Abstract
The aim of the present study was to investigate the feasibility of measuring metabolic tumor burden using [F-18] fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) treated with bendamustine–rituximab. Because the standardized uptake value is a critical parameter of tumor characterization, we carried out a phantom study of 18F-FDG PET/CT to ensure quality control for 28 machines in the 24 institutions (Japan, 17 institutions; Korea, 7 institutions) participating in our clinical study. Fifty-five patients with relapsed or refractory DLBCL were enrolled. The 18F-FDG PET/CT was acquired before treatment, after two cycles, and after the last treatment cycle. Treatment response was assessed after two cycles and after the last cycle using the Lugano classification. Using this classification, remission was complete in 15 patients (27%) and incomplete in 40 patients (73%) after two cycles of therapy, and remission was complete in 32 patients (58%) and incomplete in 23 patients (42%) after the last treatment cycle. The percentage change in all PET/CT parameters except for the area under the curve of the cumulative standardized uptake value–volume histogram was significantly greater in complete response patients than in non-complete response patients after two cycles and the last cycle. The Cox proportional hazard model and best subset selection method revealed that the percentage change of the sum of total lesion glycolysis after the last cycle (relative risk, 5.24; P = 0.003) was an independent predictor of progression-free survival. The percent change of sum of total lesion glycolysis, calculated from PET/CT, can be used to quantify the response to treatment and can predict progression-free survival after the last treatment cycle in patients with relapsed or refractory DLBCL treated with bendamustine–rituximab.
Keywords: [F-18] fluorodeoxyglucose, diffuse large B-cell lymphoma, lymphoma, metabolic tumor burden, positron emission tomography/computed tomography
Diffuse large B-cell lymphoma, a major aggressive mature B-cell lymphoma, is often refractory and the cause of high mortality. Recent studies have indicated a favorable response and the safety of bendamustine in combination with rituximab (a cytotoxic agent with alkylator) in relapsed or refractory patients with DLBCL.1,2
Sequential PET/CT using 18F-FDG is a sensitive method for evaluating response to therapy in patients with lymphoma.3 The finding that interim PET/CT is more accurate than CT alone highlights the potential use of interim PET/CT in “response-adapted” treatment strategies, where the treatment can be tailored (escalated or de-escalated) according to the individual's response to chemotherapy. As the treatment course is not complete in the setting of interim PET/CT, the emphasis is on characterizing the response as either positive or negative,4 which is not ideal because therapeutic changes in tumors occur on a continuum. Thus, it is becoming increasingly desirable to use continuous criteria to grade the tumor response.
Several methods of quantitative assessment can predict treatment response or outcome. The SUV is most commonly used to give a semiquantitative measure of response, and ΔSUVmax is considered to be a significant prognostic indicator in DLBCL.5–7 The ΔSUVmax at baseline and after two or four cycles are predictive indicators of PFS in patients receiving rituximab, whereas visual assessment of PET is not a significant predictive indicator.8 Metabolic tumor burden can express not only intensity of FDG accumulation but extent in volumetry. Some investigators have reported the greater usefulness of metabolic tumor volume or total lesion glycolysis for response assessment, because these volumetric parameters reflect metabolic tumor burdens.9–15 In contrast, genetic analyses of malignant tumors indicate intratumoral heterogeneity between individual tumors.16 Recent studies showed that the quantitative assessment of intratumoral heterogeneity could be used to evaluate malignant tumors.17–19
The purpose of the present study was to clarify the feasibility of a PET/CT volumetric approach reflecting metabolic tumor burden for assessment of therapeutic response in patients with relapsed or refractory DLBCL.
Materials and Methods
Patient eligibility
The study group was based on the multicenter, open-label, single-arm, phase II clinical study. All patients have been previously described.2 This prior article dealt with the efficacy of bendamustine–rituximab therapy, whereas this report focuses on the prognostic significance of metabolic tumor burden assessed by 18F-FDG PET/CT. The criteria for eligibility were histologically confirmed DLBCL unresponsive to, or relapsed after, prior therapy in patients aged 20–75 years. The number of prior therapies ranged from one to three. Patients were required to have a measurable lesion >1.5 cm in one dimension. Adequate hematologic, renal, hepatic, respiratory, and cardiovascular functions were required. No carry-over effects of prior therapy were allowed, and a 3-week wash-out period was required. Patients who failed to obtain CR, CR unconfirmed, or PR in any prior treatment, or who had a prior history of allogeneic hematopoietic stem cell transplantation or radioimmunotherapy, uncontrolled diabetes, pregnancy, apparent infection, spread of the lymphoma to the central nervous system, or concomitant malignancy were not eligible according to the protocol. This study was carried out in accordance with the amended Helsinki Declaration and approved by the local ethics committees of all participating institutions in Japan and Korea after all the patients had provided their informed consent to participate.
Treatment
Bendamustine 120 mg/m2 was given on days 2 and 3 in combination with rituximab 375 mg/m2 on day 1 of every 21-day treatment cycle for up to six treatment cycles. Dose reductions were carried out and described previously.2 No dose escalation was allowed after a dose reduction, and no dose reduction of rituximab was required. The use of G-CSF was permitted during cycles 2–6, as well as during cycle 1 when grade ≥3 neutropenia was confirmed. When G-CSF was given, PET study was carried out at least 3 weeks after the last dosing of G-CSF.20
Positron emission tomography/computed tomography
A phantom study was carried out in accord with previously published recommendations and guidelines21–23 to establish the necessary and sufficient conditions of PET data acquisition for quality assurance prior to clinical study in all institutions. In all, 28 PET/CT machines (nine types of machine) were used for this study in 24 institutions (Japan, 17 institutions; Korea, 7 institutions). The European Association of Nuclear Medicine/National Electrical Manufactures Association's image quality phantom (NU 2-2001) was used for cross-calibration. Patients received an i.v. injection of 3.5–5.0 MBq/kg of 18F-FDG after at least 6 h of fasting, and the injection was followed by an uptake phase of 63 ± 8 min. The patients were then placed in a supine, arm-up position, immediately after urination. Data acquisition was carried out for each patient from the top of the skull to the mid-thigh. Although the published recommendations and guidelines give no recommendation regarding the SUV,21–23 we obtained values for 12 ROIs defined in the phantom background area to evaluate the accuracy with an allowance of 1.0 ± 0.1.24
Image interpretation
The PET and CT images in all standard planes were reviewed on a dedicated workstation (PETSTAT; AdIn Research, Tokyo, Japan). Images were analyzed by two board certified nuclear medicine physicians as the central review committee. When their interpretations were discrepant, the judgment of a third board certified nuclear medicine physician was sought. Largest diameter of the lesion with the greatest amount of 18F-FDG uptake was measured. The SPD within the lesion was assessed in up to six target lesions. The percentage reduction rates of SPD (ΔSPD) were also calculated. For the visual analysis, abnormal 18F-FDG uptake was defined as substantially greater activity than in the mediastinal blood pool on attenuation-corrected images. An ROI was outlined within areas of increased 18F-FDG uptake and measured on each slice. The SUVmax was calculated after correction based on body weight. The SUL was calculated for a maximal 1.2-cm diameter ROI located within the tumor.25 As an index of metabolic tumor burden, MTV was calculated by tumor uptake above a cut-off SUVmax >2.5 as a reference.13 Total lesion glycolysis (the response score)26 was also calculated as the product of the volume obtained by PET and the average SUV as a reference of metabolic tumor burden. Intratumoral heterogeneity of 18F-FDG uptake was assessed by estimating the AUC-CSH.18 The SUVmax, SUL, MTV, TLG, and AUC-CSH of the lesion with the greatest amount of 18F-FDG uptake were measured for each patient. The ΣMTV and ΣTLG for a maximum of six target lesions per patient were also calculated. Evaluation of the metabolic response was accomplished by comparing the changes from baseline in ΔSUV, ΔSUL, ΔTLG, ΔMTV, ΔΣMTV, ΔΣTLG, and ΔAUC-CSH.
Tumor responses were assessed by PET/CT after two treatment cycles and after the last treatment cycle. Patients were classified based on the best tumor response according to the Lugano classification,4 which is visual five-point scale designed to reduce interobserver variability as: 1, no uptake; 2, uptake ≤mediastinum blood pool; 3, uptake ≤liver; 4, moderately increased uptake >liver; or 5, markedly increased uptake >liver and/or new lesions. A score of 1–3 was regarded as negative and 4 or 5 as positive. The PFS was calculated as the time from day 1 of the first cycle to either disease progression (including relapse and exacerbation), onset of another treatment, or death from any cause.
Statistical analysis
The sample size was calculated based on the expected and threshold ORRs of 45% and 25%, respectively, described previously.2 The ORR was calculated as the proportion of treated patients who achieved PR or better. Comparison of the means between groups was carried out using a three-way anova with Bonferroni's adjustment for multiple comparisons. The thresholds of PET/CT measurements to predict CR were determined by receiver operating characteristic analysis. The median PFS was estimated according to the Kaplan–Meier method, and 95% CIs were calculated using Greenwood's formula. The log–rank test was used to compare PFS between subgroups. Cox's proportional hazard model and the best subset selection method were used for multivariate analysis of factors related to PFS. A P-value < 0.05 was considered statistically significant. Statistical analysis was carried out using the PASW Statistics 19 software program (IBM SPSS, Chicago, IL, USA).
Results
After review of imaging data from 63 patients with relapsed or refractory DLBCL described previously,2 the quality of imaging data from 55 patients (41 Japanese and 14 Korean) were sufficient to be evaluated (Table1). Fifty-three patients (96%) received at least one cycle of rituximab-containing chemotherapy. The number of prior treatment cycles ranged from one to three. Eight patients (15%) had undergone autologous stem cell transplantation. In 51 patients (93%), targeted nodal lesions were identified in less than four nodal sites. Eight patients (15%) had bone marrow involvement at baseline. A high proportion of patients had low (36%) or low-intermediate (38%) IPI risk. The median number of cycles administered was four (range, 1–6).
Table 1.
Characteristics of patients with relapsed/refractory diffuse large B-cell lymphoma (n = 55) treated with bendamustine–rituximab
| Variables | n (%) |
|---|---|
| Gender | |
| Male | 22 (40) |
| Female | 33 (60) |
| Age, years | |
| <65 | 22 (40) |
| ≥65 | 33 (60) |
| Clinical stage (Ann Arbor staging) | |
| I | 3 (5) |
| I-E | 2 (4) |
| II | 16 (29) |
| II-E | 2 (4) |
| III | 14 (25) |
| III-E | 4 (7) |
| III-S | 2 (4) |
| IV | 13 (24) |
| B symptoms | |
| Yes | 6 (11) |
| No | 49 (89) |
| Prior medication | |
| Yes | 50 (91) |
| No | 5 (9) |
| Prior ASCT | |
| Yes | 8 (15) |
| No | 47 (55) |
| No. of regimens | |
| 1 | 37 (67) |
| 2 | 12 (22) |
| 3 | 6 (11) |
| Performance status | |
| 0 | 37 (67) |
| 1 | 18 (33) |
| LDH, U/L | |
| <240 | 26 (47) |
| ≥240 | 29 (53) |
| Nodal sites | |
| <4 nodular sites | 51 (93) |
| ≥4 nodular sites | 4 (7) |
| Extranodal sites | |
| <2 extranodular sites | 26 (47) |
| ≥2 extranodular sites | 29 (53) |
| Bone marrow involvement | |
| Positive | 8 (15) |
| Negative | 47 (85) |
| IPI risk category | |
| Low | 20 (36) |
| Low–intermediate | 21 (38) |
| High–intermediate | 10 (18) |
| High | 4 (7) |
ASCT, autologous stem cell transplantation; IPI, International Prognostic Index; LDH, lactate dehydrogenase.
The mean PET/CT parameters of all target lesions at baseline, two treatment cycles, and the last treatment cycle are listed in Table2. The mean LD (P = 0.002), SPD (P = 0.036), SUVmax (P = 0.004), SUL (P = 0.005), MTV (P = 0.001), ΣMTV (P < 0.0001), TLG (P = 0.001), and ΣTLG (P < 0.0001) were significantly lower in CR patients than in non-CR patients after two cycles of treatment by three-way anova with Bonferroni's adjustment. However, the mean AUC-CSH (P = 0.975) of both groups was similar after two cycles. Similarly, all PET/CT parameters (P < 0.0001–0.035) except for AUC-CSH (P = 0.413) were significantly lower in CR patients than in non-CR patients after the last cycle of treatment by three-way anova with Bonferroni's adjustment.
Table 2.
Absolute values of PET/CT parameters during treatment of patients with relapsed/refractory diffuse large B-cell lymphoma (n = 55) using bendamustine–rituximab
| Baseline | Two cycles | Last cycle | |
|---|---|---|---|
| LD, mm | 38.8 ± 3.5 | 31.8 ± 4.3 | 29.9 ± 5.5 |
| SPD, mm2 (×102) | 15.2 ± 3.1 | 14.9 ± 4.0 | 15.5 ± 6.2 |
| SUVmax, g/mL | 15.0 ± 1.1 | 6.3 ± 1.0 | 6.8 ± 1.2 |
| SUL, g/mL | 12.4 ± 1.1 | 5.3 ± 0.9 | 5.6 ± 1.0 |
| MTV, mm3 (×101) | 66.1 ± 14.5 | 34.7 ± 13.4 | 41.7 ± 10.9 |
| ΣMTV, mm3 (×101) | 105.8 ± 23.2 | 59.1 ± 22.7 | 75.1 ± 19.6 |
| TLG, g (×101) | 105.5 ± 23.2 | 61.4 ± 21.7 | 52.3 ± 16.3 |
| ΣTLG, g (×101) | 173.1 ± 38.1 | 108.7 ± 38.4 | 97.7 ± 30.4 |
| AUC-CSH (×10−1) | 5.2 ± 0.7 | 5.3 ± 0.8 | 5.3 ± 0.2 |
AUC-CSH, area under the curve of cumulative standardized uptake value (SUV)–volume histogram; LD, largest diameter; MTV, metabolic tumor volume; ΣMTV, sum of MTV for a maximum of six target lesions per patient; SPD, sum of products of the maximum perpendicular diameters; SUL, peak value of SUVmax corrected for the lean body mass; SUVmax, maximum SUV; TLG, total lesion glycolysis; ΣTLG, sum of TLG for a maximum of six target lesions per patient.
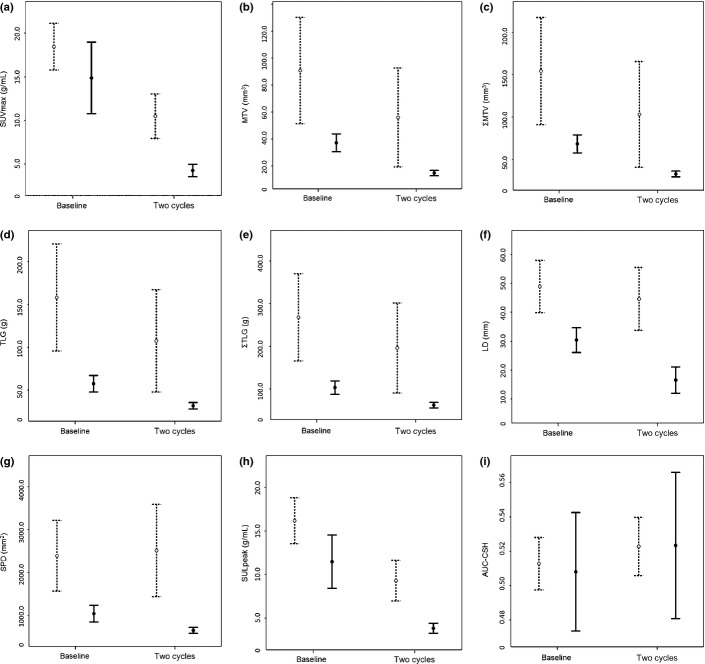
The response after two cycles of therapy was complete in 15 patients (27%) and incomplete in 40 patients (73%) using the Lugano classification. The percent changes in the PET/CT parameters ΔSUV02 (P = 0.023), ΔSUL02 (P = 0.024), ΔMTV02 (P = 0.005), ΔΣMTV02 (P = 0.002), ΔTLG02 (P = 0.004), and ΔΣTLG02 (P = 0.001), but not ΔAUC-CSH02 (P = 0.674), were significantly greater in the CR group than non-CR group after two treatment cycles by three-way anova with Bonferroni's adjustment (Fig.1).
Fig 1.
Absolute values of PET/CT parameters in complete response (CR, solid bar) and non-CR (dashed bar) groups after two cycles of treatment assessed according to the Lugano classification. Maximum standardized uptake value (SUVmax, g/mL) (a), metabolic tumor volume (MTV, mm3) (b), sum of MTV for a maximum of six target lesions per patient (ΣMTV, mm3) (c), total lesion glycolysis (TLG, g) (d), and sum of TLG for a maximum of six target lesions per patient (ΣTLG, g) (e) are shown. Large diameter (LD, mm) (f), sum of the products of the maximum perpendicular diameters (SPD mm2) (g), peak value of SUV corrected for lean body mass (SULpeak, g/mL) (h), and the area under the curve of cumulative SUV–volume histogram (AUC-CSH) (i) are shown. The absolute values of all PET/CT parameters except for AUC-CSH did not overlap between baseline and after two cycles in the CR group.
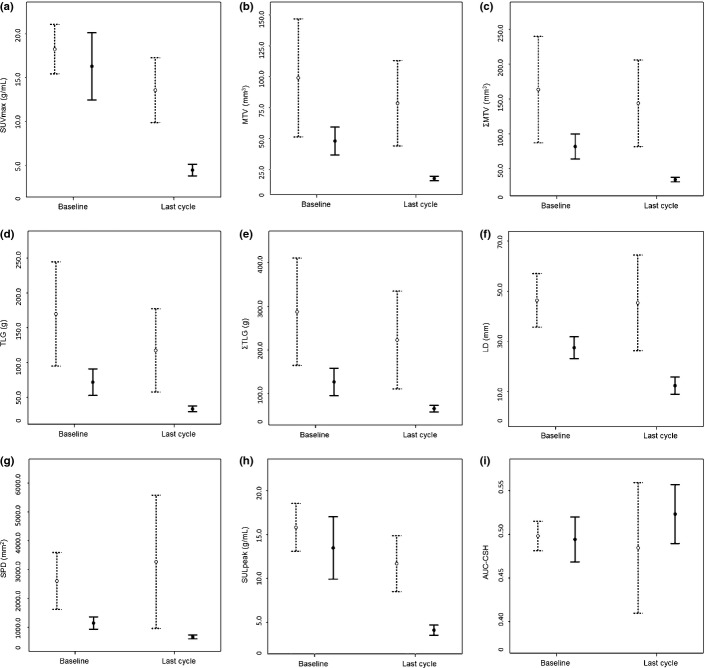
After the last cycle of therapy, evaluation by the Lugano classification revealed complete response in 32 patients (58%) and incomplete response in 23 patients (42%). The percent changes in the PET/CT parameters ΔSUV0L (P = 0.0015), ΔSUL0L (P = 0.003), ΔMTV0L (P = 0.001), ΔΣMTV0L (P < 0.0001), ΔTLG0L (P < 0.0001), and ΔΣTLG0L (P < 0.0001), but not ΔAUC-CSH0L (P = 0.267), were significantly greater in the CR group than the non-CR group RC by three-way anova with Bonferroni's adjustment (Fig.2).
Fig 2.
Absolute values of PET/CT parameters in the complete response (CR, solid bar) and non-CR (dash bar) groups after the last cycle of treatment assessed according to the Lugano classification. Maximum standardized uptake value (SUVmax, g/mL) (a), metabolic tumor volume (MTV, mm3) (b), sum of MTV for a maximum of six target lesions per patient (ΣMTV, mm3) (c), total lesion glycolysis (TLG, g) (d), and sum of TLG for a maximum of six target lesions per patient (ΣTLG, g) (e) are shown. Large diameter (LD, mm) (f), sum of the products of the maximum perpendicular diameters (SPD, mm2) (g), peak value of SUV corrected for lean body mass (SULpeak, g/mL) (h), and the area under the curve of cumulative SUV–volume histogram (AUC-CSH) (i) are shown. The absolute values of all PET/CT parameters except for AUC-CSH did not overlap between baseline and after the last cycles in the CR group.
The performance of percent change in PET/CT measurements for predicting CR after two cycles and the last cycle of therapy is summarized in Table3. The ΔΣTLG02 and ΔΣTLG0L showed the highest sensitivity and specificity for predicting CR, respectively. However, the P-values of ΔΣMTV02 and ΔΣTLG02 showed marginal significance because of the small patient population.
Table 3.
Diagnostic accuracy of PET/CT parameters to discriminate between complete response and non-complete response groups of patients with relapsed/refractory diffuse large B-cell lymphoma (n = 55) after two cycles and the last cycle of treatment with bendamustine–rituximab
| Threshold, % | AUC | P-value | Sensitivity, % | Specificity, % | |
|---|---|---|---|---|---|
| Baseline – two cycles | |||||
| ΔSUV02 | 66.0 | 0.770 | 0.0230 | 80.0 | 60.0 |
| ΔSUL02 | 65.0 | 0.700 | 0.0300 | 80.0 | 60.0 |
| ΔMTV02 | 68.0 | 0.776 | 0.0400 | 73.3 | 62.0 |
| ΔΣMTV02 | 66.0 | 0.867 | 0.0490 | 73.3 | 60.0 |
| ΔTLG02 | 68.0 | 0.784 | 0.0300 | 74.6 | 63.8 |
| ΔΣTLG02 | 67.0 | 0.875 | 0.0470 | 80.0 | 65.0 |
| ΔAUC-CSH02 | −3.7 | 0.419 | 0.3590 | 53.3 | 55.0 |
| Baseline – last cycle | |||||
| ΔSUV0L | 75.0 | 0.728 | 0.0010 | 73.8 | 67.8 |
| ΔSUL0L | 70.0 | 0.697 | 0.0140 | 72.7 | 66.7 |
| ΔMTV0L | 65.0 | 0.758 | 0.0010 | 80.1 | 69.7 |
| ΔΣMTV0L | 61.0 | 0.865 | 0.0010 | 77.3 | 75.8 |
| ΔTLG0L | 67.0 | 0.765 | 0.0010 | 77.3 | 75.8 |
| ΔΣTLG0L | 66.0 | 0.878 | <0.0001 | 81.8 | 75.8 |
| ΔAUC-CSH0L | −6.0 | 0.591 | 0.2570 | 63.6 | 54.5 |
AUC, area under the curve; Δ, percentage change; 02, from baseline to after 2 cycle; 0L, from baseline to after last cycle; SUV, maximum standardized uptake value; SUL, standardized uptake value corrected by lean body mass; MTV, metabolic tumor volume; ΣMTV, sum of MTV for a maximum of 6 target lesions per patient; TLG, total lesion glycolysis; ΣTLG, sum of TLG for a maximum of 6 target lesions per patient; AUC-CSH, area under the curve of cumulative SUV-volume histogram.
The ORR, CR rate, PR rate, stable disease rate, and progressive disease rate were 58.2% (95% CI, 45.2–71.2%), 41.8% (95% CI, 28.8–54.8%), 16.4% (95% CI, 6.6–26.2%), 12.7% (95% CI, 3.9–21.5%), and 29.1% (95% CI, 17.1–41.1%), respectively. The median PFS was 155 days (range, 20–576 days). After a median follow-up of 185 days (range, 19–575 days), disease progression was observed in 31 patients (56.4%; 95% CI, 43.3–69.5%). The median PFS was achieved. The estimated PFS rate at 1 year was 39.2% (95% CI, 26.3–52.1%) in all patients.
Univariate analyses of potential prognostic factors showed an association of PFS with B symptoms, prior medication, ECOG performance status, nodal sites, IPI risk category, the Lugano classification, and all percent changes in PET/CT parameters except for ΔAUC-CSH0L (Table S1). Gender, age, clinical stage, prior autologous stem cell transplantation, number of regimens, serum lactate dehydrogenase, extranodal sites, and bone marrow involvement lacked predictive value. An analysis of factors related to disease progression was carried out using a Cox proportional hazard model and the best subset selection method. In order of relative risk, ΔΣTLG0L was identified as an independent predictor of PFS (threshold 66.0%; relative risk, 5.24; 95% CI, 1.76–15.60; P = 0.003) (Fig.3). Other percent changes in PET/CT measurements were not identified as independent predictors.
Fig 3.
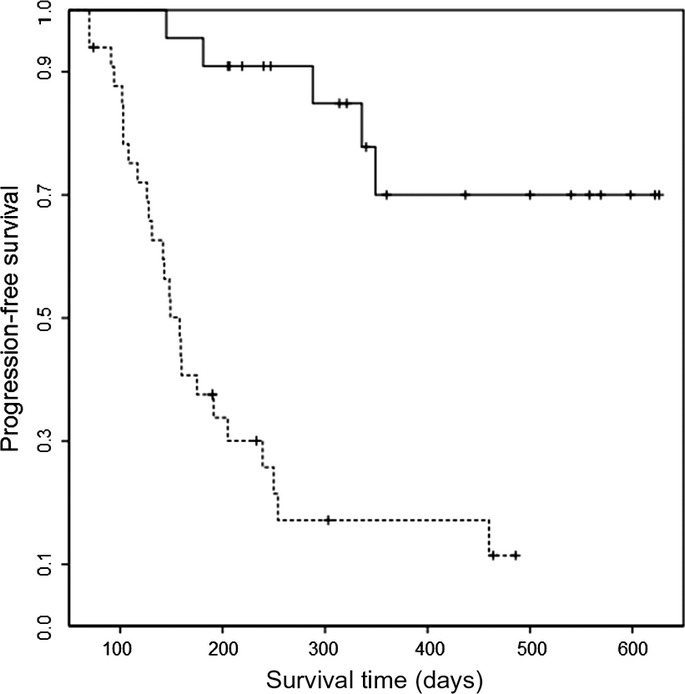
Kaplan–Meier curves of progression-free survival by the percentage change of the sum of total lesion glycolysis after the last cycle of treatment (cut-off, 66.0%; log–rank test, P < 0.0001). The solid line indicates the group showing ≥66.0%; the dotted line is the group showing >66.0%.
Discussion
In this study, we documented the feasibility of quantitating metabolic tumor burden with PET/CT to assess therapeutic response in patients with relapsed or refractory DLBCL. Although several studies have described the results of response evaluation based on visual score,3,4 ΔSUV,5–8 and volumetric measurements,9–15 direct comparisons between these indices have not been carried out in a single study. Univariate and multivariate analyses identified ΔΣTLG among the various PET/CT measurements as an independent predictor of PFS after the last treatment cycle. To the best of our knowledge, this is the first prospective multicenter study comparing various PET/CT measurements reflecting metabolic tumor burden for assessment of treatment response as well as application of the Lugano classification.
Semiquantification as a technique for interpreting PET/CT images has generally been used for analysis of malignant lymphomas. The semiquantification of PET images is useful in defining minimal uptake and a more objective way to interpret therapeutic response than visual analysis alone.27 Visual dichotomous assessment is subjective and occasionally difficult to make because FDG uptake is a continuous variable. Although scans can be semiquantitated by several PET/CT measurements, which of these measurements would be useful to the community physician is not clear.
The semiquantitative measure ΔSUV may be useful in response assessment. Investigators have reported the utility of ΔSUV on interim PET/CT for early response assessment.8,28 Although ΔSUV is a simple quantitative parameter and suitable for clinical application with appropriate standardization of PET/CT methodology, whether it can be used in early assessment of aggressive non-Hodgkin's lymphoma remains controversial. False-positive findings occasionally encountered on interim PET/CT in patients treated with rituximab may contribute to the controversy. Wahl et al.25 proposed the use of SULpeak (i.e., SUV corrected for lean body mass) for semiquantitation and to maintain quality control, which is a PERCIST1.0 criterion. However, ΔSUL may be more difficult to measure when tumor volume is decreased by treatment. Moreover, ΔSUL is mathematically equivalent to ΔSUV when the image is smoothed because SULpeak is defined at the hottest point in the tumor focus (1 cm3 spherical ROI).25
As tumor burden may help to define treatment response and clinical outcome, some investigators have evaluated PET/CT volumetric parameters as assessment measures.9–15 The MTV is a PET/CT volumetric parameter calculated by selecting a tumor with SUVmax >2.5 or using a threshold-based method with the liver or mediastinum as a reference.25 Several studies have reported that absolute baseline MTV of the most intensively labeled lesion is an independent predictor of PFS in DLBCL.12–14 Tseng et al.14 reported that the ratio of interim to baseline MTV is a prognostic index in Hodgkin's lymphoma. Although MTV could be a prognostic indicator for assessment of malignant lymphoma, the conclusion that MTV is an indicator is based on retrospective analysis of selected datasets. Prospective multicenter studies in homogenous populations receiving the same treatment will be required to determine whether MTV has any advantage over existing methods of visual analysis and ΔSUV.
Total lesion glycolysis (the volume obtained by PET images × the average SUV within the tumor) is another volumetric parameter. The ΔTLG (the ratio of the metabolic rate of the tumor at baseline to its metabolic rate after treatment) was originally called the Larson–Ginsberg Index. It corresponds to the change in cell mass of the target lesion and reflects the global response of the entire tumor to treatment.26 In a study of patients with DLBCL, ΔTLG was shown to be a strong predictor of survival.9 Importantly, other investigators showed that ΣTLG reflects treatment response in DLBCL and predicts PFS.15 However, similar to MTV, TLG will need to be compared to other preexisting PET/CT parameters in a prospective study with homogenous populations receiving the same treatment in order to clarify its prognostic significance. Univariate and multivariate analyses of our data extended the results of previous studies and showed that ΔΣTLG can predict PFS after the last treatment cycle.9,15
The accuracy of PET/CT measurements depends on technical and physiological factors that must be standardized for widespread application.30 In addition, investigators should be attentive to the need for scan parameter adjustment in advance of image acquisition because of variability among PET scanners.31 Therefore, we carried out a phantom study in all participating institutions to determine the optimal scan parameters appropriate for each institution prior to the clinical study according to routine use recommendations and guidelines.21–24,29–31
There is no known relationship between intratumoral FDG metabolic heterogeneity and treatment response in malignant lymphoma. In some studies, the segmentation, intensity–volume histograms, and AUC-CSH have been used to characterize intratumoral heterogeneity of tracer uptake.16–18 Watabe et al.32 reported that the mean AUC-CSH of lesions in 12 patients with malignant lymphoma was 0.60, but these lesions appeared homogeneous on visual analysis. Brooks and Grigsby suggested that inclusion of tumor volumes below 45 cm3 could bias comparisons of intratumoral uptake heterogeneity metrics derived from data from the current generation of whole-body PET scanners.19 The results of the current investigations would suggest that the AUC-CSH has limited power to discriminate between CR and non-CR groups in receiver operating characteristic analysis and is not significantly associated with PFS after two treatment cycles and the last treatment cycle. Although we did not assess the difference between nodal and extranodal target lesions in our AUC-CSH analysis, further study may be needed to confirm the clinical relevance of intratumoral heterogeneity in malignant lymphoma.
One of the potential criticisms of our study might be the lack of intra- and interobserver variability in our various PET/CT parameters. Some investigators have suggested that intra- and interobserver agreement between visual scores and PET/CT parameters should be assessed for accuracy and reproducibility.27,32 However, we carried out a phantom study prior to collecting patients' data for standardization of PET/CT acquisition. Assessment of variability should be used for quantitative assessment and accurate measurement depending on technical and physiological factors. The segmentation of the ROI for determining SUVmean and derived quantities such as TLG might be required for quantitation.
There are other potential limitations to our study. Our study focused on patients with DLBCL who relapsed after prior treatment and the data may not be extrapolated to untreated cases. Numerically, our study was relatively small (55 patients analyzed) and may not have been sufficiently powered to allow for statistical comparison of some of the covariates. Sample size might have also led to false positives on interim PET/CT, and false positive results should be taken into consideration during therapy.8
The phantom studies were carried out to qualify image quality, but we did not qualify the quantitativity of PET images for analysis of AUC-CSH. Further study is warranted to elucidate the feasibility of AUC-CSH in the clinical setting. Furthermore, we used three-way anova with Bonferroni's correction as the demonstrable method in this study population. However, we did not consider enough to be corrected only for this method.
In conclusion, the changes in metabolic tumor burden have prognostic implications in patients with relapsed or refractory DLBCL treated with bendamustine–rituximab therapy. Although further research is required to determine the role of ΔΣTLG as a surrogate marker for survival in this population, use of this measurement may facilitate prioritization of bendamustine–rituximab therapy for patients with relapsed or refractory DLBCL.
Acknowledgments
This work was supported in part by grants from Scientific Research Expenses for Health and Welfare Programs and a Grant-in-Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan. This work was also supported by Symbio Pharmaceuticals and Eisai Company.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- AUC-CSH
area under the curve of the cumulative SUV–volume histogram
- CI
confidence interval
- CR
complete response
- CT
computed tomography
- Δ
percentage change
- DLBCL
diffuse large B-cell lymphoma
- 18F-FDG
[F-18] 2-fluoro-2-deoxy-D-glucose
- G-CSF
granulocyte colony-stimulating factor
- IPI
International Prognostic Index
- LD
largest diameter
- MTV
metabolic tumor volume
- ORR
overall response rate
- PFS
progression-free survival
- PR
partial response
- ROI
region of interest
- Σ
sum of
- SPD
sum of the products of the maximum perpendicular diameters
- SUL
SUVmax corrected for lean body mass
- SUV
standardized uptake value
- SUVmax
maximum value of SUV
- TLG
total lesion glycolysis
Supporting Information
Additional supporting information may be found in the online version of this article:
Table S1. Results of univariate analyses in prediction of progression-free survival (PFS) after the last treatment cycle.
References
- Ogura M, Ando K, Taniwaki M, et al. Feasibility and pharmacokinetic study of bendamustine hydrochloride in combination with rituximab in relapsed or refractory aggressive B cell non-Hodgkin's lymphoma. Cancer Sci. 2011;102:1687–92. doi: 10.1111/j.1349-7006.2011.01994.x. [DOI] [PubMed] [Google Scholar]
- Ohmachi K, Niitsu N, Uchida T, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2013;31:2103–9. doi: 10.1200/JCO.2012.46.5203. [DOI] [PubMed] [Google Scholar]
- Itti E, Lin C, Dupuis J, Paone G, et al. Prognostic value of interim F-18 FDG PET in patients with diffuse large B-cell lymphoma: SUV-based assessment at 4 cycles of chemotherapy. J Nucl Med. 2009;50:527–33. doi: 10.2967/jnumed.108.057703. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and Non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;20:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzani PL, Gandolfi L, Broccoli A, et al. Midtreatment 18F-fluorodeoxyglucose positron-emission tomography in aggressive non-Hodgkin lymphoma. Cancer. 2011;117:1010–8. doi: 10.1002/cncr.25579. [DOI] [PubMed] [Google Scholar]
- Yang D-H, Min J-J, Song H-C, et al. Prognostic significance of interim (18)F-FDG PET/CT after three or four cycles of R-CHOP chemotherapy in the treatment of diffuse large B-cell lymphoma. Eur J Cancer. 2011;47:1312–8. doi: 10.1016/j.ejca.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Terasawa T, Lau J, Bardet S, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin's lymphoma and diffuse large B-cell lymphoma: a systematic review. J Clin Oncol. 2009;27:1906–14. doi: 10.1200/JCO.2008.16.0861. [DOI] [PubMed] [Google Scholar]
- Casasnovas RO, Meignan M, Berriolo-Riedinger A, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood. 2011;118:37–43. doi: 10.1182/blood-2010-12-327767. [DOI] [PubMed] [Google Scholar]
- Kim TM, Paeng JC, Chun IK, et al. Total lesion glycolysis in positron emission tomography is a bettwerpredictor of outcome than the International prognostic index for patients with diffuse large B cell lymphoma. Cancer. 2012;119:1195–202. doi: 10.1002/cncr.27855. [DOI] [PubMed] [Google Scholar]
- Berkowitz A, Basu S, Srinivas S, et al. Determination of whole-body metabolic burden as a quantitative measure of disease activity in lymphoma: a novel approach with fluorodeoxyglucose-PET. Nucl Med Commun. 2009;29:521–6. doi: 10.1097/MNM.0b013e3282f813a4. [DOI] [PubMed] [Google Scholar]
- Rossi M, Korkola P, Pertovaara H, et al. PET imaging in a longitudinal non-Hodgkin's lymphomastudy: association with tumor volume. Acta Radiol. 2011;52:995–1002. doi: 10.1258/ar.2011.110099. [DOI] [PubMed] [Google Scholar]
- Song MK, Chung JS, Shin HJ, et al. Prognostic value of metabolic tumor volume on PET/CT in primary gastrointestinal diffuse large B cell lymphoma. Cancer Sci. 2012;103:477–82. doi: 10.1111/j.1349-7006.2011.02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MK, Chung JS, Shin HJ, et al. Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol. 2012;91:697–703. doi: 10.1007/s00277-011-1357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng D, Rachakonda LP, Su Z, et al. Interim-treatment quantitative PET parameters predict prgression and death among patients with Hodgin's disease. Radiat Oncol. 2012;7:5. doi: 10.1186/1748-717X-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani SA, Heidari P, Halpern EF, et al. Baseline total lesion glycolysis measured with 18F-FDG PET/CT as a predictor of progression-free survival in diffuse large B cell lymphoma: a pilot study. Am J Nucl Med Mol Imaging. 2013;3:272–81. [PMC free article] [PubMed] [Google Scholar]
- Huang B, Chan T, Kwong DLW, et al. Nasopharyngeal carcinoma: investigation of intratumoral heterogeneity with FDG PET/CT. AJR Am J Roentogenol. 2012;199:169–74. doi: 10.2214/AJR.11.7336. [DOI] [PubMed] [Google Scholar]
- Vriens D, Disselhorst JA, Oyen WJ, et al. Quantitative assessment of heterogeneity in tumor metabolism using FDG-PET. Int J Radiat Oncol Biol Phys. 2012;82:e725–31. doi: 10.1016/j.ijrobp.2011.11.039. [DOI] [PubMed] [Google Scholar]
- van Velden FHP, Cheebsumon P, Yaqub M, et al. Evaluation of a cumulative SUV-volume histogram method for parameterizing heterogeneous intratumoral FDG uptake in non-small cell lung cancer PET studies. Eur J Nucl Med Mol Imaging. 2011;38:1636–47. doi: 10.1007/s00259-011-1845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks FJ, Grigsby PW. The effect of small tumor volumes on studies of intratumoral heterogeneity of tracer uptake. J Nucl Med. 2014;55:37–42. doi: 10.2967/jnumed.112.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka K, Hosono M, Usami K, et al. Fluorodeoxyglucose uptake in the bone marrow after granulocyte-stimulating factor administration in patients with non-Hodgkin's lymphoma. Nucl Med Commun. 2011;32:678–83. doi: 10.1097/MNM.0b013e328346b32a. [DOI] [PubMed] [Google Scholar]
- Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–66. [PubMed] [Google Scholar]
- Boellaard R, O'Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200. doi: 10.1007/s00259-009-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermann JS, Saffer JR, Karp JS, et al. Qualification of PET scanners for use in multicenter cancer clinical trials: the American College of Radiology Imaging Network experience. J Nucl Med. 2009;50:1187–93. doi: 10.2967/jnumed.108.057455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukukita H, Senda M, Terauchi T, et al. Japanese guideline for the oncology FDG-PET/CT data acquisition protocol: synopsis of Version 1.0. Ann Nucl Med. 2010;24:325–34. doi: 10.1007/s12149-010-0377-7. [DOI] [PubMed] [Google Scholar]
- Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S–50S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson SM, Erdi Y, Akhurst T, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging: the visual response score and the change in total lesion glycolysis. Clin Pos Imag. 1999;2:159–71. doi: 10.1016/s1095-0397(99)00016-3. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Hutching M, Rigacci L, et al. Early interm 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–52. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- Itti E, Meignan M, Berriolo-Riedinger A, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and ΔSUVmax. Eur J Nucl Med Mol Imaging. 2013;40:1312–20. doi: 10.1007/s00259-013-2435-6. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Delbeke D, Guiberteau MJ, et al. Concurrent PET/CT with an integrated imagingsystem: intersociety dialogue from the joint working group of the American College of Radiology, the Society of Nuclear Medicine, and the Society of Computed Body Tomography and Magnetic Resonance. J Nucl Med. 2005;46:1225–39. [PubMed] [Google Scholar]
- Horning SJ, Juweid ME, Schoder H, et al. Interim positron emission tomography scans in diffuse large B-cell lymphoma: an independent expert nuclear medicine evaluation of the Eastern Cooperative Oncology Group E3404 study. Blood. 2010;115:775–7. doi: 10.1182/blood-2009-08-234351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe T, Tatsumi M, Watabe H, et al. Intratumoral heterogeneity of F-18 FDG uptake differentiates between gastrointestinal stromal tumors and abdominal malignant lymphomas on PET/CT. Ann Nucl Med. 2012;26:222–7. doi: 10.1007/s12149-011-0562-3. [DOI] [PubMed] [Google Scholar]
- Biggi A, Gallamini A, Chauvie S, et al. International validation study for interim PET in ABVD-treated, advanced-stage Hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. J Nucl Med. 2013;54:683–90. doi: 10.2967/jnumed.112.110890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Results of univariate analyses in prediction of progression-free survival (PFS) after the last treatment cycle.