Abstract
Eating disorders (ED) are characterized by a persistent disturbance of eating that impairs health or psychosocial functioning. They are associated with increased rates of medical complications and mortality. Insulin omission is a unique purging behavior available to individuals with type 1 diabetes mellitus (T1DM). The standard treatment regimen for T1DM requires a major focus on food and eating patterns. Moreover, intensive insulin therapy is associated with increasing body weight. These factors, combined with the psychological burden of chronic disease management and depression, may contribute to ED. The comorbidity of ED in T1DM patients is associated with poorer glycemic control and consequently higher rates of diabetes complications. Early recognition and adequate treatment of ED in T1DM is essential.
Keywords: Type 1 diabetes, Eating disorders, Insulin omission
Core tip: Intentional insulin omission for the purpose of preventing weight gain is a unique behavior available to individuals with type 1 diabetes mellitus (T1DM). It is classified as either an inappropriate compensatory feature of bulimia nervosa or as a purging disorder component of other specified feeding or eating disorder (ED). Its prevalence increases with age, affecting up to 40% of young adult females with T1DM. The comorbid of ED in T1DM patients is associated with higher rates of short and long-term diabetes complications. A high index of suspicion is needed since ED behaviors are often well hidden and denied. Treatment involves a complex interplay of psychosocial, dietary and medical aspects and requires a multidisciplinary team.
DEFINITIONS OF EATING DISORDERS
Eating disorders (ED) are characterized by a persistent disturbance of eating that impairs health or psychosocial functioning[1,2]. Based upon the fifth edition of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM-V), which was released in May 2013, ED are divided into eight mutually exclusive categories[3]. These categories are based upon observed symptoms and include anorexia nervosa (AN), bulimia nervosa (BN), binge eating disorder, avoidant/restrictive food intake disorder, pica, rumination disorder, other specified feeding or eating disorder (OSFED) and unspecified feeding or eating disorder. Some diagnoses include a dimensional component that enables clinicians to specify the severity of illness[4-6].
In patients with type 1 diabetes mellitus (T1DM), intentional insulin omission or reduction for the purpose of preventing weight gain is recognized as either an inappropriate compensatory feature of bulimia nervosa or as a purging disorder, a component of OSFED. The latter is relevant when the recurrent purging behavior to influence weight or shape (e.g., insulin omission) occurs in the absence of binge eating.
The two categories: “eating disorders not otherwise specified (EDNOS)” and “disturbed eating behavior (DEB)”, compromised a wide spectrum of eating disorder pathologies, at a frequency or severity that does not merit a formal ED diagnosis. Though these categories are not included in DSM-V, they appear in the current review, due to their use by several studies involving individuals with diabetes.
ARE ADOLESCENTS WITH T1DM AT INCREASED RISK TO DEVELOP ED?
This question should be answered for the specific ED studied, according to the tools used for assessment and the prevalence rates in the general population.
Diagnostic tools of ED in persons with T1DM
The diagnosis of ED in individuals with T1DM is difficult, since eating behaviors are often well hidden and denied. The use of different questionnaires for assessing prevalence rates of ED makes comparisons across studies difficult. Furthermore, as Markowitz et al[7] pointed out, that general diagnostic questionnaires for detecting ED are not appropriate for individuals with T1DM for two main reasons. Firstly, these questionnaires do not identify eating disorder behaviors that are unique to T1DM, such as insulin omission[7]. Secondly, such questionnaires may inflate the prevalence of eating problems in those with T1DM, because behaviors that are considered disturbed, such as particular concern about diet, reduced intake of certain food groups, and eating when not hungry, are integral to diabetes care[8].
Prevalence rates of ED among persons with T1DM
Prevalence rates of ED among persons with TIDM vary according to the different ED categories and the populations studied. Some studies examined prevalence rates of AN and BN only, while others examined the prevalence of insulin omission only, and some reported the prevalence of a combination of all ED together. Some studies reported the prevalence of full-threshold diagnoses of ED, whereas others also included subclinical ED. Reported prevalence rates differ also according to characteristics of the study populations, such as age range, and whether males were included or only females.
Prevalence of AN: A meta-analysis of controlled studies that defined ED in females according to DSM III-R or DSM IV criteria reported the prevalence of AN in T1DM subjects was not significantly different from that of controls[9].
Prevalence of BN: This same meta-analysis showed a significantly higher prevalence of BN among the females with T1DM than those without diabetes[9].
Overall prevalence of AN, BN and EDNOS: In a recently published meta-analysis[10] of six studies of adolescents[11-16], 7.0% of the 825 individuals with T1DM had ED, compared with 2.8% of the 2282 individuals without T1DM.
Prevalence of DEB: In a meta-analysis[10] of five studies[11,15-18], a higher proportion of adolescents with T1DM than without T1DM were classified as having DEB (39.3% vs 32.5%). The prevalence rate of DEB increased significantly with weight and age, from 7.2% in the underweight group to 32.7% in the obese group, and from 8.1% in the youngest age-group (11-13 years) to 38.1% in the oldest age-group (17-19 years)[19].
Prevalence of insulin omission in T1DM: Insulin omission is a unique purging behavior available to individuals with T1DM. As presented in Table 1, insulin omission for weight loss is identified mainly in females; its prevalence increases with age, affecting up to 40% of young adult females with T1DM.
Table 1.
Prevalence rates of insulin omission according to age range and gender
| Prevalence of insulin omission (%) | Age range (yr) | Number and sex | Ref. |
| 2 | 9-13 | 101 females | Colton et al[12] |
| 26.2 4.5 | 11-19 | 390 females 380 males | Wisting et al[19] |
| 11 | 12-19 | 361 females | Jones et al[11] |
| 14 | 12–19 | 356 females | Rodin et al[61] |
| 10 1 | 12-21 | 70 females 73 males | Neumark-Sztainer et al[24] |
| 34 | 16-22 | 91 females | Rydall et al[46] |
| 40 | 18-30 | 59 females | Stancin et al[74] |
| 11 0 | 12-56 | 141 females 58 males | Philippi et al[75] |
PREDISPOSING FACTORS FOR DEVELOPING ED
We suggest a three level model to describe the development of disturbed eating in adolescents with T1DM. This model comprises a hierarchically arranged continuum of potential effects on health problems (Figure 1). The first circle in the model, the premorbid status, includes a tendency to overweight, and factors relating to personality and family characteristics and dynamics. The second circle comprises factors arising at diagnosis of diabetes, such as the age of diabetes onset and satisfaction from weight loss. The third circle comprises factors associated with the chronic management of diabetes, such as recurrent hypoglycemic episodes, strict insulin treatment and carbohydrate counting.
Figure 1.
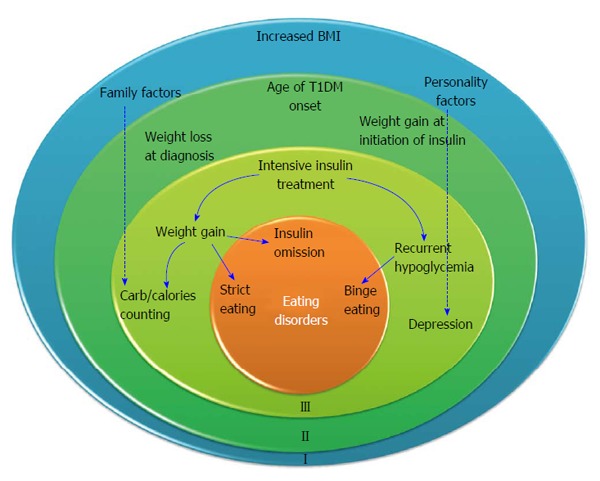
Three level models describing the development of eating disorders in adolescents with type 1 diabetes mellitus. The outer circle delineates the premorbid status. The middle circle delineates weight changes at diagnosis and with initiation of treatment. The inner circle includes factors associated with the management of T1DM that predispose to eating disorders. Dashed arrows imply that the specific factor extends on all levels. T1DM: Type 1 diabetes mellitus; BMI: Body mass index.
FIRST CIRCLE - PREMORBID FACTORS
Increased body weight and a tendency to gain weight
It has been suggested that the single question “Have you ever been overweight?” may be sufficient to screen for those with T1DM who are at a high risk for disordered eating attitudes/behaviors[20]. Indeed, adolescent girls with T1DM who reported ever being overweight endorsed more disordered eating attitudes and behaviors[21]. In addition, a higher body mass index percentile among teenagers and adults with T1DM, particularly among females, one to two years prior to the onset of ED, was found to be associated with disordered eating[22]. Furthermore, youth at risk for disordered eating have been shown to have poorer diet quality, including a higher intake of total fat and saturated fat[23].
Low self-esteem and body dissatisfaction
Higher levels of weight dissatisfaction tended to be associated with unhealthy weight control/disordered eating among adolescent females[24]. Low self-esteem predicts eating disturbances in the general population. Lower self-esteem was associated with disturbed eating behavior in girls with T1DM[25]. Of note, low self-esteem and body dissatisfaction could also arise along the course of the disease. In a longitudinal study the development of DEB in girls with T1DM, was predicted by lower self-esteem related to physical appearance, and lower global self-esteem[26].
Personality characteristics
Perfectionism has been associated with attitudinal aspects of eating disorders such as weight preoccupation. Borderline personality characteristics were related to insulin omission[27]. Adolescent girls with T1DM and an ED showed deficits in self-regulation and narcissistic gain from illness[28], and tended to blame themselves for the situation[29].
Comparing adolescents with ED and adolescents with T1DM and ED, those with T1DM were less pathologically compromised, scored lower on several affective areas and had a lower prevalence of depression and anxiety[30].
Family characteristics and dynamics
Several factors involving family characteristics and dynamics are associated with an increased risk for ED. Firstly, in many families of adolescent girls with T1DM, interactions within the family center around food and weight. The prevalence of ED was found to be higher in families in which parents tended to make negative comments about eating or weight[31]. Secondly, mothers who engaged in dieting and binge-eating themselves were more likely to have daughters with disturbed eating behavior. Maternal weight and shape concerns and impaired mother-daughter relationships significantly predicted eating disturbances in girls with T1DM, accounting for 57% of the variance[32]. Finally, family dysfunctioning has been identified as an important risk factor for eating disorders among the general population. Specifically, among girls with T1DM, less support from, poorer communication with, and less trust in their relationships with their parents, were reported among those with eating disturbances than among those without[33]. Families of girls with ED more often reported having infrequent family meals than did families without ED[34].
SECOND CIRCLE - FACTORS ASSOCIATED WITH DISEASE ONSET
Age of diabetes onset
The age of onset of T1DM that is most closely related to the subsequent development of a severe eating disorder such as AN and BN was studied in 53 women with ED compared with 49 with T1DM with T1DM who had no eating disorder - related problem[35]. It was demonstrated that for diabetes onset age between 7 to 18 years, the density of the “eating disorder” group was higher, but for the younger and older onset ages the densities were lower. Thus the development of T1DM in preadolescence or adolescence seems to place girls at risk for the subsequent development of AN or BN. Similarly, frequent insulin reduction or omission was reported in type 1 diabetic patients with later disease onset (mean age at onset between 8 and about 17 years)[36]. It was suggested that later age at diabetes onset, in particular during pubertal age with hormonal changes and gain in weight and fat mass may be associated with a greater risk for ED especially in female patients.
Weight issues
The cycle of weight loss at disease onset and subsequent weight gain with the initiation of insulin treatment is a risk factor for the development of ED in susceptible patients[11].
THIRD CIRCLE - FACTORS ASSOCIATED WITH THE CHRONIC MANAGEMENT OF DIABETES
Intensive insulin treatment
Intensive insulin treatment conveys an increased risk of weight gain[37]. A model of disordered eating and T1DM proposed by Goebel-Fabbri et al[38,39] suggests that the increased weight gain causes negative feelings. Fear of further weight gain can lead to insulin restriction for caloric purging or weight regulation[40].
Dietary restraint
Diabetes management imposes dietary restraint. This may lead to the yearning and craving of “forbidden foods”, and result in binging, without administration of the appropriate insulin dose[41].
Hypoglycemic episodes
The delicate balance between dietary regimen, insulin and exercise that is integral to T1DM management can result in recurrent hypoglycemic episodes. Hypoglycemia is accompanied by intense hunger and by eating sweetened foods and drinks that are normally forbidden. Patients may subsequently feel guilty about consuming these foods and restrict eating, which may result in yet another hypoglycemic episode. This vicious cycle of dietary restriction, over-eating and guilt is similar to that experienced by individuals with bulimia[42].
Chronic disease and depression
The risk of significant depressive symptoms in individuals with T1DM has been assessed as about double that of the general population[43]. Depression may increase the susceptibility for developing disturbed eating behavior. Indeed, among girls with T1DM, those diagnosed with depression scored higher on the Eating Disorder Examination than did those without depression; 75% and 45%, respectively[44]. Furthermore, anxiety disorders and eating disorders co-occur in the general population, and 21% of children and adolescents with T1DM were reported to screen positively for anxiety[45].
CLINICAL SIGNS OF ED AMONG INDIVIDUALS WITH T1DM
The diagnosis of subclinical or clinical eating disorders in individuals with T1DM is not easily established. Due to the frequent concealment and denial of eating disorder behaviors, a high index of suspicion is needed. In addition to the predisposing characteristics described above, a number of clinical signs should alert health care providers to the possibility of ED.
Poor glycemic control
Among females with T1DM, mean HbA1c levels were shown to be significantly higher in those with disturbed eating than among those with non-disturbed eating[46]. HbA1c level of patients who confessed intentional under-dosing of insulin was 9.0% ± 1.6% compared with 7.8% ± 1.2% of compliant patien[47]. Similarly, in a nationwide population-based study, conducted in Norway, a significantly higher HbA1c was documented in patients reporting insulin restriction (9.0% ± 1.7%) than in nonrestrictors (8.3% ± 1.2%)[19] In a meta-analysis, eating problems, including both DEB and ED, were found to be associated with poorer glycemic control[10]. Using a computer model of data mining, we recently showed that adolescent females with intentional insulin omission were discriminated by HbA1c > 9.2% and by more than 20% of HbA1c measurements above the 90th percentile[48].
Recurrent episodes of diabetic ketoacidosis
Intentional insulin omission has long been recognized as a cause of recurrent episodes of recurrent episodes of diabetic ketoacidosis (DKA) in adolescents with T1DM[49]. Thus, any recurrent episode of DKA in established diabetes should arouse suspicion[50].
Recurrent hypoglycemic episodes
Recurrent hypoglycemic episodes may occur among individuals with T1DM and ED, mainly among those participating in binge eating and self-induced vomiting. Deliberately inducing hypoglycemia to justify eating sweets and high carbohydrate meals were described in individuals with T1DM. Eighteen percent of a cohort of males and females with T1DM, aged 10-22 years, reported intentional overdosing of insulin[47]. The most common reason (49%) stated was the desire for uncontrolled binge eating, followed by self-destructive behavior in stressful situations.
Other characteristics and signs of ED
Other characteristic and clinical signs of ED are: frequently missed medical appointments, refusal to be weighed, preoccupation with appearance, a tendency to vegetarianism, and the calculating of caloric values and weighing of foods. Apparently, due to the availability of insulin omission as a weight loss strategy, females with diabetes are less likely to use other unhealthy weight control behaviors like vomiting, laxatives or diuretics, and skipping meals or fasting, compared to their peers without diabetes[51].
DIAGNOSIS
Several questionnaires were developed specifically for adolescents with T1DM. The revised Diabetes Eating Problem Survey (DEPS-R) is a 16-item diabetes-specific self-report measure of disordered eating that was designed specifically to identify patients with T1DM who are at risk for early intervention for disordered eating behaviors[7]. The mSCOFF is a simple 5 item screening tool, with demonstrated reliability and validity, which can easily be used during a follow-up visit[52]. Using data mining methods, a clinical prediction model that provides a decision support system for the detection of intentional insulin omission for weight loss in adolescent females with T1DM was developed[48]. The model is based on identifying a pattern of HbA1c levels indicative of intentional insulin omission. After a period of apparently stable HbA1c levels, the onset of insulin omission is characterized by both high HbA1c levels and wide fluctuations between clinical visits. A typical example of the course of HbA1c levels in a patient who developed ED is depicted in Figure 2.
Figure 2.
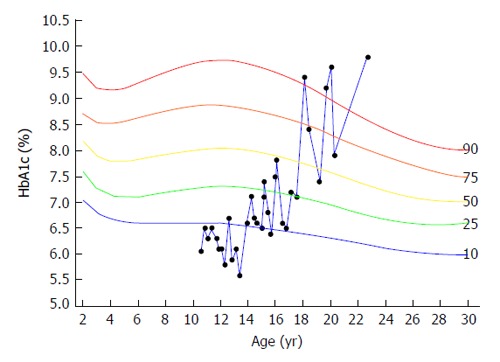
Typical example of the course of HbA1c levels in a patient, with intentional insulin omission for weight loss, plotted on the HbA1c percentile reference curves. HbA1c levels were around the 10th percentile from age 10 to 14 years. From age 18 the pattern changed and was characterized by sharp increases to levels above the 90th percentile, sharp decreases and multiple measurements above the 90th percentile.
MORBIDITY AND MORTALITY
ED result in poor metabolic control and cause short and long-term complications.
Short term complications
Insulin omission is associated with recurrent events of DKA, and disturbed eating behavior is associated with recurrent episodes of severe hypoglycemia[53]. Data from the diabetes patienten verlaufsdokumentation study, including 52215 T1DM males and females aged 8 to 30 years, revealed significantly higher rates of severe hypoglycemic episodes in adolescents with AN, BN or EDNOS, than with no ED: 12.1, 18.0, 12.9 and 5.7, respectively[54]. Moreover, rates of hypoglycemia with coma, and DKA with hospitalization were higher, and the duration of hospital stay longer, among those with eating disorders[54].
Long term complications
Long-term complications have been shown to be markedly increased among individuals with both T1DM and ED[54]. Data from the DPV study revealed that both hypertension and dyslipidemia were more common in persons with T1DM and either EDNOS or BN than in those with T1DM without ED[54].
Of T1DM patients who developed serious microvascular complications, 21% had a probable clinical ED, 47% had a history of DEB, and 48% had a history of insulin omission. The development of two or more serious complications was associated with the presence of a probable clinical eating disorder[55].
Different ED are associated with varying prevalence rates of morbidities. For example, data from the DPV study revealed a 2.5-fold higher risk for retinopathy in T1DM patients with BN than in those without ED. Patients with EDNOS also had a higher risk but without statistical significance, whereas those with AN had no increased risk for retinopathy[54].
The duration of ED may also affect diabetes complications. The duration of severe insulin omission was the factor most closely associated with the development of retinopathy and nephropathy in T1DM females[56].
As seen in Table 2, higher rates of retinopathy[46] and nephropathy[57] were documented in young TIDM patients with ED than in those without ED.
Table 2.
Prevalence rates of retinopathy and nephropathy among type 1 diabetes mellitus patients with eating disorders
| Complication | Average T1DM duration (yr) | T1DM patients with severe ED (%) | T1DM patients with moderate ED (%) | T1DM patients without ED (%) |
| Retinopathy[46] | 11 ± 4 | 86 | 42 | 24 |
| Nephropathy[46] | 11 ± 4 | 43 | 20 | 18 |
| Nephropathy[57] | 28 | 25 | 10 | |
| Foot problems[57] | 28 | 25 | 12 |
ED: Eating disorders; T1DM: Type 1 diabetes mellitus.
Mortality
Several studies documented increased relative risk of death among patients with ED. Mortality rates per 1000 person years were reported as 2.2 in girls with T1DM, 7.3 in girls with ED and 34.6 in girls with both T1DM and an ED[58]. During an 11-year period, self-reported insulin restriction at baseline increased the relative risk of death by 3.2 times in women, mean age 45 years, mean diabetes duration 28 years; those with ED were younger when they died (aged 44 vs 58 years, P < 0.01)[57]. In a 12-year follow-up of a cohort of 14 women with T1DM and ED (12 with AN), 5 died (36%)[59]. The median age of the cohort was 37 years (range 25-46), with a median duration of diabetes of 26 years (14-33). The age of death of the 5 patients was 30 years (25-42).
PREVENTION
Promoting the understanding and awareness among healthcare providers of factors involved in the development of ED in young vulnerable individuals with T1DM may help prevent these disorders[60]. These factors include the weight gain with the initiation of insulin treatment[11], the dietary restraint necessitated by diabetes management, and recurrent hypoglycemic episodes. Diabetes treatment should therefore afford flexibility where possible, and non-depriving approaches to eating[61]. As current clinical practice includes a focus on measuring body weight, health care professionals should be sensitive to preoccupation with body weight. Interventions aimed at increasing self-esteem and body acceptance, as well as family-based interventions aimed to improve family management of obesity and diabetes, may also help to diminish the risk of developing ED in individuals with diabetes[62].
Girls aged between 10 and 12 with a diagnosis of T1DM for at least 1 year, attended two group sessions, each of 4-h duration[63]. The content of the sessions targeted perfectionism, media literacy, self-esteem. The program was found to have impact on self-efficacy relating to diabetes management. As lower T1DM regimen adherence is associated with lower self-efficacy in adolescents, it was concluded that a brief interactive program had a favorable impact on protective factors for disordered eating.
Among college-age women at highest risk for an eating disorder an internet-based intervention, has successfully reduced weight/shape concerns and prevented eating disorders[64]. Specifically guided discussion group resulted in reduction in weight/shape concern. We did not find similar reports among adolescent girls with T1DM, however it is possible that online interventions may be successfully used to reduce eating disorder risk factors and preventing eating disorders in this group.
TREATMENT
Treatment of ED in patients with T1DM is challenging, especially since the clinical improvement achieved with insulin treatment is associated with weight gain. Compliance to the therapeutic regimen may therefore be poor.
Treatment should mirror the three circle model presented above, and address the factors predisposing to the development of ED (Figure 3).
Figure 3.
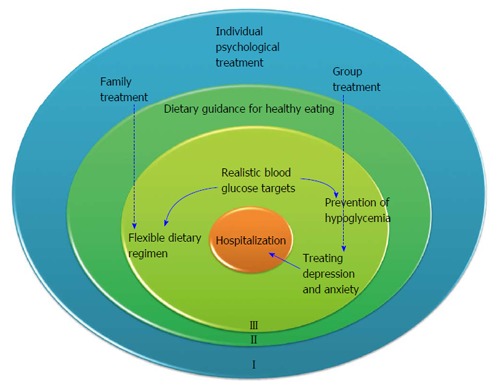
Three level models describing treatment modalities of eating disorders in adolescents with type 1 diabetes mellitus. The outer circle addresses treatment for premorbid variables. The middle circle delineates. The inner circle includes factors associated with the management of T1DM that predispose to eating disorders. T1DM: Type 1 diabetes mellitus.
Treating the first circle - premorbid variables
Increased body weight and a tendency to gain weight should be addressed by prudent dietary management: Since a tendency to gain weight was found to be associated with ED[22], particular attention should be given to the constellation of excess weight and preoccupation with food.
Low self-esteem, body dissatisfaction and personality variables should be addressed by individual psychological treatment or group therapy: The concurrence of eating behavior disorder and mood disorder in patients with T1DM supports the importance of treating depression as a means of treating ED. If depression and anxiety are suspected, a psychiatric consultation is needed. Psychiatrists have a distinct role in the diabetes care of children under their care, specifically in working through issues associated with the burden of the disease[65]. The impact of anti-depressant and anti-anxiety medications on recovery from eating disorders in adolescents with T1DM has not been studied systematically.
The effect of psychoeducation on adolescents with T1DM and ED is controversial. A psychoeducation approach with six weekly group sessions was associated with reductions in dieting, in body dissatisfaction and with preoccupation with thinness and eating; the reduction in disturbed eating behaviour was maintained for at least 6 mo[66]. In another study, a six week intervention was as effective as a wait list control group[67].
Group cognitive-behavioral therapy intervention has been shown to improve glycemic control, well-being and diabetes related stress among poorly controlled adult T1DM patients[68], and among patients with T1DM and depression[69]; however, it was not studied in patients with insulin omission.
As family dynamics may influence the development of ED[34], family education is an important component of the treatment. However, we could not find data on the impact of family intervention as a treatment modality for adolescents with T1DM and ED.
Treating the second circle - factors associated with disease onset
Current T1DM management involves carbohydrate counting, which may lead to excessive preoccupation with food and diets. The cycle of weight loss at disease onset, and subsequent weight gain with the initiation of insulin treatment, is a risk factor for the development of ED in susceptible patients[11]. Dietary treatment should focus on healthy choices and low calorie alternatives with a high satiety index.
Treating the third circle - factors associated with the chronic management of diabetes
Intensive insulin treatment, dietary restraint, and hypoglycemic episodes should be addressed by the diabetes management team: As good metabolic control is associated with weight gain, changes in target blood glucose levels should be gradual. Setting higher than standard target blood glucose ranges (preprandial 120 to 150 mg/dL and postprandial < 200 mg/dL) may yield more benefit in the long run. In contrast, achieving excellent control may result in marked weight gain. Moreover, since low glucose target levels are associated with an increased risk of recurrent hypoglycemic episodes, which may result in additional increased calorie intake, setting higher target levels may be a better initial objective. In girls who were skipping insulin dosing at almost every meal, starting with adequate insulin injection at a single meal is sometimes helpful as a first step. Caregivers should be aware that patients often switch from one disturbed behavior to another, ie from insulin omission to restrictive eating or binge eating; thus, a prescribed diet should be flexible.
Treatment with an insulin pump was postulated as a means of enabling better physiological insulin delivery to adolescents with T1DM than achieved with multiple daily injections. Patients with insulin pumps require less insulin and thus gain less weight. Furthermore, the occurrence of hypoglycemic episodes is decreased, and thus the need to consume extra calories. A recently published multicenter study demonstrated a decrease in disturbed eating behaviors, as assessed by the DEPS-R, in youth with T1DM, 6 mo after pump initiation[70]. Moreover, among adolescent girls with T1DM and ED, the mean HbA1c level was significantly lower in those who were treated by insulin pumps than in those who were treated with multiple daily injections (9.07% ± 1.33% vs 10.40% ± 2.01%; P = 0.04)[71].
Failure of outpatient treatment, the presence of a severe psychopathological state and poor glycemic control are key elements in the decision for hospitalization. Information is limited regarding the long-term outcomes of hospitalized adolescents with ED. Significantly lower levels of HbA1c and lower psychological test scores related to eating disorder psychopathology, depressiveness and anxiety were reported for 9 patients who were hospitalized, compared to 10 who were not[72]. At 3-year follow-up, 78% of the hospitalized patients in that study no longer fulfilled any criteria for clinical or subclinical ED.
Thus, treatment of ED in individuals with T1DM involves a complex interplay of psychosocial, dietary and medical aspects and requires a multidisciplinary team. It is important to realize that adolescents with T1DM and ED tend to have lower motivation to change their eating habits[73]. Furthermore, they are characterized by giving up easily when confronted with frustration and with low levels of accomplishment. These personality characteristics may explain the high dropout rate from therapy (79%) among patients with T1DM and ED[73].
CONCLUSION
Deliberate insulin omission as a weight loss strategy is recognized as either an inappropriate compensatory feature of bulimia nervosa or as a purging disorder, a component of OSFED. About one-third of individuals with T1DM intentionally omit insulin. Diagnosis of eating disorders in individuals with T1DM is difficult, since eating disorder behaviors are often well hidden. Weight loss that is related to deteriorated glycemic control and recurrent DKA should raise suspicion. Early diagnosis is essential, as the combination of eating disorders and diabetes is associated with increased morbidity and mortality.
ACKNOWLEDGMENTS
We thank Ms. Cindy Cohen for her editorial assistance.
Footnotes
P- Reviewer: Codoner-Franch P, Marchesini G S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Conflict-of-interest: None.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 28, 2014
First decision: November 27, 2014
Article in press: January 19, 2015
References
- 1.Treasure J, Claudino AM, Zucker N. Eating disorders. Lancet. 2010;375:583–593. doi: 10.1016/S0140-6736(09)61748-7. [DOI] [PubMed] [Google Scholar]
- 2.Zipfel S, Löwe B, Reas DL, Deter HC, Herzog W. Long-term prognosis in anorexia nervosa: lessons from a 21-year follow-up study. Lancet. 2000;355:721–722. doi: 10.1016/S0140-6736(99)05363-5. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 4.Wildes JE, Marcus MD. Incorporating dimensions into the classification of eating disorders: three models and their implications for research and clinical practice. Int J Eat Disord. 2013;46:396–403. doi: 10.1002/eat.22091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavender JM, Crosby RD, Wonderlich SA. Dimensions in the eating disorders: past, present, and future. Commentary on Wildes and Marcus: Incorporating dimensions into the classification of eating disorders. Int J Eat Disord. 2013;46:404–407. doi: 10.1002/eat.22121. [DOI] [PubMed] [Google Scholar]
- 6.Pike KM. Classification, culture, and complexity: a global look at the diagnosis of eating disorders: Commentary on Wildes and Marcus: Incorporating dimensions into the classification of eating disorders. Int J Eat Disord. 2013;46:408–411. doi: 10.1002/eat.22122. [DOI] [PubMed] [Google Scholar]
- 7.Markowitz JT, Butler DA, Volkening LK, Antisdel JE, Anderson BJ, Laffel LM. Brief screening tool for disordered eating in diabetes: internal consistency and external validity in a contemporary sample of pediatric patients with type 1 diabetes. Diabetes Care. 2010;33:495–500. doi: 10.2337/dc09-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young-Hyman DL, Davis CL. Disordered eating behavior in individuals with diabetes: importance of context, evaluation, and classification. Diabetes Care. 2010;33:683–689. doi: 10.2337/dc08-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannucci E, Rotella F, Ricca V, Moretti S, Placidi GF, Rotella CM. Eating disorders in patients with type 1 diabetes: a meta-analysis. J Endocrinol Invest. 2005;28:417–419. doi: 10.1007/BF03347221. [DOI] [PubMed] [Google Scholar]
- 10.Young V, Eiser C, Johnson B, Brierley S, Epton T, Elliott J, Heller S. Eating problems in adolescents with Type 1 diabetes: a systematic review with meta-analysis. Diabet Med. 2013;30:189–198. doi: 10.1111/j.1464-5491.2012.03771.x. [DOI] [PubMed] [Google Scholar]
- 11.Jones JM, Lawson ML, Daneman D, Olmsted MP, Rodin G. Eating disorders in adolescent females with and without type 1 diabetes: cross sectional study. BMJ. 2000;320:1563–1566. [PMC free article] [PubMed] [Google Scholar]
- 12.Colton P, Olmsted M, Daneman D, Rydall A, Rodin G. Disturbed eating behavior and eating disorders in preteen and early teenage girls with type 1 diabetes: a case-controlled study. Diabetes Care. 2004;27:1654–1659. doi: 10.2337/diacare.27.7.1654. [DOI] [PubMed] [Google Scholar]
- 13.Smith FM, Latchford GJ, Hall RM, Dickson RA. Do chronic medical conditions increase the risk of eating disorder? A cross-sectional investigation of eating pathology in adolescent females with scoliosis and diabetes. J Adolesc Health. 2008;42:58–63. doi: 10.1016/j.jadohealth.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Svensson M, Engström I, Aman J. Higher drive for thinness in adolescent males with insulin-dependent diabetes mellitus compared with healthy controls. Acta Paediatr. 2003;92:114–117. doi: 10.1111/j.1651-2227.2003.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 15.Engström I, Kroon M, Arvidsson CG, Segnestam K, Snellman K, Aman J. Eating disorders in adolescent girls with insulin-dependent diabetes mellitus: a population-based case-control study. Acta Paediatr. 1999;88:175–180. doi: 10.1080/08035259950170358. [DOI] [PubMed] [Google Scholar]
- 16.García-Reyna NI, Gussinyer S, Raich RM, Gussinyer M, Tomàs J, Carrascosa A. [Eating disorders in young adolescents with type 1 diabetes] Med Clin (Barc) 2004;122:690–692. doi: 10.1016/s0025-7753(04)74357-2. [DOI] [PubMed] [Google Scholar]
- 17.Pinar R. Disordered eating behaviors among Turkish adolescents with and without Type 1 diabetes. J Pediatr Nurs. 2005;20:383–388. doi: 10.1016/j.pedn.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Alice Hsu YY, Chen BH, Huang MC, Lin SJ, Lin MF. Disturbed eating behaviors in Taiwanese adolescents with type 1 diabetes mellitus: a comparative study. Pediatr Diabetes. 2009;10:74–81. doi: 10.1111/j.1399-5448.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- 19.Wisting L, Frøisland DH, Skrivarhaug T, Dahl-Jørgensen K, Rø O. Disturbed eating behavior and omission of insulin in adolescents receiving intensified insulin treatment: a nationwide population-based study. Diabetes Care. 2013;36:3382–3387. doi: 10.2337/dc13-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz JT, Lowe MR, Volkening LK, Laffel LM. Self-reported history of overweight and its relationship to disordered eating in adolescent girls with Type 1 diabetes. Diabet Med. 2009;26:1165–1171. doi: 10.1111/j.1464-5491.2009.02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domargård A, Särnblad S, Kroon M, Karlsson I, Skeppner G, Aman J. Increased prevalence of overweight in adolescent girls with type 1 diabetes mellitus. Acta Paediatr. 1999;88:1223–1228. doi: 10.1080/080352599750030329. [DOI] [PubMed] [Google Scholar]
- 22.Hyrich KL, Watson KD, Lunt M, Symmons DP. Changes in disease characteristics and response rates among patients in the United Kingdom starting anti-tumour necrosis factor therapy for rheumatoid arthritis between 2001 and 2008. Rheumatology (Oxford) 2011;50:117–123. doi: 10.1093/rheumatology/keq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tse J, Nansel TR, Haynie DL, Mehta SN, Laffel LM. Disordered eating behaviors are associated with poorer diet quality in adolescents with type 1 diabetes. J Acad Nutr Diet. 2012;112:1810–1814. doi: 10.1016/j.jand.2012.06.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumark-Sztainer D, Patterson J, Mellin A, Ackard DM, Utter J, Story M, Sockalosky J. Weight control practices and disordered eating behaviors among adolescent females and males with type 1 diabetes: associations with sociodemographics, weight concerns, familial factors, and metabolic outcomes. Diabetes Care. 2002;25:1289–1296. doi: 10.2337/diacare.25.8.1289. [DOI] [PubMed] [Google Scholar]
- 25.Colton PA, Olmsted MP, Daneman D, Rydall AC, Rodin GM. Natural history and predictors of disturbed eating behaviour in girls with Type 1 diabetes. Diabet Med. 2007;24:424–429. doi: 10.1111/j.1464-5491.2007.02099.x. [DOI] [PubMed] [Google Scholar]
- 26.Olmsted MP, Colton PA, Daneman D, Rydall AC, Rodin GM. Prediction of the onset of disturbed eating behavior in adolescent girls with type 1 diabetes. Diabetes Care. 2008;31:1978–1982. doi: 10.2337/dc08-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock-BarZiv SM, Davis C. Personality factors and disordered eating in young women with type 1 diabetes mellitus. Psychosomatics. 2005;46:11–18. doi: 10.1176/appi.psy.46.1.11. [DOI] [PubMed] [Google Scholar]
- 28.Grylli V, Wagner G, Berger G, Sinnreich U, Schober E, Karwautz A. Characteristics of self-regulation in adolescent girls with type 1 diabetes with and without eating disorders: a cross-sectional study. Psychol Psychother. 2010;83:289–301. doi: 10.1348/147608309X481180. [DOI] [PubMed] [Google Scholar]
- 29.Grylli V, Wagner G, Hafferl-Gattermayer A, Schober E, Karwautz A. Disturbed eating attitudes, coping styles, and subjective quality of life in adolescents with Type 1 diabetes. J Psychosom Res. 2005;59:65–72. doi: 10.1016/j.jpsychores.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Powers MA, Richter S, Ackard D, Gerken S, Meier M, Criego A. Characteristics of persons with an eating disorder and type 1 diabetes and psychological comparisons with persons with an eating disorder and no diabetes. Int J Eat Disord. 2012;45:252–256. doi: 10.1002/eat.20928. [DOI] [PubMed] [Google Scholar]
- 31.Maharaj S, Rodin G, Connolly J, Olmsted M, Daneman D. Eating problems and the observed quality of mother-daughter interactions among girls with type 1 diabetes. J Consult Clin Psychol. 2001;69:950–958. [PubMed] [Google Scholar]
- 32.Maharaj SI, Rodin GM, Olmsted MP, Connolly JA, Daneman D. Eating disturbances in girls with diabetes: the contribution of adolescent self-concept, maternal weight and shape concerns and mother-daughter relationships. Psychol Med. 2003;33:525–539. doi: 10.1017/s0033291702007213. [DOI] [PubMed] [Google Scholar]
- 33.Maharaj SI, Rodin GM, Olmsted MP, Daneman D. Eating disturbances, diabetes and the family: an empirical study. J Psychosom Res. 1998;44:479–490. doi: 10.1016/s0022-3999(97)00273-0. [DOI] [PubMed] [Google Scholar]
- 34.Mellin AE, Neumark-Sztainer D, Patterson J, Sockalosky J. Unhealthy weight management behavior among adolescent girls with type 1 diabetes mellitus: the role of familial eating patterns and weight-related concerns. J Adolesc Health. 2004;35:278–289. doi: 10.1016/j.jadohealth.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Takii M, Uchigata Y, Kishimoto J, Morita C, Hata T, Nozaki T, Kawai K, Iwamoto Y, Sudo N, Kubo C. The relationship between the age of onset of type 1 diabetes and the subsequent development of a severe eating disorder by female patients. Pediatr Diabetes. 2011;12:396–401. doi: 10.1111/j.1399-5448.2010.00708.x. [DOI] [PubMed] [Google Scholar]
- 36.Baechle C, Castillo K, Straßburger K, Stahl-Pehe A, Meissner T, Holl RW, Giani G, Rosenbauer J. Is disordered eating behavior more prevalent in adolescents with early-onset type 1 diabetes than in their representative peers? Int J Eat Disord. 2014;47:342–352. doi: 10.1002/eat.22238. [DOI] [PubMed] [Google Scholar]
- 37.McErlane F, Foster HE, Davies R, Lunt M, Watson KD, Symmons DP, Hyrich KL. Biologic treatment response among adults with juvenile idiopathic arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2013;52:1905–1913. doi: 10.1093/rheumatology/ket248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goebel-Fabbri AE, Fikkan J, Connell A, Vangsness L, Anderson BJ. Identification and treatment of eating disorders in women with type 1 diabetes mellitus. Treat Endocrinol. 2002;1:155–162. doi: 10.2165/00024677-200201030-00003. [DOI] [PubMed] [Google Scholar]
- 39.Goebel-Fabbri AE. Disturbed eating behaviors and eating disorders in type 1 diabetes: clinical significance and treatment recommendations. Curr Diab Rep. 2009;9:133–139. doi: 10.1007/s11892-009-0023-8. [DOI] [PubMed] [Google Scholar]
- 40.Thompson CJ, Cummings JF, Chalmers J, Gould C, Newton RW. How have patients reacted to the implications of the DCCT? Diabetes Care. 1996;19:876–879. doi: 10.2337/diacare.19.8.876. [DOI] [PubMed] [Google Scholar]
- 41.Rodin GM, Daneman D. Eating disorders and IDDM. A problematic association. Diabetes Care. 1992;15:1402–1412. doi: 10.2337/diacare.15.10.1402. [DOI] [PubMed] [Google Scholar]
- 42.Verrotti A, Catino M, De Luca FA, Morgese G, Chiarelli F. Eating disorders in adolescents with type 1 diabetes mellitus. Acta Diabetol. 1999;36:21–25. doi: 10.1007/s005920050140. [DOI] [PubMed] [Google Scholar]
- 43.Dantzer C, Swendsen J, Maurice-Tison S, Salamon R. Anxiety and depression in juvenile diabetes: a critical review. Clin Psychol Rev. 2003;23:787–800. doi: 10.1016/s0272-7358(03)00069-2. [DOI] [PubMed] [Google Scholar]
- 44.Colton PA, Olmsted MP, Daneman D, Rodin GM. Depression, disturbed eating behavior, and metabolic control in teenage girls with type 1 diabetes. Pediatr Diabetes. 2013;14:372–376. doi: 10.1111/pedi.12016. [DOI] [PubMed] [Google Scholar]
- 45.Bernstein CM, Stockwell MS, Gallagher MP, Rosenthal SL, Soren K. Mental health issues in adolescents and young adults with type 1 diabetes: prevalence and impact on glycemic control. Clin Pediatr (Phila) 2013;52:10–15. doi: 10.1177/0009922812459950. [DOI] [PubMed] [Google Scholar]
- 46.Rydall AC, Rodin GM, Olmsted MP, Devenyi RG, Daneman D. Disordered eating behavior and microvascular complications in young women with insulin-dependent diabetes mellitus. N Engl J Med. 1997;336:1849–1854. doi: 10.1056/NEJM199706263362601. [DOI] [PubMed] [Google Scholar]
- 47.Schober E, Wagner G, Berger G, Gerber D, Mengl M, Sonnenstatter S, Barrientos I, Rami B, Karwautz A, Fritsch M. Prevalence of intentional under- and overdosing of insulin in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011;12:627–631. doi: 10.1111/j.1399-5448.2011.00759.x. [DOI] [PubMed] [Google Scholar]
- 48.Pinhas-Hamiel O, Hamiel U, Greenfield Y, Boyko V, Graph-Barel C, Rachmiel M, Lerner-Geva L, Reichman B. Detecting intentional insulin omission for weight loss in girls with type 1 diabetes mellitus. Int J Eat Disord. 2013;46:819–825. doi: 10.1002/eat.22138. [DOI] [PubMed] [Google Scholar]
- 49.Golden MP, Herrold AJ, Orr DP. An approach to prevention of recurrent diabetic ketoacidosis in the pediatric population. J Pediatr. 1985;107:195–200. doi: 10.1016/s0022-3476(85)80124-4. [DOI] [PubMed] [Google Scholar]
- 50.Goebel-Fabbri AE. Diabetes and eating disorders. J Diabetes Sci Technol. 2008;2:530–532. doi: 10.1177/193229680800200326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ackard DM, Vik N, Neumark-Sztainer D, Schmitz KH, Hannan P, Jacobs DR. Disordered eating and body dissatisfaction in adolescents with type 1 diabetes and a population-based comparison sample: comparative prevalence and clinical implications. Pediatr Diabetes. 2008;9:312–319. doi: 10.1111/j.1399-5448.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 52.Zuijdwijk CS, Pardy SA, Dowden JJ, Dominic AM, Bridger T, Newhook LA. The mSCOFF for screening disordered eating in pediatric type 1 diabetes. Diabetes Care. 2014;37:e26–e27. doi: 10.2337/dc13-1637. [DOI] [PubMed] [Google Scholar]
- 53.Weinert LS, Scheffel RS, Severo MD, Cioffi AP, Teló GH, Boschi A, Schaan BD. Precipitating factors of diabetic ketoacidosis at a public hospital in a middle-income country. Diabetes Res Clin Pract. 2012;96:29–34. doi: 10.1016/j.diabres.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Scheuing N, Bartus B, Berger G, Haberland H, Icks A, Knauth B, Nellen-Hellmuth N, Rosenbauer J, Teufel M, Holl RW. Clinical characteristics and outcome of 467 patients with a clinically recognized eating disorder identified among 52,215 patients with type 1 diabetes: a multicenter german/austrian study. Diabetes Care. 2014;37:1581–1589. doi: 10.2337/dc13-2156. [DOI] [PubMed] [Google Scholar]
- 55.McErlane F, Beresford MW, Baildam EM, Thomson W, Hyrich KL. Recent developments in disease activity indices and outcome measures for juvenile idiopathic arthritis. Rheumatology (Oxford) 2013;52:1941–1951. doi: 10.1093/rheumatology/ket150. [DOI] [PubMed] [Google Scholar]
- 56.Takii M, Uchigata Y, Tokunaga S, Amemiya N, Kinukawa N, Nozaki T, Iwamoto Y, Kubo C. The duration of severe insulin omission is the factor most closely associated with the microvascular complications of Type 1 diabetic females with clinical eating disorders. Int J Eat Disord. 2008;41:259–264. doi: 10.1002/eat.20498. [DOI] [PubMed] [Google Scholar]
- 57.Goebel-Fabbri AE, Fikkan J, Franko DL, Pearson K, Anderson BJ, Weinger K. Insulin restriction and associated morbidity and mortality in women with type 1 diabetes. Diabetes Care. 2008;31:415–419. doi: 10.2337/dc07-2026. [DOI] [PubMed] [Google Scholar]
- 58.Nielsen S, Emborg C, Mølbak AG. Mortality in concurrent type 1 diabetes and anorexia nervosa. Diabetes Care. 2002;25:309–312. doi: 10.2337/diacare.25.2.309. [DOI] [PubMed] [Google Scholar]
- 59.Walker JD, Young RJ, Little J, Steel JM. Mortality in concurrent type 1 diabetes and anorexia nervosa. Diabetes Care. 2002;25:1664–1665. doi: 10.2337/diacare.25.9.1664-a. [DOI] [PubMed] [Google Scholar]
- 60.Daneman D, Olmsted M, Rydall A, Maharaj S, Rodin G. Eating disorders in young women with type 1 diabetes. Prevalence, problems and prevention. Horm Res. 1998;50 Suppl 1:79–86. doi: 10.1159/000053110. [DOI] [PubMed] [Google Scholar]
- 61.Rodin G, Olmsted MP, Rydall AC, Maharaj SI, Colton PA, Jones JM, Biancucci LA, Daneman D. Eating disorders in young women with type 1 diabetes mellitus. J Psychosom Res. 2002;53:943–949. doi: 10.1016/s0022-3999(02)00305-7. [DOI] [PubMed] [Google Scholar]
- 62.Nansel TR, Anderson BJ, Laffel LM, Simons-Morton BG, Weissberg-Benchell J, Wysocki T, Iannotti RJ, Holmbeck GN, Hood KK, Lochrie AS. A multisite trial of a clinic-integrated intervention for promoting family management of pediatric type 1 diabetes: feasibility and design. Pediatr Diabetes. 2009;10:105–115. doi: 10.1111/j.1399-5448.2008.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilksch SM, Starkey K, Gannoni A, Kelly T, Wade TD. Interactive programme to enhance protective factors for eating disorders in girls with type 1 diabetes. Early Interv Psychiatry. 2013;7:315–321. doi: 10.1111/eip.12012. [DOI] [PubMed] [Google Scholar]
- 64.Kass AE, Trockel M, Safer DL, Sinton MM, Cunning D, Rizk MT, Genkin BH, Weisman HL, Bailey JO, Jacobi C, et al. Internet-based preventive intervention for reducing eating disorder risk: A randomized controlled trial comparing guided with unguided self-help. Behav Res Ther. 2014;63C:90–98. doi: 10.1016/j.brat.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dahan A, McAfee SG. A proposed role for the psychiatrist in the treatment of adolescents with type I diabetes. Psychiatr Q. 2009;80:75–85. doi: 10.1007/s11126-009-9099-1. [DOI] [PubMed] [Google Scholar]
- 66.Olmsted MP, Daneman D, Rydall AC, Lawson ML, Rodin G. The effects of psychoeducation on disturbed eating attitudes and behavior in young women with type 1 diabetes mellitus. Int J Eat Disord. 2002;32:230–239. doi: 10.1002/eat.10068. [DOI] [PubMed] [Google Scholar]
- 67.Alloway SC, Toth EL, McCargar LJ. Effectiveness of a group psychoeducation program for the treatment of subclinical disordered eating in women with type 1 diabetes. Can J Diet Pract Res. 2001;62:188–192. [PubMed] [Google Scholar]
- 68.Amsberg S, Anderbro T, Wredling R, Lisspers J, Lins PE, Adamson U, Johansson UB. A cognitive behavior therapy-based intervention among poorly controlled adult type 1 diabetes patients--a randomized controlled trial. Patient Educ Couns. 2009;77:72–80. doi: 10.1016/j.pec.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 69.Markowitz SM, Carper MM, Gonzalez JS, Delahanty LM, Safren SA. Cognitive-behavioral therapy for the treatment of depression and adherence in patients with type 1 diabetes: pilot data and feasibility. Prim Care Companion CNS Disord. 2012;14:pii: PCC.11m01220. doi: 10.4088/PCC.11m01220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Battaglia MR, Alemzadeh R, Katte H, Hall PL, Perlmuter LC. Brief report: disordered eating and psychosocial factors in adolescent females with type 1 diabetes mellitus. J Pediatr Psychol. 2006;31:552–556. doi: 10.1093/jpepsy/jsj047. [DOI] [PubMed] [Google Scholar]
- 71.Pinhas-Hamiel O, Graph-Barel C, Boyko V, Tzadok M, Lerner-Geva L, Reichman B. Long-term insulin pump treatment in girls with type 1 diabetes and eating disorders--is it feasible? Diabetes Technol Ther. 2010;12:873–878. doi: 10.1089/dia.2010.0049. [DOI] [PubMed] [Google Scholar]
- 72.Takii M, Uchigata Y, Komaki G, Nozaki T, Kawai H, Iwamoto Y, Kubo C. An integrated inpatient therapy for type 1 diabetic females with bulimia nervosa: a 3-year follow-up study. J Psychosom Res. 2003;55:349–356. doi: 10.1016/s0022-3999(02)00629-3. [DOI] [PubMed] [Google Scholar]
- 73.Custal N, Arcelus J, Agüera Z, Bove FI, Wales J, Granero R, Jiménez-Murcia S, Sánchez I, Riesco N, Alonso P, et al. Treatment outcome of patients with comorbid type 1 diabetes and eating disorders. BMC Psychiatry. 2014;14:140. doi: 10.1186/1471-244X-14-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stancin T, Link DL, Reuter JM. Binge eating and purging in young women with IDDM. Diabetes Care. 1989;12:601–603. doi: 10.2337/diacare.12.9.601. [DOI] [PubMed] [Google Scholar]
- 75.Philippi ST, Cardoso MG, Koritar P, Alvarenga M. Risk behaviors for eating disorder in adolescents and adults with type 1 diabetes. Rev Bras Psiquiatr. 2013;35:150–156. doi: 10.1590/1516-4446-2012-0780. [DOI] [PubMed] [Google Scholar]


