Abstract
The glycine receptor (GlyR) is the predominant inhibitory neurotransmitter receptor in the brainstem and spinal cord but is also found in higher brain regions. GlyR function is affected by a variety of allosteric modulators including drugs of abuse, such as ethanol and inhalants and the ubiquitous divalent cation zinc. Two-electrode voltage-clamp experiments were conducted on Xenopus laevis oocytes expressing wild-type α1 homomeric glycine receptors to compare the degree of enhancement produced by zinc on GlyR activated by two agonists (glycine vs. taurine) that vary markedly in their efficacies. Zinc potentiation of both glycine- and taurine-evoked currents was the same at the concentrations of agonists that produced the same currents, corresponding to 6% of the maximal effect of glycine compared to 23% of the maximal effect of taurine. Similar results were seen with 50 and 200 mM ethanol. A direct comparison of agonist concentration-response curves showed that zinc enhancement was greater, overall, for taurine-activated than glycine-activated receptors. In addition, zinc only enhanced taurine- but not glycine-activated GlyR when agonists were applied at saturating concentrations. These data suggest that zinc affects taurine affinity, as well as the probability of channel opening at sub-maximal taurine concentrations, and that the magnitude of allosteric modulation at the GlyR depends on the efficacy of the agonist tested. This has implications for mutagenesis studies in which changes in the degree of allosteric modulation observed may result from mutation-induced changes in agonist efficacy.
Keywords: Zinc, Ethanol, Allosteric modulation, Glycine receptor, electrophysiology, Xenopus oocytes
1. Introduction
The glycine receptor (GlyR) is a member of the Cys-loop family of ligand-gated ion channels. It is the primary inhibitory receptor in the brainstem and spinal cord but also plays important roles in higher brain regions, including the hippocampus, nucleus accumbens and prefrontal cortex (Baer et al. 2009; Jonsson et al. 2012a, 2009b; Lynch 2004). GlyRs are pentameric in structure with the five subunits arranged around a central anion-conducting channel. Thus far, four alpha subunits and one beta subunit have been identified, of which α1-3 and β are found in humans. GlyRs can express either as homomeric receptors composed solely of α subunits, or as αβ heteromeric receptors with a stoichiometry of 2α:3β (Betz et al. 1993; Bowery and Smart 2006; Lynch 2004). In the spinal cord a developmental switch occurs from prenatal α2 homomers to α1β receptors in the adult. However, in the forebrain it appears that the α2 subunit continues to be expressed into adulthood along with the β subunit (Jonsson et al., 2012).
GlyR activity is affected by a large variety of allosteric modulators including zinc, alcohols, anesthetics and inhaled drugs of abuse (Beckstead et al. 2000; Harvey et al. 1999; Mihic et al. 1997), making it a promising clinical target for the treatment of alcohol and drug addiction (Söderpalm and Ericson, 2013; Tipps et al. 2010). Zinc is present endogenously at nanomolar concentrations known to enhance GlyR function. It exhibits biphasic actions at GlyRs, potentiating currents at concentrations <10 μM, while higher concentrations produce inhibition (Harvey et al. 1999; Laube et al. 2000).
Taurine is a partial agonist of the GlyR, with approximately 5% the efficacy of glycine (Lape et al. 2008), and is believed to be an important GlyR agonist in a number of brain regions (Albrecht and Schousboe 2005). For example, Mori et al. (2002) showed that an uptake inhibitor of taurine induced a strychnine-sensitive chloride current in hippocampal organotypic slice cultures. Previous research has largely focused on allosteric modulation at glycine-activated receptors. Modulators shift glycine concentration-response curves either to the left or to the right but have no effects at maximally-effective glycine concentrations. However, Kirson et al. (2013a, 2012b) showed that ethanol, volatile anesthetics, inhaled drugs of abuse and zinc are able to enhance currents elicited by maximally-effective concentrations of taurine, but not glycine. This suggested that these modulators had an effect on the probability of taurine-activated channel opening (Po), which would already be near maximum when a saturating concentration of glycine was tested. Most studies of allosteric modulation are performed using concentrations of agonists that are low on their concentration-response curves, since it is at these agonist concentrations that the greatest modulatory effects are seen. In this study we investigated whether agonist efficacy also affects the magnitude of zinc and ethanol enhancement of GlyR function seen under those conditions.
2. Results
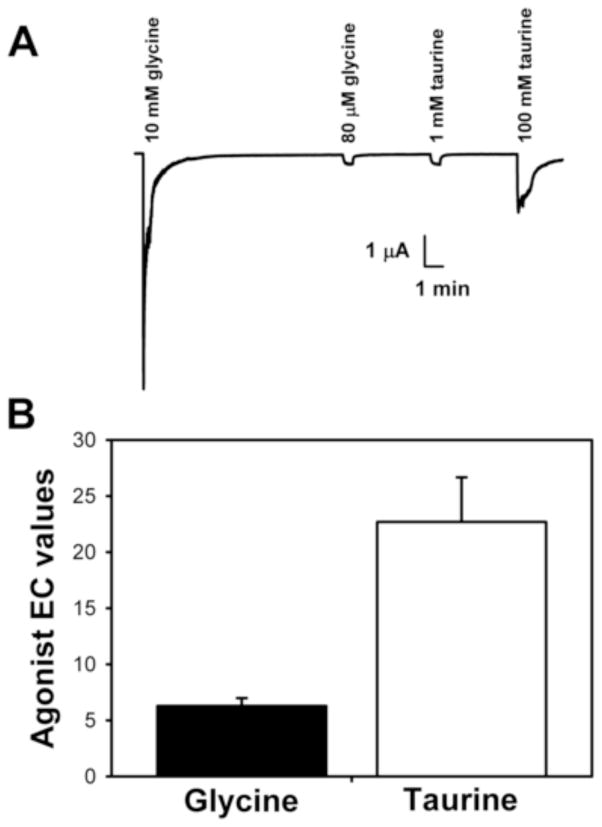
Zinc was tested for its enhancing effects of α1 homomeric GlyR currents elicited by submaximal concentrations of glycine or taurine. Concentrations of each agonist were first identified that produced equal currents, corresponding to 5 – 10% of the maximally-effective glycine response (EC5-10 glycine). In order to do so the EC5-10 concentration of glycine was first identified in each oocyte and then the concentration of taurine producing a similar current was determined (Fig. 1A). Where that concentration of taurine fell on the taurine concentration-response curve was next determined, relative to a maximally-effective concentration of taurine (100 mM). Concentrations of 84 ± 4 μM glycine had EC values of 6.28 ± 0.70 relative to 10 mM glycine, while concentrations of 1.2 ± 0.2 mM taurine, producing currents of the same magnitude in each oocyte as glycine, had EC values of 22.69 ± 3.98 relative to 100 mM taurine. The concentrations of taurine used thus fell significantly higher on their concentration-response curves than the concentrations of glycine did on theirs [t(4) = 4.06, p < 0.005] (Fig. 1B).
Figure 1.
Low concentrations of glycine and taurine, eliciting similar currents, correspond to different effective concentrations on their respective concentration-response curves. (A) Sample tracings showing the identification of concentrations of glycine and taurine that elicited similar currents, between 5–10% of the current produced by a saturating (10 mM) concentration of glycine. The glycine and taurine concentrations were chosen so as to elicit similar absolute currents. (B) Despite producing similar absolute currents the concentrations of glycine and taurine identified fell at markedly different points on their respective concentration-response curves. Data are shown as mean + S.E.M. of 5 oocytes.
We next compared the enhancing effects of 100 nM zinc on currents produced by a low concentration of glycine with zinc effects on the same currents produced by the partial agonist taurine. In this experiment, zinc was co-applied with concentrations of glycine or taurine producing 5–10% of the maximally effective glycine response; i.e., the absolute currents produced by glycine and taurine were similar (Fig 2A). Zinc-potentiated currents were compared to those produced by agonist alone as shown in Fig. 2B. Co-application with zinc resulted in a significant enhancement of both glycine- and taurine-mediated currents [F(1,23) = 24.12, p < 0.001]. There was, however, no difference in the degree of zinc enhancement seen between glycine and taurine [F(1,23) = 1.19, p > 0.28].
Figure 2.
Zinc enhances glycine- and taurine-activated GlyR to a similar extent despite the two agonists being tested at different effective concentrations. A) Sample tracings showing that concentrations of glycine and taurine producing similar absolute currents also result in similar degrees of enhancement by zinc. B) A concentration of glycine producing approximately 5–10% (EC5-10) of the maximal effect of 10 mM glycine was first identified in each oocyte. A series of taurine concentrations were then applied to identify a concentration of taurine that produced the same absolute current as that EC5-10 glycine concentration. These concentrations of glycine and taurine were applied, first alone in the absence of zinc, and then co-applied with 100 nM zinc following a 30 s preincubation with zinc. Zinc potentiated glycine and taurine-mediated currents to the same degree. Peak currents were measured and used to calculate the degree of current enhancement produced by zinc. Data are shown as mean + S.E.M. of 6 oocytes. C) Percent current enhancement of glycine- and taurine-mediated currents by 100 nM zinc for individual oocytes plotted against the agonist EC values relative to a saturating (10 mM) concentration of glycine; 100% on the ordinate represents a doubling of the current seen in the absence of zinc. Data are shown for 6 oocytes.
When data from individual oocytes were plotted (Fig. 2C), there was a large degree of variation in zinc enhancement seen. Previous studies revealed that the buffers used in our studies contain nanomolar levels of contaminating zinc, sufficient to affect GlyR function (Cornelison and Mihic, 2014). We hypothesized that the degree of variability within our data set might be attributable to variations in this amount of contaminating zinc between the different preparations of buffers used in this experiment. In order to minimize the effects that variable background levels of zinc might have on our results, we repeated our previous experiment using a much higher concentration of zinc (2.5 μM) applied with a fixed (2.5 mM) concentration of the zinc chelator, tricine. This resulted in much lower inter-experiment variability in zinc enhancement but, again, no differences were seen in zinc effects on glycine- vs. taurine-activated GlyR (Figs. 3A,B) [F(1,15) = 0.62, p > 0.44].
Figure 3.
In the presence of the chelator tricine, zinc enhances glycine- and taurine-activated GlyR to a similar extent despite the two agonists being tested at different effective concentrations, relative to the maximal effects produced by each agonist. A) A concentration of glycine producing approximately 5–10% (EC5-10) of the maximal effect of 10 mM glycine was first identified in each oocyte. A series of taurine concentrations were then applied to identify a concentration of taurine that produced the same absolute current as that EC5-10 glycine concentration. These concentrations of glycine and taurine were applied (in the presence of 2.5 mM tricine), first in the absence of zinc, and then co-applied with 2.5 μM zinc following a 30 s preincubation with zinc. Zinc potentiated glycine and taurine currents to the same degree. Peak currents were measured and used to calculate the degree of current enhancement produced by zinc. Data are shown as mean + S.E.M. of 4 oocytes. B) Percent current enhancement of glycine- and taurine-mediated currents by 2.5 μM zinc + 2.5 mM tricine for individual oocytes plotted against the agonist EC values relative to a saturating (10 mM) concentration of glycine; 100% on the ordinate represents a doubling of the current seen in the absence of zinc. Data are shown for 4 oocytes.
We next determined how the percent enhancement of taurine-activated GlyR currents by zinc varied with taurine and glycine concentration. The average zinc percent potentiation and taurine EC values were plotted on the ordinate against taurine concentration (Fig. 4A). A concentration of 2.5 μM zinc (+ 2.5 mM tricine) enhanced taurine responses in a manner that was dependent on the concentration of taurine used [F(5,38) = 2.55, p < 0.05], with less enhancement seen at higher taurine concentrations. It should be noted, however, that even at saturating taurine concentrations, zinc still had a potentiating effect. When glycine was the agonist (Fig. 4B), zinc again enhanced GlyR currents in a concentration-dependent manner [F(4,19) = 16.29, p < 0.001], but this time with negligible enhancement seen at higher glycine concentrations. Zinc produced no enhancement at EC50 glycine concentrations and above, in contrast to the clear enhancement still seen at the corresponding taurine concentrations. When zinc enhancement was plotted against each agonist’s EC value (relative to its own maximal effect), greater zinc-mediated potentiation was seen at all concentrations of taurine (Fig. 4C). If only data obtained using low (less than EC20) concentrations of taurine and glycine are compared, even then significantly greater zinc enhancement is seen with taurine [t(28) = 3.21, p <0.004].
Figure 4.
Zinc enhances taurine- and glycine-mediated GlyR currents in a concentration-dependent fashion. A) A taurine concentration-response curve (open triangles) was determined and ordinate values are shown on the right axis. At each taurine concentration tested, the percent enhancement produced by 2.5 μM zinc (in the presence of 2.5 mM tricine) was also determined (open circles) and ordinate values are shown on the left axis. B) A glycine concentration-response curve (filled triangles) was determined and ordinate values are shown on the right axis. At each glycine concentration tested, the percent enhancement produced by 2.5 μM zinc (in the presence of 2.5 mM tricine) was also determined (closed circles) and ordinate values are shown on the left axis. Data are shown as mean ± S.E.M. of 5–7 oocytes. C) Zinc enhancement of GlyR function plotted against the glycine and taurine EC values. In this plot each current elicted by taurine was expressed as a percentage of the effect produced by a maximally-effective concentration of taurine, to yield the taurine EC value. Each current elicted by glycine was expressed as a percentage of the effect produced by a maximally-effective concentration of glycine, to yield the glycine EC value.
In order to determine if the effects seen in our previous experiments were unique to zinc, we tested another allosteric modulator, ethanol, in place of zinc. Co-application of 200 mM ethanol had a significant potentiating effect on glycine-and taurine-mediated currents [F(1,19) = 123.22, p < 0.001]. However, again there was no difference seen in the degree of potentiation between glycine and taurine [F(1,19) = 0.001, p > 0.97] (Fig. 5A), despite the significant difference [t(8) = 3.2, p <0.014] in their respective agonist EC values (Fig. 5B, inset). All assays in this experiment were performed using a single preparation of MBS so that background, contaminating levels of zinc would be constant throughout the experiment. Data shown for individual oocytes (Fig. 5B) show less variability than seen previously when the concentration of contaminating zinc was not controlled (Fig. 2C). A lower concentration (50 mM) of ethanol was also tested using this same experimental paradigm, and similar results were found. Ethanol enhanced glycine-mediated currents by 15.6 ± 2.2%, and taurine-mediated currents by 18.1 ± 5%, when the two agonists produced currents of the same magnitude, and thus when the concentrations of taurine used fell significantly higher on their concentration-response curves than did the concentrations of glycine on theirs.
Figure 5.
Ethanol enhances glycine- and taurine-activated GlyR to a similar extent despite the two agonists being tested at different effective concentrations. A concentration of glycine producing approximately 5–10% of the maximal effect of glycine (EC5-10) was first identified in each oocyte. A series of taurine concentrations were then applied to identify a concentration of taurine that produced the same absolute currents as the EC5-10 glycine. Ethanol potentiated these two currents to the same degree. Concentrations of either glycine or taurine producing currents equivalent to EC5-10 glycine were first applied. These same agonist concentrations were then co-applied with 200 mM ethanol following a 30 s preincubation with ethanol. Peak currents were measured and used to calculate the degree of current enhancement produced by ethanol. A) Summary of the potentiating effects of 5–10% maximal glycine- and taurine-evoked currents by 200 mM ethanol. Data are shown as mean + S.E.M. of 5 oocytes. B) Percent current enhancement of glycine-mediated and taurine-mediated currents by 200 mM ethanol for individual oocytes plotted against the agonist EC values relative to a saturating (10 mM) concentration of glycine; 100% on the ordinate represents a doubling of the current seen in the absence of ethanol. Data are shown for 5 oocytes. The inset graph shows that low concentrations of glycine and taurine, eliciting similar currents, correspond to very different effective concentrations on their respective concentration-response curves. Data are shown as mean + S.E.M. of 5 oocytes.
3. Discussion
The GlyR is responsible for mediating much of the neuronal inhibition in the brainstem and spinal cord, although these receptors are also found in a number of higher brain regions (Baer et al. 2009; Jonsson et al. 2012a, 2009b; Lynch 2004). A variety of structurally-diverse allosteric modulators are known to affect GlyR function including divalent cations, alcohols, anesthetics and numerous drugs of abuse (Beckstead et al. 2000; Harvey et al. 1999; Kirson et al. 2013a, 2012b; Mihic et al. 1997). Although glycine has long been thought to be the endogenous agonist acting at these receptors, taurine, the second most abundant amino acid in the brain, may also play a role in many brain regions (Albrecht et al. 2005; Mori et al. 2002). Allosteric modulation of glycine-activated receptors has been quite extensively studied, but not as much is known about modulation of GlyR activated by taurine.
Depending on whether they act as positive or negative allosteric modulators at the GlyR, compounds such as ethanol, inhalants or zinc either leftshift or rightshift glycine concentration-response curves (Beckstead et al., 2000; Miller et al. 2005; Welsh et al. 2010). The greatest percent enhancing effects of these agents are thus seen when low concentrations of agonists are tested, and this is likely due to an enhancement of glycine affinity for its receptors. However, these allosteric modulators have minimal effects when applied with saturating concentrations of glycine (Fig. 4B). In contrast, Kirson et al. (2012) showed that ethanol, volatile anesthetics and inhaled drugs of abuse produce marked enhancement of currents elicited by maximally-effective concentrations of taurine. The same phenomenon was later also shown using 100 nM zinc (Kirson et al. 2013). The effects of these modulators at saturating concentrations of taurine cannot be due to their enhancement of taurine affinity but must instead be due to their increasing the probability of channel opening (Po) subsequent to binding. In contrast, since a saturating concentration of glycine produces a Po of approximately 0.95 (Lape et al. 2008), there is little opportunity for enhancement of Po by modulators at glycine-activated receptors. Even when very low concentrations of glycine (3 μM) are used, the intra-burst Po is still about 0.7 (Welsh et al. 2009).
We first compared how 100 nM zinc affected GlyR responses to low concentrations of glycine vs. taurine by determining the concentrations of glycine and taurine that produced the same currents. These concentrations corresponded to markedly different agonist EC values on their respective concentration-response curves (Fig. 1). We expected that the degree of zinc potentiation would be lower for taurine-activated GlyR than receptors activated by glycine as the concentration of taurine used fell much higher on its concentration-response curve than glycine did on its. Surprisingly, our data showed the same degree of enhancement for both agonists (Fig. 2B). This also suggested that, even at lower effective concentrations of taurine, allosteric modulators might have greater effects on taurine-activated than on glycine-activated GlyR.
A direct comparison of zinc enhancement of taurine- and glycine-activated receptors showed that considerably greater enhancement was seen in the former (Fig. 4). The most parsimonious explanation is that allosteric modulators have minimal effects on intra-burst Po at all concentrations of glycine, since they are already high, instead acting primarily to decrease the rate of glycine unbinding and thus prolonging burst durations (Laube et al. 2000; Welsh et al. 2009). This is no longer a factor at a saturating glycine concentration, since a glycine molecule would bind almost immediately upon the unbinding of another, explaining the lack of ethanol and zinc effects at maximally-effective glycine concentrations. When a saturating concentration of taurine is applied the same reasoning holds true: modulators are still not able to exert any effects via enhancing taurine affinity. However, by affecting intra-burst properties, they are able to affect Po, which they clearly do as shown in Figs. 4A and 4C. At lower taurine concentrations the modulators may affect both taurine unbinding to enhance burst durations and also enhance intra-burst Po. This could explain why zinc produces greater enhancement of taurine-activated GlyR than glycine-activated GlyR at low agonist concentrations (Fig. 4C) and also why similar degrees of ethanol enhancement are seen with EC8 glycine and EC34 taurine (Fig. 5).
In conclusion, our data demonstrate that the nature and magnitude of allosteric potentiation of GlyR function depends on the efficacy of the agonist that gates the receptor. Positive allosteric modulators such as ethanol and zinc appear to act solely by increasing the affinities of binding of high efficacy agonists such as glycine, but have minimal effects on intra-burst Po and thus produce no enhancement at saturating concentrations of agonist. The effects of modulators on the actions of low efficacy agonists like taurine are more complex. They enhance currents mediated by saturating concentrations of taurine, by a mechanism unrelated to enhancement of taurine affinity. In addition, as the taurine concentration is decreased, the degree of allosteric modulation increases above the levels seen with glycine. This may reflect the modulators affecting both intra-burst Po as well as taurine binding affinity.
The possible influence of agonist efficacy on allosteric modulation should be considered when comparing modulator effects on recombinant receptors. If an agonist varies significantly in efficacy on two recombinant receptors, this could result in differences in the magnitude of allosteric modulation observed when receptors are tested using low effective concentrations of the agonist. There is a considerable body of research dealing with the identification of specific amino acid residues responsible for modulation of receptor function by allosteric modulators such as metals, inhaled anesthetics and alcohols. Our current findings suggest that, if a receptor subunit mutation alters the efficacy of the agonist being tested, it may also have additional effects on the magnitude of allosteric modulation observed.
4. Experimental Procedure
4.1 Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Xenopus laevis frogs were obtained from Xenopus Express (Homosassa, FL) and housed at 19C on a 12-hour light/dark cycle.
4.2 Oocyte isolation and cDNA injection
Partial ovarectomies were performed under an IACUC animal protocol approved at the University of Texas at Austin. Stage V and VI oocytes were isolated by manually removing the thecal and epithelial layers with forceps and placed in isolation medium containing 108 mM NaCl, 1 mM EDTA, 2 mM KCl and 10 mM HEPES. Isolated oocytes were placed in collagenase buffer [0.5 mg/mL type 1A collagenase, 83 mM NaCl, 2 mM MgCl2, and 5 mM HEPES] for 10 minutes to remove the follicular layer. Oocytes were then injected with human glycine α1-receptor subunit cDNA (1.5 ng in 30 nL) in a modified pBK-cytomegalovirus vector (Mihic et. al, 1997) via the “blind” method of Colman (1984) using a micropipette (10–15 μm tip size) attached to an electronic microdispenser. Oocytes were stored in the dark at 19 C in 96-well plates containing modified Barth’s saline (MBS) [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 10 mM HEPES, 0.82 mM MgSO4•7H2O, 0.33 mM Ca(NO3)2, 0.91 mM CaCl2 at pH 7.5] with the addition of 2 mM sodium pyruvate, 0.5 mM theophylline, 10 U/ml penicillin, 10 mg/l streptomycin and 50 mg/l gentamicin, that had been sterilized by passage through a 0.22 μm filter.
4.3 Two-electrode voltage-clamp electrophysiology
Oocytes typically expressed GlyR within 24 hours. All electophysiology measurements were made 1–5 days following cDNA injection. Oocytes were placed in a 100 μL bath with their animal poles facing upwards. Cells were pierced with two high-resistance (0.5–10 MΩ) glass electrodes filled with 3 M KCl and voltage-clamped at −70 mV using an OC-725C oocyte clamp (Warner Instruments, Hamden, CT). They were then perfused with MBS at a rate of 2 ml/min using a Masterflex USA peristaltic pump (Cole Parmer Instrument Co., Vernon Hills, IL) through 18-gauge polyethylene tubing. All solutions were prepared in one of the following: MBS, MBS + 100 nM zinc, MBS + 200 mM ethanol, or MBS + 2.5 μM zinc with 2.5 mM tricine. Drug applications lasted 30 s for submaximal glycine and taurine concentrations and 15 s when maximally-effective agonist concentrations were used; washouts periods lasted 3–15 minutes, as appropriate, for each agonist application. Data were acquired using a Powerlab 4/30 digitizer with LabChart version 7 software (ADInstruments, Bella Vista, NSW, Australia).
4.4 Data Analysis
Peak currents were measured and used in data analysis. Currents observed in the presence of agonist were compared with currents elicited by co-application of agonist and modulator. Experimental values are listed as the mean ± S.E.M. Statistical significance was determined using t-tests, paired t-tests and 2-way ANOVAs as indicated. Statistical testing was performed using SigmaPlot version 11.0 (Systat Software, San Jose, CA).
Highlights.
Glycine and taurine are agonists that vary in their efficacies at the GlyR.
GlyR function is affected by allosteric modulators such as zinc and ethanol.
Zinc enhancement was greater for taurine-activated than glycine-activated receptors.
The degree of allosteric modulation depends on the efficacy of the agonist tested.
Acknowledgments
This research was supported by funds provided by the Waggoner Center for Alcohol and Addiction Research.
Abbreviations
- ECx
effective concentration producing x% of maximal agonist effect
- GlyR
glycine receptor
- MBS
Modified Barth’s Saline
- Po
channel open-state probability
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht J, Schousboe A. Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res. 2005;30:1615–1621. doi: 10.1007/s11064-005-8986-6. [DOI] [PubMed] [Google Scholar]
- Baer K, Waldvogel HJ, Faull RL, Rees MI. Localization of glycine receptors in the human forebrain, brainstem and cervical spinal cord: an immunohistochemical review. Front Mol Neurosci. 2009;2:1–11. doi: 10.3389/neuro.02.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Weiner JL, Eger EI, 2nd, Gong DH, Mihic SJ. Glycine and gamma-aminobutyric acidA receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol. 2000;57:1199–1205. [PubMed] [Google Scholar]
- Betz H, Langosch D, Rundström N, Bormann J, Kuryatov A, Kuhse J, Schmieden V, Matzenbach B, Kirsch J. Structure and biology of inhibitory glycine receptors. Ann NY Acad Sci. 1993;707:109–115. doi: 10.1111/j.1749-6632.1993.tb38046.x. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Smart TG. GABA and glycine as neurotransmitters: a brief history. Brit J Pharmacol. 2006;147:S109–S119. doi: 10.1038/sj.bjp.0706443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison GL, Mihic SJ. Contaminating levels of zinc found in commonly-used labware and buffers affect glycine receptor currents. Brain Res Bull. 2014;100:1–5. doi: 10.1016/j.brainresbull.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, Thomas P, James CH, Wilderspin A, Smart TG. Identification of an inhibitory Zn2+ binding site on the human glycine receptor alpha 1 subunit. J Physiol. 1999;520:53–64. doi: 10.1111/j.1469-7793.1999.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S, Kerekes N, Hyytia P, Ericson M, Söderpalm B. Glycine receptor expression in the forebrain of male AA/ANA rats. Brain Res. 2009;1305:S27–S36. doi: 10.1016/j.brainres.2009.09.053. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Morund J, Pickering C, Adermark L, Ericson M, Söderpalm B. Changes in glycine receptor subunit expression in forebrain regions of the Wistar rat over development. Brain Res. 2012;1446:12–21. doi: 10.1016/j.brainres.2012.01.050. [DOI] [PubMed] [Google Scholar]
- Kirson D, Todorovic J, Mihic SJ. Positive allosteric modulators differentially affect full versus partial agonist activation of the glycine receptor. JPET. 2012;342:61–70. doi: 10.1124/jpet.112.191486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson D, Cornelison GL, Philpo AE, Todorovic J, Mihic SJ. Physiological concentrations of zinc reduce taurine-activated GlyR responses to drugs of abuse. Neuropharmacol. 2013;75:286–294. doi: 10.1016/j.neuropharm.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R, Colquhoun D, Sivilotti LG. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 2008;454:722–727. doi: 10.1038/nature07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Kinetic and mutational analysis of Zn2+ modulation of recombinant human inhibitory glycine receptors. J Physiol. 2000;522:215–230. doi: 10.1111/j.1469-7793.2000.t01-1-00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Trudell JR, Goldstein BE, Harris RA, Mihic SJ. Zinc enhances ethanol modulation of the α1 glycine receptor. Neuropharmacol. 2010;28:676–681. doi: 10.1016/j.neuropharm.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Trudell JR, McCracken ML, Harris RA. Zinc-dependent modulation of α2- and α3-glycine receptor subunits by ethanol. Alcohol Clin Exp Res. 2013;37:2002–2010. doi: 10.1111/acer.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Miller PS, Beato M, Harvey RJ, Smart TG. Molecular determinants of glycine receptor αβ subunit sensitivities to Zn2+-mediated inhibition. J Physiol. 2005;566:657–670. doi: 10.1113/jphysiol.2005.088575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Gahwiler BH, Gerber U. Beta-alanine and taurine as endogenous agonists at glycine receptors in rat hippocampus in vitro. J Physiol. 2002;539:191–200. doi: 10.1113/jphysiol.2001.013147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderpalm B, Ericson M. Neurocircuitry involved in the development of alcohol addiction: the dopamine system and its access points. Curr Top Behav Neurosci. 2013;13:127–161. doi: 10.1007/7854_2011_170. [DOI] [PubMed] [Google Scholar]
- Tipps ME, Lawshe JE, Ellington AD, Mihic SJ. Identification of novel specific allosteric modulators of the glycine receptor using phage display. J Biol Chem. 2010;285:22840–22845. doi: 10.1074/jbc.M110.130815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh BT, Kirson D, Allen HM, Mihic SJ. Ethanol enhances taurine-activated glycine receptor function. Alcohol Clin Exp Res. 2010;34:1634–9. doi: 10.1111/j.1530-0277.2010.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]