Abstract
Background
Endothelial dysfunction present in patients with peripheral artery disease (PAD) may be better understood by measuring the temporal dynamics of blood flow and oxygen saturation during reactive hyperemia than by conventional static measurements.
Methods and Results
Perfusion, Intravascular Venous Oxygen saturation, and T2* (PIVOT), a recently developed MRI technique, was used to measure the response to an ischemia-reperfusion paradigm in ninety-six patients with PAD of varying severity, and ten healthy controls. Perfusion, venous oxygen saturation (SvO2), and T2* were each quantified in the calf at two second temporal resolution, yielding a dynamic time course for each variable. Compared to healthy controls, patients had a blunted and delayed hyperemic response. Moreover, patients with lower ankle-brachial index had: 1) a more delayed reactive hyperemia response time, manifesting as an increase in time to peak perfusion in the gastrocnemius, soleus, and peroneus muscles, and in the anterior compartment; 2) an increase in the time to peak T2* measured in the soleus muscle; and 3) a prolongation of the posterior tibial vein SvO2 washout time. Intra- and inter-session repeatability was also assessed. Results indicated that time to peak perfusion and time to peak T2* were the most reliable extracted time course metrics.
Conclusions
Perfusion, dynamic SvO2, and T2* response times following induced ischemia are highly correlated with PAD disease severity. Combined imaging of peripheral microvascular blood flow and dynamics of oxygen saturation with PIVOT may be a useful tool to investigate the pathophysiology of PAD.
Keywords: peripheral artery disease, magnetic resonance imaging, vascular reactivity, perfusion, blood-oxygen level dependence
Peripheral artery disease (PAD) is most commonly a manifestation of atherosclerosis in vessels supplying the lower limbs, and causes significant morbidity and mortality in the United States.1-4 In PAD, atherosclerotic plaque encroaches on the peripheral artery lumen, decreasing blood flow and vascular reactivity of the large arteries.5,6 Collateral arteries vasodilate in order to meet the baseline metabolic demand of skeletal muscle; however, the collateral vasculature may be inadequate to quickly and adaptively accommodate increases in blood flow demand, such as those that occur during exercise.5,7,8 This effect can result in an oxygen supply-demand mismatch, causing patients to experience claudication.4
The presence of flow-limiting stenoses can be quickly and non-invasively detected by measuring the ankle-brachial index (ABI), the ratio of systolic blood pressures in the ankle and brachial artery.4,9 An ABI of less than 0.9 confirms the diagnosis of PAD, and an ABI of less than 0.4 is suggestive of critical limb ischemia.4,10 Interventions such as cilostazol11 or exercise rehabilitation12 lessen claudication symptomatology, however are not necessarily associated with a clinically significant improvement in the ABI. Additionally, the ABI is not sensitive to primary microvascular impairment, endothelial dysfunction, or alterations in vascular reactivity that may coexist with the macrovascular lesions.5,13 Thus, there is a compelling need for the development of diagnostic tools that would allow for the interrogation of the contribution of vascular dysfunction to PAD.
Peripheral vascular function can be interrogated by monitoring the dynamics of blood flow and oxygenation in response to a stressor, analogous to cardiac stress testing. This can be accomplished using an ischemia-reperfusion paradigm, which induces reactive hyperemia. In such a paradigm, proximal arterial occlusion is sustained for several minutes using a blood pressure cuff secured around the subject’s thigh. During the period of arterial occlusion, blood flow in the arteries, veins, and capillaries is halted, however oxygen extraction continues in the stagnant blood of the capillaries. Because the venous blood is static, there is no change in venous oxygen saturation in the large draining veins. However, following cuff release, hyperemia ensues with an increase in arterial flow through the large arteries and collateral arteries due to endothelium-mediated vasodilation primarily from release of nitric oxide.14 The oxygenated arterial blood travels to the capillary bed, causing tissue oxygenation to recover as well. At the onset of hyperemia, venous oxygen saturation (SvO2) sharply decreases as the deoxygenated blood from the capillary bed travels to the large draining veins.15 Hyperperfusion of oxygenated arterial blood causes SvO2 to rise and eventually surpass the baseline value during the period that blood velocity exceeds the oxygen extraction rate. The kinetics of the hyperemic response provide information about vascular reactivity and endothelial function.16
Several magnetic resonance imaging (MRI) techniques can non-invasively evaluate the dynamic changes that occur in blood flow and oxygenation during the hyperemic response. Specifically, prior studies have investigated perfusion using arterial spin labeling (ASL),17-19 dynamics of SvO2 using MR susceptometry-based oximetry,15,20 and changes in the T2* signal (commonly known as blood-oxygen-level dependent (BOLD) response),21-23 which provides a relative measure of muscle capillary bed oxygenation.24,25 In response to induced ischemia, the kinetics of each of these variables is associated with the presence of PAD. Compared to healthy controls, patients exhibit a blunted and delayed reperfusion,26 and a reduced rate of recovery of tissue oxygenation, as evidenced by both direct measurement of SvO2,15,27 and of relative changes in T2*.21
More recently, we developed a technique termed Perfusion, Intravascular Venous Oxygen saturation, and T2* (PIVOT) which allows for dynamic and simultaneous quantification of perfusion, SvO2, and skeletal muscle T2*.28 The purpose of this work was to evaluate the hyperemic response in patients with PAD and healthy controls using PIVOT. We hypothesize that PIVOT will be able to detect PAD severity-dependent changes in macro- and microvascular function, and that the relationship between these impairments will help us to better understand the pathophysiologic processes that underlie the disease.
Methods
Subjects
Ninety-six patients with intermittent claudication and a diagnosis of PAD and ten healthy controls were recruited to participate in the study. Upon enrollment, each subject was classified into a disease severity group based on ABI, where healthy subjects had an ABI > 0.90 (10 subjects; 3 males), mild disease corresponded to an ABI range of 0.7 – 0.89 (28 patients; 18 males); moderate disease to an ABI range of 0.50 – 0.69 (45 patients; 28 males); and severe disease to an ABI of less than 0.50 (17 patients; 13 males). Additional characteristics of the study participants are shown in Table 1.
Table 1.
Characteristics of study participants.
| Healthy ABI >0.9 | Mild ABI 0.7 to 0.89 | Moderate ABI 0.5 to 0.69 | Severe ABI <0.5 | All patients ABI<0.9 | |
|---|---|---|---|---|---|
|
| |||||
| Total | 10 | 28 | 49 | 19 | 96 |
| Repeat visit | 9 | 11 | 23 | 8 | 42 |
| Gender (M/F) | 3/7 * | 18/10 | 29/20 | 15/4 | 62/34 |
| Age | 59.5 (3.9) * | 66.8 (6.3) | 69.4 (8.1) | 71.3 (8.6) | 69.0 (7.8) |
| BMI | 26.2 (4.9) | 27.6 (3.9) | 27.4 (4.0) | 27.5 (4.0) | 27.5 (3.9) |
| Systolic BP | 123 (21) * | 142 (18) | 141 (19) | 137 (17) | 141 (18) |
| Diabetes | 0 * | 10 | 15 | 6 | 31 |
| Smoking status (Never/Past/Current) | 5/5/0 * | 2/19/7 | 4/32/13 | 2/7/10 | 8/58/30 |
Mean (standard deviation) or counts are shown.
Indicates a significant difference between healthy subjects and all PAD patients.
Experimental protocol
Prior to participation in the study, each subject provided written informed consent. The Institutional Review Board of the University of Pennsylvania approved all aspects of this study.
PAD patient protocol
Each patient reported to the testing center for two separate visits no more than one month apart. Some subjects were selected to return for a third visit, approximately three months after the second. On the first visit, screening and medical history questionnaires were completed, and the ABI was measured bilaterally using Doppler sonography according to the current standards.29 Patients were instructed to refrain from strenuous exercise for 3 days prior to the second visit, and to avoid alcohol and caffeine for 24 hours prior. Upon reporting for the second visit, patients underwent a vascular function MRI scanning session. The MRI protocol consisted of dynamic imaging of blood flow and oxygenation of the mid-calf using PIVOT throughout an ischemia-reperfusion paradigm. If selected to return, the third visit was identical to the second.
Healthy subject protocol
Healthy subjects reported to the testing center for two identical visits, separated by 1 day to 1 week. Each MRI session included two identical PIVOT scans throughout periods of ischemia-reperfusion.
MRI scan protocol
All imaging was performed on a 3T MR imaging system (Siemens, Erlanger, Germany). For every patient, the leg with the lower ABI was scanned, and for each healthy control, the right leg was scanned. The maximum girth of the calf was centered in an 8-channel transmit-receive knee coil (In vivo, Inc. Gainesville, FL). PIVOT data were continuously collected throughout one minute of baseline, five minutes of proximal arterial occlusion, and six minutes following cuff deflation. Proximal arterial occlusion was achieved using a pneumatic tourniquet system (Hokanson, Inc., Bellevue, WA) with a cuff placed on the mid-thigh. After one minute of baseline scanning, the cuff was rapidly inflated to 75 mmHg above the measured systolic blood pressure, or 250 mmHg, whichever was lower. For all healthy subjects, the ischemia-reperfusion paradigm was repeated within the same scan session to assess intra-session repeatability.
As described previously,28 PIVOT allows for continuous, simultaneous measurement of perfusion, intravascular SvO2, and skeletal muscle T2* using an interleaved dual-slice pulsed ASL (PASL) and multi-echo GRE sequence. Briefly, perfusion quantification is accomplished using SATuration Inversion-Recovery (SATIR),18 a Flow-Alternating Inversion Recovery (FAIR) ASL variant, in which slice-selective and non-selective adiabatic inversion pulses are used to achieve tag and control conditions, respectively. Image acquisition follows the post-labeling delay (PLD), which is immediately followed by a slice-selective saturation pulse to reset the magnetization. In PIVOT, a keyhole30 RF-spoiled multi-echo GRE sequence acquires data at a slice located downstream from the PASL slice during the PLD. Only multi-echo GRE data acquired during the PLD following the slice-selective inversion are used for SvO2 and T2* analysis, however the multi-echo GRE interleave was run during every PLD to control for potential magnetization transfer effects. From these data, SvO2 is derived using susceptometry-based oximetry,15,20 and T2* is calculated by fitting the signal magnitude to a monoexponential function.
For the PASL interleave, images were acquired with the following parameters: partial-Fourier GRE-EPI readout; field of view (FOV) = 250 × 250 mm2; acquired matrix = 80 × 50, reconstructed to 80 × 80; slice thickness = 1 cm; slice location = isocenter; TR/TE = 1 s/8.05 ms; PLD = 952 ms. For the multi-echo GRE interleave, images were acquired with: FOV = 96 × 96 mm2; keyhole acquired matrix = 96 × 24, slice thickness = 1 cm; slice location = 3 cm distal from isocenter; TR/TE1/TE2/TE3/TE4/TE5 = 38.12/3.78/6.99/12.32/19.32/26.32 ms. For SvO2 data analysis, the acquired matrix was keyhole30 reconstructed to 96 × 96 pixels using outer k-space data from a fully sampled reference image obtained immediately after the dynamic PIVOT acquisition; only dynamic data were used for T2* analysis. Each variable was quantified at 2-second temporal resolution.
Image analysis
Image analysis was performed using MATLAB (The MathWorks, Inc., Natick, MA). Prior to data processing, the time series of images were motion corrected with rigid-body transformations using NIH ImageJ software (developed by Wayne Rasbands, National Institutes of Health, Bethesda, MD). Perfusion was quantified in regions of interest (ROIs) in the gastrocnemius, soleus, and peroneus muscles, as well as in the anterior compartment (AC) – which includes the tibialis anterior and extensor digitorum longus muscles (Figure 1a). For the assessment of repeatability, a whole-leg ROI was used, which comprised of all four muscle groups. As described previously,28 an ROI was delineated in the muscle of interest using a high-resolution scout image as a reference for muscle boundaries. The average signal-intensity in the defined ROI following slice-selective (MSS) or non-selective (MNS) inversion was determined. Using the model described by Raynaud, et al.,18 perfusion (f, in mL/min/100g) was quantified for each tag-control pair as:
| [1] |
where λ is the tissue-partition coefficient (λ=0.9 mL/g), T is the post-labeling delay, T1 is the longitudinal relaxation time of blood, which in this model is assumed to be equivalent to that of tissue (T1 = T1muscle = 1420 ms31 ≈ T1blood). The perfusion offset was corrected by subtracting the average perfusion during the period of cuff occlusion from each time point, as perfusion is assumed to be zero during the period of proximal arterial occlusion.32 For each muscle, the peak perfusion and time to peak perfusion (TTPPerf) were recorded from the dynamic time course data (Figure 1c).
Figure 1.
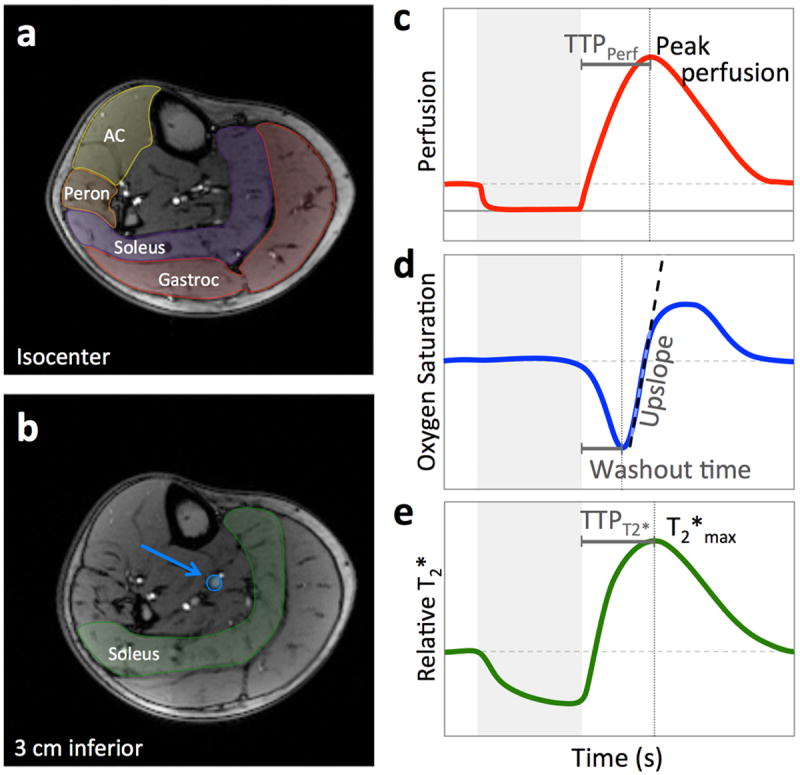
Anatomic images corresponding to PASL (a) and multi-echo GRE (b) slice locations. As shown in (a), perfusion is quantified in regions of interest in the gastrocnemius (red), soleus (purple), peroneus (orange), and anterior compartment (AC) (yellow). From the multi-echo GRE data, SvO2 is quantified in the larger posterior tibial vein (blue arrow), and T2* is calculated from a region of interest in the soleus (green) shown in (b). Each variable is quantified every two seconds, yielding a dynamic time course. Schematics of the perfusion (c), SvO2 (d), and T2* (e) time courses are shown, with the extracted metrics indicated. The grey box in c-e indicates the period of proximal arterial occlusion.
Using the first two echoes of the multi-echo GRE, SvO2 was quantified in the larger posterior tibial vein (Figure 1b). As described previously,15 SvO2 was calculated by measuring the difference in phase accrual (Δϕ) between echoes spaced apart by ΔTE in the blood versus surrounding reference tissue as:
| [2] |
where γ is the proton gyromagnetic ratio, Δχdo the susceptibility difference between fully oxygenated and deoxygenated blood (Δχdo = 4·0.27 ppm33,34), Hct the hematocrit measured by venipuncture, B0 the main magnetic field strength, and θ the angle of the vessel with respect to B0 (measured from axial scout images). The washout time, equal to the time between cuff release and the minimum SvO2, and the upslope, calculated as the maximum slope during recovery, were recorded (Figure 1d).
T2* was measured within an ROI in the soleus muscle (Figure 1b) by fitting multi-echo GRE data from echoes 2-5 to a monoexponential function. Signal intensity in the ROI was first averaged for each echo, then the data were fit to a monoexponential function and T2* was computed at each time point. The T2* time course data were normalized to the average baseline value, and relative T2*max, and time to peak T2* (TTPT2*) were recorded from the time course (Figure 1e).
Statistical Analysis
All statistical analyses were performed with JMP software (JMP®, Version 11. SAS Institute Inc., Cary, NC). Data normality was evaluated using the Shapiro-Wilk test. For data from the normal or log-normal distribution, two-sample Student’s t-tests with equal variance were used to determine whether differences existed between healthy subjects (ABI ≥ 0.9) and patients with PAD (ABI < 0.9). Non-parametric Wilcoxon rank tests were used to assess differences of non-normally distributed variables. In patients with PAD, correlations between the ABI and each time course metric and between pairs of time course metrics were calculated using Pearson’s correlation coefficient (if data were normally distributed) or Spearman’s rank correlation coefficient (if data were not normally distributed). Holm’s adjustment for multiple comparisons was applied to all tests and correlations to maintain the family-wise error rate of 0.05. Thus, for all tests, pholms < 0.05 was considered to be significant.
In healthy controls, intra-session and inter-session (1 day to 1 week between scans) repeatability was assessed. Inter-session repeatability was also assessed in a subset of patients with PAD (approximately 3 months between scans). In all cases, the intraclass correlation coefficients (ICC) and mean within-subject coefficients of variation (CVW) were calculated to assess measurement repeatability.
Results
In six subjects, a cuff pressure of 75 mmHg above systolic blood pressure did not result in a full occlusion, likely due to calcification of the feeding arteries. Insufficient occlusion was determined based on appearance of venous pooling in the EPI images and T2* time course. In the remaining 100 subjects, several subjects’ MRI images were unanalyzable due to motion contamination, partial volume effects, low SNR, or insufficient vein size. Table 2 shows the number of patients for each variable that were of sufficient quality for analysis. Notably, for all subjects in whom a full occlusion was achieved, at least one variable was fit for analysis.
Table 2.
Synopsis of number of subjects with analyzable images for each measured variable.
| Healthy ABI >0.9 | Mild ABI 0.7 to 0.89 | Moderate ABI 0.5 to 0.69 | Severe ABI <0.5 | All study participants | |
|---|---|---|---|---|---|
|
| |||||
| Total | 10 | 27 | 45 | 18 | 100 |
| Gastrocnemius Perfusion | 10 | 26 | 40 | 15 | 91 |
| Soleus Perfusion | 10 | 26 | 41 | 14 | 91 |
| Peroneus Perfusion | 10 | 25 | 40 | 9 | 84 |
| AC Perfusion | 10 | 23 | 36 | 13 | 82 |
| SvO2 | 10 | 20 | 34 | 13 | 77 |
| Relative T2* | 10 | 27 | 44 | 18 | 99 |
For visualization purposes, average time courses were generated for all healthy subjects and patients with mild, moderate, and severe PAD disease burden for perfusion in the gastrocnemius muscle (Figure 2a), posterior tibial vein SvO2 (Figure 2b), and relative T2* in the soleus muscle (Figure 2c). The typical ischemia-hyperemia response is seen in each variable’s time course. During the period of arterial occlusion, T2* decreases as the stagnant blood in the capillary bed becomes increasingly desaturated. After cuff release, SvO2 initially drops as the deoxygenated blood from the capillary bed advances into the large draining veins in the measurement slice. Concurrently, perfusion increases in order to repay the oxygen debt incurred during the ischemic period. This hyperperfusion of oxygenated blood causes T2* and SvO2 to recover and surpass their baseline values. As blood flow and venous oxygen saturation normalize, so too does T2*. From the average time courses a striking difference is seen between healthy subjects and patients with PAD, and furthermore, TTPPerf, washout time, and TTPT2* are increasingly prolonged as disease severity worsens.
Figure 2.
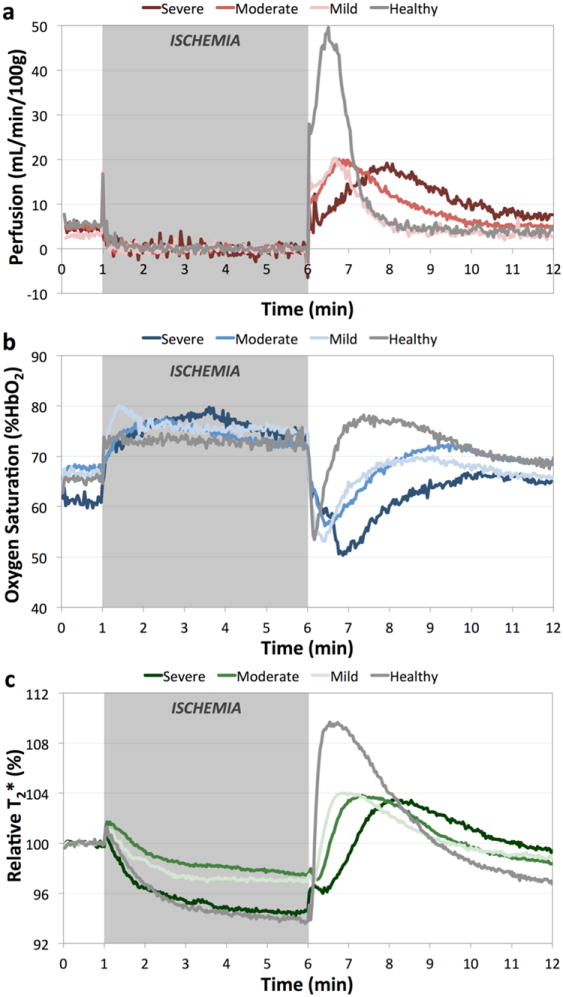
Group-average time courses for healthy subjects (grey), and patients with mild (light colored), moderate (medium colored), and severe (dark colored) PAD for gastrocnemius muscle perfusion (a), posterior tibial vein SvO2 (b), and relative T2* in the soleus muscle (c). Grey box indicates the period of proximal arterial occlusion. With increasing disease severity, there is a delay in the reactive hyperemia response evident in each variable that was measured.
The group-wise average (standard deviation) of each time course metric for the measured variables are summarized in Table 3. TTPPerf in the peroneus, T2*max, and TTPT2* were not from the normal distribution, all other variables were from the log-normal distribution. Each variable, with the exception of SvO2 upslope, was significantly different between healthy controls and patients with PAD. With the presence of PAD, there was a significant prolongation of the hyperemic response time, and a reduction in the magnitude of the hyperemic response. SvO2 washout time increased from 11 s in healthy subjects to 39 s in PAD patients. TTPPerf was delayed as well – averaged over all muscle groups it increased from 24 s to 72 s in patients with disease. Peak perfusion decreased from 52.6 mL/min/100g to 25.6 mL/min/100g with disease. TTPT2* in the soleus muscle increased from 33 s to 91 s between healthy subjects and PAD patients, and relative T2*max decreased from 110% to 106%. Averaged (standard deviation) over all subjects, baseline T2* was 19 (2) ms, and averaged across all muscle groups the perfusion offset during ischemia was 7.6 (2.7) mL/min/100g.
Table 3.
Associations of the presence of PAD with PIVOT time course metrics.
| Healthy ABI >0.9 (n =10) | Patients with PAD ABI<0.9 (n = 90) | |
|---|---|---|
| ABI | 1.11 (0.10) | 0.60 (0.14) * |
| Gastrocnemius Peak Perfusion (mL/min/100g) | 54.0 (14.5) | 28.3 (12.9) * |
| Gastrocnemius TTPPerf (s) | 29 (10) | 77 (53) * |
| Soleus Peak Perfusion (mL/min/100g) | 74.9 (33.0) | 28.9 (13.9) * |
| Soleus TTPPerf (s) | 25 (13) | 72 (49) * |
| Peroneus Peak Perfusion (mL/min/100g) | 41.2 (11.3) | 20.8 (12.4) * |
| Peroneus TTPPerf (s) | 24 (9) | 74 (47) * |
| AC Peak Perfusion (mL/min/100g) | 43.4 (16.0) | 24.5 (14.1) † |
| AC TTPPerf (s) | 16 (5) | 64 (50) * |
| Washout time (s) | 11 (4) | 39 (23) * |
| Upslope (%HbO2/s) | 0.91 (0.40) | 0.51 (0.22) |
| T2*max (%) | 111 (4) | 106 (3) ‡ |
| TTPT2* (s) | 35 (12) | 91 (55) * |
pholms<0.001;
pholms<0.01;
pholms<0.05
Inter-variable correlations were assessed in PAD patients only. The correlations between ABI and time course metrics reinforce some of the findings from the group-wise analysis. A significant correlation was found between the ABI and response time following cuff release (Figure 3 a, c, e). As the ABI decreases, an increase in the time to peak perfusion in all muscles (gastrocnemius r = -0.60, pholms<0.0001; soleus r = -0.66, pholms<0.0001; peroneus r = -0.43, pholms<0.0001; TA r = -0.55, pholms<0.0001), an increase in the time to peak T2* (r = -0.55, pholms<0.0001), and an increase in the washout time (r = -0.59, pholms<0.0001) were observed. However, no statistically significant correlation was detected between ABI and peak perfusion (r = 0.08), SvO2 upslope (r = 0.14), or T2*max. (r = -0.04) (Figure 3 b, d, f).
Figure 3.
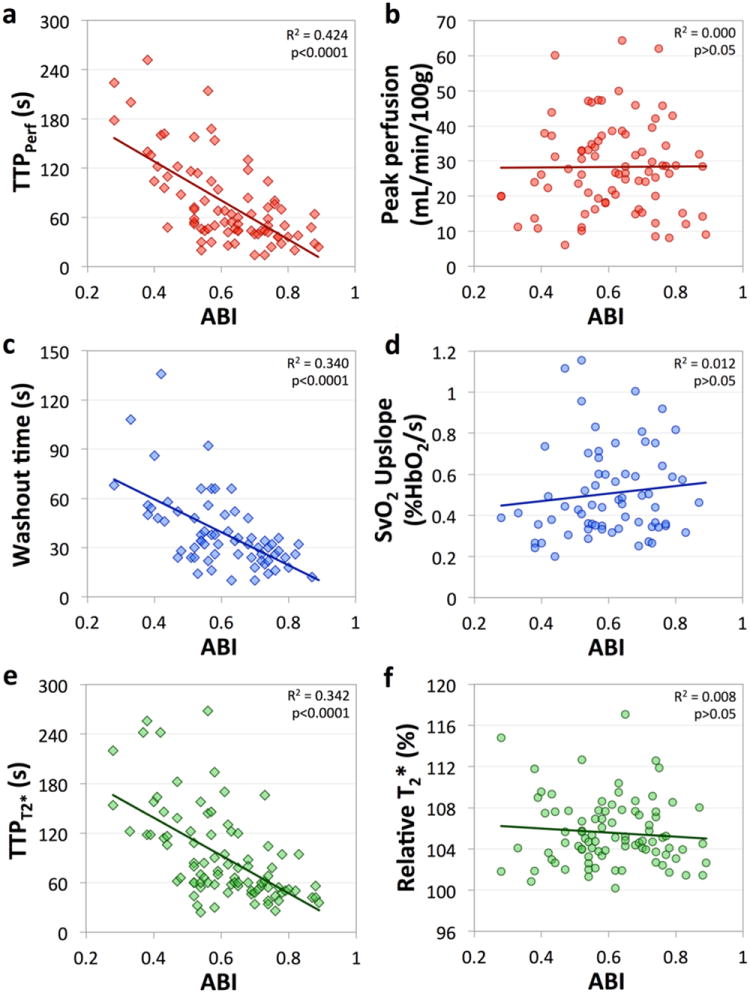
Correlation plots for ABI versus gastrocnemius muscle time to peak perfusion (a) and peak perfusion (b), SvO2 washout time (c) and upslope (d), and time to peak T2* (e) and relative T2*max (f) measured in the soleus. As disease severity worsens, the reactive hyperemia response time is progressively prolonged. However, no correlation was observed between the magnitude of the hyperemic response and the ABI.
In addition to the negative correlation between the hyperemic response time and ABI, significant positive correlations were detected between most pairs of timing metrics. For example, as TTPPerf in the gastrocnemius muscle increased, so too did TTPPerf in the other muscles (averaged over all muscle ROIs, r = 0.71, pholms<0.0001), TTPT2* in the soleus (r = 0.57; p holms<0.0001), and SvO2 washout time (r = 0.53; p holms<0.0001). The single exception was the lack of a correlation between the peroneus TTPPerf and SvO2 washout time (r = 0.26). Positive correlations were also detected between peak perfusion measured in the gastrocnemius and soleus (r = 0.53; pholms<0.0001) or anterior compartment (r = 0.40; pholms<0.02). The other significant correlation detected was between relative T2*max and TTPPerf in the peroneus (r=0.43; pholms<0.005).
In healthy subjects, both intra-session and inter-session repeatability were assessed (Table 4). The intra-session ICC was greater than 0.7 for all measured variables, with the exception of TTPT2* (r = 0.61). One healthy subject was lost to follow-up, thus the number of healthy subjects in whom inter-session repeatability was assessed was 9. Though lower than intra-session repeatability, the inter-session repeatability in the healthy subjects was still high for peak perfusion (r = 0.84) and time to peak perfusion (r = 0.74). The ICC, however, was lower for SvO2 upslope and washout time, T2*max, and TTPT2*. In all cases, the mean within-subject CV in healthy subjects was less than 25%. In patients with PAD, the ICCs of the perfusion time course metrics and TTPT2* were approximately equal to or better than the ICC of the ABI measurement (r = 0.53). The ICC for the SvO2 washout time and upslope, and T2*max, however, was low (r < 0.35). The mean within-subject CV in patients with PAD was approximately 10% greater than in healthy subjects for all measured variables.
Table 4.
Assessment of repeatability in healthy subjects and patients with PAD.
| Healthy subjects – Intra-session
| ||||
|---|---|---|---|---|
| Scan 1 | Scan 2 | ICC | CVW | |
|
| ||||
| Leg Peak Perfusion (mL/min/100g) | 51.5 (13.5) | 51.6 (14.3) | 0.95 | 4.1% |
| Leg TTPPerf (s) | 23 (8) | 21 (7) | 0.76 | 11.0% |
| Washout time (s) | 11 (4) | 11 (5) | 0.90 | 8.8% |
| Upslope (%HbO2/s) | 0.9 (0.4) | 0.9 (0.5) | 0.79 | 18.5% |
| T2*max (%) | 111 (4) | 112 (4) | 0.78 | 1.1% |
| TTPT2* (s) | 35 (12) | 35 (11) | 0.61 | 15.3% |
|
| ||||
| Healthy subjects – Inter-session
| ||||
| Session 1 | Session 2 | ICC | CVW | |
|
| ||||
| Leg Peak Perfusion (mL/min/100g) | 52.1 (14.2) | 50.3 (17.1) | 0.78 | 11.8% |
| Leg TTPPerf (s) | 23 (8) | 21 (7) | 0.71 | 10.9% |
| Washout time (s) | 12 (4) | 14 (9) | 0.61 | 23.4% |
| Upslope (%HbO2/s) | 0.9 (0.5) | 0.8 (0.4) | 0.57 | 24.4% |
| T2*max (%) | 111 (4) | 110 (3) | 0.58 | 2.0% |
| TTPT2* (s) | 37 (12) | 36 (12) | 0.58 | 16.5% |
|
| ||||
| Patients with PAD – Inter-session
| ||||
| Session 1 | Session 2 | ICC | CVW | |
|
| ||||
| ABI | 0.59 (1.5) | 0.59 (1.7) | 0.53 | 11.8% |
| Leg Peak Perfusion (mL/min/100g) | 23.3 (8.9) | 35.5 (9.5) | 0.52 | 20.3% |
| Leg TTPPerf (s) | 68 (42) | 72 (44) | 0.78 | 22.0% |
| Washout time (s) | 34 (17) | 30 (16) | 0.13 | 31.9% |
| Upslope (%HbO2/s) | 0.5 (0.2) | 0.7 (0.4) | 0.08 | 36.7% |
| T2*max (%) | 106 (3) | 106 (3) | 0.33 | 1.9% |
| TTPT2* (s) | 90 (49) | 84 (39) | 0.57 | 23.4% |
Discussion
In this study, peripheral vascular function was investigated in patients with PAD and healthy controls using PIVOT, a recently developed MRI technique, allowing for simultaneous measurement of the dynamics of blood flow and oxygen saturation throughout an ischemia-reperfusion paradigm. Compared to healthy controls, PAD patients exhibited a blunted and delayed hyperemic response, as measured by perfusion, SvO2, and T2*. Moreover, the results showed a significant correlation between PAD disease severity and the temporal dynamics of recovery of blood flow and oxygenation following induced ischemia. With decreasing ABI, the time required to accommodate the hyperperfusion of oxygen-rich blood to the ischemic muscle was prolonged; there was an increase in the time needed for desaturated blood to flow out of the capillary bed into the large draining veins; and a delay in the time to peak T2*, indicating a decrease in the rate of recovery of oxygen saturation in the capillary bed. These data are thus suggestive of disease-severity-dependent impairment of vascular function in PAD.
Previous studies have used an ischemia-reperfusion model to investigate peripheral vascular function in patients with PAD compared to healthy controls, however these studies were limited to the measurement of a single variable – perfusion26 or T2*21, or two variables – SvO2 and bulk arterial blood flow15,27. Separately measuring all of the variables in a single study would require multiple scans. However, with PIVOT, perfusion, SvO2, and T2* are measured simultaneously, allowing for the direct investigation of the temporal relationships between perfusion, oxygen saturation, and T2* at no additional scan time or patient discomfort. PIVOT was previously evaluated by comparing results from PIVOT to those obtained with the traditional PASL or multi-echo GRE sequences in a cohort of young healthy subjects. No bias was detected between PIVOT and the individual measurement methods, suggesting that the simultaneous measurement of perfusion, SvO2, and T2* at high temporal resolution is possible with PIVOT.28
Data in the present study show that the temporal dynamics during reactive hyperemia are associated with the presence of PAD and are tightly correlated with disease severity, thus high temporal resolution sampling of the response is extremely important. PIVOT provides 2-second temporal resolution of each of the measured variables, higher sampling rate than prior studies investigating perfusion alone (16-second temporal resolution),17,26 or SvO2 and bulk arterial flow (5-second temporal resolution of SvO2).27 Temporal resolution of 1-second was used in previous studies investigating only T2* in skeletal muscle.21,35
Unlike perfusion and SvO2, the origin of the BOLD signal is less physiologically straightforward. BOLD signal depends upon blood oxygenation in the capillary bed, as well as microvascular flow, blood volume, cellular pH, and vessel diameter and orientation.24 So, while relative T2* can be quickly acquired, and in this study was the most reliable variable to measure (relative T2* was analyzable in 99/100 subjects), the multifactorial contrast mechanism and the fact that the measured response is relative, rather than in physiologic units, makes the interpretation of the BOLD signal more complicated. To reduce potential confounds due to blood volume on the measured T2*, care was taken to ensure that the patient’s leg was at heart height, and each patient was supine for approximately 15 minutes prior to the onset of ischemia.36 Additionally, arterial occlusion was induced using a pneumatic tourniquet system capable of fully inflating in approximately 0.3 second, thus occluding venous and arterial flow nearly simultaneously.
Hyperemia can also be induced via exercise rather than induced ischemia. Exercise is more physiologically relevant to PAD, however muscles may be stressed to varying degrees, depending on which muscle group is activated (e.g. plantar flexion vs. dorsiflexion), and total work is subject-effort dependent. Conversely, tourniquet-induced ischemia is a global stimulus, more uniformly affecting all muscle groups downstream from the site of inflation, and more consistent between subjects.
A prior study by Wu, et al. measured post-ischemic skeletal muscle perfusion with a continuous ASL (CASL) technique in patients with PAD and healthy controls.26 Compared to the results from this study, there is good agreement in the trend of timing of the hyperemic response and disease severity – with the presence of PAD, and with increasing disease severity, the time to peak perfusion is progressively prolonged.26 Wu, et al. also found an association between the peak perfusion and disease severity,26 whereas in this study an association between peak perfusion and disease presence, but not severity was detected. CASL provides higher signal-to-noise ratio of the perfusion signal at the cost of lower temporal resolution (16 seconds with CASL26 compared to 2 seconds in this study). As such, relative to this study much higher peak perfusions were observed in Wu, et al.’s investigation for all disease severity subgroups. For instance, in the gastrocnemius muscle, peak perfusion of approximately 60 mL/min/100g was reported in patients with PAD, compared to 28.3 mL/min/100g reported in the present study. Using an invasive, but highly precise method, Lassen, et al. investigated the clearance rate of radioactive xenon-133 to determine perfusion in patients and healthy controls.37 In that study, the reported peak perfusion and time to peak perfusion are in agreement with the results of the present study – 51.8 mL/min/100g versus 17.1 mL/min/100g, and 22 s versus 138 s for healthy subjects versus patients, respectively. Prior studies investigating perfusion in young healthy subjects using the same PASL variant, either SATIR alone18,36 or using the combined PIVOT technique,28 found peak perfusion during reactive hyperemia to be similar to those values reported in the present work.
Results of intravascular SvO2 measured in the posterior tibial vein are in relative agreement with Langham, et al.’s work investigating SvO2 in the femoral vein of PAD patients compared to young healthy subjects and age-matched healthy controls.27 As in the results of this study, Langham, et al. reported a prolonged washout time in patients with PAD compared to their healthy peers. In the present study, a correlation between the ABI and washout time in the posterior tibial vein was additionally detected. Langham, et al. also observed a reduction in upslope in patients compared to controls, which was not observed in this study. This deviation could be attributed to the different vessels investigated by the two studies – posterior tibial vein in this study, compared to the larger and more superior femoral vein in the work by Langham, et al. A larger vein would result in higher signal-to-noise ratio of the measured SvO2, thereby increasing measurement precision. In this study, it was necessary to acquire SvO2 data downstream from the perfusion measurement location so as to not disturb the magnetization of inflowing blood.
Finally, the present results are in general agreement with previous BOLD studies in the leg.21,35 The average T2*max of patients with PAD of 106% is slightly lower than the relative T2*max of 110.5% reported by Ledermann, et al.21 However, these authors normalized T2* to the average T2* value of the first 3 seconds following cuff release, whereas for the present study, T2* was normalized to the baseline average T2*. T2* decreases during the ischemic period, therefore normalizing the data to the post-ischemic period would increase the T2* range and thus increase the relative T2*max. In agreement with Ledermann et al.’s study, the current study showed that with decreasing ABI there is an increase in TTPT2*.
The inter-variable correlations were investigated to better understand the relationship between blood flow and oxygenation in the capillary bed and large draining veins. Strong positive correlations were detected between the various timing metrics, however, no significant correlations were observed between the response time and response magnitude (e.g. peak perfusion in the gastrocnemius muscle was not significantly correlated with its time to peak perfusion).
In addition to the benefit of assessing inter-variable relationships, the acquisition of simultaneous perfusion, SvO2, and T2* measurements with PIVOT increases the likelihood that at least one of the variables is of sufficient quality for analysis. For example, if a patient has a very small posterior tibial vein, the measure of SvO2 would not be possible. However, perfusion and T2* data could still be used to investigate the hyperemic response. Alternatively, in some patients perfusion was very noisy due to partial-volume effects from unperfused intramuscular fibrous inclusions. Yet the T2* and SvO2 data were still able to provide insight into vascular function. In the present study, though only 71 subjects had analyzable images for all three variables, in all 100 subjects in whom a complete cuff occlusion was achieved, at least one of the variables was fit for analysis.
The dynamics of the hyperemic response are a function of upstream arterial occlusions, diffuse atherosclerosis, and endothelium-mediated vascular reactivity. Thus, the assessment of the response with several variables provides complementary, rather than independent information. The combination of results from perfusion and SvO2 could, for example, be used to investigate the relative metabolic response. Furthermore, by modeling the combined PIVOT data, there may be added sensitivity to detect a response to a therapeutic intervention.
The intra-session repeatability in healthy subjects shows that the measured time course metrics have excellent precision in the short term. The hyperemic response measured for relative T2* had a broad peak, unlike the sharp peak of the perfusion measurement (as shown in Figure 2). The TTPT2* was, therefore, more sensitive to time course noise than TTP perfusion, resulting in a reduction in repeatability. This pattern was observed for both intra- and inter-session repeatability. Compared to intra-session repeatability, inter-session repeatability was slightly lower. However, it is likely that there are true differences in the reactive hyperemia response due to normal physiologic variation. Care was taken to minimize known factors that impact vascular reactivity - all subjects were instructed to refrain from caffeine intake and vigorous exercise prior to scanning, and scans were conducted at the same time of day. The measured differences are, therefore, a combination of true physiologic variation, measurement error (as is present for intra-session repeatability), and due to the potential impact of slight changes in slice location. Generally, the within-subject CV was still quite small, and similar to the reported results in a previous investigation of inter- and intra-session repeatability of PIVOT-derived time course metrics in young healthy subjects.28
The inter-session repeatability in patients with PAD was acceptable for some variables, and poor for others (SvO2 washout time and upslope, and T2*max). Overall, the variables were similarly repeatable in healthy subjects and patients with PAD. Given that the ABI measurement differed between the two scan sessions, there may be true pathophysiologic progression or regression of disease in some patients. The ICCs of peak perfusion, TTP perfusion and TTPT2* were on the order of the ICC of the ABI measurement. The relatively low within-subject coefficient of variation suggests that although the measured response may differ between subjects, the span over which they differ is small compared to the range of values expected in healthy subjects versus patients with PAD. Time to peak perfusion and time to peak T2* both had relatively good repeatability, and were sensitive to disease presence and severity, making these metrics the most reliable for detection of alterations in vascular function.
There are several limitations to this study. Some variables have inter-session ICCs of <0.6, suggesting that the measurement of vascular function by PIVOT during reactive hyperemia is not suitable for clinical monitoring of a single patient. However, the present method could be well suited to investigate the response to therapy in a cohort of patients with PAD.
The moderate disease severity group was much larger than either the mild or severe disease severity groups, though this is less of a limitation when using correlations to investigate the relationship between disease severity and the MRI-measured variables. Additionally, patients recruited for this study were not restricted to either aortoiliac or femoropopliteal artery PAD. Angiographic data were not available for these patients, thus the site of obstruction is not known.
Furthermore, all correlations of the MRI data to disease severity were based on the ABI. Even though the ABI is the clinical standard for diagnosis of PAD, it is not without limitation. The ABI may be falsely elevated in patients with severely calcified arteries,38 or in situations of undiagnosed brachial or subclavian artery stenosis.39 Direct measurement of a patient’s functional capacity via exercise testing, including the 100 foot walking time or the claudication onset time and peak walking time of the Gardner protocol40, may provide more physiologically relevant information than the measurement of the ABI alone. However, these physiologic measures are subjective, based upon an individual’s pain tolerance or effort. There have been mixed results in prior studies as to whether the physiologic measures of disease severity correlate with the ABI. McDermott, et al. showed that maximum treadmill walking time did correlate with the ABI;41 however, Szuba, et al. found no correlation.42 Assessment of the correlation between the MRI time course metrics and physiologic testing results would be of great interest.
The variables measured indicate impaired vascular function, related to both macrovascular lesions as well as microvascular dysfunction. Additional measurement of bulk arterial blood flow during the ischemia-reperfusion paradigm would allow for assessment of temporal dynamics of blood flow in the macrovasculature and microvasculature. Comparison of the temporal dynamics in the large artery and capillary bed could help to separate the macrovascular and microvascular responses. This can be accomplished by velocity-encoding the multi-echo GRE interleave of PIVOT, though the temporal resolution of the sequence must be decreased to accommodate double the number of phase encoding segments of the multi-echo GRE.43 Additionally, the MRI data may be complemented with the measurement of hemoglobin oxygen saturation using continuous-wave near infrared spectroscopy (NIRS)44,45.
In conclusion, earlier studies investigating peripheral vascular function in patients with PAD were limited to the investigation of only one or two variables. Using PIVOT, simultaneous measurement of perfusion, SvO2, and T2* can be achieved. Results indicate that increasing PAD disease severity is associated with a prolongation of response time following induced ischemia. Time to peak perfusion and time to peak T2* were both highly repeatable measures and were sensitive to the presence and severity of PAD. In the future, PIVOT could be used as a means to monitor disease progression and evaluate treatment response in patients with PAD.
Acknowledgments
We would like to thank Elizabeth Beothy and Scott Welden for their assistance with patient recruitment.
Sources of Funding
This work was supported by an award from the American Heart Association and NIH Grants R01 HL075649, R01 HL109545, and K25 HL111422.
Footnotes
Disclosures
None.
References
- 1.Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71:510–515. doi: 10.1161/01.cir.71.3.510. [DOI] [PubMed] [Google Scholar]
- 2.Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: The Rotterdam study. Atheroscler Thromb Vasc Biol. 1998;18:185–192. doi: 10.1161/01.atv.18.2.185. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WRC, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, White CJ, White J, White RA, Antman EM, Smith SC, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B American Association for Vascular Surgery, Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, ACC/AHA Task Force on Practice Guidelines, American Association of Cardiovascular and Pulmonary Rehabilitation, National Heart, Lung, and Blood Institute, Society for Vascular Nursing, (null), Vascular Disease Foundation. ACC/AHA 2005 Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): Executive summary - A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) Journal of the American College of Cardiology. 2006;47:1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Faxon DP, Fuster V, Libby P, Beckman JA, Hiatt WR, Thompson RW, Topper JN, Annex BH, Rundback JH, Fabunmi RP, Robertson RM, Loscalzo J, American Heart Association Atherosclerotic Vascular Disease Conference: Writing Group III: Pathophysiology. Circulation. 2004;109:2617–2625. doi: 10.1161/01.CIR.0000128520.37674.EF. [DOI] [PubMed] [Google Scholar]
- 6.Harris LM, Faggioli GL, Shah R, Koerner N, Lillis L, Dandona P, Izzo JL, Snyder B, Ricotta JJ. Vascular Reactivity in Patients with Peripheral Vascular-Disease. American Journal of Cardiology. 1995;76:207–212. doi: 10.1016/s0002-9149(99)80066-6. [DOI] [PubMed] [Google Scholar]
- 7.Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, Strandness DE, Taylor LM. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: A critical review. Circulation. 1996;94:3026–3049. doi: 10.1161/01.cir.94.11.3026. [DOI] [PubMed] [Google Scholar]
- 8.Winsor T, Payne JH, Rudy N, Beatty JO. Collateral circulation in health and disease. AMA Arch Surg. 1957;74:20–28. [PubMed] [Google Scholar]
- 9.Lijmer JG, Hunink MG, van den Dungen JJ, Loonstra J, Smit AJ. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol. 1996;22:391–398. doi: 10.1016/0301-5629(96)00036-1. [DOI] [PubMed] [Google Scholar]
- 10.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. New England Journal of Medicine. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 11.Mohler ER, Beebe HG, Salles-Cuhna S, Zimet R, Zhang P, Heckman J, Forbes WP. Effects of cilostazol on resting ankle pressures and exercise-induced ischemia in patients with intermittent claudication. Vasc Med. 2001;6:151–156. doi: 10.1177/1358836x0100600305. [DOI] [PubMed] [Google Scholar]
- 12.Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, Thum CC, Goldberg S, Jaff MR, Steffes MW, Comerota AJ, Ehrman J, Treat-Jacobson D, Walsh ME, Collins T, Badenhop DT, Bronas U, Hirsch AT for the CLEVER Study Investigators. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: Six-month outcomes from the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) study. Circulation. 2012;125:130–139. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Criqui MH, Denenberg JO. The generalized nature of atherosclerosis: how peripheral arterial disease may predict adverse events from coronary artery disease. Vasc Med. 1998;3:241–245. doi: 10.1177/1358836X9800300311. [DOI] [PubMed] [Google Scholar]
- 14.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. Journal of the American College of Cardiology. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 15.Langham MC, Floyd TF, Mohler ER, Magland JF, Wehrli FW. Evaluation of cuff-induced ischemia in the lower extremity by magnetic resonance oximetry. Journal of the American College of Cardiology. 2010;55:598–606. doi: 10.1016/j.jacc.2009.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: From research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu WC, Wang J, Detre JA, Wehrli FW, Mohler E, Ratcliffe SJ, Floyd TF. Hyperemic flow heterogeneity within the calf, foot, and forearm measured with continuous arterial spin labeling MRI. AJP: Heart and Circulatory Physiology. 2008;294:H2129–H2136. doi: 10.1152/ajpheart.01399.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raynaud JS, Duteil S, Vaughan JT, Hennel F, Wary C, Leroy-Willig A, Carlier PG. Determination of skeletal muscle perfusion using arterial spin labeling NMRI: Validation by comparison with venous occlusion plethysmography. Magn Reson Med. 2001;46:1–7. doi: 10.1002/mrm.1192. [DOI] [PubMed] [Google Scholar]
- 19.Pollak AW, Meyer CH, Epstein FH, Jiji RS, Hunter JR, DiMaria JM, Christopher JM, Kramer CM. Arterial Spin Labeling MR Imaging Reproducibly Measures Peak-Exercise Calf Muscle Perfusion. JACC: Cardiovascular Imaging. 2012;5:1224–1230. doi: 10.1016/j.jcmg.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández-Seara MA, Techawiboonwong A, Detre JA, Wehrli FW. MR susceptometry for measuring global brain oxygen extraction. Magn Reson Med. 2006;55:967–973. doi: 10.1002/mrm.20892. [DOI] [PubMed] [Google Scholar]
- 21.Ledermann HP, Schulte AC, Heidecker HG, Aschwanden M, Jager KA, Scheffler K, Steinbrich W, Bilecen D. Blood oxygenation level-dependent magnetic resonance imaging of the skeletal muscle in patients with peripheral arterial occlusive disease. Circulation. 2006;113:2929–2935. doi: 10.1161/CIRCULATIONAHA.105.605717. [DOI] [PubMed] [Google Scholar]
- 22.Potthast S, Schulte A, Kos S, Aschwanden M, Bilecen D. Blood Oxygenation Level-Dependent MRI of the Skeletal Muscle during Ischemia in Patients with Peripheral Arterial Occlusive Disease. Fortschr Röntgenstr. 2009;181:1157–1161. doi: 10.1055/s-0028-1109786. [DOI] [PubMed] [Google Scholar]
- 23.Wigmore DM, Damon BM, Pober DM, Kent-Braun JA. MRI measures of perfusion-related changes in human skeletal muscle during progressive contractions. Journal of Applied Physiology. 2004;97:2385–2394. doi: 10.1152/japplphysiol.01390.2003. [DOI] [PubMed] [Google Scholar]
- 24.Damon BM, Gore JC. Physiological basis of muscle functional MRI: Predictions using a computer model. Journal of Applied Physiology. 2004;98:264–273. doi: 10.1152/japplphysiol.00369.2004. [DOI] [PubMed] [Google Scholar]
- 25.Noseworthy MD, Bulte DP, Alfonsi J. BOLD magnetic resonance imaging of skeletal muscle. Seminars in Musculoskeletal Radiology. 2003;7:307–315. doi: 10.1055/s-2004-815678. [DOI] [PubMed] [Google Scholar]
- 26.Wu W-C, Mohler E, Ratcliffe SJ, Wehrli FW, Detre JA, Floyd TF. Skeletal muscle microvascular flow in progressive peripheral artery disease: Assessment with continuous arterial spin-labeling perfusion magnetic resonance imaging. Journal of the American College of Cardiology. 2009;53:2372–2377. doi: 10.1016/j.jacc.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langham MC, Englund EK, Mohler ER, III, Li C, Rodgers ZB, Floyd TF, Wehrli FW. Quantitative CMR markers of impaired vascular reactivity associated with age and peripheral artery disease. Journal of Cardiovascular Magnetic Resonance. 2013;15:1–10. doi: 10.1186/1532-429X-15-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Englund EK, Langham MC, Li C, Rodgers ZB, Floyd TF, Mohler ER, Wehrli FW. Combined measurement of perfusion, venous oxygen saturation, and skeletal muscle T2* during reactive hyperemia in the leg. Journal of Cardiovascular Magnetic Resonance. 2013;15:1–13. doi: 10.1186/1532-429X-15-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FGR, Hiatt WR, Jonsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D on behalf of the American Heart Association Council on Peripheral Vascular Disease, Council on Epidemiology and Prevention, Council on Clinical Cardiology, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Measurement and Interpretation of the Ankle-Brachial Index: A Scientific Statement From the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 30.van Vaals JJ, Brummer ME, Dixon WT, Tuithof HH, Engels H, Nelson RC, Gerety BM, Chezmar JL, Boer den JA. “Keyhole” method for accelerating imaging of contrast agent uptake. J Magn Reson Imaging. 1993;3:671–675. doi: 10.1002/jmri.1880030419. [DOI] [PubMed] [Google Scholar]
- 31.Gold GE, Han E, Stainsby J, Wright G, Brittain J, Beaulieu C. Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. American Journal of Roentgenology. 2004;183:343–351. doi: 10.2214/ajr.183.2.1830343. [DOI] [PubMed] [Google Scholar]
- 32.Wu W-C, Wang J, Detre JA, Ratcliffe SJ, Floyd TF. Transit delay and flow quantification in muscle with continuous arterial spin labeling perfusion-MRI. J Magn Reson Imaging. 2008;28:445–452. doi: 10.1002/jmri.21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spees WM, Yablonskiy DA, Oswood MC, Ackerman J. Water proton MR properties of human blood at 1.5 Tesla: Magnetic susceptibility, T1, T2, T2* and non-Lorentzian signal behavior. Magn Reson Med. 2001;45:533–542. doi: 10.1002/mrm.1072. [DOI] [PubMed] [Google Scholar]
- 34.Jain V, Abdulmalik O, Propert KJ, Wehrli FW. Investigating the magnetic susceptibility properties of fresh human blood for noninvasive oxygen saturation quantification. Magn Reson Med. 2011;68:863–867. doi: 10.1002/mrm.23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Versluis B, Backes WH, van Eupen MGA, Jaspers K, Nelemans PJ, Rouwet EV, Teijink JAW, Mali WPTM, Schurink G-W, Wildberger JE, Leiner T. Magnetic resonance imaging in peripheral arterial disease: reproducibility of the assessment of morphological and functional vascular status. Investigative Radiology. 2011;46:11–24. doi: 10.1097/RLI.0b013e3181f2bfb8. [DOI] [PubMed] [Google Scholar]
- 36.Duteil S, Wary C, Raynaud JS, Lebon V, Lesage D, Leroy-Willig A, Carlier PG. Influence of vascular filling and perfusion on BOLD contrast during reactive hyperemia in human skeletal muscle. Magn Reson Med. 2006;55:450–454. doi: 10.1002/mrm.20760. [DOI] [PubMed] [Google Scholar]
- 37.Lassen NA, Lindbjerg J, Munck O. Measurement of Blood-Flow Through Skeletal Muscle by Intramuscular Injection of Xenon-133. Lancet. 1964;1:686–689. doi: 10.1016/s0140-6736(64)91518-1. [DOI] [PubMed] [Google Scholar]
- 38.Stein R, Hriljac I, Halperin JL, Gustavson SM, Teodorescu V, Olin JW. Limitation of the resting ankle–brachial index in symptomatic patients with peripheral arterial disease. Vasc Med. 2006;11:29–33. doi: 10.1191/1358863x06vm663oa. [DOI] [PubMed] [Google Scholar]
- 39.Mohler ER., III Peripheral arterial disease: identification and implications. Arch Intern Med. 2003:2306–2314. doi: 10.1001/archinte.163.19.2306. [DOI] [PubMed] [Google Scholar]
- 40.Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, Goldberg AP. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: A randomized controlled trial. J Am Geriatr Soc. 2001;49:755–762. doi: 10.1046/j.1532-5415.2001.49152.x. [DOI] [PubMed] [Google Scholar]
- 41.McDermott MM, Ferrucci L, Guralnik JM, Dyer AR, Kiang Liu, Pearce WH, Clark E, Yihua Liao, Criqui MH. The ankle-brachial index is associated with the magnitude of impaired walking endurance among men and women with peripheral arterial disease. Vasc Med. 2010;15:251–257. doi: 10.1177/1358863X10365181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szuba A, Oka RK, Harada R, Cooke JP. Limb hemodynamics are not predictive of functional capacity in patients with PAD. Vasc Med. 2006;11:155–163. doi: 10.1177/1358863x06074828. [DOI] [PubMed] [Google Scholar]
- 43.Englund EK, Rodgers ZB, Langham MC, Mohler ER, III, Floyd TF, Wehrli FW. Simultaneous measurement of microvascular and macrovascular blood flow and oxygenation in the leg. Proceedings of the International Society for Magnetic Resonance in Medicine. 2014;22:722. doi: 10.1002/mrm.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chance B, Nioka S, Kent J, McCully K, Fountain M, Greenfeld R, Holtom G. Time-Resolved Spectroscopy of Hemoglobin and Myoglobin in Resting and Ischemic Muscle. Anal Biochem. 1988;174:698–707. doi: 10.1016/0003-2697(88)90076-0. [DOI] [PubMed] [Google Scholar]
- 45.Mesquita RC, Putt M, Chandra M, Yu G, Xing X, Han SW, Lech G, Shang Y, Durduran T, Zhou C, Yodh AG, Mohler ER. Diffuse optical characterization of an exercising patient group with peripheral artery disease. J Biomed Opt. 2013;18:57007. doi: 10.1117/1.JBO.18.5.057007. [DOI] [PMC free article] [PubMed] [Google Scholar]


