Abstract
Almost all cancers show intrinsic and/or evasive resistance to vascular endothelial growth factor (VEGF) inhibitors by multiple mechanisms. Serum angiopoietin-2 (Ang2) level has been proposed as a potential biomarker of VEGF inhibitor response in several cancers. From these clinical observations, the Ang2 and Tie2 (its receptor) axis has been focused on as a promising target. Here, we show a novel strategy to circumvent the resistance by combining multi-tyrosine kinase inhibitors lenvatinib (VEGF receptor, fibroblast growth factor receptor, and RET inhibitor) and golvatinib (E7050; c-Met, Tie2, and EphB4 inhibitor). Tie2 identifies a highly pro-angiogenic macrophage subset, Tie2-expressing macrophages (TEM). Angi-Tie2 and EphB4-EphrinB2 signaling plays critical roles in pericyte-mediated vessel stabilization. In vitro analyses suggested that golvatinib combined with lenvatinib inhibited pericyte-mediated vessel stabilization and TEM differentiation. In thyroid and endometrial cancer models, golvatinib and lenvatinib inhibited pericyte network development and TEM infiltration, resulting in severe perfusion disorder and massive apoptosis. Body weight loss was tolerable, and no macroscopic change was observed. These preclinical studies suggest that modulation of the tumor microenvironment by a strategic and well-tolerated combination of multi-targeting tyrosine kinase inhibitors may sensitize cancer to VEGF inhibitors.
Keywords: Ang2, angiogenesis, golvatinib, lenvatinib, microenvironment
Clinical studies have shown that serum Ang2 levels are decreased in patients treated with VEGF inhibitors and are increased at the time of disease progression,1 and that lower levels of Ang2 correlate with treatment response.2 Lenvatinib is a tyrosine kinase inhibitor of VEGFR1–3, fibroblast growth factor receptor 1–4, platelet-derived growth factor receptor α, RET, and KIT kinases and is currently undergoing several clinical trials, including differentiated thyroid cancer and endometrial cancer.3,4 Although Ang2 was originally identified as a physiological Tie2 antagonist for Ang1, several groups have shown that its effects depend on context. In stressed endothelial cells, especially in cancer, Ang2 can activate Tie2 signaling and contribute to vessel stabilization.5,6 Vessel stabilization is tightly associated with pericyte assembly, which is regulated by Ang1-Tie2 and EphrinB2-EphB4 signaling.7–9 It is reported that tumor vessels acquire pericyte coverage during anti-angiogenesis therapy and that such stabilized vessels are less sensitive to VEGF inhibitors.10,11 Activation of TEMs, a subset of tumor-associated macrophages, is also regulated by Ang2-Tie2 signaling in response to anti-angiogenesis therapy.12,13 Tie2-expressing macrophages stimulate angiogenesis by releasing non-VEGF angiogenic factors and by downregulating anti-angiogenic signaling.14
Materials and Methods
Compounds
Lenvatinib (E7080) (4-[3-chloro-4-(N′-cyclopropylureido)phenoxy]-7-methoxyquinoline-6-carboxamide mesylate) and golvatinib (E7050) N-[2-fluoro-4-({2-[4-(4-methylpiperazin-1-yl)piperidin-1-yl] carbonylaminopyridin-4-yloxy) phenyl]-N′-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide (2R,3R)-tartrate were synthesized by Eisai Co., Ltd (Tsukuba, Japan).
Cell preparation
K1 (human thyroid cancer) and A2780 (human ovarian cancer) cell lines were obtained from Dainippon Sumitomo Pharma (Tokyo, Japan). AN3CA (human endometrial cancer), IMR90, and MRC5 (human normal fibroblast) cell lines were obtained from ATCC (Manassas, VA, USA). Human brain vascular pericytes were obtained from ScienCell Carlsbad, CA, USA. MKN45 (human gastric cancer) cell line was obtained from the Japanese Collection of Research Bioresources Cell Bank Tokyo, Japan. Human umbilical vascular endothelial cells were isolated from human umbilical cords and cultured in EGM-2 (Eidia) Tokyo, Japan. Human Ang2, AcGFP, and red fluorescent protein were subcloned into pCLXSN retroviral expression vectors (Imgenex) San Diego, CA, USA. K1, HUVEC, and HBVP cells were infected with the retroviral vectors according to the manufacturer's instructions.
Animal studies
Nude mice (CAnN.Cg-Foxn1nu/CrlCrlj; female, 5–6 weeks old) were obtained from Charles River Laboratories Yokohama, Japan. Cancer cells (5 × 106 cells/head) were implanted s.c. When the tumor volume reached 100–300 mm3, mice were randomized into treatment groups. Lenvatinib or golvatinib were given orally once a day. The tumor volume was calculated by using the following formula: tumor volume (mm3) = 1/2 × length (mm) × width (mm)2. For the isolation of splenic macrophages, BALB/cAnNCrlCrlj mice (female, 5–6 weeks old) were obtained from Charles River Laboratories. All of the animal experiments were carried out in accordance with the guidelines for animal experiments of Eisai Co., Ltd.
Histological analysis
Tumors were embedded in OCT compound (Sakura) Tokyo, Japan. Cryosections were prepared and stained with APC-conjugated anti-CD31 antibody (BD Bioscience) San Jose, CA, Japan, anti-SMA antibody (Abcam) Cambridge, MA, USA, Alexa Fluor 568-conjugated anti-rabbit IgG antibody (Invitrogen) Carlsbad, CA, USA, PE-conjugated anti-CD11b antibody (eBioscience) San Diego, CA, USA, Alexa Fluor 488-conjugated anti-F4/80 antibody (AbD Serotech), biotin-conjugated anti-mannose receptor, C-type 1 (Mrc1) antibody (BioLegend) San Diego, CA, USA, or Alexa Fluor 555-conjugated streptavidin (Invitrogen). In some experiments, tumor-bearing mice were perfused with Hoechst 33342 (Invitrogen), and cryosections were stained by using a TUNEL-based In Situ Cell Death Detection Kit, TMR Red (Roche) Mannheim, Germany. Fluorescence images were obtained with a Biorevo BZ-9000 microscope (Keyence) Osaka, Japan. CD31, SMA, Hoechst, or TUNEL fluorescence was quantitated by using BZ-II Analyzer software (Keyence). CD11b, F4/80, or Mrc1 fluorescence was quantitated in five view fields per tumor at 10× magnification.
Flow cytometric analysis
Tumors were enzymatically digested with collagenase type I (1.6 kU/mL; Sigma) St. Louis, MO, USA and DNase type I (1.5 kU/mL; Sigma). Digested tumor suspension was stained with PE-conjugated anti-CD11b antibody (BD Bioscience), PE-conjugated anti-F4/80 antibody (AbD Serotech), or PE-conjugated anti-CD31 antibody (BioLegend), and analyzed by using a FACS Aria II system (BD Bioscience). Cells were gated according to forward/side scatter, and dead cells were excluded by using propidium iodide (Sigma) staining. In some experiments, cells were sorted from the mixture of all the tumors in each group. The whole RNA was extracted, and cDNA was obtained by using a Cells-to-CT kit (Applied Biosystems) Foster City, CA, USA for quantitative PCR analysis.
Cell-free kinase assay
Cell-free kinase activities were determined by Carna Biosciences. Briefly, the compound solution and substrate/ATP/metal solution were mixed and incubated for 1 or 5 h at room temperature. The reaction mixture was applied to a LabChip3000 system (Caliper Life Science) Hopkinton, MA, USA, and the product and substrate peptide peaks were separated and quantitated. The kinase reaction was evaluated by the product ratio calculated from peak heights of product and substrate peptides.
Cell-based kinase assay
Cellular kinase activities were determined by ProQinase Freiburg, Germany. Briefly, phosphorylation of Tie2 was assessed in Chinese hamster ovary cells stably overexpressing human Tie2. Phosphorylation of Tie2 was stimulated with 10 mM sodium orthovanadate. Phosphorylation of EphB4 was assessed in mouse embryonic fibroblasts expressing human EphB4. Phosphorylation of EphB4 was stimulated with 2 μg/mL EphrinB2-Fc. Phosphorylation of c-Met was assessed in MKN45 cells that have constitutively active c-Met. Quantification of substrate phosphorylation was assessed using sandwich ELISA by using a substrate-specific capture antibody and an anti-phosphotyrosine detection antibody.
Endothelial network formation assay based on 2D coculture
The HUVEC/AcGFP cells and HBVPs were mixed to densities of 1.3 × 104 and 1.87 × cells/mL, respectively, with EGM-2. Cell suspensions were dispensed at 100 μL/well in collagen type-I-coated 96-well plates and cultured for 10 days. Compound- or vehicle-containing EGM-2 was added, and the cells were incubated for an additional 4 days. Fluorescence images of the HUVEC/AcGFP network were obtained with an IN Cell Analyzer 1000 (GE Healthcare) Waukesha, WI, USA. The obtained images were analyzed by using Angiogenesis Image Analyzer software version 2.0 (Kurabo) Osaka, Japan to measure the length of the endothelial network. For the quantification of Ang1, Ang2, and HGF protein levels, the culture supernatants were collected at day 14. The concentration was determined by using a Quantikine Ang1 ELISA kit, a Quantikine Ang2 ELISA kit, or a Quantikine HGF ELISA kit (R&D) Minneapolis, MN, USA.
Sprouting assay based on 3D coculture
Spheroids containing HUVEC/AcGFP cells and HBVPs were prepared by culturing 10 000 HUVEC/AcGFP cells and 5000 HBVPs in hanging drops for 24 h. Spheroids were suspended in 2.1 mg/mL collagen (Cellmatrix Type IA; Nitta Gelatin) Osaka, Japan and dispensed into 24-well plates. Endothelial sprouting was stimulated with EGM-2 for 10 days. Compound- or vehicle-containing EGM-2 was added, and the cells were incubated for an additional 4 days. Fluorescence images of HUVEC/AcGFP cells were obtained by using a Biorevo BZ-9000 microscope.
In vitro macrophage assay
Mononuclear cells were separated from spleens of BALB/cAnNCrlCrlj mice by using Lymphoprep (Axis-Shield) Oslo, Norway and then spread onto culture plates. Non-adherent cells were removed after 1 h, and macrophage-enriched adherent cells were cultured for 24 h. The cells were then cultured with recombinant mouse Ang2 (200 ng/mL; R&D), lenvatinib, or golvatinib in a normoxic or hypoxic atmosphere for an additional 24 h. For quantitative PCR analysis, cDNA was obtained by using a Cells-to-CT kit.
Quantitative PCR analysis
Real-time quantitative PCR was carried out with an ABI7900 system (Applied Biosystems). The following TaqMan probes were used: EphB4, Hs00174752_m1; Efnb2, Hs00187950_m1; Tie2, Mm00443243_m1; Mrc1, Mm00485148_m1; Ang2, Mm00657574_s1; Vegfa, Mm01281449_m1; Fgf2, Mm00433287_m1; Hgf, Mm01135193_m1; Igf1, Mm00439560_m1; Il10, Mm00439614_m1; Il12a, Mm00434165_m1; and Gapdh, Mm99999915_g1. Mean Ct values were obtained from triplicate reactions. Relative expression levels were calculated by using the 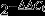 method.
method.
Quantification of VEGF and HGF protein levels in culture supernatants of cancer cells
For the quantification of VEGF protein levels, cancer cells (5 × 103 cells/cm2) were seeded on 24-well plates and incubated for 48 h. The medium was then changed, and the cells were incubated for an additional 48 h in a normoxic or hypoxic atmosphere. For the quantification of HGF protein levels, cancer cells (5 × 103 cells/cm2) were seeded and incubated for 72 h. The concentration of VEGF or HGF in those culture supernatants was determined by using a Quantikine VEGF ELISA Kit or a Quantikine HGF ELISA kit, respectively.
Cell proliferation assay
Cells (1500 cells/100 μL/well) were seeded on 96-well culture plates with various concentrations of golvatinib and cultured for 3 days, then WST-8 reagent (Dojindo) Kumamoto, Japan was added to each well. The absorbance for each well was measured at 450 nm by using a microplate reader.
Results
Golvatinib treatment inhibited HUVEC network stabilization and Tie2 upregulation in macrophage
Golvatinib was originally developed as a tyrosine kinase inhibitor of c-Met,15,16 but showed inhibitory activities against a variety of kinases in the cell-free kinase assay (Table S1). Among angiogenesis-related kinases, golvatinib had especially high activities to Tie2 and EphB4. Therefore, we focused on Tie2 and EphB4 as secondary targets, which have the possibility to potentiate VEGF inhibitors' efficacy. The cell-based kinase assay also showed that golvatinib was a potent inhibitor of Tie2 and EphB4 (Fig.1a). Next, we examined the effect of golvatinib on pericyte-mediated vessel stabilization and TEM differentiation. First, we quantitated the network length of HUVECs on a monolayer of HBVPs or human fibroblasts (IMR90 or MRC5) with lenvatinib (Fig. S1a). Using this system, we could focus the effect on network stability or maturation, because network formation of HUVECs was complete at the time we started the assay. The HUVECs on HBVPs showed lower sensitivity to lenvatinib than those on the other cells, suggesting that they underwent pericyte-mediated network stabilization. The lenvatinib/golvatinib combination disrupted the lenvatinib-resistant HUVEC network (Fig.1b). Higher EphB4 expression was observed on HUVECs rather than on HBVPs, and higher EphrinB2 expression was observed on HBVPs than on HUVECs (Fig. S1b). This might suggest that the EphB4-EphrinB2 intercellular signaling pathway is activated in the HUVEC–HBVP coculture system. However, a high level of Ang1 and HGF proteins was also observed in the supernatant of HUVEC–HBVP, suggesting active Tie2 and c-Met signaling (Fig. S1c). From these results, golvatinib probably inhibited all of these signalings and showed sensitization to lenvatinib. We next established a HUVEC/HBVP spheroid sprouting assay. Addition of HBVPs to HUVEC spheroids induced the formation of a stable network (Fig. S1d). Although lenvatinib treatment inhibited only the extension at the network's periphery, the lenvatinib/golvatinib combination broadly disrupted the whole network (Fig.1c). These results suggest that golvatinib inhibits pericyte function by inhibiting EphB4 and/or Tie2 and sensitizes the lenvatinib-resistant network. Next, we determined the effect of golvatinib on TEM differentiation. Hypoxia and Ang2 upregulate Tie2 expression in macrophages.13,17 Therefore, Tie2 expression in mouse splenic macrophages was examined by using recombinant Ang2 and a hypoxic atmosphere. Golvatinib inhibited the Tie2 upregulation induced by hypoxia and Ang2 (Fig.1d). These data suggest that golvatinib can inhibit TEM activation by inhibiting Ang2-Tie2 signaling.
Fig 1.
Golvatinib treatment inhibits pericyte-mediated network stabilization of HUVECs and Tie2 upregulation in mouse macrophages. (a) Kinase activities of c-Met, Tie2, and EphB4 were assessed using a cell-based kinase assay in the presence of golvatinib. (b) HUVEC/Aequorea coerulescens green fluorescent protein (AcGFP) network formation assay on a human brain vascular pericyte (HBVP) monolayer. Drug treatment was started at day 10. HUVEC network length was quantitated at day 14 and relative values are shown. Data are presented as mean ± SD (n = 9). **P < 0.01, ***P < 0.001 versus lenvatinib single treatment, Student's t-test. (c) HUVEC/AcGFP sprouting assay based on 3D coculture with HBVP. Drug treatment was started at day 10. Fluorescence images of HUVEC/AcGFP were obtained at days 10 and 14. Scale bar = 300 μm. (d) Macrophages isolated from mouse spleen were cultured with mouse angiopoietin-2 (Ang2) and lenvatinib, golvatinib, or lenvatinib/golvatinib in combination in a normoxic or hypoxic atmosphere for 24 h. Tie2mRNA expression level was analyzed by quantitative PCR and relative values are shown. Experiments were carried out three times, and a representative result is shown.
Golvatinib treatment sensitized tumors to lenvatinib
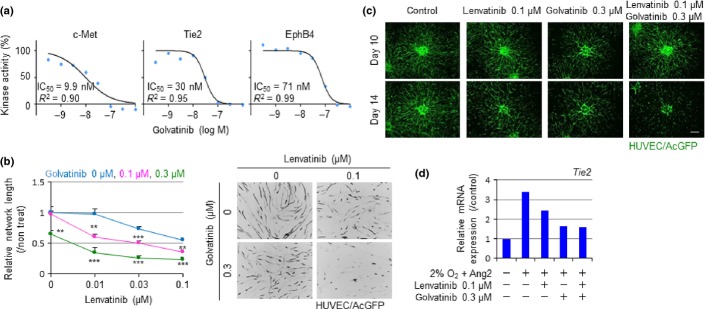
The lenvatinib/golvatinib combination was then evaluated in K1/Ang2 and AN3CA xenograft models. The K1 differentiated thyroid cancer cell line, which is highly sensitive to lenvatinib in a mouse xenograft model, was transduced with Ang2. Both VEGF and Ang2 signaling are known to play critical and cooperative roles in tumor hypoxic environments.18 AN3CA cells showed high VEGF secretion in hypoxic conditions (Fig. S2a). To exclude the involvement of c-Met inhibition by golvatinib, we confirmed that golvatinib treatment did not strongly inhibit cell proliferation (Fig. S2b), and that these cells did not secrete HGF (Fig. S2c). Golvatinib treatment showed a combination effect with lenvatinib in both models (Fig.2a). Body weight loss was tolerable, and no macroscopic abnormalities were observed (Fig. S3a). Quantitative PCR analysis of CD31+ endothelial cells sorted from AN3CA tumor showed that lenvatinib treatment induced endothelial cell Ang2 expression (Fig. S3b). This was probably the result of the endothelial response to lenvatinib-induced hypoxia. To mark functional vessels, the DNA-binding dye Hoechst was perfused into mice. Tumor cryosections were then stained using a TUNEL method. A significant decrease of Hoechst perfusion and increase of cell apoptosis were observed in the lenvatinib/golvatinib combination group of both models (Fig.2b). Interestingly, double staining of Hoechst and TUNEL showed cell apoptosis only in non-perfused areas (Fig.2c), suggesting that the antitumor effect of the combination can be explained by the inhibition of tumor angiogenesis.
Fig 2.
Golvatinib treatment sensitizes tumors to lenvatinib with a significant decrease in blood perfusion. (a) Nude mice bearing K1/angiopoietin-2 (Ang2) or AN3CA tumors were treated with vehicle, lenvatinib 10 mg/kg, golvatinib 100 mg/kg, or lenvatinib/golvatinib in combination. Tumor volume was measured using calipers on the indicated days and relative values are shown. Data are presented as mean ± standard deviation (n = 6). *P < 0.05, ***P < 0.001, ****P < 0.0001 versus vehicle at the end of treatment, Student's t-test. #P < 0.05, two-way anova. (b) Hoechst perfusion and TUNEL assay of tumors. Fluorescent areas were quantitated and relative values are shown. Data are presented as mean ± SD (n = 6). *P < 0.05, **P < 0.01 versus vehicle, Student's t-test. Scale bar = 2 mm. (c) Hoechst and TUNEL double staining images of tumor treated with lenvatinib/golvatinib in combination.
Golvatinib/lenvatinib in combination inhibited pericyte network development in vivo
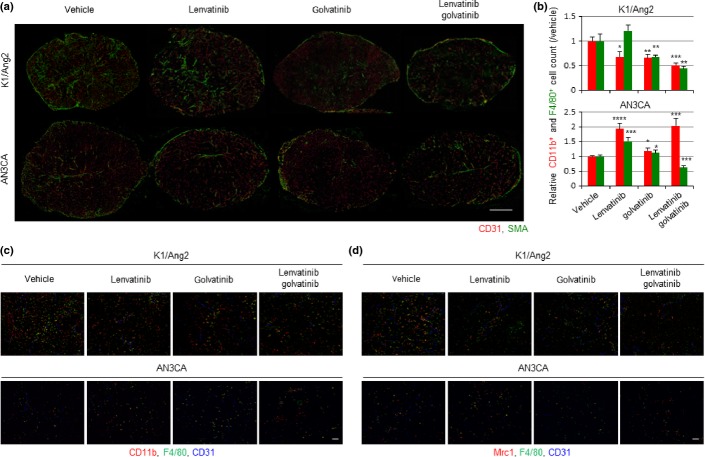
In both models, treatment with lenvatinib or golvatinib reduced the CD31+ endothelial network, and the combination resulted in the largest reduction (Figs3a,S4a). In the K1/Ang2 tumor, golvatinib treatment drastically reduced the SMA+ pericyte network. The other group reported that EphB4-specific blockade also induced pericyte network disruption and decreased interaction between endothelial cells and pericytes.8 These results indicate that lenvatinib further disrupted the endothelial network destabilized by pericyte depletion. Compared to K1/Ang2 tumor, AN3CA tumor showed a poor pericyte network, which was not significantly affected by drug treatment (Figs3a,S4a). Golvatinib-induced pericyte depletion could be one of the reasons for the sensitization to lenvatinib in the K1/Ang2 model, but not in the AN3CA model.
Fig 3.
Golvatinib treatment in combination with lenvatinib inhibits pericyte development and Tie2-expressing macrophage infiltration in vivo. (a) Double immunostaining of CD31 and smooth muscle actin (SMA) in tumor. Scale bar = 2 mm. (b) Flow cytometric analysis of digested tumor. The numbers of live CD11b+ and F4/80+ cells were counted and relative values are shown. Data are presented as mean ± SD (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus vehicle, Student's t-test. (c) Triple immunostaining of CD11b, F4/80, and CD31 in tumor. Scale bar = 100 μm. (d) Triple immunostaining of mannose receptor, C-type 1 (Mrc1), F4/80, and CD31 in tumor. Scale bar = 100 μm.
Golvatinib/lenvatinib in combination inhibited Tie2-expressing macrophage infiltration in vivo
We next evaluated the effect of treatment on tumor-infiltrating macrophages. In K1/Ang2 tumor, a significant decrease in myeloid cells (CD11b+) was observed in all treatment groups by using immunohistochemical and flow cytometric analyses (Figs3b,c,S4b). However, a significant decrease in macrophages (F4/80+ in flow cytometry, CD11b+F4/80+ in immunohistochemistry) was observed only in the golvatinib treatment groups. It appears that forced expression of Ang2 results in active recruitment of myeloid cells in coordination with VEGF signaling, and macrophage recruitment is mainly regulated by Ang2-Tie2 signaling. In the AN3CA tumor, lenvatinib-induced hypoxia stimulated VEGF expression in AN3CA cells and Ang2 expression in endothelial cells (Figs S2a,S3b), leading to enhanced myeloid cell and macrophage recruitment (Figs3b,c,S4b). In the combination group, Ang2-Tie2 signal inhibition by golvatinib probably decreased macrophage population specifically. Next, we examined the immunohistochemistry of Mrc1 and quantitative PCR using F4/80+ macrophage sorted from tumor. Tie2-expressing macrophages can be distinguished from Tie2− tumor-associated macrophages by their surface marker, Mrc1.19 In the K1/Ang2 xenograft model, a significant decrease in F4/80+Mrc1+ macrophages was observed in all treatment groups (Figs3d,S4c). Because F4/80+ macrophage recruitment was also decreased in lenvatinib-treated tumor, there was no significant change in the ratio of F4/80+Mrc1+ macrophages to total F4/80+ macrophages. Golvatinib-treated tumor showed significant decreases in both the area and ratio of F4/80+Mrc1+ macrophages (Figs3d,S4c). In the AN3CA xenograft model, lenvatinib treatment increased the area and ratio of F4/80+Mrc1+ macrophages, and the lenvatinib/golvatinib combination negated that increase.
Golvatinib/lenvatinib in combination altered the gene expression pattern of macrophage to a Tie2-expressing macrophage-like pattern in vivo
The results of quantitative PCR analyses supported the immunohistochemistry data. Macrophages in golvatinib-treated K1/Ang2 tumor showed lower expression of Mrc1 and angiogenic growth factors (Fig.4). In the AN3CA tumor, lenvatinib treatment increased Mrc1 expression in macrophages, and the lenvatinib/golvatinib combination decreased Mrc1 and angiogenic factor expression (Fig.4). In reference to the M1-M2 paradigm, TEM has been reported to show some phenotypes of M2-polarized macrophages.14,19 Therefore, we examined the expression of IL10, an M2 marker, and IL12α, an M1 marker, in macrophages. Interleukin-12α is an anti-angiogenic factor. In K1/Ang2 tumor, the lenvatinib/golvatinib combination decreased IL10 expression in macrophages, and golvatinib treatment increased IL12α expression (Fig.4). In AN3CA tumor, treatment with lenvatinib or golvatinib increased IL10 expression and decreased IL12α expression, and combination treatment negated the changes. Together, these results show that the lenvatinib/golvatinib combination inhibited Ang2- or hypoxia-induced TEM activation in both models, resulting in sensitization to lenvatinib.
Fig 4.
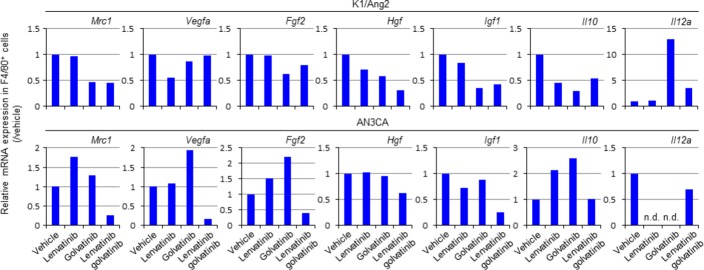
Golvatinib/lenvatinib in combination alters the gene expression pattern of F4/80+ macrophages to a Tie2-expressing macrophage-like pattern in vivo. F4/80+ macrophages were sorted from the mixture of all the tumors in each group at the end of treatment using flow cytometry. The whole RNA was extracted, and Mrc1,Vegfa,Fgf2,Hgf,Igf1,Il10, and Il12amRNA expression levels were analyzed using quantitative PCR and relative values are shown. n.d., not determined.
Discussion
Although VEGF inhibitors provide significant clinical benefit, they often require dose reduction or withdrawal due to their severe toxicity.20 Furthermore, almost all cancers can show resistance to VEGF inhibitors through various mechanisms.21 Even in preclinical models, K1/Ang2 and AN3CA cells showed different resistance profiles regarding the contribution of pericytes and TEMs. Simultaneous intervention in all possible mechanisms would be the ideal strategy. Indeed, neutralization of Ang2 alone results in increased interaction between endothelial cells and pericytes.6,13
Clinical studies have shown that the level of serum Ang2 is a key indicator of VEGF inhibitor resistance, which can manifest as intrinsic refractoriness2,22 or evasive escape1 against VEGF inhibitors. In K1/Ang2 tumor-bearing mice, high serum Ang2 levels were maintained in non-treated mice, and TEM activation and pericyte network development were observed. That is, this tumor is a model of intrinsic refractoriness with a high baseline level of Ang2. However, in AN3CA tumors, lenvatinib treatment increased both Ang2 expression in tumor endothelial cells and TEM infiltration. Serum Ang2 was also slightly increased in lenvatinib-treated mice in this model (data not shown). These observations might be considered one aspect of evasive escape, because the serum Ang2 level is reported to increase at the time of disease progression in the clinic.1
Our preclinical study suggests that the combination of lenvatinib and golvatinib sensitizes certain cancers through a range of mechanisms, which may allow the clinical dose to be reduced. Inhibition of c-Met by golvatinib would also be favorable for the sensitization because HGF was reported as a resistant factor.2 From these aspects, several phase II clinical trials of golvatinib combination are currently underway with the aim of studying the effect of combination therapy on antitumor activity.23
Disclosure Statement
All authors are employees of Eisai Co., Ltd.
Glossary
- AcGFP
Aequorea coerulescens green fluorescent protein
- Ang2
angiopoietin-2
- HBVP
human brain vascular pericyte
- HGF
hepatocyte growth factor
- IL
interleukin
- Mrc1
mannose receptor, C-type 1
- PE
phycoerythrin
- SMA
smooth muscle actin
- TEM
Tie2-expressing macrophage
- VEGF
vascular endothelial growth factor
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1.Coculture with human brain vascular pericytes (HBVPs) stabilizes HUVEC network. (a) HUVEC/Aequorea coerulescens green fluorescent protein (AcGFP) network formation assay on HBVP, IMR90, or MRC5 monolayers. Drug treatment was started at day 10. HUVEC network length was quantitated at day 14 and relative values are shown. Data are presented as mean ± SD (n = 9). *P < 0.05, ****P < 0.0001 versus HBVP, Student's t-test. (b) mRNA expressions of EphB4 and EphrinB2 were determined by quantitative PCR. (c) Protein levels of angiopoietin-1 (Ang1), Ang2, and hepatocyte growth factor (HGF) in culture supernatants were determined by ELISA. (d) HUVEC/AcGFP sprouting assay with or without HBVP coculture. Fluorescence images of HUVEC/AcGFP networks were obtained on the indicated days. Scale bar = 300 μm.
Fig. S2. K1/angiopoietin-2 (Ang2) and AN3CA cells are not involved in hepatocyte growth factor (HGF)–c-Met signaling. (a) Culture supernatants were collected from AN3CA cells cultured in a normoxic or hypoxic atmosphere for 48 h. Vascular endothelial growth factor (VEGF) protein level was quantitated and is shown as a value relative to that obtained in a normoxic atmosphere. (b) Cell proliferations of K1/Ang2, AN3CA, and MKN45 were assessed in the presence of golvatinib at the indicated concentrations. (c) Culture supernatants were collected from A2780, K1/Ang2, and AN3CA after culture for 72 h. HGF protein levels were quantitated and are shown in the bar graph.
Fig. S3. Golvatinib/lenvatinib combination treatment shows antitumor effects with high tolerability. (a) Representative images of tumors. (b) Relative body weight curves of tumor-bearing mice during drug treatment. (c) CD31+ endothelial cells were sorted from AN3CA tumor at the end of treatment using flow cytometry. Ang2 mRNA expression was analyzed with quantitative PCR and relative values are shown.
Fig. S4. Golvatinib treatment in combination with lenvatinib inhibits pericyte development and Tie2-expressing macrophage infiltration in vivo. (a) CD31+ and SMA+ fluorescent areas were quantitated and relative values are shown. Data are presented as mean ± SD (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle, Student's t-test. (b) CD11b+ and CD11b+F4/80+ fluorescent areas were quantitated and relative values are shown. Data are presented as mean ± SD (n = 4). **P < 0.01 versus vehicle, Student's t-test. (c) F4/80+ and F4/80+Mrc1+ fluorescent areas were quantitated and relative values are shown. The ratio of F4/80+Mrc1+ area to total F4/80+ area is also shown. Data are presented as mean ± SD (n = 4). *P < 0.05, *P < 0.01 versus vehicle, Student's t-test.
Table S1. IC50 values of golvatinib against various kinases in a cell-free system
References
- van der Veldt AA, Vroling L, de Haas RR, et al. Sunitinib-induced changes in circulating endothelial cell-related proteins in patients with metastatic renal cell cancer. Int J Cancer. 2012;131:E484–93. doi: 10.1002/ijc.26456. [DOI] [PubMed] [Google Scholar]
- Miyahara K, Nouso K, Tomoda T, et al. Predicting the treatment effect of sorafenib using serum angiogenesis markers in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1604–11. doi: 10.1111/j.1440-1746.2011.06887.x. [DOI] [PubMed] [Google Scholar]
- Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14(17):5459–65. doi: 10.1158/1078-0432.CCR-07-5270. [DOI] [PubMed] [Google Scholar]
- Boss DS, Glen H, Beijnen JH, et al. A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br J Cancer. 2012;106:1598–604. doi: 10.1038/bjc.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C, Pasnikowski E, Burova E, et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci U S A. 2006;103(42):15491–6. doi: 10.1073/pnas.0607538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly C, Eichten A, Castanaro C, et al. Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it limits the effects of VEGF inhibition. Cancer Res. 2013;73:108–18. doi: 10.1158/0008-5472.CAN-12-2064. [DOI] [PubMed] [Google Scholar]
- Augustin HG, Young Koh G, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165–77. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Djokovic D, Trindade A, Gigante J, et al. Combination of Dll4/Notch and Ephrin-B2/EphB4 targeted therapy is highly effective in disrupting tumor angiogenesis. BMC Cancer. 2010;10:641. doi: 10.1186/1471-2407-10-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erber R, Eichelsbacher U, Powajbo V, et al. EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. EMBO J. 2006;25(3):628–41. doi: 10.1038/sj.emboj.7600949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellberg C, Ostman A, Heldin CH. PDGF and vessel maturation. Recent Results Cancer Res. 2010;180:103–14. doi: 10.1007/978-3-540-78281-0_7. [DOI] [PubMed] [Google Scholar]
- Korff T, Kimmina S, Martiny-Baron G, Augustin HG. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001;15:447–57. doi: 10.1096/fj.00-0139com. [DOI] [PubMed] [Google Scholar]
- Huang H, Lai JY, Do J, et al. Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration, and tumor growth. Clin Cancer Res. 2011;17:1001–11. doi: 10.1158/1078-0432.CCR-10-2317. [DOI] [PubMed] [Google Scholar]
- Mazzieri R, Pucci F, Moi D, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–26. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Tal AO, Scholz A, et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010;70(13):5270–80. doi: 10.1158/0008-5472.CAN-10-0012. [DOI] [PubMed] [Google Scholar]
- Wang W, Li Q, Takeuchi S, et al. Met kinase inhibitor E7050 reverses three different mechanisms of hepatocyte growth factor-induced tyrosine kinase inhibitor resistance in EGFR mutant lung cancer. Clin Cancer Res. 2012;18(6):1663–71. doi: 10.1158/1078-0432.CCR-11-1171. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Matsushima T, Kawano S, et al. Lenvatinib in combination with golvatinib overcomes hepatocyte growth factor pathway-induced resistance to vascular endothelial growth factor receptor inhibitor. Cancer Sci. 2014;105:723–30. doi: 10.1111/cas.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C, Tazzyman S, Webster S, Lewis CE. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol. 2007;178(11):7405–11. doi: 10.4049/jimmunol.178.11.7405. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Farhadi M, Gaumann A, et al. Microtumor growth initiates angiogenic sprouting with simultaneous expression of VEGF, VEGF receptor-2, and angiopoietin-2. J Clin Invest. 2002;109:777–85. doi: 10.1172/JCI14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci F, Venneri MA, Biziato D, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–14. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- Tesarova P, Tesar V. Proteinuria and hypertension in patients treated with inhibitors of the VEGF signalling pathway–incidence, mechanisms and management. Folia Biol (Praha) 2013;59(1):15–25. [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goede V, Coutelle O, Neuneier J, et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br J Cancer. 2010;103(9):1407–14. doi: 10.1038/sj.bjc.6605925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil BH, Bendell JC, Modiano MR, et al. Phase I/II study of E7050 (golvantinib) in combination with sorafenib in patients (pts) with advanced hepatocellular carcinoma (HCC): Phase I results. J Clin Oncol. 2013;31 (Suppl: abstr 294) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1.Coculture with human brain vascular pericytes (HBVPs) stabilizes HUVEC network. (a) HUVEC/Aequorea coerulescens green fluorescent protein (AcGFP) network formation assay on HBVP, IMR90, or MRC5 monolayers. Drug treatment was started at day 10. HUVEC network length was quantitated at day 14 and relative values are shown. Data are presented as mean ± SD (n = 9). *P < 0.05, ****P < 0.0001 versus HBVP, Student's t-test. (b) mRNA expressions of EphB4 and EphrinB2 were determined by quantitative PCR. (c) Protein levels of angiopoietin-1 (Ang1), Ang2, and hepatocyte growth factor (HGF) in culture supernatants were determined by ELISA. (d) HUVEC/AcGFP sprouting assay with or without HBVP coculture. Fluorescence images of HUVEC/AcGFP networks were obtained on the indicated days. Scale bar = 300 μm.
Fig. S2. K1/angiopoietin-2 (Ang2) and AN3CA cells are not involved in hepatocyte growth factor (HGF)–c-Met signaling. (a) Culture supernatants were collected from AN3CA cells cultured in a normoxic or hypoxic atmosphere for 48 h. Vascular endothelial growth factor (VEGF) protein level was quantitated and is shown as a value relative to that obtained in a normoxic atmosphere. (b) Cell proliferations of K1/Ang2, AN3CA, and MKN45 were assessed in the presence of golvatinib at the indicated concentrations. (c) Culture supernatants were collected from A2780, K1/Ang2, and AN3CA after culture for 72 h. HGF protein levels were quantitated and are shown in the bar graph.
Fig. S3. Golvatinib/lenvatinib combination treatment shows antitumor effects with high tolerability. (a) Representative images of tumors. (b) Relative body weight curves of tumor-bearing mice during drug treatment. (c) CD31+ endothelial cells were sorted from AN3CA tumor at the end of treatment using flow cytometry. Ang2 mRNA expression was analyzed with quantitative PCR and relative values are shown.
Fig. S4. Golvatinib treatment in combination with lenvatinib inhibits pericyte development and Tie2-expressing macrophage infiltration in vivo. (a) CD31+ and SMA+ fluorescent areas were quantitated and relative values are shown. Data are presented as mean ± SD (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle, Student's t-test. (b) CD11b+ and CD11b+F4/80+ fluorescent areas were quantitated and relative values are shown. Data are presented as mean ± SD (n = 4). **P < 0.01 versus vehicle, Student's t-test. (c) F4/80+ and F4/80+Mrc1+ fluorescent areas were quantitated and relative values are shown. The ratio of F4/80+Mrc1+ area to total F4/80+ area is also shown. Data are presented as mean ± SD (n = 4). *P < 0.05, *P < 0.01 versus vehicle, Student's t-test.
Table S1. IC50 values of golvatinib against various kinases in a cell-free system