is a target for regulation by retinoic acid and HOX homeoproteins. EMBO J. 1992 Jan;11(1):265–277. doi: 10.1002/j.1460-2075.1992.tb05049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncinelli E., Simeone A., Acampora D., Mavilio F. HOX gene activation by retinoic acid. Trends Genet. 1991 Oct;7(10):329–334. doi: 10.1016/0168-9525(91)90423-n. [DOI] [PubMed] [Google Scholar]
- Conlon R. A., Rossant J. Exogenous retinoic acid rapidly induces anterior ectopic expression of murine Hox-2 genes in vivo. Development. 1992 Oct;116(2):357–368. doi: 10.1242/dev.116.2.357. [DOI] [PubMed] [Google Scholar]
- Dekker E. J., Pannese M., Houtzager E., Boncinelli E., Durston A. Colinearity in the Xenopus laevis Hox-2 complex. Mech Dev. 1993 Jan;40(1-2):3–12. doi: 10.1016/0925-4773(93)90083-a. [DOI] [PubMed] [Google Scholar]
- Duboule D. The vertebrate limb: a model system to study the Hox/HOM gene network during development and evolution. Bioessays. 1992 Jun;14(6):375–384. doi: 10.1002/bies.950140606. [DOI] [PubMed] [Google Scholar]
- Gaunt S. J., Singh P. B. Homeogene expression patterns and chromosomal imprinting. Trends Genet. 1990 Jul;6(7):208–212. [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Marshall H., Nonchev S., Sham M. H., Muchamore I., Lumsden A., Krumlauf R. Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature. 1992 Dec 24;360(6406):737–741. doi: 10.1038/360737a0. [DOI] [PubMed] [Google Scholar]
- Mavilio F. Regulation of vertebrate homeobox-containing genes by morphogens. Eur J Biochem. 1993 Mar 1;212(2):273–288. doi: 10.1111/j.1432-1033.1993.tb17660.x. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Papalopulu N., Lovell-Badge R., Krumlauf R. The expression of murine Hox-2 genes is dependent on the differentiation pathway and displays a collinear sensitivity to retinoic acid in F9 cells and Xenopus embryos. Nucleic Acids Res. 1991 Oct 25;19(20):5497–5506. doi: 10.1093/nar/19.20.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöpperl H., Featherstone M. S. An autoregulatory element of the murine Hox-4.2 gene. EMBO J. 1992 Oct;11(10):3673–3680. doi: 10.1002/j.1460-2075.1992.tb05452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Arcioni L., Andrews P. W., Boncinelli E., Mavilio F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990 Aug 23;346(6286):763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Nigro V., Faiella A., D'Esposito M., Stornaiuolo A., Mavilio F., Boncinelli E. Differential regulation by retinoic acid of the homeobox genes of the four HOX loci in human embryonal carcinoma cells. Mech Dev. 1991 Mar;33(3):215–227. doi: 10.1016/0925-4773(91)90029-6. [DOI] [PubMed] [Google Scholar]
- Tabin C. J. Retinoids, homeoboxes, and growth factors: toward molecular models for limb development. Cell. 1991 Jul 26;66(2):199–217. doi: 10.1016/0092-8674(91)90612-3. [DOI] [PubMed] [Google Scholar]
- Zappavigna V., Renucci A., Izpisúa-Belmonte J. C., Urier G., Peschle C., Duboule D. HOX4 genes encode transcription factors with potential auto- and cross-regulatory capacities. EMBO J. 1991 Dec;10(13):4177–4187. doi: 10.1002/j.1460-2075.1991.tb04996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




