Summary
Cancer immunotherapies under development have generally focused on either stimulating T-cell immunity or driving antibody-directed effector functions of the innate immune system such as antibody-dependent cell-mediated cytotoxicity (ADCC). We find that a combination of an anti-tumor antigen antibody and an untargeted IL-2 fusion protein with delayed systemic clearance induces significant tumor control in aggressive isogenic tumor models via a concerted innate and adaptive response involving neutrophils, NK cells, macrophages, and CD8+ T-cells. This combination therapy induces an intratumoral “cytokine storm” and extensive lymphocyte infiltration. Adoptive transfer of anti-tumor T-cells together with this combination therapy leads to robust cures of established tumors and establishment of immunological memory.
Introduction
The compelling promise of immunotherapies is to counter heterogeneously mutating tumors with the adaptive immune response, and in particular, the benefits of combining multiple therapies are particularly appealing (van Elsas et al., 1999; Overwijk, 2005; Stagg et al., 2007). One of the earliest such combinations tested was the cytokine IL-2 together with monoclonal antibodies against tumor antigens.
Antibodies such as trastuzumab, rituximab, and cetuximab have achieved tremendous clinical successes (Weiner et al., 2009) and their capability to enlist innate effector functions is a critical component of their therapeutic efficacy (Ferris et al., 2010). In mechanistic studies in xenograft mouse models, innate effector cells expressing activating FcγR, particularly NK cells, were shown to be required for therapeutic efficacy of monoclonal antibodies (Clynes et al., 2000; Sliwkowski et al., 1999) and lymphoma patients expressing higher-affinity alleles of FcγRIII responded better to rituximab therapy (Weng and Levy, 2003), consistent with a major contribution of ADCC to antibody therapy. Encouragingly, cell culture bioassay studies demonstrated that IL-2 enhanced NK cell activity against antibody-coated tumor cells (Carson et al., 2001; Eisenbeis et al., 2004). Unfortunately, these results did not translate clinically as such combinations consistently failed to provide significant clinical benefit over antibody alone (Khan et al., 2006; Mani et al., 2009; Poiré et al., 2010).
T-cells play an unexpectedly critical role in anti-tumor antigen antibody therapy, although their importance is often not observed due to studies being performed in immunodeficient mice. In studies of antibody therapy in immunocompetent mice with isogenic tumors, therapeutic effects vanish when CD8+ T-cells are depleted (Abès et al., 2010; Dyall et al., 1999; Park et al., 2010; Stagg et al., 2011; Vasovíc et al., 1997; Wang et al., 2012). We imagined that IL-2 treatment might be exploited to amplify monoclonal antibody therapy not simply via the previously assumed NK-mediated ADCC, but also by boosting the CD8+ T-cell adaptive response, since IL-2 exerts significant pleiotropic effects on regulatory, helper, and cytolytic memory T-cells (Liao et al., 2013).
However, given the poor clinical results of combining IL-2 with monoclonal antibodies, we hypothesized that the signaling resulting from parenteral IL-2 administration may be temporally limited because IL-2 is rapidly cleared when intravenously administered in bolus doses (Konrad et al., 2009), leading to highly oscillatory cytokine exposure. The cellular response to such IL-2 spikes can be dramatically different than the response to more stable concentration trajectories (Rao et al., 2005). Both the duration and strength of IL-2 signaling determines the balance between effector and memory cytolytic T-cell development (Feau et al., 2011; Kalia et al., 2010; Pipkin et al., 2010), a balance critical to the success of immunotherapies such as adoptive cell therapy (June, 2007). It is noteworthy that in previous clinical trials combining IL-2 and antibodies, IL-2 was administered as a subcutaneous low-dose pulse either once per day (Mani et al., 2009; Poiré et al., 2010) or three times per week (Khan et al., 2006). Consequently, these patients’ T-cells were exposed to short bursts of IL-2 signaling. Therefore, we sought to develop a means by which sufficiently sustained IL-2 signaling could be provided, such that simultaneous dosing with an antitumor antigen monoclonal antibody might provide the synergistic therapeutic effect that has thus far remained elusive.
Results
Extending IL-2 Serum Exposure via Multiple Injections
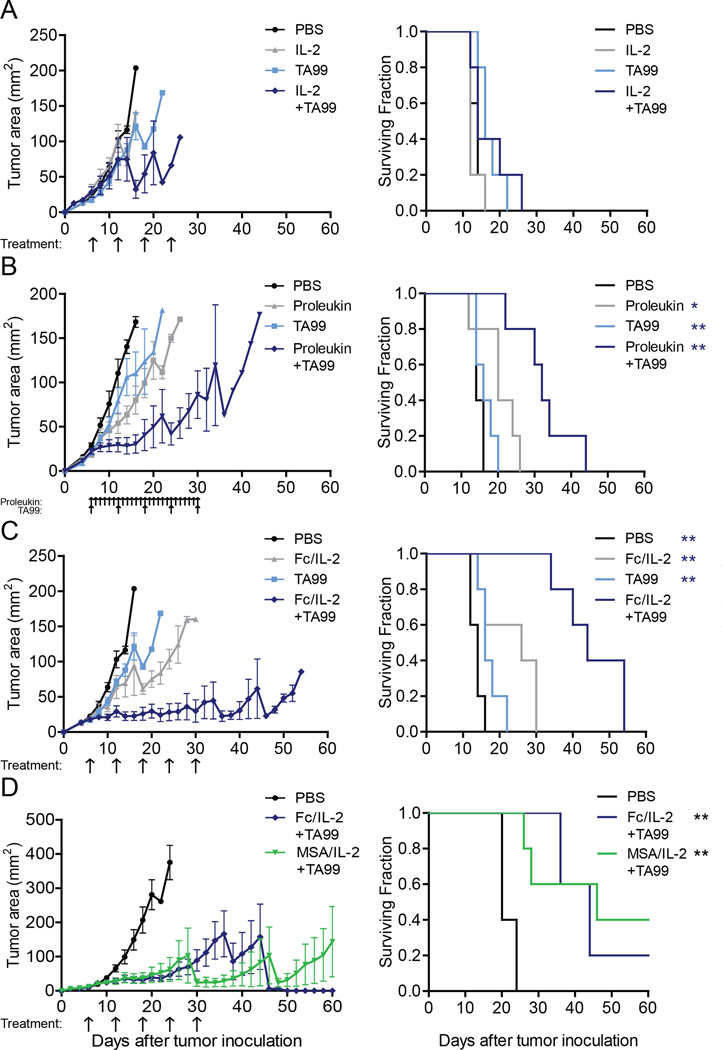
To explore the effects of differing IL-2 exposure combined with a monoclonal antibody targeting a tumor antigen, we first treated established B16F10 melanoma with IL-2 or the anti-TYRP-1 antibody, TA99. TYRP-1 is a melanocyte marker but becomes surface expressed on B16F10. With infrequent IL-2 exposure, neither agents dosed separately nor together provided survival benefit (Figure 1A). However, when extended daily dosing was performed, a notable synergistic effect was observed when TA99 was added, significantly extending survival (Figure 1B). Despite this promising response, daily IL-2 dosing resulted in poor body condition for many of the mice treated (Data not shown) and such a regimen could be arduous and expensive for clinical translation. Therefore, we sought to achieve similar synergistic effects through a form of IL-2 that provides similar long-term IL-2 exposure but requiring fewer and smaller doses.
Figure 1. Extended serum half-life IL-2 combined with a tumor-specific antibody controls tumor growth.
(A–D) Tumor growth and survival curves of C57BL/6 mice bearing subcutaneous B16F10 tumors treated with (A) PBS, murine IL-2 and/or TA99; (B) PBS, Proleukin and/or TA99; (C) PBS, Fc/IL-2 and/or TA99; (D) PBS, Fc/IL-2+TA99, or MSA/IL-2+TA99. Arrows indicate points of treatment. n = 5 per group. *p < 0.05, **p < 0.01 v. corresponding color group in legend. Data are mean ± SEM. See also Figure S1 and Table S1.
Extending IL-2 Circulation Lifetime via Fc Fusion
To increase the persistence of IL-2 exposure in vivo, we fused wild-type murine IL-2 to the Fc fragment of murine IgG2a, termed Fc/IL-2 (Figure S1A). We introduced the D265A mutation to the Fc—previously shown to abrogate FcγR binding and complement activation (Baudino et al., 2008)—to eliminate cytotoxic effects on the IL-2R+ cells we wished to stimulate. We chose a monovalent heterodimeric form of the fusion protein in order to avoid avidity effects from two IL-2 molecules on the same protein. Fc/IL-2 possessed similar specific bioactivity to murine IL-2 produced either from E. coli or the clinical drug Proleukin (Figure S1B). Fc/IL-2 demonstrated an increase in serum duration, with a beta half-life nearly three times that of wild type murine IL-2 (Figure S1C and Table S1). Fc/IL-2 exhibited significant degradation after in vitro incubation in serum at 37 °C for 48 hours (Figure S1D).
Fc/IL-2 and TA99 Synergize to Control B16F10 Growth
We proceeded to evaluate the efficacy of Fc/IL-2 against established B16F10 tumors and its potential synergism with TA99. Cohorts were administered equimolar Fc/IL-2 to that of the IL-2 given in the infrequent exposure case with identical dosing schedules. Impressively, administration of Fc/IL-2 with TA99 (Fc/IL-2+TA99) resulted in a strong synergy between the two agents that fully controlled B16F10 growth during treatment, extending survival further than either single agent (Figure 1C). The dosing of Fc/IL-2+TA99 appeared well-tolerated by the mice which continued to gain weight during treatment (Figure S1E) and showed no changes in pulmonary wet weight (Figure S1F), although splenomegaly (Figure S1G) and an acute increase in the serum level of liver enzymes (Figure S1H) and various inflammatory cytokines (Figure S1I) was observed.
To confirm that the synergistic efficacy was solely due to the serum half-life extension of Fc/IL-2 as opposed to immunological effects conferred by the Fc region, we fused IL-2 to mouse serum albumin (MSA/IL-2) to generate an alternative form of IL-2 with extended serum half-life (Figure S1C; Table S1) and bioactivity (Figure S1B), as albumin is similarly recycled by FcRn (Anderson et al., 2006). MSA/IL-2+TA99 and Fc/IL-2+TA99 were essentially identical in efficacy (Figure 1D), suggesting that any form of IL-2 with increased serum half-life, or perhaps a continuous infusion of IL-2, will exhibit this synergistic therapeutic effect.
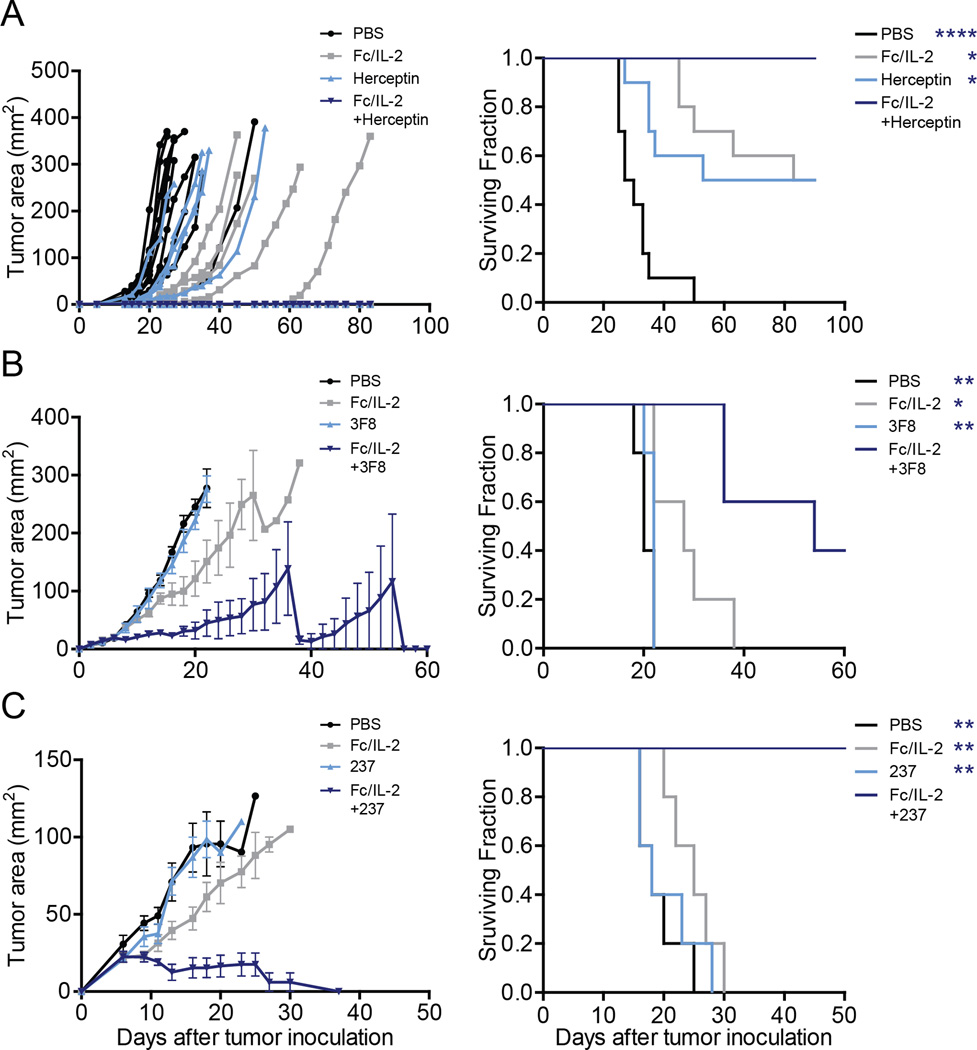
We further tested three additional tumor models with three different monoclonal antibodies to demonstrate the generalizability of this strategy:
D5-HER2 melanoma in hmHER2 transgenic mice (Wang et al., 2012) using Fc/IL-2 and trastuzumab (Herceptin) (Figure 2A);
RM9 prostate cancer (Baley et al., 1995) in C57BL/6 mice using Fc/IL-2 and 3F8 (Zhang et al., 1998), a murine IgG3 antibody targeting the GD2 antigen expressed on RM9 (Figure 2B);
Ag104 fibrosarcoma in C3H/HeN mice using Fc/IL-2 and 237 mAb, a murine IgG2a targeting the OTS8 antigen found on Ag104A (Ward et al., 1989) (Figure 2C).
Figure 2. Extended serum half-life IL-2 combined with a tumor-specific antibody is generalizable.
Tumor growth and survival curves of
(A) hmHER2 Tg mice bearing subcutaneous D5-HER2 tumors treated with PBS, Fc/IL-2 and/or Herceptin. n = 10 per group.
(B) C57BL/6 mice bearing subcutaneous RM9 tumors treated with PBS, Fc/IL-2 and/or 3F8 mAb. n = 5 per group.
(C) C3H mice bearing subcutaneous Ag104A tumors treated with PBS, Fc/IL-2 and/or 237 mAb. n = 5 per group.
*p < 0.05, **p < 0.01, ****p < 0.0001 v. corresponding color group in legend. Data are mean ± SEM.
In every case, the combination of Fc/IL-2 with an anti-tumor IgG showed superior efficacy to either agent alone, even where the antibody itself provided no benefit (Figure 2). Thus, the strong tumor-suppressive effect is generalized to four different tumor models with a unique anti-tumor antigen and antibody pairing plus a serum-persistent form of IL-2.
Fc/IL-2+TA99 Promotes Effector Cell Infiltration
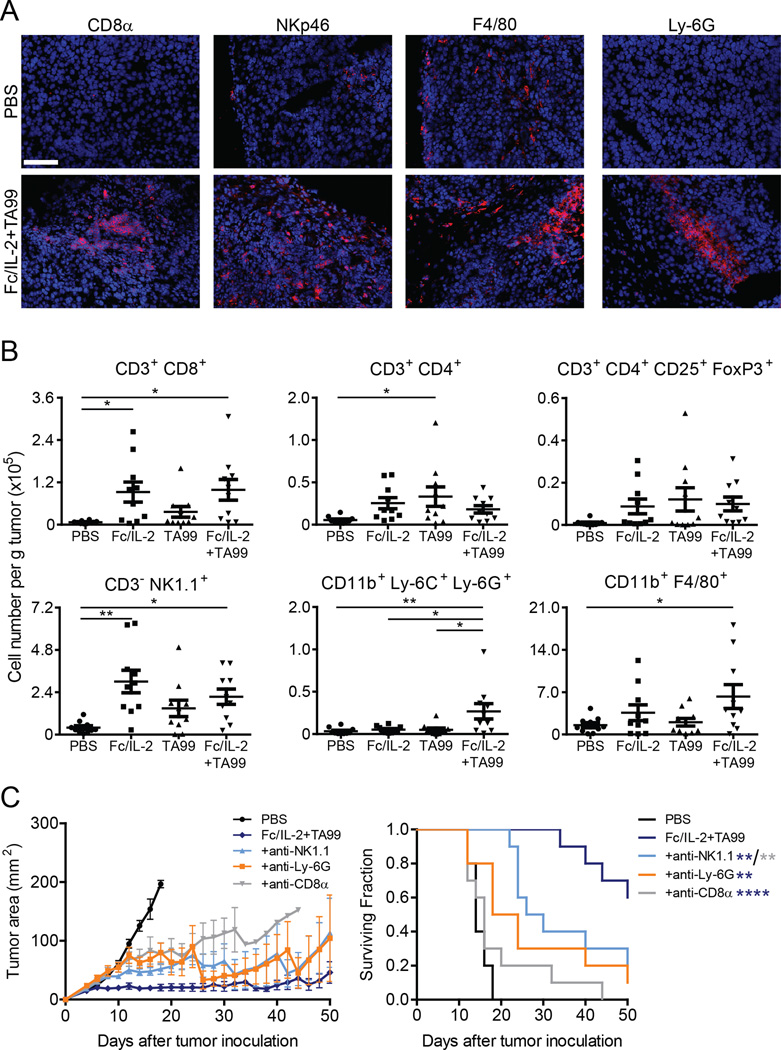
We hypothesized that the strong control of tumor growth observed was due to a combination of adaptive and innate immune activation localized to the tumor microenvironment. H&E staining of tumor sections from mice treated with either Fc/IL-2 or TA99 alone showed some lymphocyte infiltration restricted to the edges (Figure S2A–b and c); however, Fc/IL-2+TA99 treatment resulted brisk lymphocyte infiltration both at the periphery (Figure S2A–d) and within the tumor mass (Figure S2B), as well as widespread necrosis (Figure S2C). Additionally, we observed instances of direct lymphocyte engagement with a tumor cell (Figure S2B). Immunofluorescence of tumor sections similarly showed robust infiltration of these immune cells (Figure 3A).
Figure 3. Fc/IL-2+TA99 induces immune infiltrates within B16F10 tumors.
(A) Representative immunofluorescence of immune cell infiltrates into B16F10 treated with PBS or Fc/IL-2+TA99. Hoescht 33258 (Blue), indicated lineage marker (Red). Magnification 20×. Scale bar 100 microns.
(B) Analysis of immune cell infiltrates into treated B16F10 tumors, normalized to total tumor mass, with lineage markers as indicated. n = 10 per group. *p < 0.05, **p < 0.01 between indicated groups.
(C) Tumor growth and survival curves of C57BL/6 mice bearing subcutaneous B16F10 tumors treated with PBS, Fc/IL-2+TA99, and with depletions as indicated. n = 10 per group. *p < 0.05, **p < 0.01 v. corresponding color group in legend.
Data are mean ± SEM. See also Figure S2.
It has been previously reported that the presence of brisk infiltrating lymphocytes is a favorable prognostic indicator in melanoma (Clemente et al., 1996). This apt observation prompted us to further identify and quantify infiltrating effector cells using flow cytometry. The CD3+CD8+ T-cell population was significantly increased by over 10fold with Fc/IL-2 or Fc/IL-2+TA99 (Figure 3B). The CD3+CD4+ T-cell and CD3+CD4+CD25+FoxP3+ Treg population were elevated with all three treatment groups (Figure 3B). As expected of an IL-2 based therapy, the increase in Treg cells was proportional to that of CD8+ T-cells, as the ratio of these two cells was unaffected (Figure S2D). The CD3−NK1.1+ NK cell population was also increased significantly by over 5-fold with Fc/IL-2 or Fc/IL-2+TA99 (Figure 3B). Interestingly, an increase in the CD11b+Ly-6C+Ly-6G+ neutrophil population was unique to the combination of Fc/IL-2+TA99 (Figure 3B), and only Fc/IL-2+TA99 showed a statistically significant difference in the CD11b+F4/80+ macrophage population relative to PBS-treated control (Figure 3B).
We next sought to determine the contribution of distinct effectors to therapeutic efficacy through antibody-mediated depletions, where confirmation of systemic depletion was obtained using samples from the spleen, blood, or peritoneal cavity (Figure S2E). CD8+ T-cell depletion, neutrophil depletion, or NK cell all attenuated therapeutic efficacy, suggesting an important role for each of these cell types in controlling tumor growth (Figure 3C). Depletion of the tumor-resident CD11b+F4/80+ macrophage population by an anti-CSF-1R antibody had no apparent effect on the efficacy of the treatment (Figure S2F). Nevertheless, this population might simultaneously contribute to and hinder the therapeutic effect via a mix of “M1 antitumor” or “M2 pro-tumor” macrophages, respectively. To briefly investigate this, we looked at the macrophage polarization through the ratio of CD206 and MHC-II expression (Gabrilovich et al., 2012), indicative of macrophages tending towards M2 or M1 polarization, respectively. This ratio was slightly lower in Fc/IL-2+TA99 treated tumors but not statistically significant (Figure S2G).
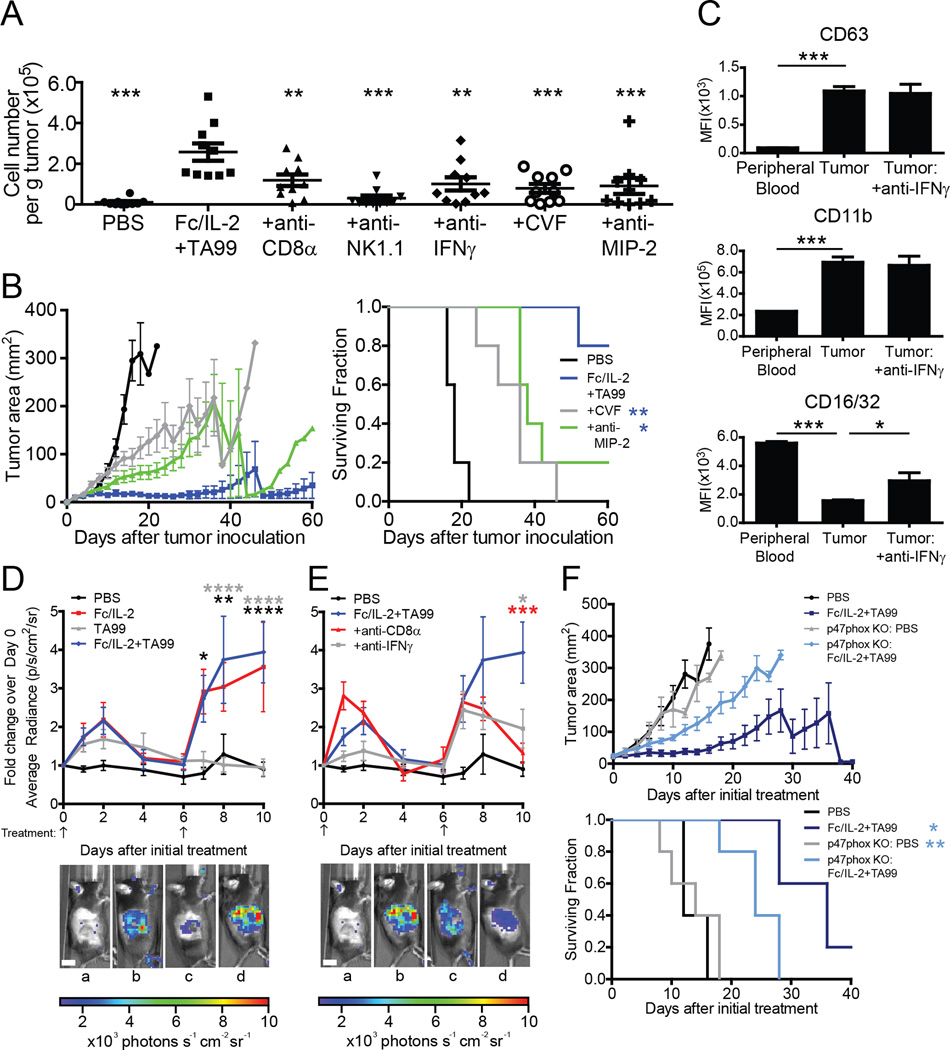
Fc/IL-2+TA99 Promotes Intratumoral Cytokine Storm with NK Cells Inducing Macrophage-Derived MIP-2
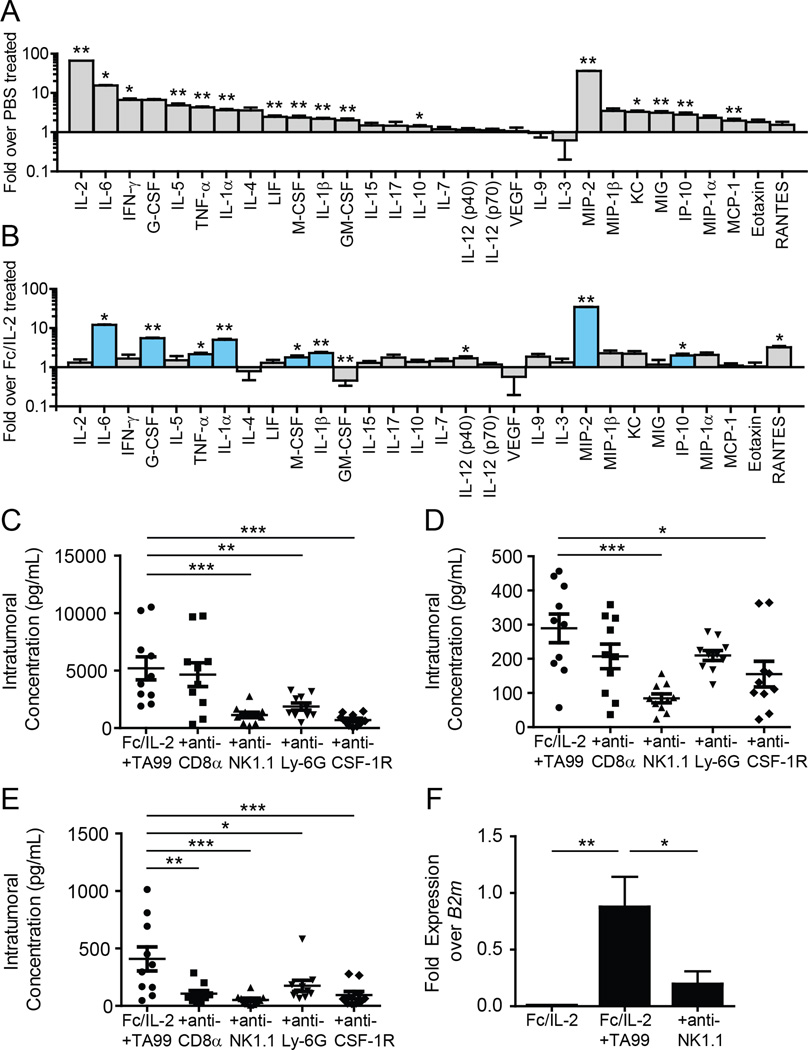
Given the extensive intratumoral immune cell infiltration with Fc/IL-2+TA99, we explored changes in intratumoral cytokine and chemokine levels induced by these infiltrating effector cells through multiplex Luminex assays. Nearly all cytokines and chemokines measured exhibited significant increases in intratumoral concentration when tumor-bearing mice were treated with Fc/IL-2+TA99 relative to PBS (Figure 4A). The set of elevated cytokines observed in our system broadly overlaps with those seen in clinical presentation of “cytokine storm” (Suntharalingam et al., 2006; Winkler et al., 1999), indicative of a broad and strong immune activation within the tumor. The CXCR2 ligand MIP-2 (CXCL2) showed a striking increase of nearly 40-fold (Figure 4A), an observation consistent with the observed increase in neutrophil infiltration (Figure 3B), as MIP-2 is a powerful neutrophil attractant (Wolpe et al., 1989).
Figure 4. Fc/IL-2+TA99 induces intratumoral cytokine storm and uniquely increases several inflammatory and neutrophil-related factors.
(A–B) Luminex analysis of intratumoral concentrations of a panel of cytokines and chemokines in tumors treated with (A) PBS or Fc/IL-2+TA99; (B) Fc/IL-2 or Fc/IL- 2+TA99. Blue bars indicate factors elevated only in Fc/IL-2+TA99 treated tumors. *p < 0.05, **p < 0.01 v. Fc/IL-2+TA99.
(C–E) Intratumoral concentrations of (C) MIP-2, (D) G-CSF, and (E) IL-6 in tumors treated with Fc/IL-2+TA99 and with depletions as indicated. n = 10 per group.
(F) Cxcl2 expression in CD11b+F4/80+ macrophages sorted from tumors treated with the indicated conditions, normalized to beta-2 microglobulin (B2m) expression. n = 5 per group.
*p < 0.05, **p < 0.01, ***p < 0.001 between indicated groups.
Data are mean ± SEM. See also Figure S3.
We next wished to understand how the antibody TA99 was able to drastically improve the mild efficacy of Fc/IL-2 alone. Fc/IL-2 or Fc/IL-2+TA99 induced nearly equivalent intratumoral levels of many different cytokines and chemokines, but there were 8 different cytokines and chemokines that appeared to be elevated in the tumor only with Fc/IL-2+TA99 treatment (Figure 4B; shown in blue). These included inflammatory cytokines such as IL-6, IL-1α, and IL-1β, but also neutrophil-related factors such as G-CSF and MIP-2 (Figure 4B). These results further suggest the importance of neutrophils in the therapeutic efficacy of Fc/IL-2+TA99, and highlight that only with the two agents together is this synergistic effect achieved.
To further understand which cells might be mediating these different cytokines, we monitored changes in intratumoral cytokine and chemokine levels when specific effector cells were depleted. CD8+ T-cells, NK cells, neutrophils, and macrophages all appeared to either directly or indirectly affect the levels of various factors (Figure S3A). Our data suggested that no single cell type was entirely responsible for the increase in any given cytokine or chemokine, but likely a complex interdependent network of cells was involved. We focused on the neutrophil-related factors MIP-2 and G-CSF, as well as the inflammatory factor IL-6, which was the most elevated inflammatory cytokine. In the case of MIP-2, depletion of NK cells, macrophages, or neutrophils led to a sharp decrease in the intratumoral concentration of this chemokine (Figure 4C), whereas G-CSF production seemed to be dependent on the presence of NK cells and macrophages (Figure 4D). Finally, IL-6 appeared to be dependent on the presence of all the interrogated effector cells to various degrees (Figure 4E).
While MIP-2 can be produced by macrophages (Wolpe et al., 1989), and neutrophils can also release MIP-2 (as well as IL-6) from pre-formed granules (Lacy and Stow, 2011), it was interesting to note that depletion of NK cells also reduced intratumoral MIP-2 levels (Figure 4C). NK cells are not known to generate MIP-2, and indeed, sorted intratumoral CD3−NK1.1+ NK cells showed little expression of Cxcl2 as measured by qPCR (Figure S3B). We hypothesized that NK cells may be modulating the production of MIP-2 by macrophages. When Cxcl2 expression in intratumoral CD11b+F4/80+ macrophages was quantified, we observed a significant upregulation of the gene when treating with Fc/IL-2+TA99 (Figure 4F). Furthermore, with NK cell depletion, we observed a significant downregulation of Cxcl2 expression in these macrophages (Figure 4F), suggesting that NK cells influence macrophage production of MIP-2, but solely upon Fc/IL-2+TA99 treatment.
Infiltration and Function of Neutrophils and Contribution of Respiratory Burst
A striking aspect of the synergy of Fc/IL-2+TA99 was the increase in the neutrophil population above all other treatment conditions (Figure 3B). We further explored the causes of this pronounced infiltration by depleting different cell types or neutralizing different factors that may be involved in boosting neutrophil infiltration or function. Both CD8+ T-cell and NK cell depletion resulted in a statistically significant drop of intratumoral neutrophils (Figure 5A). As NK cells modulated intratumoral MIP-2 levels via macrophages (Figure 4C and F), it follows that the absence of NK cells may impair neutrophil infiltration. Moreover, both CD8+ T-cells and NK cells contributed to intratumoral IFNγ (Figure S3A) which has been shown to play a role in neutrophil longevity (Pelletier et al., 2010). Indeed, intratumoral neutrophils also decreased when IFNγ was neutralized (Figure 5A).
Figure 5. Neutrophil infiltration and function modulated by a suite of immunological elements; oxidative burst contributes to therapeutic efficacy.
(A) Analysis of CD11b+Ly-6C+Ly-6G+ neutrophil infiltration into B16F10 tumors treated with PBS, Fc/IL-2+TA99, and with depletions/neutralizations as indicated, normalized to total tumor mass. Complement depleted by cobra venom factor (CVF). n = 10 per group. *p < 0.05, **p < 0.01, ***p < 0.001 v. Fc/IL-2+TA99.
(B) Tumor growth and survival curves of C57BL/6 mice bearing subcutaneous B16F10 tumors treated with PBS, Fc/IL-2+TA99, and with depletions/neutralizations as indicated. n = 5 per group. *p < 0.05, **p < 0.01 v. Fc/IL-2+TA99.
(C) Analysis of degranulation and activation markers of neutrophils in mice treated with Fc/IL-2+TA99. n = 5 per group. *p < 0.05, ***p < 0.001 between indicated groups.
(D–E) B16F10 tumors were treated with indicated treatments and depletions/neutralizations as applicable. On days of imaging, luminol was injected and average radiant luminescence was quantified. Representative images on day 8 are shown. In (D): a) PBS; b) Fc/IL-2; c) TA99; d) Fc/IL-2+TA99. In (E): a) PBS; b) Fc/IL-2+TA99; c) Fc/IL-2+TA99+anti-CD8α; d) Fc/IL-2+TA99+anti-IFNγ. Arrows indicate points of treatment. n = 5 per group. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 Fc/IL-2+TA99 v. corresponding color group in legend. Scale bar 1 cm.
(F) Tumor growth and survival curves of C57BL/6 mice or p47phox KO mice bearing subcutaneous B16F10 tumors treated with PBS or Fc/IL-2+TA99. n = 5 per group. *p < 0.05, **p < 0.01 v. p47phox KO: Fc/IL-2+TA99.
Data are mean ± SEM. See also Figure S4.
We proceeded to investigate other potential factors promoting neutrophil infiltration. C5a of the complement system is known to be a neutrophil chemoattractant (Guo and Ward, 2005) and depletion of complement via cobra venom factor (CVF) did decrease intratumoral neutrophils (Figure 5A). Antibody-mediated neutralization of MIP-2 also led to a decrease in intratumoral neutrophils (Figure 5A), suggesting that both of these neutrophil chemoattractants play a role in neutrophil infiltration into the tumor. Furthermore, blockade of either factor during Fc/IL-2+TA99 treatment resulted in a significant reduction in treatment efficacy (Figure 5B).
Functionally, we found that intratumoral neutrophils appear to be releasing cytosolic vesicles and granules, given the high surface expression of CD63 and CD11b (Kuijpers et al., 1991) compared to neutrophils in the peripheral blood (Figure 5C). Moreover, the activation state of the neutrophils was affected by IFNγ, as observed by surface expression of CD16. While neutrophil surface expression of CD32 remains unchanged under activating conditions, CD16 surface expression is drastically reduced (Huizinga et al., 1988; Kuijpers et al., 1991). This effect is observed with Fc/IL-2+TA99, as intratumoral neutrophils showed decreased surface CD16/32 compared to peripheral blood neutrophils, but neutralization of IFNγ partially reversed this trend (Figure 5C). Another effector function of neutrophils is the production of reactive nitrogen species via iNOS (Fang, 2004). We found that only Fc/IL-2+TA99 treatment led to an increase in iNOS, but those levels were reduced when either neutrophils were depleted or IFNγ was neutralized, suggesting that neutrophils may be a potential but not necessarily exclusive source of iNOS, and that IFNγ is important for the induction of iNOS (Figure S4A). Furthermore, iNOS transcription was nearly undetectable in neutrophils from peripheral blood, but was significantly increased in intratumoral neutrophils (Figure S4B). Overall, it would appear that Fc/IL-2+TA99 led to the infiltration and activation of neutrophils, releasing factors that contribute to inflammation and tumor killing.
Another well-known cytotoxic function of neutrophils is the generation of reactive species through respiratory burst (Fang, 2004) and we hypothesized that neutrophils might contribute to tumor killing via respiratory burst whose activity can be quantified from the in vivo injection of luminol (Gross et al., 2009). Surprisingly, both Fc/IL-2 and Fc/IL-2+TA99 treated mice exhibited increased luminescent signal over initial background measurement (Figure 5D). Although the respiratory burst activity appeared to also be a result of synergy, as depletion of CD8+ T-cells or neutralization of IFNγ resulted in a decrease in luminol-derived chemiluminescence (Figure 5E), it seemed unlikely that the source of respiratory burst was from neutrophils, since Fc/IL-2 alone did not result in increased neutrophil infiltration (Figure 3B). This was confirmed upon neutrophil depletion, as the chemiluminescence signal was essentially unchanged (Figure S4F). Instead, we identified eosinophils as a likely source of this respiratory burst given an increase in intratumoral IL-5 and CD11b+Siglec-F+ eosinophils with both Fc/IL-2 and Fc/IL-2+TA99 (Figure S4C–D). Moreover, depletion of eosinophils via anti-IL-5 antibody reduced luminol-derived chemiluminescence as well as dimished the therapeutic efficacy of Fc/IL-2+TA99 (Figure S4E–G). Given the apparent importance of respiratory burst, we proceeded to investigate its contribution to the efficacy of Fc/IL-2+TA99 regardless of the cellular source. We employed p47phox KO mice, which lack the p47phox subunit necessary for NADPH oxidase (Jackson et al., 1995), leading to a lack of luminol-derived chemiluminescence (Figure S4H). Indeed, the efficacy of the therapy was reduced in the treatment of these mice (Figure 5F), exhibiting the importance of respiratory burst in tumor control.
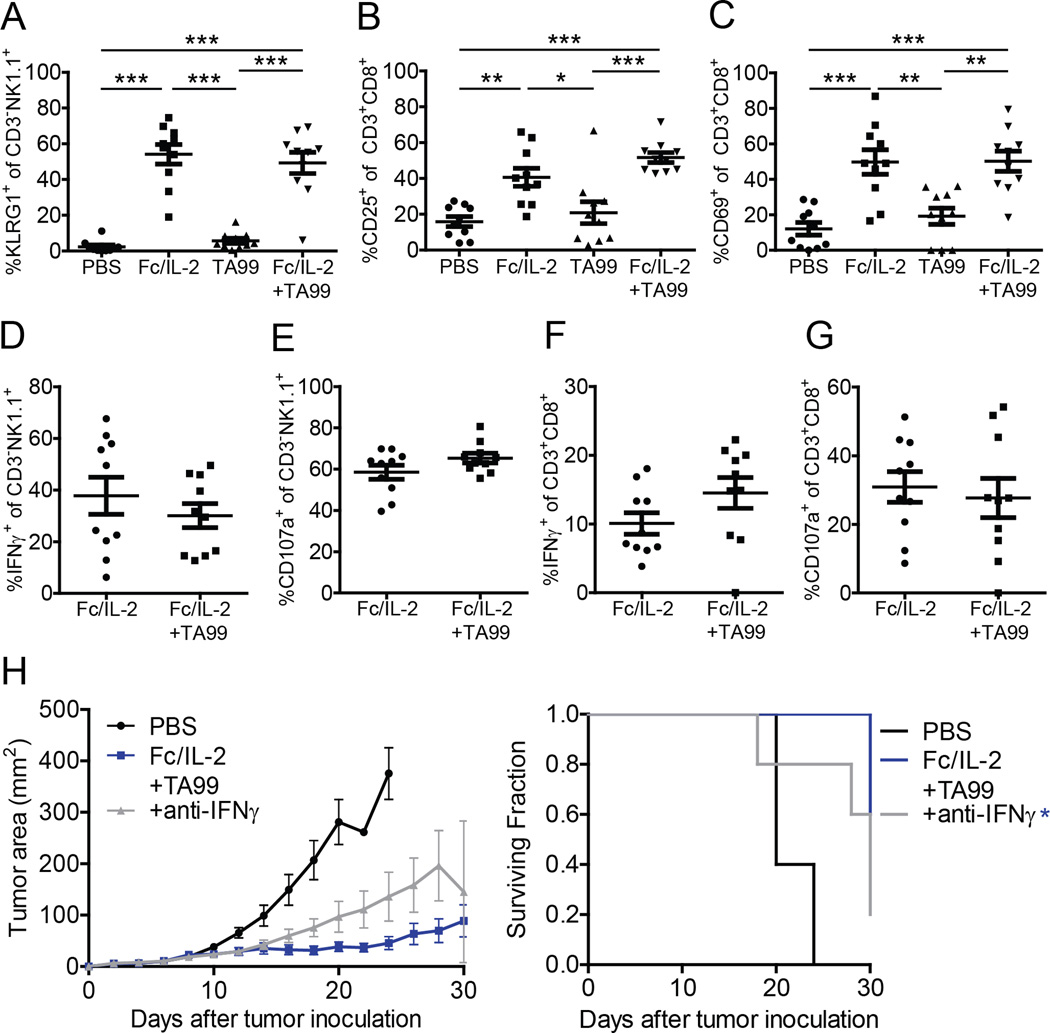
Fc/IL-2 Promotes NK Cell and CD8+ T-Cell Activation and Cytotoxic Function
While NK cells and CD8+ T-cells potentiated neutrophil infiltration and function, these cells themselves were likely contributing directly to therapeutic efficacy. As Fc/IL-2 or Fc/IL-2+TA99 significantly increased intratumoral NK cells and CD8+ T-cells, we proceeded to investigate how Fc/IL-2 in either case may affect their effector functions. Fc/IL-2 significantly activated intratumoral NK cells as measured through the activation marker KLRG-1 (Huntington et al., 2007) (Figure 6A). Fc/IL-2 also activated intratumoral CD8+ T-cells as measured through the activation markers CD25, CD69, and CD71 (Neckers and Cossman, 1983; Schwartz, 2003), with CD25 and CD69 being highly significant (Figure 6B–C) while CD71 showed a weaker trend (Figure S5A). The immune check-point receptors PD-1 and CTLA-4 are also markers for T-cell activation (Keir et al., 2008; Walunas et al., 1994), but no differences were identified 3 days after treatment (Figure S5B–C). Thus, although Fc/IL-2 alone led to significantly increased CD8+ T-cell infiltration and activation in the tumor, it was apparent that T-cells alone were insufficient to account for the strong tumor growth inhibition caused by Fc/IL-2+TA99 (Figure 1C).
Figure 6. Fc/IL-2 or Fc/IL-2+TA99 have comparable effect on NK cells and CD8+ T-cells.
(A–C) Analysis of NK cell or CD8+ T-cell activation markers, as a percentage of the intratumoral NK cell or CD8+ T-cell population, respectively. The following conditions were investigated (A) CD3NK1.1+KLRG1 + cells; (B) CD3+CD8+CD25+ cells; (C) CD3+CD8+CD69+ cells.
(D–G) Analysis of IFNγ-producing or degranulating NK cells or CD8+ T-cells, as a percentage of the intratumoral NK cell or CD8+ T-cell population, respectively. The following conditions were investigated (D) CD3-NK1.1+IFNγ+ cells; (E) CD3-NK1.1+CD107a+ cells; (F) CD3+CD8+IFNγ+ cells; (G) CD3+CD8+CD107a+ cells. n = 10 per group. *p < 0.05, **p < 0.01, ***p < 0.001 between indicated groups.
(H) Tumor growth and survival curves of C57BL/6 mice bearing subcutaneous B16F10 tumors treated with PBS, Fc/IL-2+TA99, or Fc/IL-2+TA99 with anti-IFNγ antibody. n = 5 per group. *p < 0.05 v. Fc/IL-2+TA99.
Data are mean ± SEM. See also Figure S5.
We next wanted to investigate the cytotoxic function of NK cells and CD8+ T-cells. With Fc/IL-2 or Fc/IL-2+TA99, NK cells appeared to secrete IFNγ and degranulate as measured through CD107a (Betts et al., 2003), although surprisingly both treatment conditions were comparable (Figure 6D–E), suggesting that the presence of an antitumor antibody in vivo does not directly affect these particular functions assayed 2 days after treatment. A similar observation was made for CD8+ T-cells (Figure 6F–G), although infiltrating CD8+ T-cells did exhibit reactivity against B16F10 as demonstrated by ELISPOT (Figure S5D).
Given that CD8+ T-cells produced IFNγ during anti-tumor responses, it was of interest to determine the contribution of IFNγ in controlling tumor growth. Administration of anti-IFNγ antibody demonstrated an intermediate reduction in therapeutic efficacy (Figure 6H). The previously observed increase in intratumoral TNFα (Figure 4A) could potentially be generated by infiltrating CD8+ T-cells as well, but neutralization of this cytokine did not appear to reduce the efficacy of the therapy (Figure S5E). It has also been shown that IFNγ upregulates MHC-I expression on B16F10 (Ju et al., 2005). Similarly, in our system, we confirmed via immunohistochemistry that an IFNv-dependent increase of MHC-I expression on B16F10 occurred when tumors were treated with Fc/IL-2+TA99 (Figure S5F).
Adoptive Cell Transfer of pmel-1 Combined with Fc/IL-2+TA99 Confers Robust Cures and Expansion of pmel-1 Population
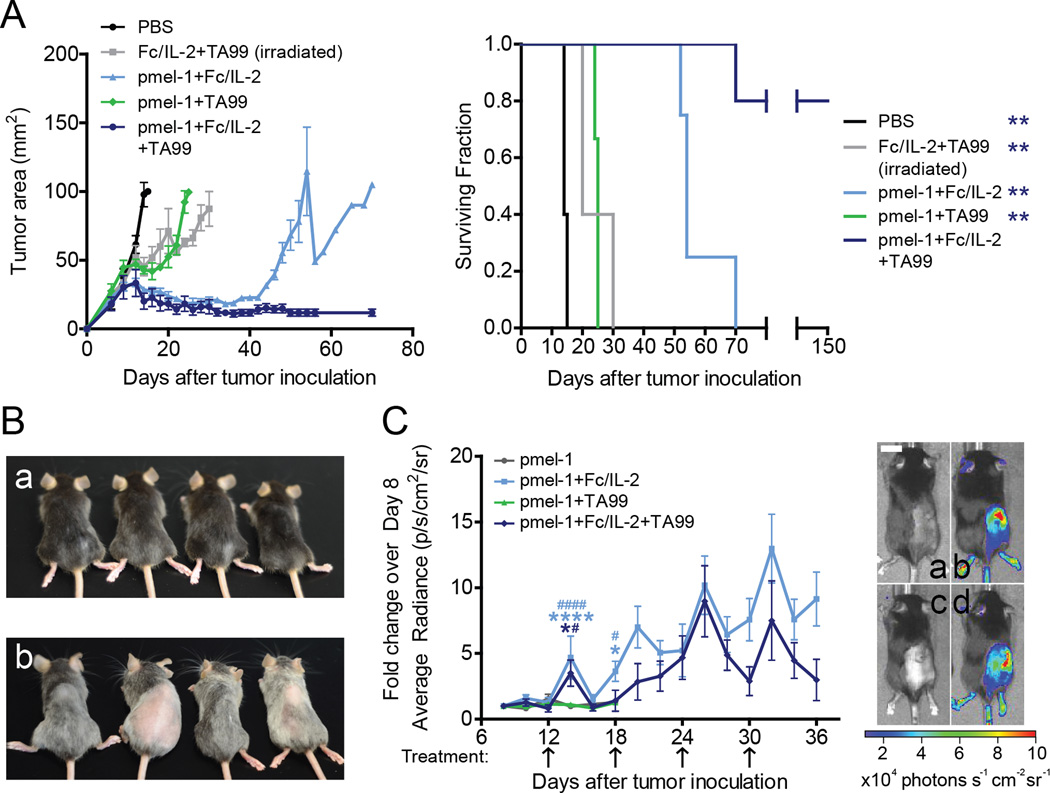
Given the importance of CD8+ T-cells in the Fc/IL-2+TA99 therapy, yet rareness of durable cures following cessation of treatment, we tested whether increasing the number of tumor specific CD8+ T-cells would be sufficient to generate complete cures. To accomplish this, mice received adoptive transfer of 107 pmel-1 CD8+ T-cells and were treated with Fc/IL-2 and/or TA99, as previously described. While irradiated mice treated with Fc/IL-2+TA99 or pmel-1+TA99 showed only a slight initial delay in tumor growth, mice treated with pmel-1+Fc/IL-2 showed significant survival advantage yet all tumors eventually grew out (Figure 7A). However, all 5 mice treated with the triple combination of pmel-1 +Fc/IL-2+TA99 experienced complete regression of their tumors with 4 out of 5 continuing to survive up to 150 days (Figure 7A). Moreover, these mice developed prominent vitiligo, suggesting that a strong melanocyte-specific T-cell response was generated (Figure 7B).
Figure 7. Pmel-1+Fc/IL-2+TA99 induces long-term control of B16F10 growth through activation and expansion of pmel-1 cells.
(A) Tumor growth and survival curves of C57BL/6 mice bearing subcutaneous B16F10 tumors were treated with indicated combinations of Fc/IL-2, TA99, and 107 CD8+ pmel-1 T-cells. All mice received total body irradiation 5 days after tumor inoculation. n = 3–5 per group. **p < 0.01 v. pmel-1+Fc/IL-2+TA99.
(B) Representative pictures 100 days after tumor inoculation showing vitiligo after treatment. a) Non-treated, non-tumor bearing and age-matched; b) pmel-1+Fc/IL-2+TA99 treated.
(C) Identical to (A), except CD8+ T-cells were derived from luciferase expressing pmel-1 mice. On days of imaging, D-luciferin was injected and average radiant luminescence was quantified. Representative pictures on day 14 are shown: a) pmel-1; b) pmel-1+Fc/IL-2; c) pmel-1+TA99; d) pmel-1+Fc/IL-2+TA99. Arrows indicate point of treatment. n = 5 per group. *p < 0.05, ****p < 0.0001 pmel-1 v. corresponding color group in legend; #p < 0.05, ####p < 0.0001 pmel-1+TA99 v. corresponding color group in legend. Scale bar 1 cm.
Data are mean ± SEM. See also Figure S6.
To further understand the activity of the pmel-1 CD8+ T-cells when combined with Fc/IL-2 and/or TA99, the experiment was repeated with pmel-1 CD8+ T-cells expressing luciferase, allowing us to monitor this population by bioluminescence. Without any Fc/IL-2, the signal was very weak, suggesting that the adoptively transferred T-cell population did not expand in vivo (Figure 7C). Conversely, any treatment with Fc/IL-2 resulted in cumulatively increasing luminescence (Figure 7C). In these cases, the pmel-1 CD8+ T-cells appeared to survive and expand within these mice, with luciferase signal observed up to 140 days after initial adoptive transfer (Figure S6A). To test the memory response of these mice, 105 B16F10 cells were injected subcutaneously into the opposite flank and no palpable tumors were formed for up to 30 days after rechallenge (Data not shown). The capability of Fc/IL-2 treatment to sustain survival of adoptively transferred T-cells was consistent with the increased infiltration and activation of endogenous T-cells by such treatments (Figure 3B, Figure 6B–C). Also analogous is the insufficiency of this T-cell effect to produce long-term efficacy in the absence of the TA99 antibody treatment. It is clear that even a huge population of melanocyte-specific CD8+ T-cells sustained by Fc/IL-2 alone was insufficient to induce durable cures without the necessary contribution of an anti-tumor antibody. In this case, however, neutrophils appeared to be dispensable when the third component of pmel-1 CD8+ T-cells was introduced (Figure S6B).
Discussion
There have been determined clinical attempts to combine anti-tumor antigen antibodies with pulsed IL-2 treatment in Phase I (Bajorin et al., 1990; Bleumera et al., 2006; Eisenbeis et al., 2004; Fleming et al., 2002; Repka et al., 2003) and Phase II (Khan et al., 2006; Mani et al., 2009; Poiré et al., 2010) clinical trials, all without evidence of efficacy. PEGylated IL-2 with increased serum half-life has also been tested clinically as monotherapy, and found not to be clinically superior (Yang et al., 1995). This suggests that a critical aspect missing may be more persistent IL-2 signaling, and in fact the clinical administration of anti-GD2 antibody, continuous infusion of IL-2, subcutaneous GM-CSF, and isotretinoin led to increased survival in children with high-risk neuroblastoma (Yu et al., 2010). Indeed, our own combination of an anti-tumor antibody and a fusion protein bestowing prolonged IL-2 signaling, despite its greater simplicity, demonstrated significant efficacy in various syngeneic murine tumor models.
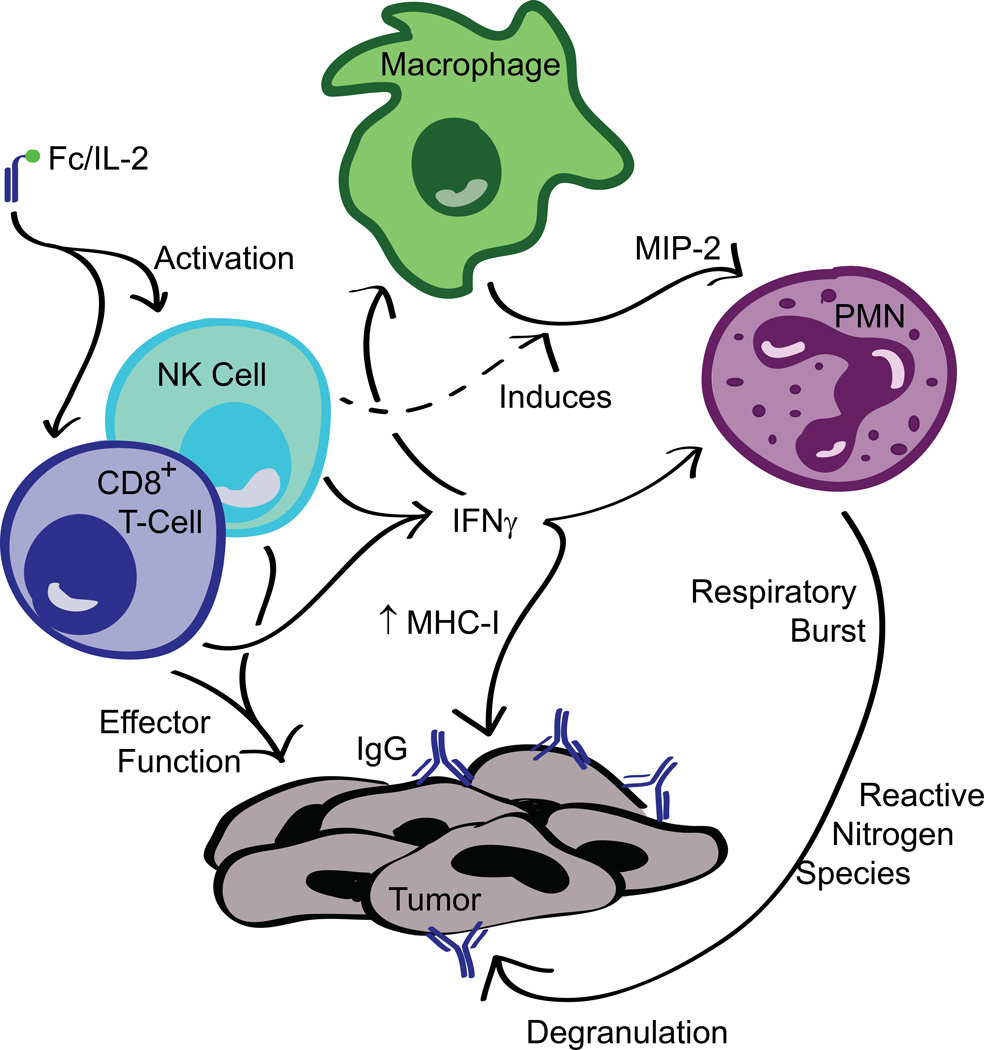
The demonstrated key components of the immune response induced by our combination immunotherapy are summarized in Figure 8. We find that our model reflects many observations of critical interactions between various effectors during administration of cancer immunotherapy reported in the literature. In syngeneic tumor models, the efficacy of antibody treatments has previously been shown to be dependent on and can synergize with CD8+ T-cell activity (Abès et al., 2010; Park et al., 2010; Stagg et al., 2011). Stimulation of CD8+ T-cells provides not only the benefit of direct tumor killing, but also the modulation of PMNs for ADCC, such as through the release of cytokines like IFNγ known to promote neutrophil activity (Pelletier et al., 2010). Although a concern with IL-2 administration could be the expansion of the intratumoral Treg population, we find that this does not dramatically affect the efficacy of our therapy. Indeed, despite the unchanged CD8/Treg ratio (Figure 2SD), the enhanced response by the innate immune system via the anti-tumor antibody may aid in overcoming immunosuppression by Tregs. However, increased Treg numbers may limit robust overall cures from this two-agent system.
Figure 8. Mechanistic model of the immune response induced by Fc/IL-2+TA99 against B16F10.
Combination of Fc/IL-2 and anti-tumor IgG administration induces a synergistic innate and adaptive immune response. Fc/IL-2 increases the sustained exposure of NK cells and CD8+ T-cells to IL-2 signaling, activating them. The presence of three factors— anti-tumor antibodies, Fc/IL-2, and NK cells—induces macrophages to release the neutrophil chemoattractant, MIP-2. This chemokine recruits neutrophils which, expressing FcγR, likely engage the tumor via the opsonizing antibodies and perform ADCC. These and other PMNs have their cytotoxic function bolstered by IFNγ released by the activated NK cells and CD8+ T-cells. Finally, NK cells and CD8+ T-cells perform direct effector function against the tumor cells as well. Overall, this complex network of immune responses results in controlled B16F10 growth during the course of treatment.
Significant interaction between neutrophils and other effector cells in our own system was evident, as infiltration and activation of neutrophils was dependent on IFNγ, and by association, the cells that produce it. The production of the neutrophil chemoattractant MIP-2 (Wolpe et al., 1989) by macrophages was also shown to be important for efficacy, despite reports that associate the human homologue IL-8 with increased cancer progression (Waugh and Wilson, 2008), potentially through the recruitment of N2 polarized neutrophils by Tregs or the tumors themselves (Fridlender and Albelda, 2012). However, in our system, it would appear that the combination of Fc/IL-2+TA99 induces MIP-2 release by macrophages for the purpose of recruiting neutrophils which were clearly anti-tumor in this context. In addition, many other cytokines in the intratumoral cytokine storm induced by Fc/IL-2+TA99 are known to enhance neutrophil activity, such as TNFα and GM-CSF (Pelletier et al., 2010), or IL-1β and G-CSF (Colotta et al., 1992).
Interestingly, IL-6 was highly elevated as well, where in many cases, tumor-derived IL-6 has been associated with increased disease progression (Allavena et al., 2000; Balkwill et al., 2005; Pollard, 2004). Nevertheless, IL-6 is a pleiotropic cytokine, critical for transitioning from the innate to the adaptive response as it promotes CD3+ T-cell trafficking and survival (Jones, 2005). In our immunotherapy, we observed IL-6 derivation from various effector cells including neutrophils, which would suggest that in addition to tumor killing, neutrophils may also be functioning to modulate the intratumoral T-cell response as well. Overall, there are many indications that through the administration of these two agents simultaneously, the tumor microenvironment may undergo a repolarization, such that with the presence of many inflammatory and pleiotropic cytokines and chemokines, the observed response elicited from the immune system becomes one that is anti-tumor.
To date, little focus has been placed on eliciting strong neutrophil responses against tumors. Tumor-associated neutrophils are often considered as pro-tumor “N2” polarized neutrophils (Fridlender and Albelda, 2012) and the immunosuppressive granulocytic-subtype of myeloid derived suppressor cells also possess a similar phenotype (Gabrilovich et al., 2012). However, there are examples where neutrophils are required for antibody therapeutic effects in xenograft tumor control studies (Eisenbeis et al., 2003; Schneider-Merck et al., 2010; Siders et al., 2010). In fact, it seems intuitive to utilize the underappreciated neutrophil to mount an anti-tumor response given that they are the most abundant of circulating leukocytes, and in other forms of immune challenge, neutrophils are the first responders, inducing massive cell-killing. Moreover, neutrophils have been shown to release chemotactic factors to recruit other effector cells (Lillard et al., 1999), produce cytokines which can modulate T-cells (Cassatella, 1995). While a robust T-cell response is ultimately required for tumor rejection and immunological memory, the innate arm of the immune system may provide necessary support to boost existing T-cell-focused immunotherapies. This was demonstrated when we combined the existing Fc/IL-2+TA99 with adoptive T-cell transfer.
We surmised and confirmed that creating an overwhelming melanocyte-specific CD8+ T-cell response via adoptive cell transfer provided the sustained effector function necessary for complete cures in combination with Fc/IL-2+TA99. Although Fc/IL-2 treatment alone maintained the survival of adoptively transferred pmel-1 cells, it is significant that in the absence of the antibody to bridge tumors to innate effectors, all pmel-1+Fc/IL-2 treated mice ultimately succumbed to the tumor. Thus, in this system, antibody-mediated effects remained critical for providing tumor killing that contributed to long-term T-cell-mediated efficacy.
In this study, we have uncovered a mechanism of tumor control induced by Fc/IL-2+TA99 that differs qualitatively from a predominant importance of NK cells, a view that had previously been held for combination treatments using anti-tumor antibodies and IL-2. In particular, the potential for strong synergistic interaction of T-cells and neutrophils has been noted in infectious and autoimmune disease (Müller et al., 2009), but has been largely unappreciated as a contributor to tumor immunotherapy (Buonocore et al., 2008). Thus, while work in the immunotherapy space has traditionally been focused on boosting only an anti-tumor T-cell or NK cell response, a more powerful approach may be to identify ways to conscript neutrophils and other innate effector cells to synergize with existing immunotherapies that stimulate T-cell activity.
Experimental Procedures
More detailed procedures can be found in the Supplemental Information.
Mice
C57BL/6NTac mice (Taconic), C3H/HeNTac mice (Taconic), and Pmel-1 (B6.Cg-Thy1a/Cy Tg(TcraTcrb8Rest/J) (The Jackson Laboratory) were aged between 6–10 weeks of age before tumor induction. All animal work was conducted under the approval of the MIT Division of Comparative Medicine, in accordance with federal, state, and local guidelines.
Tumor Inoculation and Treatment
For induction of B16F10 tumors, 106 cells in 100 µL of PBS were injected subcutaneously into the flanks of C57BL/6 mice. Retro-orbital injection of PBS, IL-2 (6 µg; PeproTech), Fc/IL-2 (25 µg), MSA/IL-2 (30 µg), and/or TA99 (100 µg) was done on day 6, 12, 18, 24, and 30 after tumor inoculation for a total of five treatments. Additional details, models, depletions, neutralizations, and adoptive cell transfer are described in Supplemental Information.
Flow Cytometry
Tumor inoculation and treatment were done as previously described. Single cell suspensions were prepared, stained, and analyzed as described in Supplemental Information.
Intratumoral Cytokine Analysis
Tumor inoculation and treatment were done as previously described. Tumors were homogenized, protein concentrations normalized, and evaluated in triplicate using the Mouse 32-Plex Cytokine/Chemokine Panel Luminex Assay as performed by Eve Technologies (Eve Technologies, Calgary, AB, Canada). More detailed information described in Supplemental Information.
Statistics
Statistical analysis was performed with GraphPad Prism 5 software (GraphPad). More detailed information described in Supplemental information.
Supplementary Material
Significance.
Weekly intravenous administration of two proteins—an anti-tumor antigen antibody and an IL-2 fusion protein with extended serum half-life—significantly increases tumor infiltration of neutrophils, NK cells, macrophages, and CD8+ T-cells, and controls growth of aggressive isogenic tumors. Our work supports a paradigm of stimulating concerted anti-tumor action from both adaptive and innate immunity in order to achieve durable tumor control.
Acknowledgements
We thank Dr. Bronson for pathological analysis and the staff of the Swanson Biotechnology Center at the Koch Institute for technical assistance. This study was supported by the U.S. National Cancer Institute (Grant No. NCI CA174795). E.F.Z. was supported by the NIH/NIGMS Biotechnology Training Program. S.A.G. and C.F.O were supported by a NSF Graduate Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
E.F.Z., S.A.G., and C.F.O. designed and performed experiments. B.H.K., R.S., M.J.K., K.D.M., A.A., and R.T.W. performed experiments. M.C.M. provided analysis of histology. M.T.S., J.S.K., M.B.Y., D.J.I., L.M.W., and G.D. provided critical reagents and advice. E.F.Z., S.A.G, C.F.O., and K.D.W. interpreted data and wrote the manuscript.
References
- Abès R, Gélizé E, Fridman WH, Teillaud J-L. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. 2010;116:926–934. doi: 10.1182/blood-2009-10-248609. [DOI] [PubMed] [Google Scholar]
- Allavena P, Sica A, Vecchi A, Locati M, Sozzani S, Mantovani A. The chemokine receptor switch paradigm and dendritic cell migration: its significance in tumor tissues. Immunol. Rev. 2000;177:141–149. doi: 10.1034/j.1600-065x.2000.17714.x. [DOI] [PubMed] [Google Scholar]
- Anderson CL, Chaudhury C, Kim J, Bronson CL, Wani MA, Mohanty S. Perspective-- FcRn transports albumin: relevance to immunology and medicine. Trends Immunol. 2006;27:343–348. doi: 10.1016/j.it.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Bajorin DF, Chapman PB, Wong G, Coit DG, Kunicka J, Dimaggio J, Cordon-cardo C, Urmacher C, Dantes L, Templeton MA, et al. Phase I evaluation of a combination of monoclonal antibody R24 and interleukin 2 in patients with metastatic melanoma. Cancer Res. 1990;50:7490–7495. [PubMed] [Google Scholar]
- Baley Pa, Yoshida K, Qian W, Sehgal I, Thompson TC. Progression to androgen insensitivity in a novel in vitro mouse model for prostate cancer. J. Steroid Biochem. Mol. Biol. 1995;52:403–413. doi: 10.1016/0960-0760(95)00001-g. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Baudino L, Shinohara Y, Nimmerjahn F, Furukawa J, Nakata M, Martínez-Soria E, Petry F, Ravetch JV, Nishimura S, Izui S. Crucial role of aspartic acid at position 265 in the CH2 domain for murine IgG2a and IgG2b Fc-associated effector functions. J. Immunol. 2008;181:6664–6669. doi: 10.4049/jimmunol.181.9.6664. [DOI] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price Da, De Rosa SC, Douek DC, Roederer M, Koup Ra. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Bleumera I, Oosterwijka E, Oosterwijk-Wakkaa JC, Völlera MCW, Melchiorb S, Warnaarc SO, Malac C, Beckb J, Mulders PFA. A clinical trial With chimeric monoclonal antibody WX-G250 and low dose interleukin-2 pulsing scheme for advanced renal cell carcinoma. J. Urol. 2006;175:57–62. doi: 10.1016/S0022-5347(05)00040-6. [DOI] [PubMed] [Google Scholar]
- Buonocore S, Haddou NO, Moore F, Florquin S, Paulart F, Heirman C, Thielemans K, Goldman M, Flamand V. Neutrophil-dependent tumor rejection and priming of tumoricidal CD8+ T cell response induced by dendritic cells overexpressing CD95L. J. Leukoc. Biol. 2008;84:713–720. doi: 10.1189/jlb.0108075. [DOI] [PubMed] [Google Scholar]
- Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, Baselga J, Caligiuri Ma. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur. J. \Immunology. 2001;31:3016–3025. doi: 10.1002/1521-4141(2001010)31:10<3016::aid-immu3016>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- Clemente C, Mihm MJ, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- Dyall R, Vasovic LV, Clynes RA, Nikolić-Zugić J. Cellular requirements for the monoclonal antibody-mediated eradication of an established solid tumor. Eur. J. Immunol. 1999;29:30–37. doi: 10.1002/(SICI)1521-4141(199901)29:01<30::AID-IMMU30>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Eisenbeis CF, Caligiuri MA, Byrd JC. Rituximab : converging mechanisms of action in non-hodgkin’s lymphoma? Clin. Cancer Res. 2003;9:5810–5812. [PubMed] [Google Scholar]
- Eisenbeis CF, Grainger A, Fischer B, Baiocchi RA, Carrodeguas L, Roychowdhury S, Chen L, Banks AL, Davis T, Young D, et al. Combination immunotherapy of B-Cell Non-Hodgkin’s Lymphoma with rituximab and interleukin-2 : a preclinical and phase I study. Clin. Cancer Res. 2004;10:6101–6110. doi: 10.1158/1078-0432.CCR-04-0525. [DOI] [PubMed] [Google Scholar]
- Van Elsas A, Hurwitz AA, Allison JP. Combination Immunotherapy of B16 Melanoma Using Anti-Cytotoxic T Lymphocyte-associated Antigen 4 (CTLA-4) and Granulocyte/Macrophate Colony-Stimulating Factor (GM-CSF)-producing Vaccines Induces Rejection of Subcutaneous and Metastatic Tumors Accompanied. J. Exp. Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells. Nat. Immunol. 2011;12:908–913. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J. Clin. Oncol. 2010;28:4390–4399. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming GF, Meropol NJ, Rosner GL, Hollis DR, Carson WE, Caligiuri M, Mortimer J, Tkaczuk K, Parihar R, Schilsky RL, et al. A phase I trial of escalating doses of trastuzumab combined with daily subcutaneous interleukin 2 : report of cancer and leukemia group B 9661. Clin. Cancer Res. 2002;8:3718–3727. [PubMed] [Google Scholar]
- Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S, Gammon ST, Moss BL, Rauch D, Harding J, Heinecke JW, Ratner L, Piwnica-Worms D. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 2009;15:455–461. doi: 10.1038/nm.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R-F, Ward Pa. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- Huizinga TWJ, van der Schoot CE, Jost C, Klaassen R, Kleijer M, von dem Borne AEGK, Roos D, Tetteroo PAT. The PI-linked receptor FcRIII is released on stimulation of neutrophils. Nature. 1988;333:667–669. doi: 10.1038/333667a0. [DOI] [PubMed] [Google Scholar]
- Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli-Esposti Ma, Smyth MJ, Tarlinton DM, Nutt SL. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J. Immunol. 2007;178:4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones Sa. Directing Transition from Innate to Acquired Immunity: Defining a Role for IL-6. J. Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- Ju S-A, Lee S-C, Kwon T-H, Heo S-K, Park S-M, Paek H-N, Suh J-H, Cho HR, Kwon B, Kwon BS, et al. Immunity to melanoma mediated by 4-1BB is associated with enhanced activity of tumour-infiltrating lymphocytes. Immunol. Cell Biol. 2005;83:344–351. doi: 10.1111/j.1440-1711.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- June CH. Principles of adoptive T cell cancer therapy. J. Clin. Invest. 2007;117:1204–1212. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan KD, Emmanouilides C, Benson DM, Hurst D, Garcia P, Michelson G, Milan S, Ferketich AK, Piro L, Leonard JP, et al. A phase 2 study of rituximab in combination with recombinant interleukin-2 for rituximab-refractory indolent non-Hodgkin’s lymphoma. Clin. Cancer Res. 2006;12:7046–7053. doi: 10.1158/1078-0432.CCR-06-1571. [DOI] [PubMed] [Google Scholar]
- Konrad MW, Hemstreet G, Hersh EM, Mansell PWA, Mertelsmann R, Kolitz JE, Bradley EC. Pharmacokinetics of recombinant interleukin 2 in humans. Cancer Res. 2009;50:2009–2017. [PubMed] [Google Scholar]
- Kuijpers TW, Tool aT, van der Schoot CE, Ginsel La, Onderwater JJ, Roos D, Verhoeven a J. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 1991;78:1105–1111. [PubMed] [Google Scholar]
- Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood. 2011;118:9–18. doi: 10.1182/blood-2010-08-265892. [DOI] [PubMed] [Google Scholar]
- Liao W, Lin J-X, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillard JW, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl. Acad. Sci. U. S. A. 1999;96:651–656. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani A, Roda J, Young D, Caligiuri Ma, Fleming GF, Kaufman P, Brufsky A, Ottman S, Carson WE, Shapiro CL. A phase II trial of trastuzumab in combination with low-dose interleukin-2 (IL-2) in patients (PTS) with metastatic breast cancer (MBC) who have previously failed trastuzumab. Breast Cancer Res. Treat. 2009;117:83–89. doi: 10.1007/s10549-008-0251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I, Munder M, Kropf P, Hänsch GM. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. 2009;30:522–530. doi: 10.1016/j.it.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Neckers LM, Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. PNAS. 1983;80:3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW. Breaking tolerance in cancer immunotherapy: time to ACT. Curr. Opin. Immunol. 2005;17:187–194. doi: 10.1016/j.coi.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier M, Micheletti A, Cassatella MA. Modulation of human neutrophil survival and antigen expression by activated CD4+ and CD8+ T cells. J. Leukoc. Biol. 2010;88:1163–1170. doi: 10.1189/jlb.0310172. [DOI] [PubMed] [Google Scholar]
- Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiré X, Kline J, Grinblatt D, Zimmerman T, Conner K, Muhs C, Gajewski T, Besien KVan, Smith SM. Phase II study of immunomodulation with granulocyte-macrophage colony-stimulating factor, interleukin-2, and rituximab following autologous stem cell transplant in patients with relapsed or refractory lymphomas. Leuk. Lymphoma. 2010;51:1241–1250. doi: 10.3109/10428194.2010.486876. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Tumor-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- Rao BM, Driver I, Lauffenburger DA, Wittrup KD. High-affinity CD25-binding IL-2 mutants potently stimulate persistent T cell growth. Biochemistry. 2005;44:10696–10701. doi: 10.1021/bi050436x. [DOI] [PubMed] [Google Scholar]
- Repka T, Chiorean EG, Gay J, Herwig KE, Kohl VK, Yee D, Miller JS. Trastuzumab and interleukin-2 in HER2-positive metastatic breast cancer : a pilot study. Clin. Cancer Res. 2003;9:2440–2446. [PubMed] [Google Scholar]
- Schneider-Merck T, Lammerts van Bueren JJ, Berger S, Rossen K, van Berkel PHC, Derer S, Beyer T, Lohse S, Bleeker WK, Peipp M, et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J. iImmunology. 2010;184:512–520. doi: 10.4049/jimmunol.0900847. [DOI] [PubMed] [Google Scholar]
- Schwartz RH. T cell anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- Siders WM, Shields J, Garron C, Hu Y, Boutin P, Shankara S, Weber W, Roberts B, Kaplan JM. Involvement of neutrophils and natural killer cells in the anti-tumor activity of alemtuzumab in xenograft tumor models. Leuk. Lymphoma. 2010;51:1293–1304. doi: 10.3109/10428191003777963. [DOI] [PubMed] [Google Scholar]
- Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin) Semin. Oncol. 1999;26:60–70. [PubMed] [Google Scholar]
- Stagg J, Johnstone RW, Smyth MJ. From cancer immunosurveillance to cancer immunotherapy. Immunol. Rev. 2007;220:82–101. doi: 10.1111/j.1600-065X.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Stagg J, Loi S, Divisekera U, Foong S, Duret H, Yagita H, Teng MW, Smyth MJ. Anti - ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti - PD-1 or anti-CD137 mAb therapy. PNAS. 2011;108:10–15. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- Vasovíc LV, Dyall R, Clynes RA, Ravetch JV, Nikolić-Žugić J. Synergy between an antibody and CD8+ cells in eliminating an established tumor. Eur. J. Immunol. 1997;27:374–382. doi: 10.1002/eji.1830270206. [DOI] [PubMed] [Google Scholar]
- Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone Ja. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Wang S, Astsaturov IA, Bingham CA, McCarthy KM, von Mehren M, Xu W, Alpaugh RK, Tang Y, Littlefield BA, Hawkins LD, et al. Effective antibody therapy induces host-protective antitumor immunity that is augmented by TLR4 agonist treatment. Cancer Immunol. Immunother. 2012;61:49–61. doi: 10.1007/s00262-011-1090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PL, Koeppen H, Hurteau T, Schreiber H. Tumor antigens defined by cloned immunological probes are highly polymorphic and are not detected on autologus normal cells. J. Exp. Med. 1989;170:217–232. doi: 10.1084/jem.170.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh DJJ, Wilson C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–1040. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng W-K, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (Rituximab, IDEC-C2B8) Blood. 1999;94:2217–2224. [PubMed] [Google Scholar]
- Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. PNAS. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JC, Topalian SL, Schwartzentruber DJ, Parkinson DR, Marincola FM, Weber JS, Seipp CA, White DE, Rosenberg SA. The use of polyethylene glycol-modified interleukin-2 (PEG-IL-2) in the treatment of patients with metastatic renal cell carcinoma and melanoma. Cancer. 1995;76:687–694. doi: 10.1002/1097-0142(19950815)76:4<687::aid-cncr2820760424>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhang S, Cheung N-KV, Ragupathi G, Livingston PO. Antibodies against GD2 Ganglioside Can Eradicate Syngeneic Cancer Micrometastases. Cancer Res. 1998;58:2844–2849. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.